Abstract
Venom of the yellow sac spider Cheiracanthium punctorium (Miturgidae) was found unique in terms of molecular composition. Its principal toxic component CpTx 1 (15.1 kDa) was purified, and its full amino acid sequence (134 residues) was established by protein chemistry and mass spectrometry techniques. CpTx 1 represents a novel class of spider toxin with modular architecture. It consists of two different yet homologous domains (modules) each containing a putative inhibitor cystine knot motif, characteristic of the widespread single domain spider neurotoxins. Venom gland cDNA sequencing provided precursor protein (prepropeptide) structures of three CpTx 1 isoforms (a–c) that differ by single residue substitutions. The toxin possesses potent insecticidal (paralytic and lethal), cytotoxic, and membrane-damaging activities. In both fly and frog neuromuscular preparations, it causes stable and irreversible depolarization of muscle fibers leading to contracture. This effect appears to be receptor-independent and is inhibited by high concentrations of divalent cations. CpTx 1 lyses cell membranes, as visualized by confocal microscopy, and destabilizes artificial membranes in a manner reminiscent of other membrane-active peptides by causing numerous defects of variable conductance and leading to bilayer rupture. The newly discovered class of modular polypeptides enhances our knowledge of the toxin universe.
Keywords: Confocal Microscopy, Peptide Biosynthesis, Protein Chemistry, Protein Purification, Protein Structure, Toxins, Electrophysiology, Membrane-active Peptide, Spider Venom, cDNA Sequence
Introduction
With over 40,000 species described to date (1), spiders represent one of the most diverse and successful groups of living organisms. Initial studies of spider venoms focused on those few dangerous to human health or threatening domestic animals (2). These include the widow spiders from the genus Latrodectus (Theridiidae) (3), the recluse spiders from the genus Loxosceles (Sicariidae) (4), the wandering spiders from the genus Phoneutria (Ctenidae) (5), and the Australian funnel-web spiders from the genera Atrax and Hadronyche (Hexathelidae) (6). Today, however, spider venoms attract more research because they represent vast evolutionary-edited natural pharmacopoeias (7). These can be sources of indispensable tools for fundamental studies. For instance, ω-agatoxins from Agelenopsis aperta and hanatoxins from Grammostola rosea are used ubiquitously as specific ligands of voltage-gated calcium and potassium channels, respectively (8, 9). At the very same time, spider toxins that specifically recognize different targets may serve as leads for drug and insecticide discovery and thus find application in medicine and green biotechnology (10–13).
Spider venoms are complex mixtures of compounds of diverse chemical nature varying from inorganic salts to high molecular weight proteins (7, 14). However, preference is usually given to a certain chemical framework, and the major active constituents of spider venoms currently known can be allocated into four large structural groups. (a) Acylpolyamine toxins that block ionotropic receptors and ion channels with average low specificity are produced by several families of spiders, most notably the orb weavers (Araneidae), but also Nephilidae and Agelenidae (15, 16). (b) Linear cytolytic peptides were found in venoms of spiders from the superfamily Lycosoidea (including Lycosidae, Ctenidae, and Oxyopidae) (17). More recently, Lachesana tarabaevi (Zodariidae) has been shown to be a unique “expert” in this type of toxin (18, 19). Most of these linear peptides are disordered in solution but form α-helices upon contact with membranes. (c) Disulfide-containing neurotoxic peptides are the most typical molecules found in spider venoms (7, 11, 14, 20). These peptides adopt compact folds with the inhibitor cystine knot (ICK)2 motif being characteristic of the majority of spider toxins (21). (d) Large proteins are usually less abundant with the exception of the neurotoxic latrotoxins from the widow spiders (3) and the necrotizing phospholipases D (sphingomyelinases D) from the recluse spiders (4).
Yellow sac spiders from the genus Cheiracanthium (Miturgidae), including the species Cheiracanthium punctorium common to Europe and Central Asia, have been erroneously suspected as inflicting skin lesions in humans similar to Loxosceles spp. In Europe in particular, C. punctorium is believed to be one of the few dangerous spider species. However, a systematic inspection of all available testimonies has proven these spiders virtually innocuous with bites causing local pain and sometimes swelling resembling bee or wasp stings with more severe effects occurring very rarely (22, 23). The venom of Cheiracanthium spiders causes cytolytic effects. It lyses erythrocytes, but its venom does not contain sphingomyelinase D, as do Loxosceles spp., whereas phospholipase A2 has been suspected to represent its active cytolytic component similar to other venomous animals, such as bees and snakes (24). In this study, we report the structure and activity of the major polypeptide toxin CpTx 1 from the venom of C. punctorium. This toxin is very unusual compared with all known animal toxins and includes two modules, each probably corresponding to a single domain ICK-type spider toxin. CpTx 1 was found to exhibit membrane-damaging and cytolytic effects, in sharp contrast to most other described disulfide-containing spider venom peptides that cause neurotoxicity.
EXPERIMENTAL PROCEDURES
Our detailed protocols on the general scheme for spider venom separation, toxin purifications, sequencing, and characterization can be found elsewhere (25).
CpTx 1 Isolation
Crude venom of the spider C. punctorium was purchased from Fauna Laboratories, Ltd. (Republic of Kazakhstan) in the form of lyophilized powder. Venom (∼5 mg corresponding to 25 μl of liquid venom) was redissolved in 100 μl of 10% acetonitrile in 0.1% aqueous trifluoroacetic acid (TFA) and fractionated on a TSK 2000SW size-exclusion chromatography (SEC) column (7.5 × 600 mm, 12.5-nm pore size, 10-μm particle size; Toyo Soda Manufacturing Co., Tokyo, Japan) equipped with a guard column (7.5 × 75 mm) in the same solvent at a flow rate of 1 ml/min. Fraction corresponding to the molecular mass range of 7–45 kDa was further separated by reverse-phase high performance liquid chromatography (RP-HPLC) on a Jupiter C5 column (4.6 × 150 mm, 30-nm pore size, 5-μm particle size; Phenomenex) using a linear gradient of acetonitrile concentration (0–20% in 10 min, 20–60% in 60 min, and 60–80% in 10 min) in 0.1% TFA at a flow rate of 1 ml/min. At each stage, eluate absorbance was monitored at 210 and 280 nm.
Mass Spectrometry
Samples were analyzed by matrix-assisted laser desorption/ionization (MALDI) time-of-flight (TOF) mass spectrometry (MS) as described previously (19). M@LDI-LR (Micromass UK Ltd.) and Ultraflex TOF-TOF (Bruker Daltonik GmbH, Bremen, Germany) spectrometers were used. Tandem mass spectrometric (MS/MS) analysis was performed on the latter instrument.
Amino Acid Sequence Analysis
N-terminal sequencing was carried out by automated stepwise Edman degradation using a Procise model 492 protein sequencer (Applied Biosystems) according to the manufacturer's protocol.
Reduction of Disulfide Bonds and Modification of Thiol Groups
Purified CpTx 1 (∼10 nmol) was dried and redissolved in 40 μl of 0.5 m Tris-HCl (pH 8.5), 6 m guanidine hydrochloride, and 3 mm EDTA. A 150-fold molar excess of 1,4-dithiothreitol was added to the mixture. The probe was sealed and incubated at 40 °C for 18 h. Then 4-vinylpyridine (a 3-fold molar excess with respect to dithiothreitol) was added. The tube was left at room temperature for 15 min in the dark. The modified polypeptide was purified by RP-HPLC on a Jupiter C5 column (4.6 × 150 mm).
