Abstract
Observations of Golgi fragmentation upon introduction of G protein βγ (Gβγ) subunits into cells have implicated Gβγ in a pathway controlling the fission at the trans-Golgi network (TGN) of plasma membrane (PM)-destined transport carriers. However, the subcellular location where Gβγ acts to provoke Golgi fragmentation is not known. Additionally, a role for Gβγ in regulating TGN-to-PM transport has not been demonstrated. Here we report that constitutive or inducible targeting of Gβγ to the Golgi, but not other subcellular locations, causes phospholipase C- and protein kinase D-dependent vesiculation of the Golgi in HeLa cells; Golgi-targeted β1γ2 also activates protein kinase D. Moreover, the novel Gβγ inhibitor, gallein, and the Gβγ-sequestering protein, GRK2ct, reveal that Gβγ is required for the constitutive PM transport of two model cargo proteins, VSV-G and ss-HRP. Importantly, Golgi-targeted GRK2ct, but not a PM-targeted GRK2ct, also blocks protein transport to the PM. To further support a role for Golgi-localized Gβγ, endogenous Gβ was detected at the Golgi in HeLa cells. These results are the first to establish a role for Golgi-localized Gβγ in regulating protein transport from the TGN to the cell surface.
Keywords: G Proteins, Heterotrimeric G Proteins, Membrane Trafficking, Protein Targeting, Signal Transduction
Introduction
Heterotrimeric G proteins, composed of α, β, and γ subunits, are involved in a wide variety of signaling responses and act by coupling heptahelical G protein-coupled receptors (GPCRs)2 to intracellular effector proteins. In its inactive state, the Gα subunit is bound to GDP and is also tightly bound to the Gβγ subunits. Upon receptor activation by ligand binding, the GPCR catalyzes the exchange of GDP for GTP on the Gα, resulting in dissociation of Gα and Gβγ. The separated subunits are then able to elicit their own signaling cascades. Hydrolysis of GTP by Gα, followed by re-association of Gα and Gβγ, completes the cycle (1, 2).
Classically, G protein signaling was thought to occur only at the cytoplasmic surface of the plasma membrane (PM); however, accumulating evidence indicates a role for G proteins at subcellular locations in addition to the PM. GPCRs and G protein subunits have been detected at a variety of subcellular locations, including endoplasmic reticulum, Golgi, and nuclei, consistent with G protein signaling at diverse organelles (3–6). G proteins can traffic to endomembrane locations while en route to the PM after synthesis or can move from the PM to endomembranes in a constitutive or activation-dependent manner (1, 7, 8). In several cases, recent work has identified effector proteins that are activated by G proteins on endomembranes. For example, yeast Gα can regulate a phosphatidylinositol 3-kinase at endosomes (9), while Gβγ has been shown to regulate endosomal Rab11a (10). Thus, it has become increasingly clear that G proteins perform non-canonical functions at subcellular locations other than the PM.
Another novel endomembrane signaling pathway that is regulated by G proteins occurs at the Golgi. A series of studies have implicated Gβγ in regulating a signaling pathway at the Golgi membrane, causing fission of PM-destined vesicles from the trans-Golgi network (TGN) (11–13). Current models indicate that key components of this Golgi pathway include: 1) diacylglycerol (DAG) production at the TGN; 2) DAG-mediated recruitment of protein kinase D (PKD) to the TGN, and activation of PKD by Golgi-localized protein kinase Cη (PKCη); and 3) PKD-mediated activation of phosphatidylinositol 4-kinase IIIβ (PI4KIIIβ) (14). Additionally, activation of PI4KIIIβ leads to Golgi recruitment of ceramide transfer protein, which in turn is phosphorylated by PKD in a negative feedback manner (15). Overactivation of some of these signaling components causes the complete fission of the Golgi, while inhibition of some of the components of this pathway decrease TGN-to-PM transport of proteins (13, 16–18). Overexpression of Gβ1γ2 also causes vesiculation of the Golgi and activates PKD in a PKCη-dependent manner, suggesting Gβγ as a critical regulator of this pathway that controls the fission of TGN-to-PM transport carriers (11–13). Moreover, a recent report demonstrated a requirement for phospholipase Cβ3 (PLCβ3), suggesting that Gβγ activates this pathway by increasing DAG at the Golgi via activation of PLCβ3 (19). Upstream regulators of Gβγ have not been defined, although it has been speculated that specific cargo might activate a Golgi-localized GPCR. Nonetheless, while several components of this Golgi-localized pathway have been demonstrated to be critical, the importance of Gβγ remains ill-defined.
Although several models propose that heterotrimeric G proteins function at Golgi membranes to regulate the fission of PM-destined vesicles (14, 18), the subcellular location where Gβγ functions has not been tested. No studies have distinguished whether Gβγ is functioning at a novel Golgi localization or resides at its typical PM location and from there activates proteins that send a signal to the Golgi. Moreover, previous work has relied on overexpressed Gβγ to demonstrate overactivation of the TGN transport carrier fission pathway, and therefore it is not clear whether endogenous Gβγ plays a role normally in protein transport. Thus, this report is focused on answering two important questions: 1) Does Gβγ function at the Golgi, rather than other subcellular locations, to activate this pathway?, and 2) Does endogenous Gβγ play a normal role at the Golgi in the transport of PM-destined cargo? Herein we demonstrate that constitutive or inducible targeting of Gβ1γ2 to Golgi membranes causes Golgi vesiculation, whereas targeting of Gβ1γ2 to other subcellular locations does not. Moreover, sequestration of endogenous Gβγ using a Golgi-directed GPCR kinase 2 (GRK2) C-terminal domain (GRK2ct) inhibits the transport of PM-destined cargo, and a novel pharmacological inhibitor of Gβγ, gallein, likewise inhibits transport from the TGN to PM. These results provide the first demonstration that Gβγ is critical for the formation of TGN-to-PM transport carriers and that Gβγ is functioning at Golgi membranes to regulate a PKD-dependent signaling pathway there.
