Abstract
A T cell response against myelin basic protein (MBP) is thought to contribute to the central nervous system (CNS) inflammation that occurs in the human demyelinating disease multiple sclerosis. To test whether MBP-reactive T cells that are normally retrieved from the circulation are capable of inducing CNS disease, MBP-reactive T cell clones were isolated from the peripheral blood of healthy, unimmunized Callithrix jacchus (C. jacchus) marmosets. This primate species is characterized by a natural chimerism of bone marrow elements between siblings that should make possible adoptive transfer of MBP-reactive T cells. We report that MBP-reactive T cell clones efficiently and reproducibly transfer CNS inflammatory disease between members of C. jacchus chimeric sets. The demyelination that is characteristic of experimental allergic encephalomyelitis induced in C. jacchus by immunization against human white matter did not occur after adoptive transfer of the MBP-reactive clones. It was noteworthy that encephalitogenic T cell clones were diverse in terms of their recognition of different epitopes of MBP, distinguishing the response in C. jacchus from that in some inbred rodents in which restricted recognition of MBP occurs. These findings are the first direct evidence that natural populations of circulating T cells directed against a CNS antigen can mediate an inflammatory autoimmune disease.
Full text
PDF

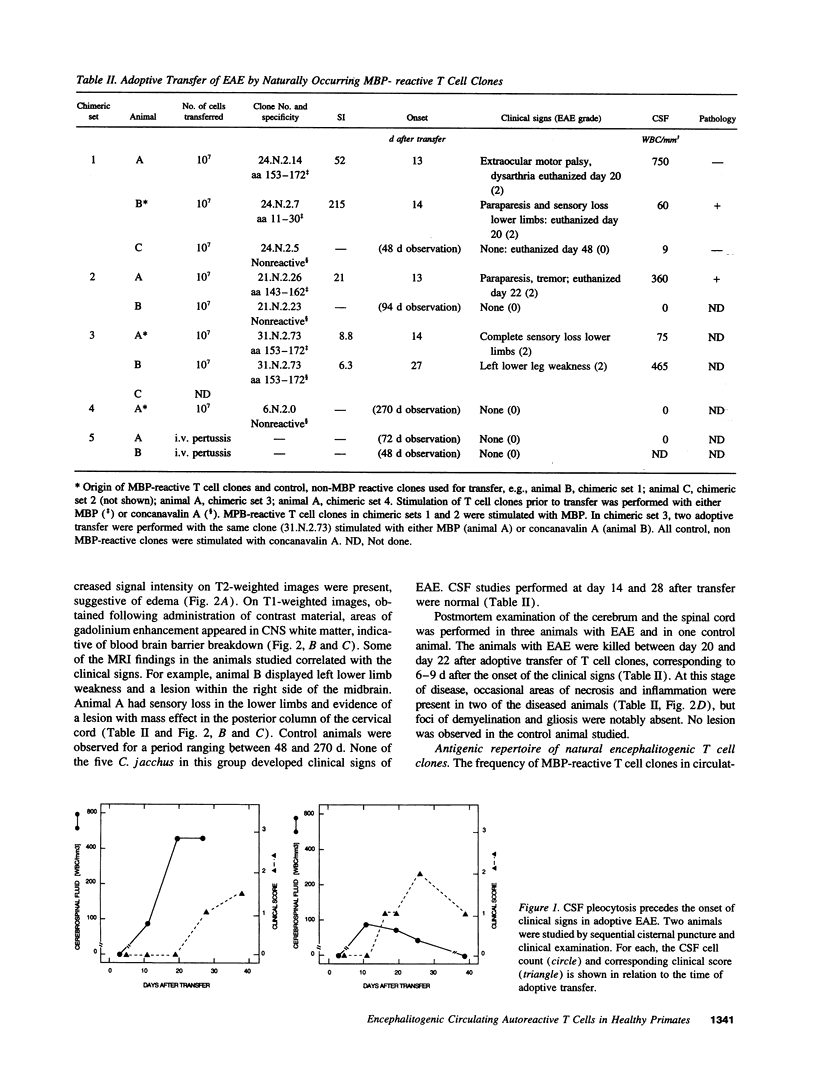
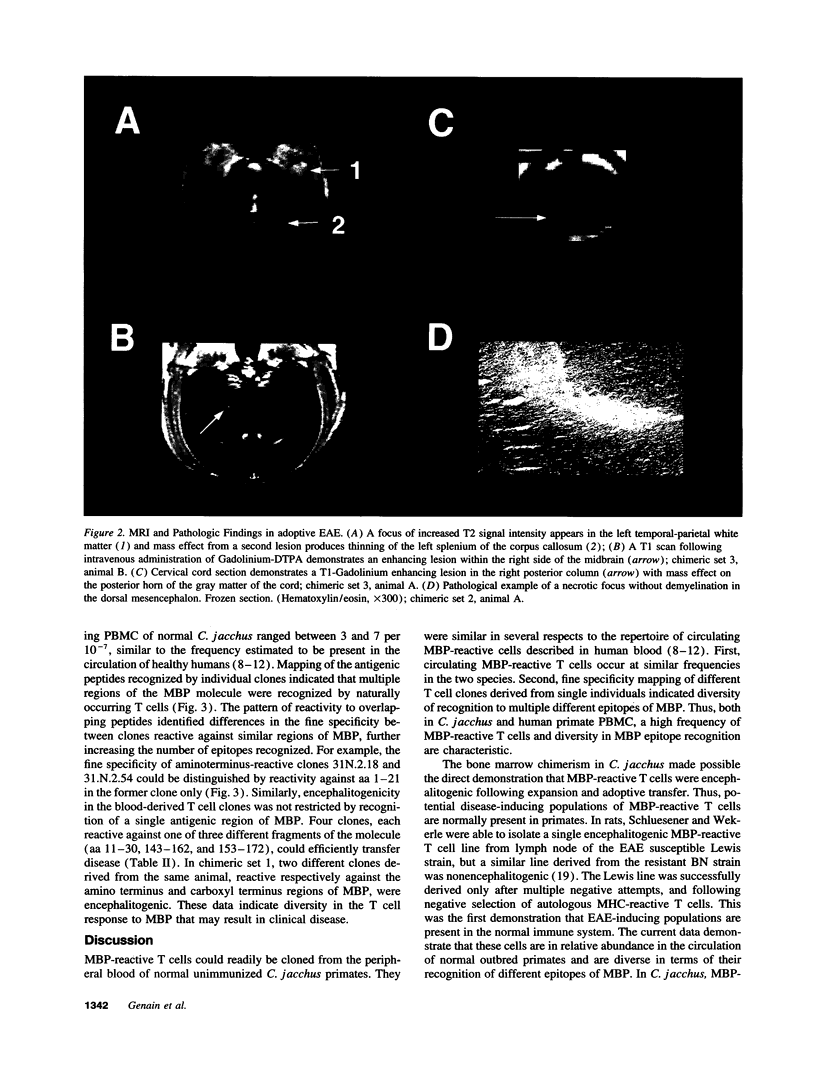



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegretta M., Nicklas J. A., Sriram S., Albertini R. J. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science. 1990 Feb 9;247(4943):718–721. doi: 10.1126/science.1689076. [DOI] [PubMed] [Google Scholar]
- Arnon R., Teitelbaum D. On the existence of suppressor cells. Int Arch Allergy Immunol. 1993;100(1):2–7. doi: 10.1159/000236379. [DOI] [PubMed] [Google Scholar]
- Brocke S., Gaur A., Piercy C., Gautam A., Gijbels K., Fathman C. G., Steinman L. Induction of relapsing paralysis in experimental autoimmune encephalomyelitis by bacterial superantigen. Nature. 1993 Oct 14;365(6447):642–644. doi: 10.1038/365642a0. [DOI] [PubMed] [Google Scholar]
- Brosnan C. F., Stoner G. L., Bloom B. R., Wisniewski H. M. Studies on demyelination by activated lymphocytes in the rabbit eye. II. Antibody-dependent cell-mediated demyelination. J Immunol. 1977 Jun;118(6):2103–2110. [PubMed] [Google Scholar]
- Chou Y. K., Vainiene M., Whitham R., Bourdette D., Chou C. H., Hashim G., Offner H., Vandenbark A. A. Response of human T lymphocyte lines to myelin basic protein: association of dominant epitopes with HLA class II restriction molecules. J Neurosci Res. 1989 Jun;23(2):207–216. doi: 10.1002/jnr.490230211. [DOI] [PubMed] [Google Scholar]