Cyanogen Bromide Cleavage
Reduced-alkylated CpTx 1 (∼1 nmol) was dried and redissolved in 80% TFA to a concentration of ∼0.5 μg/μl. Cyanogen bromide was added with a ∼100-fold molar excess, and the sample was incubated overnight at room temperature in the dark. Then 10 volumes of cool water were added, and the sample was dried and redissolved in 0.1% TFA. The mixture was analyzed directly by MALDI-MS, and the products were separated by means of RP-HPLC on a Jupiter C5 column (2 × 150 mm, 30-nm pore size, 5-μm particle size; Phenomenex) at a flow rate of 0.3 ml/min.
Endoproteinase Glu-C and Endoproteinase Arg-C Digestion
Native unreduced CpTx 1 (∼1 nmol) was subjected to proteolysis by endoproteinase Glu-C (0.3 μg; Sigma) in 50 mm ammonium bicarbonate (15 μl) for 1 h at 37 °C. Exhaustive digestion of reduced-alkylated CpTx 1 (∼4 nmol) with endoproteinase Glu-C (2 μg) was performed in the same solvent (50 μl) for 4 h at 37 °C. The same quantity of reduced-alkylated CpTx 1 (∼4 nmol) was also digested by endoproteinase Arg-C (0.4 μg; Sigma) in the same solvent (50 μl) at room temperature overnight. Peptide fragments were separated on a Jupiter C5 column (2 × 150 mm).
cDNA Library Construction, Sequencing, and Analysis
All steps were carried out in collaboration with DuPont Agriculture and Nutrition (Newark, DE) as described previously (26). Briefly, venom glands of C. punctorium were harvested and homogenized in liquid nitrogen, and cell lysis was induced by TRIzol (Invitrogen). Poly(A)+ RNA was purified on an oligo(dT)-cellulose affinity column using the mRNA purification kit (Amersham Biosciences). First strand (using Superscript II from Invitrogen) and second strand cDNA synthesis, linker addition, cloning into pBlueScript SK+ (EcoRI and XhoI sites) were all carried out according to the Stratagene cDNA kit; cDNA was purified using a cDNA column (Invitrogen). Sequencing was performed using the M13 forward primer by the ABI PRISM BigDye Terminator Cycle Sequencing Ready reaction kit with AmpliTaq DNA polymerase, FS (PerkinElmer Life Sciences) on a model 373 DNA Sequencer (Applied Biosystems).
UV Absorbance Spectroscopy
Absorption spectra were recorded on a Hitachi U-3210 spectrophotometer (Tokyo, Japan). CpTx 1 concentration was determined using the molar extinction coefficient at 280 nm (ϵ280) of 17,460 m−1 cm−1 calculated by the GPMAW program (Lighthouse Data, Odense, Denmark).
Circular Dichroism
For bicelle preparation, 100 mm 1,2-dihexanoyl-sn-glycero-3-phosphocholine (DHPC) solution was prepared in phosphate-buffered saline (PBS: 1.47 mm KH2PO4, 4.29 mm Na2HPO4, 137 mm NaCl, 2.68 mm KCl). The appropriate amount of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) was dissolved in the DHPC solution to obtain a molar DMPC/DHPC ratio of 2:1. Bicelle formation was accelerated by heating the mixture up to 45 °C and cooling down to 0 °C three times. The sample for CD measurements was prepared by mixing equal parts of bicelle suspension and 20 μm CpTx 1 solution in PBS and incubating at 40 °C for 30 min. The sample for base-line correction was obtained using a probe of DMPC/DHPC bicelles diluted 2-fold with PBS.
CD spectra were recorded using a J-810 spectropolarimeter (Jasco, Tokyo, Japan) for 10 μm CpTx 1 in PBS and DMPC/DHPC bicelle suspension as described previously (19). Secondary structure content was analyzed with the CDSSTR program (27, 28).
Insecticidal Assay
Chromatographic fractions and purified CpTx 1 were assayed by injection into flesh fly Sarcophaga carnaria maggots as described previously (19).
Cytotoxic Assay
Spodoptera frugiperda (Sf9) cells were cultured at 27 °C in TC-100 medium (Invitrogen) supplemented with 10% fetal bovine serum (HyClone) and 2 mm l-glutamine (PanEco, Moscow, Russia). CpTx 1 at a concentration of 1 and 10 μm (in several repeats) was then added to the Sf9 cells (106 cells/ml), which were incubated with for 3 h (5% CO2, 27 °C) and stained with Hoechst 33342 (10 μm) and propidium iodide (PI; 10 μm) (29) for 10 min. The stained cells were examined with a FluoView FV1000 confocal microscope (Olympus, Hamburg, Germany) using 405 nm diode and 559 nm helium-neon lasers for excitation of Hoechst 33342 and PI, respectively, and a Plan Apo objective (10×/0.4 NA) to collect emission. Cells were classified (2000–3000 cells per point) as dead (Hoechst 33342(+), PI (+)) or viable (Hoechst 33342(+), PI (−)).
Plasma Membrane Permeabilizing Activity
Sf9 cells were cultured as described above. Prior to the experiment, the cell suspension (106 cells/ml) was placed in a 12-well flexiPERM silicon chamber (Perbio Science, Erembodegem-Aalst, Belgium) attached to a cover glass, and cells were allowed to settle onto the bottom of the wells. Markers of plasma membrane permeability 5(6)-carboxyfluorescein (CF) (30) and PI were added at concentration of 50 and 10 μm, respectively. CpTx 1 was added at 10 μm corresponding to 20% cytotoxic effect.
Confocal laser scanning microscopy was used to study CpTx 1-induced CF and PI entry into the cells. The silicon chamber was mounted on the stage of a FluoView FV1000 microscope. An UPLSAPO (60×O/1.35 NA) objective (Olympus) was used to visualize cells. Excitation light was provided at a wavelength of 488 nm (for CF) and 559 nm (for PI) by argon-ion and helium-neon lasers, respectively. The beam splitter DM 405/488/559/635 was used. Fluorescence emission was collected in the range of 497–558 nm (CF) or 579–679 nm (PI). A transmitted light image was obtained using the 488 nm laser beam and recorded simultaneously with the fluorescent image. The scanning area size was 105 × 105 μm, and the pixel size was 0.066 × 0.066 μm. Pinhole diameter was 110 μm corresponding to the optical slice thickness of <0.8 μm. Sequential mode of scanning was used to collect CF and PI fluorescence unmixed.
Insect Neuromuscular Preparations
Late third stage larvae of Calliphora vicina were used in all experiments (31, 32). After dissection, the internal organs were removed so that the preparation consisted only of muscles attached to the cuticle. The ventral ganglion was removed, and the segmental nerves were stimulated through the suction electrode. Recordings of membrane potential were made by glass intracellular microelectrodes from ventral longitudinal fibers. To eliminate electrical contacts of the recorded fiber with its neighbors, the latter were dissected. This resulted in a significant increase of input resistance in the recorded cell. The preparation was perfused with saline as follows: 172 mm NaCl, 2.5 mm KCl, 0.5 mm CaCl2, 8 mm MgCl2, 2.4 mm NaHCO3, 0.3 mm NaH2PO4, 52 mm sucrose (pH 7.2). Experiments were performed at room temperature (22 °C). The excitatory postsynaptic currents were evoked by nerve stimulation and recorded by a conventional two-electrode voltage clamp using an Axoclamp-2B amplifier (Axon Instruments). The data were filtered at 2 kHz and stored on a PC.
A series of experiments was performed under voltage clamp conditions to evaluate the effect of CpTx 1 on muscle cell input resistance and the corresponding current. Holding potential was set at −60 mV and periodically (each 20 s) shifted by small hyperpolarizing steps (5-mV amplitude and 300-ms duration). Changes of input resistance were estimated from the amplitudes of current response to these steps.