EXPERIMENTAL PROCEDURES
Reagents
The rapamycin analog AP21967 and the Argent Regulated Heterodimerization Kit were generously provided by ARIAD pharmaceuticals (Cambridge, MA). Gallein was obtained from Tocris Bioscience. Fluorescein, rapamycin, and cycloheximide were from Sigma-Aldrich. The phosphatidylinositol-specific PLC inhibitors (U73122 and U73343) were from Enzo Life Sciences, and the PKD inhibitor (Gö6976) was a generous gift from Dr. Jeffery Benovic (Thomas Jefferson University).
cDNA Constructs
The expression vector for β1 and β5 (Myc- and His-tagged) was provided by Dr. David P. Siderovski (University of North Carolina) (20). Non-tagged γ2 was described previously (20). Cytosolic-γ2 (Cyto-γ2, C68S mutation) and PM-targeted γ2 (PM-γ2) were created from γ2 using QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), as described previously (21). An endoplasmic reticulum (ER)-targeting sequence from pEYFP-IBV-M1, a generous gift from Dr. Mark Philips (New York University), was amplified by PCR and inserted at the 5′ end of wild-type γ2 to create ER-γ2. The Golgi-targeting KDELr-D193N sequence from pCEFL-KDEL, a generous gift from Dr. Piero Crespo (Universidad de Cantabria, Cantabria, Spain), was amplified by PCR and inserted at the 5′ end of wild-type γ2 to create Golgi-γ2. To generate the cytoplasmic γ2-FRB, γ2-C68S was amplified by PCR and inserted into the EcoR1 and Xba1 sites of the pC4-RHE plasmid (ARIAD Pharmaceuticals) such that it contained the linker sequence GGCGGCAGCGGCGGCAGCGGG. The PM-targeted FKBP plasmid, termed LF2C, containing amino acids 1–11 of Lyn followed by two copies of FKBP and then CFP, was a generous gift from Dr. Tobias Meyer and Dr. Takanari Inoue (22) (Stanford University School of Medicine). For Golgi-targeted FKBP, KDELr-D193N was inserted into LF2C in place of the 1–11 Lyn sequence using sequential PCR (23) to include a GATAGTGCTGGTAGTGCTGGT linker after the KDELr-D193N. Using full-length GRK2 or GRK2 R587Q cDNA as template (provided by Dr. Jeffrey Benovic, Thomas Jefferson University) (24), GRK2ct and GRK2ct R587Q were created by PCR of cDNA corresponding to GRK2 amino acids 495–689, and insertion into pcDNA3. Golgi-GRK2ct and Golgi-GRK2ct R587Q were also created by sequential PCR using the KDELr-D193N sequence from pCEFL-KDEL flanked by GATAGTGCTGGTAGTGCTGGT (as a linker) and fusion to the N terminus GRK2ct. PM-GRK2ct was created using the 66-amino acid C-terminal sequence of the Rit GTPase from YFP-Rit-CT, a generous gift from Dr. Mark Philips (New York University), flanked by GATAGTGCTGGTAGTGCTGGT as a linker, and fused to the C terminus of GRK2ct. The ss-HRP, tsO45 VSV-G-GFP, and GFP-PKD expression vectors were generously provided by Dr. Pravin Sehgal (New York Medical College), Dr. Jennifer Lippincott-Schwartz (NIH), and Dr. Qiming Wang (University of Pittsburgh), respectively.
Cell Culture and Transfection
HeLa cells were grown in DMEM (Cellgro), supplemented with 10% fetal bovine serum and maintained at 37 °C in a 95% air, 5% CO2-humidified atmosphere. Cells were seeded 1 day before transfection, and 1 and 10 μg of total plasmid DNA was transfected into cells grown in 6-well and 10-cm plates, respectively, using FuGENE 6 (Roche Applied Science).
siRNA Transfection
HeLa cells were seeded 1 day before transfection, and a 25–150 nm range of siRNA was transfected into cells using HiPerFect transfection reagent (Qiagen). The β1–2 siRNA sequence was ACGACGACUUCAACUGCAA (Thermo Scientific); this sequence was well described previously for efficiently depleting β1–2 in HeLa cells (25). As a nonspecific negative control siRNA, the ON-TARGETplus Non-targeting siRNA was used (Thermo Scientific).
Immunofluorescence Microscopy
Transfected cells were fixed with 3.7% formaldehyde in phosphate-buffered saline (PBS) for 15 min and permeabilized by incubation in blocking buffer (2.5% nonfat milk in 1% Triton X-100 in Tris-buffered saline) for 20 min. Cells were then incubated with the indicated primary antibodies in blocking buffer for 1 h. Primary antibodies used included anti-TGN46 antibody (Abcam), anti-Myc antibody 9E10 (Covance), anti-GFP antibody (Rockland), anti-γ2 antibody (Santa Cruz Biotechnology), anti-β1–4 antibody (Santa Cruz Biotechnology), and anti-GRK2 antibody (a generous gift from Dr. Jeffrey Benovic). The cells were washed with blocking buffer and incubated in 1:100 dilution of one or more of the following secondary antibodies (Invitrogen) for 1 h: chicken anti-rabbit antibody conjugated with Alexa 594, donkey anti-goat antibody conjugated with Alexa 488 or goat anti-rabbit Alexa 594. The coverslips were then washed with 1% Triton X-100 in Tris-buffered saline, rinsed in distilled water, and mounted on glass slides with Prolong Anti-fade reagent (Invitrogen). Images were acquired using an Olympus BX-61 upright microscope with a 60 × 1.4 numerical aperture (NA) or 100 × 1.3 NA oil immersion objective and an ORCA-ER cooled charge-coupled device camera (Hamamatsu, Bridgewater, NJ) controlled by Slidebook version 4.0 (Intelligent Imaging Innovations, Denver, CO).