- Deibler G. E., Martenson R. E., Kies M. W. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2(2):139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- Goverman J., Woods A., Larson L., Weiner L. P., Hood L., Zaller D. M. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993 Feb 26;72(4):551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Raine C. S., Johnson A. B., Brosnan C. F., Bornstein M. B. Experimental allergic encephalomyelitis. Characterization of serum factors causing demyelination and swelling of myelin. J Neurol Sci. 1981 Apr;50(1):63–79. doi: 10.1016/0022-510x(81)90042-3. [DOI] [PubMed] [Google Scholar]
- Hao Q., Saida T., Nishimura M., Ozawa K., Saida K. Failure to transfer multiple sclerosis into severe combined immunodeficiency mice by mononuclear cells from CSF of patients. Neurology. 1994 Jan;44(1):163–165. doi: 10.1212/wnl.44.1.163. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Hsu B. L., Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991 Feb;28(2):254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- Jingwu Z., Medaer R., Hashim G. A., Chin Y., van den Berg-Loonen E., Raus J. C. Myelin basic protein-specific T lymphocytes in multiple sclerosis and controls: precursor frequency, fine specificity, and cytotoxicity. Ann Neurol. 1992 Sep;32(3):330–338. doi: 10.1002/ana.410320305. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Griffin D. E., Hirsch R. L., Wolinsky J. S., Roedenbeck S., Lindo de Soriano I., Vaisberg A. Measles encephalomyelitis--clinical and immunologic studies. N Engl J Med. 1984 Jan 19;310(3):137–141. doi: 10.1056/NEJM198401193100301. [DOI] [PubMed] [Google Scholar]
- Joshi N., Usuku K., Hauser S. L. The T-cell response to myelin basic protein in familial multiple sclerosis: diversity of fine specificity, restricting elements, and T-cell receptor usage. Ann Neurol. 1993 Sep;34(3):385–393. doi: 10.1002/ana.410340313. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Carlo D. J., Brostoff S. W., Eylar E. H. Allergic encephalomyelitis. Isolation of an encephalitogenic peptide active in the monkey. J Biol Chem. 1975 Mar 10;250(5):1718–1722. [PubMed] [Google Scholar]
- Kibler R. F., Fritz R. B., Chou F., Jen Chou C-H, Peacocke N. Y., Brown N. M., McFarlin D. E. Immune response of Lewis rats to peptide C1 (residues 68-88) of guinea pig and rat myelin basic proteins. J Exp Med. 1977 Nov 1;146(5):1323–1331. doi: 10.1084/jem.146.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Sercarz E. E. The involvement of T cell receptor peptide-specific regulatory CD4+ T cells in recovery from antigen-induced autoimmune disease. J Exp Med. 1993 Sep 1;178(3):909–916. doi: 10.1084/jem.178.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H., Brunner C., Bradl M., Linington C. Experimental allergic encephalomyelitis: the balance between encephalitogenic T lymphocytes and demyelinating antibodies determines size and structure of demyelinated lesions. Acta Neuropathol. 1988;75(6):566–576. doi: 10.1007/BF00686201. [DOI] [PubMed] [Google Scholar]