Frog Neuromuscular Preparations
The following chemicals were used: amiloride, N-methyl-d-glucamine (NMDG) chloride, and ouabain (Sigma), and tetrodotoxin (TTX; Affinity Research Products). Experiments were performed on musculus sartorius neuromuscular preparations of the common frog Rana temporaria. The preparations were placed into a plastic chamber (1.5 ml) and superfused with solution of the following composition: 113 mm NaCl, 2.5 mm KCl, 0.6 mm CaCl2, 3 mm NaHCO3, 4 mm MgCl2 (pH 7.3). In some experiments, 113 mm NaCl was replaced with 116 mm NMDG, 232 mm sucrose, 232 mm NaCl, or a combination of 113 mm NaCl and 232 mm sucrose. Solution containing a certain concentration of CpTx 1 and 0.01% bovine serum albumin (Sigma) was added to the bath. The temperature was kept at 22 °C. Glass microelectrodes filled with KCl had a resistance of 10–15 megohms. The resting membrane potential of muscle fibers was measured in 15–35 cells in control and after 30, 60, and 90 min of continuous perfusion with saline containing 50 nm of CpTx 1.
Planar Lipid Bilayer Membrane Assay
CpTx 1 was tested for the ability to cause conductance changes in planar bilayer membranes (BLM). Virtually solvent-free membranes were formed from 1,2-diphytanoyl-sn-glycero-3-phosphatidyl-choline (Avanti Polar Lipids) as described by Montal and Mueller (36). Two symmetrical chambers of a Teflon cell each with a solution volume of 1.5 ml were separated by a 15-μm-thick Teflon partition containing a round aperture of ∼100-μm diameter. Hexadecane was used for aperture pretreatment. BLM were formed from 0.1% lipid solution in pentane. The experimental cell was filled with 150 mm KCl solution buffered with 5 mm MOPS (pH 7.2). Electrical measurements were performed at room temperature (22 ± 2 °C) using a pair of Ag/AgCl electrodes connected to the cell chambers via 1.5% agarose bridges containing 2 m KCl. CpTx 1 was added to the cis-chamber 10 min after bilayer formation, and the resulting solution was mixed with a magnetic stirrer. Transmembrane currents were recorded with a custom-made amplifier and digitized with a pCLAMP-compatible board. Data collecting was performed with 2-kHz sampling frequency and low pass filtering at 200 Hz. Positive voltage means that the cis-chamber is positive with respect to the trans-chamber. Positive currents are those of cations flowing from the cis- to the trans-chamber. Clampfit 9.0 software (Molecular Devices) was used for data analysis, and Origin 7.0 (OriginLab) was used for statistical analyses.
RESULTS
Active Toxin Purification
Crude venom of the yellow sac spider C. punctorium (Miturgidae)3 was found to possess insecticidal (LD50 ∼3 μg/g in flesh fly larvae), hemolytic, cytotoxic, and membrane-damaging (33) activities. Our findings corroborate previous investigations of this and other Cheiracanthium species (24).
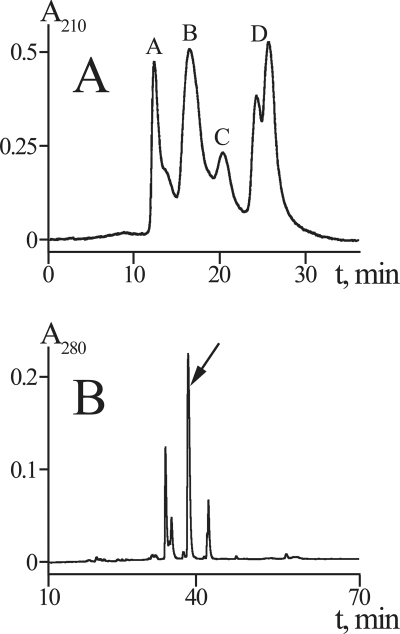
To isolate the active principle of C. punctorium venom, we used a classically simple yet effective approach that combined two methods of liquid chromatography, i.e. SEC and RP-HPLC (Fig. 1) (25). After SEC, the major insect toxicity resided in the two early eluting fractions (A, >30 kDa; B, 7–30 kDa). The high molecular weight fraction A contained several components with molecular masses of ∼30–60 kDa as estimated by electrophoresis (data not shown) and measured by MALDI-MS. In line with the previous report by Foradori et al. (24), we suspect that some of these components may exhibit phospholipase A2 activity. Fraction B contained several polypeptides with molecular masses of ∼15 kDa. RP-HPLC separation of this fraction yielded a number of peaks, the major of which were found toxic. According to analytical HPLC and MALDI-MS, the predominant peak (∼10% of crude venom protein) was represented by a single component (>95% purity) with a molecular mass of 15,100 Da that was named CpTx 1 (C. punctorium toxin 1).
FIGURE 1.
Isolation of CpTx 1. A, crude venom separation (using SEC) on a TSK 2000SW column. Pooled fractions (A–D) are labeled. B, second step chromatography (RP-HPLC) of the insecticidal fraction B on a Jupiter C5 column in a linear gradient of acetonitrile (see text for details). The major fraction corresponding to purified CpTx 1 is indicated by an arrow.
CpTx 1 Amino Acid Sequence
To determine the full amino acid sequence of the isolated toxin, we chose to follow the usual scheme of polypeptide structure analysis (25, 34). The idea was to assemble the full sequence from overlapping peptide fragments generated through various types of selective proteolysis.
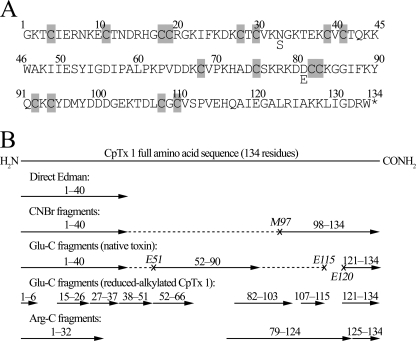
First, the presence of disulfide bonds was assessed as follows. CpTx 1 was reduced with dithiothreitol, and thiol groups were subsequently modified with 4-vinylpyridine. The molecular mass of the toxin increased to 16,800 Da, indicating the presence of 16 cysteine residues. Because modification of unreduced CpTx 1 failed to increase its molecular mass, all cysteine residues were concluded to form intramolecular disulfide bridges. Sequencing of the reduced-alkylated CpTx 1 by Edman degradation identified the first 40 N-terminal amino acid residues (cysteine residues were determined as S-pyridylethylated derivatives). Next, the following reactions of selective proteolysis were performed: (i) cyanogen bromide, (ii) endoproteinase Glu-C, (iii) endoproteinase Arg-C cleavage of the reduced-alkylated CpTx 1; and (iv) “limited” endoproteinase Glu-C digestion of the unreduced toxin. In each case, proteolytic fragments were separated by RP-HPLC; their masses were measured by MALDI-MS and their sequences established by Edman degradation (alternatively, but not exclusively, sequencing was performed by MS/MS). As a result, we were able to peptide-map the full amino acid sequence of CpTx 1 (134 residues; Fig. 2). In particular, CNBr cleavage resulted in two fragments, the larger fragment (∼12.5 kDa) corresponding to the N-terminal part of CpTx 1, and the smaller fragment (4312 Da) to its C-terminal part that was fully sequenced by Edman degradation (37 residues). Limited endoproteinase Glu-C digestion of the unreduced toxin produced four fragments as follows: N-terminal (∼5.9 kDa), C-terminal (1596 Da; 14 residues fully sequenced), and the full and C-terminally truncated forms of the central fragment (∼7.7 and ∼7.1 kDa, respectively; 39 N-terminal residues sequenced). Digestion of the reduced-alkylated CpTx 1 with the same enzyme produced a number of fragments, most of which were fully sequenced. We note that at the alkaline pH value used in our experiment, endoproteinase Glu-C was not selective for glutamic acid but also cleaved at aspartic acid residues. Finally, endoproteinase Arg-C was found to preferably cleave at Arg-124, but minor cleavage events occurred at other arginine and lysine residues, and the corresponding fragments were unambiguously assigned (see Fig. 2B).