Western Blotting
Western blots were developed using the indicated primary antibodies, secondary anti-goat, mouse or rabbit antibodies conjugated to HRP, and chemiluminescence reagent (Thermo Scientific). The imaging film, HyBlot CL, was from Denville Scientific. For detection of activated phospho-PKD, an anti-phospho-serine 744/748 PKCμ/PKD antibody was used following the manufacturer's protocol (Cell Signaling). Activated PKD was quantitated by densitometry. The signals obtained from bands in the phospho-PKD Western blot were divided by the signal from the sample of total expressed GFP-PKD to correct for any differences in expression. The resulting value from each sample was then divided by the control value to arbitrarily set the control at 1.
VSV-G Transport Assay
HeLa cells were transfected with the indicated GRK2ct plasmids and incubated 24 h at 37 °C. Cells were then washed with fresh media and transfected with a plasmid coding for tsO45 VSV-G protein with a GFP tag. 5 h after this second transfection, cells were moved to 39.5 °C and incubated overnight at this non-permissive temperature to allow the accumulation of the VSV-G protein in the ER. Then, medium was changed to serum-free medium, and cells were incubated for 2 h at 20 °C, allowing the VSV-G protein to fold properly and be transported from ER to the Golgi, and 10 μg/ml cycloheximide was added during the last hour of incubation at 20 °C. The cells were then incubated in serum-free medium for different times at the permissive 32 °C temperature and fixed to be evaluated by immunofluorescence microscopy. For experiments in which cells were treated with the inhibitor gallein, the first transfection step was omitted, and the inhibitor was added after 1.5-h incubation at 20 °C.
ss-HRP Secretion Assay
24 h after transfection with the indicated GRK2ct plasmids, HeLa cells were then transfected with the ss-HRP plasmid. 48 h after ss-HRP transfection, cells were washed and incubated with fresh media for 5 h. For experiments in which cells were treated with the inhibitor gallein, the first transfection step was omitted. The inhibitor was added 30 min before washing with fresh, serum-free medium and maintained in the serum-free medium during the subsequent 5 h. To assay for secreted HRP, 5 μl of the culture medium from a 35-mm dish was harvested and added to 50 μl of SuperSignal West Pico chemiluminescent substrate (Thermo Scientific). HRP activity was then measured using a Lumat LB 9507 luminometer. A background reading, from media of cells transfected with pcDNA3 but without ss-HRP, was subtracted from each sample.
RESULTS
A Constitutively Targeted Golgi-β1γ2 Converts the Golgi into Small Vesicles
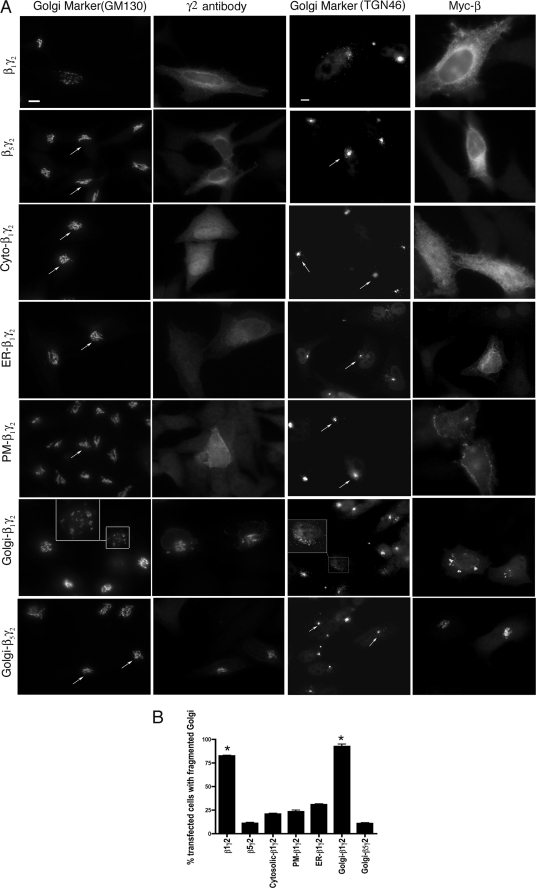
Vesiculation of Golgi membranes as a result of expression of Gβγ represents overactivation of a normal signaling pathway that controls the fission of transport carriers from the TGN (13). Here, β1γ2 and β5γ2 were transfected into HeLa cells, and the organization of the Golgi apparatus was monitored by immunofluorescence microscopy, using antibodies against TGN46 and GM130 as markers for the trans and cis Golgi network, respectively. β1γ2 caused Golgi breakdown in >75% of transfected cells, whereas β5γ2 expressing cells showed an intact Golgi in most cells, consistent with previous observations (Fig. 1, A and B) (13).
FIGURE 1.