- Liblau R., Tournier-Lasserve E., Maciazek J., Dumas G., Siffert O., Hashim G., Bach M. A. T cell response to myelin basic protein epitopes in multiple sclerosis patients and healthy subjects. Eur J Immunol. 1991 Jun;21(6):1391–1395. doi: 10.1002/eji.1830210610. [DOI] [PubMed] [Google Scholar]
- Lipton H. L. Transfer of multiple sclerosis into severe combined immunodeficiency mice. Ann Neurol. 1993 Jul;34(1):115–116. doi: 10.1002/ana.410340123. [DOI] [PubMed] [Google Scholar]
- Lisak R. P., Behan P. O., Zweiman B., Shetty T. Cell-mediated immunity to myelin basic protein in acute disseminated encephalomyelitis. Neurology. 1974 Jun;24(6):560–564. doi: 10.1212/wnl.24.6.560. [DOI] [PubMed] [Google Scholar]
- Martin R., Jaraquemada D., Flerlage M., Richert J., Whitaker J., Long E. O., McFarlin D. E., McFarland H. F. Fine specificity and HLA restriction of myelin basic protein-specific cytotoxic T cell lines from multiple sclerosis patients and healthy individuals. J Immunol. 1990 Jul 15;145(2):540–548. [PubMed] [Google Scholar]
- Massacesi L., Joshi N., Lee-Parritz D., Rombos A., Letvin N. L., Hauser S. L. Experimental allergic encephalomyelitis in cynomolgus monkeys. Quantitation of T cell responses in peripheral blood. J Clin Invest. 1992 Aug;90(2):399–404. doi: 10.1172/JCI115874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., Zhang Z. J., Sobel R. A., al-Sabbagh A., Weiner H. L. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. VI. Suppression of adoptively transferred disease and differential effects of oral vs. intravenous tolerization. J Neuroimmunol. 1993 Jul;46(1-2):73–82. doi: 10.1016/0165-5728(93)90235-q. [DOI] [PubMed] [Google Scholar]
- Oksenberg J. R., Panzara M. A., Begovich A. B., Mitchell D., Erlich H. A., Murray R. S., Shimonkevitz R., Sherritt M., Rothbard J., Bernard C. C. Selection for T-cell receptor V beta-D beta-J beta gene rearrangements with specificity for a myelin basic protein peptide in brain lesions of multiple sclerosis. Nature. 1993 Mar 4;362(6415):68–70. doi: 10.1038/362068a0. [DOI] [PubMed] [Google Scholar]