FIGURE 2.
CpTx 1 sequencing. A, amino acid sequence of CpTx 1. Microheterogeneity at positions 33 and 81 was observed, with the minor forms containing Ser-33 and Glu-81. Cysteine (half-cystine) residues are shaded; the asterisk refers to C-terminal amidation. B, peptide mapping of the full amino acid sequence of CpTx 1. Peptide sequences established by Edman degradation and/or MS/MS are represented as arrows aligned with the resulting full CpTx 1 sequence. Dashed lines refer to partial sequences of the large CNBr and Glu-C fragments released due to cleavage at Met-97 and Glu-51, Glu-115, and Glu-120 that were not established directly.
CpTx 1 is a cationic polypeptide (charge +8 at pH 7, pI ∼10). It was also found to be C-terminally amidated; the measured molecular masses of the C-terminal fragments differed by 1 Da from the respective calculated values. The sequencing procedures revealed CpTx 1 microheterogeneity at two positions with minor substitutions N33S and D81E. In both cases, the minor forms represented ∼30% of the total protein. However, at this point we could not unequivocally assign the sequences of CpTx 1 isoforms (either two additional isoforms that carry a single substitution or just one additional isoform with both substitutions simultaneously seemed equally plausible). This riddle was solved by cDNA sequencing (see below).
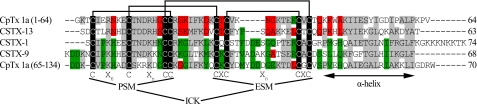
The 16 cysteine residues of CpTx 1 were found to assemble in two distinct primary structure signatures, CX6CX6CCX8CXCXnCXC. These signatures contain both the principal structural motif CX6CXnCC and the extra structural motif (ESM) CXCXnCXC characteristic of spider neurotoxins (26), and CpTx 1 is therefore allocated to the structural class 2 of spider toxins according to Kozlov and Grishin (35). Moreover, the two signatures perfectly conform to the more general ICK motif CX2–7CX3–11CX0–7CX1–17CX1–19C found in hundreds of peptides from various sources (37, 38). As proven by Glu-C digestion of the native toxin (see above), S–S bridges are formed exclusively inside the two signatures. CpTx 1 may therefore be represented as a modular polypeptide containing two disulfide-rich (most probably ICK) domains. Interestingly, sequences of the two modules share low yet significant sequence homology with each other (∼28% identical residues) (Fig. 3).
FIGURE 3.
CpTx 1 sequence homologues. Two modules (domains) of CpTx 1 corresponding to amino acid residues 1–64 and 65–134 are aligned with sequences of toxins from C. salei CSTX-1, -9, and -13. Polypeptide chain lengths are given in the right column. Note that CSTX-13 is a two-chain molecule, with no covalent bond between Gln-34 and Ala-35. Similar residues are shaded gray. Residues more common to the N-terminal domain of CpTx 1 and CSTX-13 are highlighted red; those more common to the C-terminal domain of CpTx 1 and toxins CSTX-1 and 9 are highlighted green. Invariant cysteine residues are shaded black, and the disulfide bonds assigned for CSTX toxins are drawn above. The cysteine motifs the principal structural motif (PSM) and extra structural motif (ESM), each contributing to the ICK structural fold, are identified below. The putative C-terminal α-helical region is also indicated below.
CpTx 1 exhibits a very low percent of identity with other known polypeptides, the only significant homology is found with toxins from the wolf spider Lycosa singoriensis (Lycosidae) identified from translated nucleotide sequences (39) and CSTX toxins from the venom of the wandering spider Cupiennius salei (Ctenidae) with ∼30–40% sequence identity and strict conservation of the cysteine residues (Fig. 3). Specifically, the insecticidal toxin CSTX-1 is known to block insect and mammalian calcium channels (40, 41); CSTX-9 probably acts similarly (42), and CSTX-13 is a neurotoxic enhancer that possesses lower lethal activity compared with other mentioned CSTX toxins but synergistically augments their potency (43, 44). Interestingly, the two modules of CpTx 1 cluster differentially with CSTX toxins; the C-terminal domain is more homologous to toxins CSTX-1 and CSTX-9, whereas the N-terminal part shows higher homology to the enhancer CSTX-13. The disulfide bridge arrangement has been determined for all CSTX toxins conforming to the ICK motif (42, 43), and the same cysteine pairing is proposed for the two CpTx 1 domains.
C. punctorium Venom Gland cDNA Analysis
A cDNA library was constructed for C. punctorium venom glands in collaboration with DuPont Agriculture and Nutrition (a separate paper dealing with all results in more detail is envisaged). Library analysis was performed in accordance with an earlier elaborated strategy and included not only the usual alignment and comparison with the nucleotide and polypeptide sequences deposited in public databases but also searches for structural elements and motifs specific for secreted polypeptide sequences and spider toxins (26, 35, 45, 46). As a result, putative spider toxin sequences were deduced. CpTx 1 sequence established by protein chemistry and MS was then used to query the library. Clones coding for three isoforms of this toxin were thus identified. The major isoform was named CpTx 1a and the two minor isoforms with D81E and N33S substitutions were named CpTx 1b and CpTx 1c, respectively. CpTx 1a was found to be coded by two types of mRNA corresponding to two types of precursor protein (pCpTx 1a-1 and 1a-2) that differ by two amino acid substitutions (Fig. 4). In addition, two types of precursor for CpTx 1b (pCpTx 1b-1 and 1b-2) and a precursor for CpTx 1c (pCpTx 1c) were found. No sequence containing both substitutions was detected; therefore, CpTx 1 toxin is believed to be represented by three closely homologous isoforms differing by point amino acid substitutions.
FIGURE 4.
CpTx 1a cDNA structure. The sequences of two cDNA clones coding for CpTx 1a precursors (prepropeptides) are shown. The numbering is according to the longer CpTx 1a-1 clone, and the substitutions found in CpTx 1a-2 are printed following the slash mark. The translation product of the reading frame is shown below the corresponding nucleotide sequence; the stop codon is indicated by an asterisk. The signal (pre-) peptide is in italics, and the prosequence is underlined.
pCpTx 1 precursor proteins consist of three parts (Fig. 4). (i) N-terminal signal (pre-) peptide (leader sequence) of 20 amino acid residues (identified by the SignalP 3.0 program) is cleaved off cotranslationally, on entrance to the endoplasmic reticulum. (ii) An acidic prosequence (27 residues) is removed post-translationally during the so-called limited proteolysis. The high negative charge of the prosequence (−10 at pH 7) cancels out the positive charge of the mature toxin. In spider toxins, propeptide cleavage most usually occurs at a specific site known as the processing quadruplet motif (PQM): X1X2X3R, where any Xn = E (26, 45, 46). In pCpTx 1, the PQM is 44DESR47. (iii) The mature toxin chain is located C-terminally. All precursors were found to bear an additional C-terminal dipeptide -GR, a signature of C-terminal amidation, supporting our sequencing data.