Constitutively targeted Golgi-β1γ2 converts the Golgi into small vesicles. A, Myc-tagged β1 or β5 were expressed in HeLa cells along with wild-type or mutant γ2 in the following combinations, as indicated: Myc-β1 and γ2 (β1γ2), Myc-β5 and γ2 (β5γ2), Myc-β1 and Cyto-γ2 (Cyto-β1γ2), Myc-β1 and ER-γ2 (ER-β1γ2), Myc-β1 and PM-γ2 (PM-β1γ2), Myc-β1 and Golgi-γ2 (Golgi-β1γ2), and Myc-β5 and Golgi-γ2 (Golgi-β5γ2). Cells were visualized by immunofluorescence microcopy using anti-Myc, anti-GM130, or anti-TGN46 to detect β, the cis-Golgi stacks, and the trans-Golgi stacks, respectively. Arrows indicate intact Golgi. The inset shows vesiculated Golgi. Scale bar, 10 μm. B, results from A were quantitated and plotted as the percentage of cells with a fragmented Golgi. 100 cells from each of 6 different experiments were counted as having intact or fragmented Golgi. The percentage of cells with fragmented Golgi was >75% in β1γ2-expressing cells and >90% in Golgi-β1γ2-expressing cells, whereas in control transfections (empty vector) fragmented Golgi was observed in 10% of cells (not shown). Statistical significance as compared with control was tested using unpaired t test (*, p < 0.001).
When β1γ2 is overexpressed, it localizes at the PM and endomembrane locations (Fig. 1A) (13, 20, 21, 26). Thus, the intracellular location(s) where Gβγ regulates the signaling pathway that converts the Golgi into small vesicles is not clear. To test this, mutant γ2 constructs designed to constitutively target βγ dimers to distinct subcellular locations were generated. Replacement of cysteine 68 with serine in the CaaX box of the γ2 subunit prevents geranylgeranylation and membrane association of the γ2, and therefore localizes to the cytoplasm (Cyto-γ2) (21). PM-localized γ2 was generated by introducing a series of positively charged residues (E63K, F66K, and F67K) adjacent to the CaaX box of γ2; this polybasic region plus geranylgeranylation serves to strongly target γ2 to the PM as confirmed by colocalization with a PM marker (supplemental Fig. S1). For ER-targeted γ2, we used the first transmembrane domain of the avian infectious bronchitis virus M1 protein, as described previously (supplemental Fig. S1) (27). γ2 was targeted to the Golgi membrane via fusion to a mutant KDEL receptor (KDELr) sequence. Mutation of D193N prevents KDELr from redistributing to the ER, and renders it a resident Golgi protein (supplemental Fig. S1) (28, 29). Moreover, KDELr has been shown to localize throughout the Golgi stacks (30), suggesting that KDELr fusion proteins are able to interact with other proteins at the cytoplasmic surface of various Golgi domains, including the TGN. HeLa cells were transfected with Myc-tagged β1 or β5 along with γ2, Cyto-γ2, PM-γ2, ER-γ2, or Golgi-γ2, and the organization of the Golgi apparatus was monitored by immunofluorescence microscopy. Golgi-β1γ2 caused Golgi fragmentation in >90% of transfected cells, whereas Cyto-β1γ2-, PM-β1γ2-, and ER-β1γ2-expressing cells displayed substantially less Golgi fragmentation (Fig. 1, A and B). Expression of Golgi-γ2 alone (without β1) does not cause Golgi fragmentation (supplemental Fig. S1), demonstrating that the Golgi vesiculation is specific to the Golgi-targeted β1γ2 and is not an artifact of overexpressed Golgi-γ2. Further, expression of Golgi-β5γ2 does not vesiculate the Golgi, providing additional evidence for subunit specificity for this effect (Fig. 1, A and B).
The data so far suggest that β1γ2 might regulate a signaling pathway at the Golgi membrane that leads to Golgi fragmentation. To further demonstrate that Golgi fragmentation by Golgi-β1γ2 is not simply a nonspecific effect of constitutively targeting β1γ2 to Golgi membranes, we examined the ability of GRK2ct to prevent Golgi vesiculation. GRK2ct binds Gβγ (24, 31) and has been widely used to inhibit Gβγ-mediated signaling in cells. In HeLa cells expressing Golgi-β1γ2 along with GRK2ct, the Golgi organization is intact (supplemental Fig. S2), suggesting that β1γ2, while still localized to the Golgi, is no longer free to activate its downstream effector. As a control we used GRK2ct-R587Q, a βγ binding-deficient mutant (24). GRK2ct-R587Q is not able to block Golgi fragmentation in Golgi-β1γ2-expressing cells (supplemental Fig. S2). In summary, these data suggest that β1γ2 regulates a pathway at the Golgi membrane that causes Golgi fragmentation.