- Olsson T., Zhi W. W., Höjeberg B., Kostulas V., Jiang Y. P., Anderson G., Ekre H. P., Link H. Autoreactive T lymphocytes in multiple sclerosis determined by antigen-induced secretion of interferon-gamma. J Clin Invest. 1990 Sep;86(3):981–985. doi: 10.1172/JCI114800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota K., Matsui M., Milford E. L., Mackin G. A., Weiner H. L., Hafler D. A. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990 Jul 12;346(6280):183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- PATERSON P. Y. Transfer of allergic encephalomyelitis in rats by means of lymph node cells. J Exp Med. 1960 Jan 1;111:119–136. doi: 10.1084/jem.111.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panitch H. S., McFarlin D. E. Experimental allergic encephalomyelitis: enhancement of cell-mediated transfer by concanavalin A. J Immunol. 1977 Sep;119(3):1134–1137. [PubMed] [Google Scholar]
- Pette M., Fujita K., Wilkinson D., Altmann D. M., Trowsdale J., Giegerich G., Hinkkanen A., Epplen J. T., Kappos L., Wekerle H. Myelin autoreactivity in multiple sclerosis: recognition of myelin basic protein in the context of HLA-DR2 products by T lymphocytes of multiple-sclerosis patients and healthy donors. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7968–7972. doi: 10.1073/pnas.87.20.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinelli C. B., McFarlin D. E. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2- T lymphocytes. J Immunol. 1981 Oct;127(4):1420–1423. [PubMed] [Google Scholar]
- Picus J., Aldrich W. R., Letvin N. L. A naturally occurring bone-marrow-chimeric primate. I. Integrity of its immune system. Transplantation. 1985 Mar;39(3):297–303. doi: 10.1097/00007890-198503000-00018. [DOI] [PubMed] [Google Scholar]
- Saeki Y., Mima T., Sakoda S., Fujimura H., Arita N., Nomura T., Kishimoto T. Transfer of multiple sclerosis into severe combined immunodeficiency mice by mononuclear cells from cerebrospinal fluid of the patients. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6157–6161. doi: 10.1073/pnas.89.13.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluesener H. J., Wekerle H. Autoaggressive T lymphocyte lines recognizing the encephalitogenic region of myelin basic protein: in vitro selection from unprimed rat T lymphocyte populations. J Immunol. 1985 Nov;135(5):3128–3133. [PubMed] [Google Scholar]
- Stone S. H. Transfedotr of Allergic Encephalomyelitis by Lymph Node Cells in Inbred Guinea Pigs. Science. 1961 Sep 1;134(3479):619–620. doi: 10.1126/science.134.3479.619. [DOI] [PubMed] [Google Scholar]
- Willenborg D. O., Prowse S. J. Immunoglobulin-deficient rats fail to develop experimental allergic encephalomyelitis. J Neuroimmunol. 1983 Oct;5(2):99–109. doi: 10.1016/0165-5728(83)90001-2. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., Mitchell D. J., Moore A. C., Kitamura K., Steinman L., Rothbard J. B. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986 Nov 20;324(6094):258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]