CpTx 1 Secondary Structure
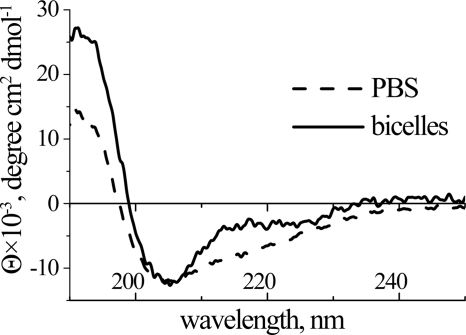
Peptides exhibiting the ICK motif are typically β-structural with 2–3 antiparallel β-strands joined by a number of reverse turns and loops of variable size, stabilized by disulfide bridges. In line with this rule, CpTx 1 main chain was found to predominantly adopt β-sheet and β-turn conformation in aqueous solution, as suggested by the CD spectrum (Fig. 5 and Table 1). In the presence of DMPC/DHPC bicelles used to mimic phospholipid membranes, changes of the spectrum were observed suggesting molecular interactions that lead to structural perturbations of the toxin; a significant increase (∼2-fold) of α-helix with a simultaneous reduction of random coil content was noted. Application of a number of algorithms resulted in prediction of two putative α-helical segments into the cysteine-free C-terminal parts of both CpTx 1 domains (∼42–57 and ∼111–134; Fig. 3). Plotted in a helical conformation, these sequences form amphiphilic structures with segregation of hydrophobic versus hydrophilic residues that may effectively interact with membranes.
FIGURE 5.
CD spectra of CpTx 1 in PBS (dashed line) and in DMPC/DHPC bicelle suspension (solid line).
TABLE 1.
Secondary structure of CpTx 1 as estimated by circular dichroism
| Solution | Secondary structure content |
|||
|---|---|---|---|---|
| α-Helix | β-Sheet | β-Turn | Random | |
| % | ||||
| PBS | 20 | 27 | 22 | 30 |
| Bicelle suspension | 40 | 25 | 16 | 19 |
CpTx 1 Biological Activity
Purified toxin was subjected to different tests and was found to exhibit a number of activities. CpTx 1 caused lethal effects on flesh fly (S. carnaria) larvae (LD50 of ∼10 μg/g); doses twice the LD50 value and higher caused instant paralysis and death, and at doses close to and lower than the LD50 value, reversible paralysis occurred.
The toxin possessed concentration-dependent cytolytic activity on insect Sf9 cells as follows: marginal (∼5%) toxicity at 0.5 μm and ∼20% cell death at 10 μm. CpTx 1 causes influx of organic cation (PI) as well as organic anion (CF) in the susceptible cells (Fig. 6). To visualize this effect accurately, confocal laser scanning microscopy was utilized, because the optical section must be thinner than the cell diameter to detect differences between intra- and extracellular marker concentrations. The peptide-induced membrane damage is accompanied by significant changes in cell morphology, including membrane blistering. At the concentration used (10 μm), CpTx 1 affects 20% of the entire cell population and causes toxicity in 30 min.
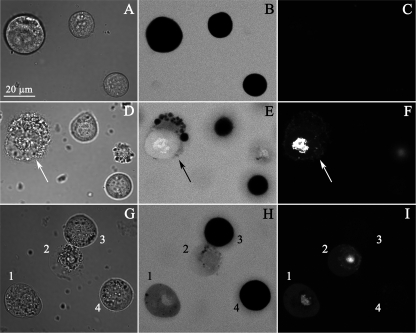
FIGURE 6.
Sf9 cells membrane lysis by CpTx 1. Upper row, A–C, before the toxin application, middle and bottom rows (D–F and G–I), 30 min after 10 μm CpTx 1 was added. Left column, A, D, and G, transmitted light images; middle column, B, E, and H, CF fluorescence confocal images; right column, C, F, and I, PI fluorescence. D, E, and F, the damaged cell is indicated by an arrow. G–I, the cells marked as 1 and 2 have lesions in the membrane, and the cells marked 3 and 4 remain impermeable for CF and PI.
The paralytic effects were further studied on fly neuromuscular preparations. At 50–200 nm, CpTx 1 caused stable and irreversible depolarization of fly muscle fibers eventually leading to contracture at higher toxin concentrations. Both the spontaneous and nerve stimulation-induced postsynaptic currents, however, were unaffected by the toxin. A similar depolarization induced by CpTx 1 was observed on frog neuromuscular preparations (Fig. 7, A and B). The average level of resting membrane potential in frog muscle fibers measured in control experiments was stable for more than 1 h (−65 to −90 mV in different cells). In the presence of 20 nm of CpTx 1, a moderate but statistically significant depolarization was observed (∼10–20 mV in 30–60 min). Increase of the toxin concentration up to 50 nm induced a much more pronounced effect that was not reversed by washing; the membrane potential drop gained ∼50 mV. Some muscle fibers demonstrated transient contractures following 7–15 min of contact with CpTx 1. In the experiments done under voltage clamp conditions, the toxin induced the rise of transmembrane current and a corresponding drop of the cell input resistance (Fig. 7C).
FIGURE 7.
CpTx 1 effects on muscle cells. A and B, CpTx 1 depolarizing effect in frog muscle fibers. Dependence is on Na+ concentration (A) and divalent cations concentration (B). Control values before toxin application (0 min) and after 30, 60, and 90 min of treatment of frog preparations with 50 nm CpTx 1 are shown as means ± S.E. (n = 5); *, p < 0.05; **, p < 0.01 in comparison with 116 mm Na+ (A) or 0.6 mm Ca2+ (B). C, effect of 100 nm CpTx 1 on input resistance and inward current recorded from Calliphora vicina muscle cell. On the abscissa, time is shown in minutes, and on the ordinate are shown the normalized averaged values (mean ± S.E., n = 5).
Removal from saline of the most parts of NaCl and its substitution by sucrose (232 mm) did not induce significant changes of the membrane potential in control. Interestingly, under these conditions of low Na+ content, the application of CpTx 1 (50 nm) for up to 90 min did not depolarize muscle fibers. To the contrary, a 2-fold increase of Na+ concentration (up to 232 mm) or addition of sucrose (232 mm, providing an equal increase of tonicity) intensified the depolarizing action of CpTx 1. Replacement of Na+ by NMDG ions, however, affected weakly the depolarizing action of the toxin (Fig. 7A). An increase of Ca2+ concentration from 0.6 to 3.6 mm substantially damped the depolarizing action of CpTx 1. 60 min of toxin application resulted in a much smaller effect, the membrane potential gained only an ∼15-mV drop compared with ∼50 mV at low calcium concentration. Addition of 1 mm Co2+ to saline (containing 0.6 mm Ca2+) slightly inhibited the depolarizing effect of the toxin, but 5 mm diminished significantly the depolarization (Fig. 7B). A 15-min pretreatment of the preparations with 1 μm TTX, 100 μm ouabain, or 1 mm amiloride had no effect on the depolarizing action of CpTx 1.
Toxin Action on Planar Bilayer Membranes
The membrane-tropic effects of CpTx 1 were explored in BLM. In control experiments, the membranes remained intact for over 100 min clamped at ±100 mV. In the presence of 50 nm of CpTx 1, however, a number of effects were observed. In ∼40% of cases, CpTx 1 induced BLM breakdown with or without prior appearance of conductance fluctuations. In the other ∼40% of cases, the toxin did not lyse the membrane, but it induced conductance in the form of numerous temporal fluctuations of variable current amplitude (|0.1|−|10| pA at ±100 mV). All effects were equally pronounced at both potential polarities. In the remaining ∼20% of experiments, the BLM remained stable as in the control. Increase of CpTx 1 concentration (≥150 nm) resulted in a higher probability of both membrane rupture (∼60%) and temporal fluctuation occurrences. Very seldom were single channel-like events observed with current amplitudes in the range of 0.2–2 pA.