An Inducibly Targeted Golgi-β1γ2 Converts the Golgi into Small Vesicles
To further examine a role for β1γ2 at the Golgi, we utilized a rapamycin-mediated heterodimerization system to rapidly and inducibly recruit βγ from the cytoplasm to the Golgi or PM. In this system, rapamycin or a rapamycin analog serves as a heterodimerizer by binding simultaneously to the FK506-binding protein (FKBP) and to the FKBP-rapamycin binding domain of FRAP (FRB) (32–35). Here FKBP was fused to KDELr-D193N or to N-terminal amino acids 1–11 of Lyn (a known PM targeting sequence) (22), to generate Golgi-FKBP or PM-FKBP, respectively. In addition, FRB was fused to the C terminus of a C68S CaaX mutant of γ2 to generate cytoplasmic γ2-FRB. HeLa cells were then transfected with PM- or Golgi-FKBP along with Myc-β1 or Myc-β5 and γ2-FRB. β1γ2-FRB and β5γ2-FRB localize to the cytoplasm, but the addition of the rapamycin analog AP21967 recruits them to the Golgi or PM in cells coexpressing Golgi-FKBP or PM-FKBP, respectively (Fig. 2, A and B). When β1γ2-FRB is recruited to Golgi membranes, Golgi morphology rapidly changes from the normal compact structure to dramatic vesiculation (Fig. 2A). Golgi vesiculation can be detected in 5 min (not shown); a time point of 15 min displays strong vesiculation and is used throughout these studies. In contrast to β1γ2-FRB, when β5γ2-FRB is inducibly recruited to Golgi membranes, the Golgi remains intact (Fig. 2A), consistent with the subunit specificity observed earlier (Fig. 1A). Inducible recruitment of β1γ2-FRB and β5γ2-FRB to the PM did not cause Golgi fragmentation (Fig. 2B and not shown), again suggesting that the PM is not the site at which Gβγ regulates a signaling pathway that leads to Golgi fragmentation. In summary, these data using the inducible recruitment of β1γ2 to Golgi membranes further confirms that the Golgi is the subcellular location at which Gβγ regulates TGN-to-PM vesicle formation.
FIGURE 2.
Rapamycin-inducible Golgi recruitment of β1γ2 causes Golgi fragmentation. HeLa cells were transfected with either Golgi-targeted-FKBP-CFP (A) or PM-targeted-FKBP-CFP (B) along withγ2-FRB and either Myc-β1 or Myc-β5. Cells were fixed before (0 min) or 15 min after the addition of 1 μm of the rapamycin analog AP21967. Cells were then processed for immunofluorescence microscopy using anti-TGN46 and anti-Myc antibodies to detect the Golgi and β1 or β5, respectively. Golgi-targeted-FKBP-CFP or PM-targeted-FKBP-CFP was also visualized due to the intrinsic fluorescence of CFP. Arrows indicate intact Golgi. The inset shows vesiculated Golgi. Scale bar, 10 μm.
PLC and PKD Inhibitors Block Golgi Vesiculation Caused by Inducibly Targeted Golgi-β1γ2
DAG is required for the recruitment of PKD to the TGN (16). More recent data suggest that PLCβ activity is necessary for DAG production and therefore recruitment/activation of PKD at Golgi membranes (19). To examine whether the Golgi fragmentation observed by inducibly targeting β1γ2 to the Golgi membrane requires PLCβ and PKD, pharmacological inhibitors were used (Fig. 3). When β1γ2-FRB was recruited to the Golgi in HeLa cells that had been treated with the PLCβ inhibitor U73122, the Golgi remained intact in the majority of cells. On the other hand, treatment of cells with U73343, an inactive analog of U73122, did not prevent Golgi vesiculation upon Golgi recruitment of β1γ2. Similar to PLCβ inhibition, the PKD inhibitor Gö6976 reduced the ability of Golgi-recruited β1γ2 to cause Golgi fragmentation. These data suggest that the Golgi vesiculation observed by inducible Golgi recruitment of β1γ2 occurs through a bona fide signaling pathway, consistent with previous observations using overexpressed wild-type β1γ2 (13, 19).
FIGURE 3.
Inhibitory effect of U73122 and Gö6976 on Golgi fragmentation caused by inducibly Golgi-targeted β1γ2. A, HeLa cells were transfected with Golgi-targeted-FKBP-CFP, Myc-β1, and cytoplasmic γ2-FRB. Cells were preincubated with 5 μm of a PLC inhibitor (U73122), its inactive analog (U73343) or a PKD inhibitor (Gö6976), as described previously (19). Cells were fixed before or after a 15-min treatment with 1 μm rapamycin, and immunofluorescence microscopy was performed using anti-TGN46 and anti-Myc antibody to detect Golgi and Myc-β, respectively. Arrowheads indicate fragmented Golgi, and arrows indicate intact Golgi. Scale bar, 10 μm. B, 100 cells were counted in each of 6 different experiments. The percentage of cells with fragmented Golgi was >80% in inducibly Golgi-targeted β1γ2 upon 15-min addition of rapamycin, whereas in cells preincubated with Gö6976 and U73122 <55% and <25% cells have fragmented Golgi, respectively. Statistical significance as compared with control (no treatment) was tested using an unpaired t test (*, p < 0.001).
Inducibly Targeted Golgi-β1γ2 Activates PKD
Next, we examined the ability of Golgi-targeted β1γ2 to activate PKD. Activation of PKD requires phosphorylation of serines 744/748. Previous work showed that expression of β1γ2 increased serine 744/748 phosphorylation of PKD, and PKCη could mediate this phosphorylation (13). Phosphospecific PKD antibodies and immunoblots were used to examine PKD activation. Lysates from HeLa cells expressing GFP-PKD, Golgi-FKBP-CFP, γ2-FRB, and Myc-β1 or Myc-β5 were prepared before or after 15-min treatment with rapamycin. Golgi recruitment of β1γ2 increased the serine 744/748 phosphorylation of PKD (Fig. 4), while Golgi recruitment of β5γ2 did not activate PKD (not shown). When Myc-β1 was not included in the transfection, such that γ2-FRB alone was recruited to the Golgi in response to rapamycin addition, PKD activation was not observed (Fig. 4). This control additionally shows that rapamycin itself does not activate PKD. These data confirm that inducibly targeted Golgi-β1γ2 is able to activate a signaling pathway at the Golgi membrane, which leads to the activation of the PKD.