DISCUSSION
Principal Component of C. punctorium Venom
Two major toxic fractions were isolated from the venom of the spider C. punctorium. The high molecular weight fraction probably contains a phospholipase (24), whereas the main component of the other fraction is a novel and unique 134-residue-long toxin CpTx 1 (represented by three closely related isoforms CpTx 1a, 1b and 1c, Fig. 2). CpTx 1 causes fast paralysis and mortality in flesh fly larvae, exerts pronounced membrane-tropic and cytolytic effects, reflecting the high insecticidal, cytotoxic, and membrane-damaging activities of the crude venom (24, 33). Certain discrepancy was noted between the measured LD50 values for the whole venom (∼3 μg/g) and purified CpTx 1 (∼10 μg/g), which either points to existence of additional powerful toxins, such as the presumed phospholipase, or may be result of synergy with other venom components, often noted for spiders (7, 17, 43, 44). Interestingly, Cheiracanthium bites produce symptoms that closely resemble bee and wasp stings (23). This observation is corroborated by assigning cytolytic function to the principal components in both Cheiracanthium and hymenopteran (17) venoms.
CpTx 1 Mode of Action
The toxin was found to affect the resting membrane potential in both insect and vertebrate muscle fibers. This effect was concentration-dependent and eventually manifested in substantial depolarization leading to muscle contracture and fiber damage. Measured under voltage clamp, CpTx 1 induced a rise of the transmembrane current and a corresponding drop of the cell input resistance (Fig. 7C). This indicates appearance in the membrane of new paths for permeable ions. To explain the toxic effect of CpTx 1, we sought to characterize its probable target, and several possible causes of membrane depolarization were tested as follows. (a) Because substitution of sucrose for Na+ ions inhibited this effect, whereas increase of Na+ concentration potentiated it (Fig. 7A), CpTx 1 seems to trigger specifically direct entry of Na+ into the muscle cells. The same effect was exhibited when instead of an increase of Na+ the appropriate amount of sucrose was added. This may be interpreted as a result of an increase in the tonicity of the outer solution, which may also potentiate the Na+ influx. (b) Substitution of Na+ by NMDG ions much less permeable through voltage-gated Na+ channels (47) and epithelial Na+ channels (ENaC) (48) did not influence significantly the CpTx 1-induced depolarization. Neither TTX, a potent blocker of Na+ channels (49), nor Co2+ (1 mm), a nonspecific blocker of Ca2+ channels (50), nor amiloride, a blocker of the ENaC family and a subset of voltage-gated Ca2+ channels (51, 52), eliminated the depolarizing effect of CpTx 1. Therefore, activation of voltage-gated Na+ and Ca2+ channels or ENaC by this toxin seems unlikely. (c) Phenomenologically, CpTx 1 effects resemble those of palytoxin from zoanthid corals, one of the most complex non-protein toxins in nature (53). Palytoxin targets the Na+/K+ pump shifting its activity from active to passive transport of Na+ and K+ that eventually disrupts the ion gradient and abolishes the membrane potential (54). The blocker of the Na+,K+-ATPase ouabain readily inhibits palytoxin activity (55) but fails to affect the action of CpTx 1. Therefore, the Na+/K+ pump does not seem to be the primary target of CpTx 1. To sum up, we excluded a number of membrane ion transport proteins (voltage-gated Na+ and Ca2+ channels, ENaC, and Na+,K+-ATPase) as possible targets of the toxin in frog neuromuscular preparations.
To the contrary, a considerable amount of evidence points to the cytoplasmic membrane lipid bilayer itself as the main target of CpTx 1. (i) Membrane environment induces structural changes of the polypeptide as determined by CD (Fig. 5 and Table 1) indicating toxin binding to the membrane. (ii) Artificial membranes are destabilized by CpTx 1, and the observed defects (numerous temporal current fluctuations of variable amplitude) are frequently seen in case of membrane-active peptides (MAPs) (56). (iii) Toxin-induced depolarization is independent of a number of membrane transport proteins and quite insensitive to the ionic composition of the extracellular medium (see above). (iv) Increase in concentration of divalent cations (3.6 mm Ca2+ or 5 mm Co2+) inhibits CpTx 1 effects (Fig. 7B), and a similar antagonism was noted in case of other MAPs; these cations compete with MAPs for binding to the membrane lipid phosphate groups (57). (v) The toxin was found to exhibit cytolytic activity characteristic of many MAPs. This activity was visualized directly by confocal laser scanning microscopy (Fig. 6). PI and CF in the ionized form are not able to penetrate the intact plasma membrane (29, 30); therefore, their appearance in a cell indicates membrane lesion formation. PI is accumulated in the nuclei, where the dye fluorescence increases dramatically due to intercalation into DNA. Simultaneous use of two dyes with charges of opposite sign further provides evidence of little selectivity of the membrane defects induced by CpTx 1.
The C-terminal parts of both domains of CpTx 1 (Fig. 3) are believed to assume α-helical conformation in the membrane environment, as suggested from secondary structure predictions and CD spectra measurements (Fig. 5 and Table 1). The putative helices are amphipathic with clusters of hydrophilic and hydrophobic residues occupying opposite sides of the cylinders. Such structures are known to effectively interact with membranes and are a hallmark of MAPs (17, 56, 57). We suggest that these fragments serve as “membrane attachment devices” of the toxin. We also note that similar sequences are present in other toxins, such as CSTX toxins from C. salei (see Fig. 3), indicating their plausible membrane-active properties. In this regard, the “membrane-access” mode of action proposed for certain spider neurotoxins deserves special attention (58). These peptides adopt amphiphilic structures due to formation of a hydrophobic patch of residues located in specific loops and β-strands of the ICK fold corresponding to fixed positions in the amino acid sequence. These hydrophobic residues are missing in CpTx 1, CSTX, and some other toxins, the noted amphipathic α-helical segment of which thus represents a novel membrane-access motif.
In conclusion, the lipid bilayer seems to represent the most probable target of CpTx 1. Most of the observed effects may be attributed to the cytolytic activity of the toxin. Existence of a specific receptor (acceptor) of this toxin cannot be totally ruled out at this point, however. Moreover, some evidence could testify in its favor; the low LD50 value determined for CpTx 1 is typical of specific neurotoxins, whereas cytolytic membrane-active peptides are usually characterized by 1 to 2 orders of magnitude higher lethal doses; there is some discrepancy between the active toxin concentrations observed ex vivo in neuromuscular preparations and in vitro in artificial membranes.
CpTx 1 Is a Unique Two-domain Modular Toxin
C. punctorium has shown profound difference from other studied spiders in terms of venom molecular composition. It is generally accepted that spider venoms may contain one of the three types of principal components: low molecular weight acylpolyamines, peptides (<10 kDa), or large proteins. The majority of investigated spider species produce venom rich in peptides having the “cystine knot” (ICK) fold (7, 11, 14, 20). MS of crude venoms typically produces a “bimodal” distribution of molecular mass species with predominance of ∼3–5- and ∼7–8-kDa species (59). In sharp contrast, C. punctorium whole venom gave a “unimodal” picture with an overwhelming dominance of ∼15-kDa constituents.
The major component of the venom, CpTx 1, was found to contain 134 residues determined by direct sequencing (Fig. 2) and further confirmed by cDNA cloning (Fig. 4). Its amino acid sequence may be considered as built up of two domains (modules), each corresponding to a putative “usual” ICK spider toxin of ∼7–8 kDa. It was directly shown by endoproteinase digestion of the native CpTx 1 that the two parts indeed represent separate domains with disulfide bonds formed only inside them. The two modules are homologous, pointing to a possible gene exon duplication followed by mutation that eventually led to the modular toxin structure. Moreover, both domains have known single domain toxin homologues found in spiders of different families (wolf spider L. singoriensis, Lycosidae, and wandering spider C. salei, Ctenidae, see Fig. 3). Curiously, one of the CpTx 1 domains shows higher similarity with CSTX-1 and -9, the principal neurotoxins from C. salei venom, whereas the other domain is more like CSTX-13, the neurotoxic enhancer, the function of which is to potentiate CSTX-1 and -9 toxicity (40–44). CpTx 1 may therefore represent a self-enhancing structure, which should be tested by comparison of the isolated modules toxicity with the full-sized polypeptide.
CpTx 1 represents the first example of a class of modular toxins with two disulfide-rich domains, thereby expanding the toxin universe. Indeed, if we consider the folding patterns realized in arachnid peptide toxins (Table 2), the following types may be noted. (a) Single domain toxins are the most widespread. Disulfide-rich toxins usually contain the ICK motif in spiders (for instance, the famous ω-agatoxins IVA and IVB from A. aperta (8)) and the cysteine-stabilized α-helix/β-sheet (CSαβ) motif in scorpions (charybdotoxin from Leiurus quinquestriatus hebraeus is a classical example (60)). Most linear toxins adopt α-helical conformation in contact with membranes (for instance, latarcins from L. tarabaevi (18) or pandinins from the scorpion Pandinus imperator (61)). (b) Two-domain modular toxins have been subsequently described, widening the known arachnid toxic arsenal. The scorpine family of modular peptides from the venom of P. imperator and other scorpions includes an N-terminal linear cationic α-helical domain, showing similarities to many MAPs, including some α-helical antimicrobial peptides (AMPs), and a C-terminal CSαβ domain, common to a wealth of peptides, including scorpion neurotoxins and plant defensins (62). More recently, cyto-insectotoxins (CITs), giant linear peptides exhibiting equally potent antimicrobial, cytolytic, and insecticidal activities, were discovered in the venom of L. tarabaevi. CITs include two linear modules each corresponding to a usual α-helical AMP (19). Together with this study, the following three classes of modular arachnid toxins are now known (note that disulfide connectivities and three-dimensional structures have yet to be established): AMP + AMP (CITs), AMP + CSαβ (scorpines), and ICK + ICK (CpTx 1).
TABLE 2.
Arachnid peptide toxins, folding patterns
On the Evolution of Modular Toxins
It seems that in spiders the modular toxins evolved from the so-called “binary” toxin precursors that are processed into two separate mature chains due to limited proteolysis at two rather than one processing motif (18, 45, 46). In CITs, a cryptic processing motif PQM, which we believe once served for separation of the two modules, is evident; the key arginine residue was mutated (EEAR → EEAQ) (19). In CpTx 1, no such sequence is at once noticed between the two domains (Fig. 2). However, the exact linker peptide 61PKPV64, immediately preceding the second domain, reverts to AETR (a typical PQM) with a “−1” frameshift in the coding DNA sequence (Fig. 4). We therefore propose that in CITs and CpTx 1 different mutation mechanisms, i.e. substitutions versus deletions/insertions, ensured the merging of two mature peptide chains of binary toxin precursors in a single toxin of modular structure.
In conclusion, we believe that the discovered two-domain modular toxin CpTx 1 from C. punctorium venom enhances our knowledge of natural toxin biodiversity. We also anticipate that the known modular toxin variability will soon be expanded.
Acknowledgments
We thank the DuPont Agriculture and Nutrition staff and especially Maureen Dolan, Will Krespan, Bill McCutchen, and Rafi Herrmann for cDNA library construction and sequencing. We are grateful to Sergey B. Akopov from the Shemyakin-Ovchinnikov Institute for the provided Sf9 cells. Our best thanks to Prof. Wolfgang Nentwig from the University of Bern and Andrey Feodorov from Fauna Laboratories, Ltd., for their professional account regarding the biology of Cheiracanthium spiders.
Note Added in Proof
When this paper had already been reviewed, a work by the Julius group describing another two-domain ICK + ICK spider toxin was published (Bohlen, C. J., Priel, A., Zhou, S., King, D., Siemens, J., and Julius, D. (2010) Cell 141, 834–845), thus confirming our expectations.
This work was supported by the Federal Agency for Education of the Russian Federation State Contract P1388, the Russian Foundation for Basic Research Grants 08-04-00326, 08-04-00454, and 09-04-00883, the Russian Scientific Schools Grants 4821.2008.4 and 3796.2010.4, and the Program of Cell and Molecular Biology of the Russian Academy of Sciences.
The protein sequences reported in this paper have been submitted to the UniProt Knowledgebase under accession numbers P86381, P86429, and P86430.
The nucleotide sequence(s) reported in this paper has been submitted to the Gen-BankTM/EBI Data Bank with accession number(s) FN594513 to FN594517.
Classification was provided by Andrey Feodorov from Fauna Laboratories, Ltd.
- ICK
- inhibitor cystine knot
- AMP
- antimicrobial peptide
- BLM
- planar bilayer membranes
- CF
- 5(6)-carboxyfluorescein
- CIT
- cyto-insectotoxin
- CSαβ
- cysteine-stabilized α-helix/β-sheet
- DHPC
- 1,2-dihexanoyl-sn-glycero-3-phosphocholine
- DMPC
- 1,2-dimyristoyl-sn-glycero-3-phosphocholine
- ENaC
- epithelial Na+ channels
- MAP
- membrane-active peptide
- NMDG
- N-methyl-d-glucamine
- PI
- propidium iodide
- PQM
- processing quadruplet motif
- RP-HPLC
- reverse-phase high performance liquid chromatography
- SEC
- size-exclusion chromatography
- TTX
- tetrodotoxin
- MS/MS
- tandem MS.
REFERENCES
- 1.Platnick N. I. (2009) The World Spider Catalog, Version 9.5, American Museum of Natural History, New York [Google Scholar]
- 2.Vetter R. S., Isbister G. K. (2008) Annu. Rev. Entomol. 53, 409–429 [DOI] [PubMed] [Google Scholar]
- 3.Grishin E. V. (1998) Toxicon 36, 1693–1701 [DOI] [PubMed] [Google Scholar]
- 4.da Silva P. H., da Silveira R. B., Appel M. H., Mangili O. C., Gremski W., Veiga S. S. (2004) Toxicon 44, 693–709 [DOI] [PubMed] [Google Scholar]
- 5.Gomez M. V., Kalapothakis E., Guatimosim C., Prado M. A. (2002) Cell. Mol. Neurobiol. 22, 579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tedford H. W., Sollod B. L., Maggio F., King G. F. (2004) Toxicon 43, 601–618 [DOI] [PubMed] [Google Scholar]
- 7.Vassilevski A. A., Kozlov S. A., Grishin E. V. (2009) Biochemistry (Moscow) 74, 1505–1534 [DOI] [PubMed] [Google Scholar]
- 8.Olivera B. M., Miljanich G. P., Ramachandran J., Adams M. E. (1994) Annu. Rev. Biochem. 63, 823–867 [DOI] [PubMed] [Google Scholar]
- 9.Swartz K. J. (2007) Toxicon 49, 213–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis R. J., Garcia M. L. (2003) Nat. Rev. Drug Discov. 2, 790–802 [DOI] [PubMed] [Google Scholar]
- 11.Estrada G., Villegas E., Corzo G. (2007) Nat. Prod. Rep. 24, 145–161 [DOI] [PubMed] [Google Scholar]