FIGURE 4.
Activation of PKD upon inducibly targeting β1γ2 to the Golgi membrane. HeLa cells were cotransfected with GFP-PKD, Golgi-FKBP-CFP, and γ2-FRB and with or without Myc-β1. The cells were lysed before or after the 15-min addition of 1 μm rapamycin. Lysates were analyzed by Western blotting to monitor the phosphorylation status of Ser-744/748 in the activation loop of the PKD (top immunoblot panel). Equivalent expression levels of GFP-PKD and Myc-β were also monitored (middle and lower immunoblot panels, respectively). PKD phosphorylation was quantitated as described under “Experimental Procedures” and shown as the mean ± S.D. (vertical bars) of three separate experiments. Statistical significance as compared with control (no Myc-β expression) was tested using unpaired t test (*, p < 0.05).
A Small Molecule Inhibitor of Gβγ Blocks VSV-G Protein Transport from TGN to the PM
Our findings so far suggest that overexpressed β1γ2 must be localized at the Golgi to activate a signaling pathway that results in fission of the Golgi. This Golgi fragmentation/vesiculation is consistent with the idea that overexpressed Gβγ overactivates a signaling pathway that normally regulates the fission of PM-destined transport vesicles from the TGN. However, the key question of whether endogenous βγ in fact regulates such a pathway at the Golgi has not been addressed. To examine this question we first utilized the well characterized vesicular stomatitis virus-G (VSV-G) transport assay and took advantage of a novel small molecule Gβγ inhibitor, gallein, that has been shown to specifically bind to Gβ effector binding sites and block the Gβγ-mediated activation of effectors such as PLCβ (36, 37). HeLa cells were transfected with a GFP-tagged temperature-sensitive mutant form of VSV-G. At the non-permissive temperature of 39.5 °C, the VSV-G protein is synthesized but not able to fold properly and therefore retained in the ER. Upon shift to 20 °C, the protein leaves the ER and stays in Golgi. Lastly, after a shift to 32 °C, VSV-G traffics from the TGN to the PM. Thus, in control HeLa cells VSV-G is found at the PM 120 min after release from the Golgi by a shift to 32 °C (Fig. 5, A and B). However, in cells treated with gallein 30 min before the shift to the 32 °C permissive temperature, VSV-G remained at the Golgi even after 120 min. Treatment of cells with fluorescein, a control compound with similar structure to gallein that does not bind to the Gβγ effector binding site (37), did not prevent the VSV-G transport to the PM (Fig. 5, A and B). These results are consistent with the involvement of endogenous Gβγ in regulating a signaling pathway that controls TGN-to-PM vesicle formation.
FIGURE 5.
Inhibition of VSV-G transport from TGN to the PM by the Gβγ inhibitor gallein. A, HeLa cells were transfected with GFP-tsO45 VSV-G. Cells were preincubated with either 10 μm gallein or fluorescein 30 min before shifting to the 32 °C permissive temperature. Cells were then fixed 120 min after the shift to 32 °C, and immunofluorescence microscopy using an anti-GFP antibody was performed to detect VSV-G-GFP localization. The arrow indicates Golgi localization of VSV-G in gallein-treated cells compared with the PM localization of VSV-G in control and fluorescein treated cells. Scale bar, 10 μm. B, 100 cells were counted in 6 different experiments. The percentage of cells that show VSV-G at the PM was <35% in gallein-treated cells. Statistical significance compared with control was tested using unpaired t test (*, p < 0.001).
Sequestration of Gβγ with GRK2ct Blocks VSV-G Transport from TGN to the PM
To further confirm that endogenous Gβγ regulates TGN-to-PM vesicle transport, we used GRK2ct to sequester endogenous Gβγ and block its signaling activity. HeLa cells were transfected with GRK2ct and the temperature-sensitive mutant of VSV-G-GFP. As described above, VSV-G is found at the PM 120 min after releasing from the TGN by shifting HeLa cells to 32 °C. In cells expressing GRK2ct, VSV-G remained at the Golgi even after 120 min at 32 °C. However, GRK2ctR587Q, a mutant unable to bind Gβγ, did not prevent the transport of VSV-G from the Golgi to the PM (Fig. 6, A and B). These data suggest that GRK2ct is able to block VSV-G transport to the PM by binding to Gβγ and preventing it from interacting with critical effectors.
FIGURE 6.
Inhibition of VSV-G transport from TGN to the PM by Gβγ sequestration. A, HeLa cells were transfected with GRK2ct, GRK2ct R587Q, Golgi-GRK2ct, Golgi-GRK2ct R587Q, or PM-GRK2ct along with GFP-tsO45 VSV-G. Following the protocol described under “Experimental Procedures,” cells were fixed immediately before (0 min) or 120 min after the shift to 32 °C. VSV-G and GRK2ct localization was detected by immunofluorescence microscopy using anti-GFP and anti-GRK2 antibodies. VSV-G is retained in the Golgi in GRK2ct- and Golgi-GRK2ct-expressing cells after 120-min incubation at the permissive temperature, compared with the cell-surface localization of VSV-G in cells expressing GRK2ct R587Q, Golgi-GRK2ct R587Q, and PM-GRK2ct. Emanating tubules (indicated by insets) were observed in GRK2ct- and Golgi-GRK2ct-expressing cells. Arrowheads indicate plasma membrane localization of VSV-G. Scale bar, 10 μm. B, 100 cells were counted in 6 different experiments. The percentage of cells that show VSV-G at the PM were <30% and <20% in cells expressing GRK2ct and Golgi-GRK2ct, respectively. Statistical significance was tested using unpaired t test (*, p < 0.001).