- 12.Fitches E., Edwards M. G., Mee C., Grishin E., Gatehouse A. M., Edwards J. P., Gatehouse J. A. (2004) J. Insect Physiol. 50, 61–71 [DOI] [PubMed] [Google Scholar]
- 13.Khan S. A., Zafar Y., Briddon R. W., Malik K. A., Mukhtar Z. (2006) Transgenic Res. 15, 349–357 [DOI] [PubMed] [Google Scholar]
- 14.Rash L. D., Hodgson W. C. (2002) Toxicon 40, 225–254 [DOI] [PubMed] [Google Scholar]
- 15.Grishin E. V., Volkova T. M., Arseniev A. S. (1989) Toxicon 27, 541–549 [DOI] [PubMed] [Google Scholar]
- 16.Mellor I. R., Usherwood P. N. (2004) Toxicon 43, 493–508 [DOI] [PubMed] [Google Scholar]
- 17.Kuhn-Nentwig L. (2003) Cell. Mol. Life. Sci. 60, 2651–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozlov S. A., Vassilevski A. A., Feofanov A. V., Surovoy A. Y., Karpunin D. V., Grishin E. V. (2006) J. Biol. Chem. 281, 20983–20992 [DOI] [PubMed] [Google Scholar]
- 19.Vassilevski A. A., Kozlov S. A., Samsonova O. V., Egorova N. S., Karpunin D. V., Pluzhnikov K. A., Feofanov A. V., Grishin E. V. (2008) Biochem. J. 411, 687–696 [DOI] [PubMed] [Google Scholar]
- 20.Grishin E. (1999) Eur. J. Biochem. 264, 276–280 [DOI] [PubMed] [Google Scholar]
- 21.Mouhat S., Jouirou B., Mosbah A., De Waard M., Sabatier J. M. (2004) Biochem. J. 378, 717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furman D. P., Reeves W. C. (1957) Calif. Med. 87, 114. [PMC free article] [PubMed] [Google Scholar]
- 23.Vetter R. S., Isbister G. K., Bush S. P., Boutin L. J. (2006) Am. J. Trop. Med. Hyg. 74, 1043–1048 [PubMed] [Google Scholar]
- 24.Foradori M. J., Smith S. C., Smith E., Wells R. E. (2005) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 141, 32–39 [DOI] [PubMed] [Google Scholar]
- 25.Vassilevski A. A., Kozlov S. A., Egorov T. A., Grishin E. V. (2010) Methods Mol. Biol. 615, 87–100 [DOI] [PubMed] [Google Scholar]
- 26.Kozlov S., Malyavka A., McCutchen B., Lu A., Schepers E., Herrmann R., Grishin E. (2005) Proteins 59, 131–140 [DOI] [PubMed] [Google Scholar]
- 27.Manavalan P., Johnson W. C., Jr. (1987) Anal. Biochem. 167, 76–85 [DOI] [PubMed] [Google Scholar]
- 28.Sreerama N., Woody R. W. (2000) Anal. Biochem. 287, 252–260 [DOI] [PubMed] [Google Scholar]
- 29.Stohr M., Vogt-Schaden M. (1979) in Flow Cytometry (Laerum O. D., Lindmo T., Thorud E. eds) Vol. IV, pp. 96–99, Universitetsforlaget, Bergen, Norway [Google Scholar]
- 30.Grimes P. A., Stone R. A., Laties A. M., Li W. (1982) Arch. Ophthalmol. 100, 635–639 [DOI] [PubMed] [Google Scholar]
- 31.Magazanik L. G., Fedorova I. M., Kovalevskaya G. I., Pashkov V. N., Bulgakov O. V., Grishin E. V. (1992) Neuroscience 46, 181–188 [DOI] [PubMed] [Google Scholar]
- 32.Fedorova I. M., Magazanik L. G., Tikhonov D. B. (2009) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 149, 275–280 [DOI] [PubMed] [Google Scholar]
- 33.Nikolsky A. S., Billen B., Vassilevski A. A., Filkin S. Y., Tytgat J., Grishin E. V. (2009) Biochemistry (Moscow), Supplement Series A: Membrane and Cell Biology 3, 245–253 [Google Scholar]
- 34.Darbre A. (1986) Practical Protein Chemistry: A Handbook, Wiley, Chichester, NY [Google Scholar]
- 35.Kozlov S., Grishin E. (2005) Toxicon 46, 672–686 [DOI] [PubMed] [Google Scholar]
- 36.Montal M., Mueller P. (1972) Proc. Natl. Acad. Sci. U.S.A. 69, 3561–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norton R. S., Pallaghy P. K. (1998) Toxicon 36, 1573–1583 [DOI] [PubMed] [Google Scholar]
- 38.Craik D. J., Daly N. L., Waine C. (2001) Toxicon 39, 43–60 [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y., Chen J., Tang X., Wang F., Jiang L., Xiong X., Wang M., Rong M., Liu Z., Liang S. (2010) Zoology (Jena) 113, 10–18 [DOI] [PubMed] [Google Scholar]
- 40.Kuhn-Nentwig L., Schaller J., Nentwig W. (1994) Toxicon 32, 287–302 [DOI] [PubMed] [Google Scholar]
- 41.Kubista H., Mafra R. A., Chong Y., Nicholson G. M., Beirão P. S., Cruz J. S., Boehm S., Nentwig W., Kuhn-Nentwig L. (2007) Neuropharmacology 52, 1650–1662 [DOI] [PubMed] [Google Scholar]
- 42.Schalle J., Kämpfer U., Schürch S., Kuhn-Nentwig L., Haeberli S., Nentwig W. (2001) Cell. Mol. Life Sci. 58, 1538–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wullschleger B., Kuhn-Nentwig L., Tromp J., Kämpfer U., Schaller J., Schürch S., Nentwig W. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 11251–11256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wullschleger B., Nentwig W., Kuhn-Nentwig L. (2005) J. Exp. Biol. 208, 2115–2121 [DOI] [PubMed] [Google Scholar]
- 45.Kozlov S. A., Grishin E. V. (2007) Toxicon 49, 721–726 [DOI] [PubMed] [Google Scholar]
- 46.Kozlov S. A., Vassilevski A. A., Grishin E. V. (2009) in Protein Biosynthesis (Esterhouse T. E., Petrinos L. B. eds) pp. 225–248, Nova Biomedical Books, New York [Google Scholar]
- 47.Chua M., Betz W. J. (1991) Biophys. J. 59, 1251–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wehner F., Bondarava M., ter Veld F., Endl E., Nurnberger H. R., Li T. (2006) Acta Physiol. 187, 21–25 [DOI] [PubMed] [Google Scholar]
- 49.Narahashi T. (2008) Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 84, 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerber U., Gähwiler B. H. (1991) Neurosci Lett. 134, 53–56 [DOI] [PubMed] [Google Scholar]
- 51.Kleyman T. R., Cragoe E. J., Jr. (1988) J. Membr. Biol. 105, 1–21 [DOI] [PubMed] [Google Scholar]
- 52.Tang C. M., Presser F., Morad M. (1988) Science 240, 213–215 [DOI] [PubMed] [Google Scholar]
- 53.Ecault E., Sauviat M. P. (1991) Br. J. Pharmacol. 102, 523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu C. H. (2009) Toxicon 54, 1183–1189 [DOI] [PubMed] [Google Scholar]
- 55.Artigas P., Gadsby D. C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 12613–12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bechinger B., Lohner K. (2006) Biochim. Biophys. Acta 1758, 1529–1539 [DOI] [PubMed] [Google Scholar]
- 57.Yeaman M. R., Yount N. Y. (2003) Pharmacol. Rev. 55, 27–55 [DOI] [PubMed] [Google Scholar]
- 58.Lee S. Y., MacKinnon R. (2004) Nature 430, 232–235 [DOI] [PubMed] [Google Scholar]
- 59.Escoubas P., Sollod B., King G. F. (2006) Toxicon 47, 650–663 [DOI] [PubMed] [Google Scholar]
- 60.Miller C. (1995) Neuron 15, 5–10 [DOI] [PubMed] [Google Scholar]
- 61.Corzo G., Escoubas P., Villegas E., Barnham K. J., He W., Norton R. S., Nakajima T. (2001) Biochem. J. 359, 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diego-García E., Schwartz E. F., D'Suze G., González S. A., Batista C. V., García B. I., de la Vega R. C., Possani L. D. (2007) Peptides 28, 31–37 [DOI] [PubMed] [Google Scholar]