To clarify the membrane location where endogenous Gβγ regulates this pathway, we generated Golgi- and PM-targeted GRK2ct using KDELrD193N and the 66-amino acid C terminus of Rit GTPase (a known PM-targeting motif) (38), respectively, to sequester endogenous Gβγ at these locations. In HeLa cells expressing Golgi-GRK2ct, VSV-G remained at the Golgi after shifting cells to the permissive temperature. However, Golgi-GRK2ctR587Q did not prevent the transport of VSV-G from the Golgi to the PM, suggesting that the inhibition of VSV-G transport observed with Golgi-targeted GRK2ct is due to sequestration of endogenous Gβγ at the Golgi and therefore blockade of its signaling activity. When GRK2ct was targeted to the PM, VSV-G transport from TGN-to-PM was not inhibited (Fig. 6, A and B), which further confirms that Gβγ at the Golgi membrane and not at the PM is involved in regulating transport to the PM.
Previous studies have shown that inhibition of components of the protein transport signaling pathway, such as PKD, at the Golgi membrane causes Golgi tubulation, with the emanated tubules containing cargo that is destined for the PM (13, 39–41). Here we also observed Golgi tubulation in cells expressing GRK2ct. Even more extensive tubulation was observed when GRK2ct was targeted to the Golgi membrane (Fig. 6A). Such tubulation upon sequestration of Gβγ is another indication for inhibition of the protein transport pathway at TGN. These data suggest that GRK2ct is able to sequester endogenous Gβγ and block its binding to downstream signaling components involved in the formation of PM-destined vesicles in TGN. Furthermore, inhibition of VSV-G transport using Golgi-GRK2ct suggests that endogenous Gβγ at the Golgi membrane, rather than other subcellular locations, regulates this pathway.
Gallein and GRK2ct Block Secretion of ss-HRP
To further ascertain the involvement of Gβγ in protein transport, we examined transport to the cell surface of another model protein, ss-HRP, horseradish peroxidase containing a signal sequence from human growth hormone (42–44). In this system, soluble HRP is secreted from cells into the media and is measured by chemiluminescence. Depletion of PKD in HeLa cells inhibits the secretion of ss-HRP (45). Here, HeLa cells were transfected with ss-HRP and cotransfected with GRK2ct constructs or treated with gallein (Fig. 7). As in the VSV-G protein transport assay, ss-HRP secretion was strongly inhibited by expression of GRK2ct and Golgi-GRK2ct or by incubation with gallein. The control βγ binding-deficient GRK2ctR587Q and Golgi-GRK2ctR587Q constructs or the fluorescein control did not inhibit ss-HRP secretion. These findings further confirm a role for endogenous Gβγ at the Golgi in regulating the transport and secretion of cargo that is destined for the cell surface.
FIGURE 7.
Inhibition of ss-HRP secretion by Gallein and GRK2ct. HeLa cells were transfected with ss-HRP expression vector for 24 h before washing cells and then incubating another 5 h before assaying secreted HRP activity in the media. In addition, cells were transfected 24 h before the ss-HRP transfection with empty vector pcDNA3 (as control), GRK2ct, GRK2ctR587Q, Golgi-GRK2ct, or Golgi-GRK2ctR587Q, as indicated, or cells were treated with 10 μm gallein and fluorescein for a total of 5.5 h before measuring secreted HRP activity as described under “Experimental Procedures.” Bars represent the mean ± S.D. of HRP activity in the medium from three experiments each performed in triplicate. Statistical significance compared with control (pcDNA3) was tested using unpaired t test (*, p < 0.001). The lower panel shows a Western blot using cell lysates and an anti-GRK2 antibody to demonstrate similar expression levels of GRK2ct, GRK2ctR587Q, Golgi-GRK2ct, and Golgi-GRK2ctR587Q.
Endogenous G Protein β Subunits Localize at the Golgi
Previous studies have used biochemical fractionation to detect Gβ and Gα at isolated Golgi membrane fractions (46–50); however, there is a lack of immunofluorescence microscopy evidence for localization of Gβγ at the Golgi in cells. Thus, we examined whether a pool of Gβγ could be detected at the Golgi in HeLa cells. A previous study had shown that Gβ1 and Gβ2 are expressed at similar levels in HeLa cells and together they account for 80% of the Gβ subunit pool (25). To examine the subcellular localization of endogenous Gβ in HeLa cells we used an antibody that detects Gβ1–4 subunits. Fig. 8A shows a perinuclear staining of Gβ that colocalizes with the Golgi marker (GM130). To clarify the Gβ1–2 Golgi localization, a specific siRNA was used to deplete Gβ1–2. This Gβ1–2 was used previously to show effective depletion of Gβ1–2 in HeLa cells (25). Western blotting showed a reduction of >75% in the level of Gβ1–2; however, control siRNA did not affect Gβ1–2 expression. In addition, the level of HSP90 was not affected by Gβ1–2-specific siRNA (Fig. 8B). Importantly, upon depletion of Gβ1–2 we observed the loss of Golgi staining of Gβ, which was not affected in control siRNA-treated cells. To confirm the siRNA results, we performed a second knockdown using a combination of Gβ1 and Gβ2 siRNAs, as used previously (25), and similarly observed a loss of Gβ staining at the Golgi (supplemental Fig. S3). Additionally, Golgi tubulation was observed in Gβ1–2 knockdown cells, further confirming the tubulation and inhibition of transport that was observed by GRK2ct and gallein inhibition (Figs. 6 and 7). The data in Fig. 8 provide evidence that endogenous Gβ is found at the Golgi in HeLa cells, where it is available to regulate TGN-to-PM transport.
FIGURE 8.
Golgi localization of endogenous Gβ. A, HeLa cells were non-transfected (C) or transfected with Gβ1,2 or control siRNA for 48 h. Cells were visualized by immunofluorescence microcopy using anti-β and anti-GM130 antibodies to detect endogenous Gβ and the Golgi apparatus, respectively. Overlapped regions between red and green signals exhibit yellow color in the merged images on the right. Emanating tubules (indicated by arrows) were observed in Gβ1,2 knockdown cells. Scale bar, 10 μm. B, HeLa cells transfected with Gβ1,2 or control siRNA were lysed 48 h after transfection. Lysates were analyzed by Western blotting to monitor the endogenous level of Gβ (top immunoblot panel) and HSP90 as loading control (lower immunoblot panel).
DISCUSSION
In this report, we have demonstrated that Golgi-localized Gβγ plays a critical role in regulating a Golgi-localized signaling pathway that is involved in the formation of PM-targeted secretory vesicles. Although previous reports had implicated Gβγ in this pathway (11–13, 19), the critical subcellular location of Gβγ had not been defined nor was there any evidence that endogenous Gβγ normally functioned in a Golgi-delimited pathway that controls the formation of Golgi-to-PM transport carriers. Overexpressed β1γ2 causes Golgi vesiculation (Fig. 1) (13, 19); however, because overexpressed β1γ2 is found throughout the cell, it was necessary to specifically target it to discrete subcellular locations to determine its site of action. Thus, we expressed β1γ2 subunits that were constitutively targeted to different locations and found that Golgi-targetedβ1γ2 was able to overactivate the vesicle formation pathway and cause a completely fragmented Golgi (Fig. 1). On the other hand, PM-, ER-, and Cytosolic-β1γ2 did not cause Golgi fragmentation. In addition, we used an inducible heterodimerization system to show that recruitment of β1γ2 from the cytoplasm to Golgi membranes caused a rapid vesiculation of the Golgi (Fig. 2). β1γ2 activated PKD upon recruitment to the Golgi, and, using inhibitors, it was demonstrated that the Golgi fragmentation required PKD and PLC (Figs. 3 and 4). We also used two model proteins that are trafficked from the Golgi to PM to demonstrate a role for endogenous Gβγ (Figs. 5–7). A novel pharmacological inhibitor, gallein, as well as the Gβγ-sequestering molecule GRK2ct, were able to inhibit transport of VSV-G and ss-HRP. Importantly, GRK2ct was able to inhibit transport when specifically targeted to the Golgi, but not when targeted to the PM. Lastly, immunofluorescence microscopy confirmed endogenous Gβ Golgi localization in HeLa cells (Fig. 8). In summary, our results firmly establish the importance of this novel G protein signaling pathway at the Golgi.
The work presented here builds on previous studies that showed that Gβγ was capable of causing fragmentation of the Golgi. Early reports demonstrated that the addition of purified Gβγ to permeabilized cells caused a dramatic fragmentation of the Golgi, and this led to the suggestion that Gβγ might be involved in regulating protein transport (11, 12). More recently, it was shown that overexpression of Gβγ by transient transfection of cells also caused vesiculation of the Golgi (13, 19). The developing model suggested that Gβγ was thus functioning at a novel location, the Golgi, to activate a pathway leading to Golgi vesiculation. However, little information existed as to the subcellular location where Gβγ was acting to regulate this pathway. It remained possible that Gβγ was signaling from its classic location at the PM, and intermediary proteins or second messengers were responsible for transmitting a signal to the Golgi. Our studies in which Gβγ was constitutively or inducibly targeted to the Golgi to cause vesiculation clarify that indeed Gβγ must localize at the Golgi to activate a pathway leading to overactive fission of transport carriers. Gβγ was also previously implicated in the pathway leading to Golgi vesiculation through the study of ilimaquinone, a sea sponge metabolite, that had the intriguing property of converting the Golgi into small vesicles when added to cells (11, 51). Interestingly, the ilimaquinone-mediated Golgi fragmentation was inhibited by addition of purified Gα to the permeabilized cells, suggesting that Gα might be sequestering Gβγ (11). Nonetheless, the exact molecular target of ilimaquinone and the relationship of ilimaquinone effects on Golgi disruption to the pathway regulating the normal generation of TGN-to-PM transport carriers remain unclear. Our results using molecular and pharmacological inhibitors show for the first time a role for endogenous Gβγ in regulating TGN-to-PM vesicle formation at the Golgi membrane.
Taken together, a number of studies indicate that a bona fide signaling pathway exists on the cytoplasmic surface of Golgi membranes to control the fission of PM-destined transport vesicles. All of the known components of this pathway appear to function downstream of Gβγ. Early studies identified the importance of PKD (12). PKD is required for the TGN-to-PM vesicle formation pathway and is recruited to the TGN through binding to DAG (16, 39, 40, 52). More recent data suggest that PKD is activated by another protein kinase, PKCη (13), and the activity and recruitment of PKD and PKCη depend on the DAG levels at the TGN. How DAG levels at the Golgi are regulated has not been clearly defined, although a recent report supports the idea that PLCβ3 is responsible for generating DAG at the Golgi (19). PLCβ3 can be activated by Gβγ, and thus the current model proposes that Gβγ activates PLCβ3 to increase Golgi DAG; DAG serves to recruit and activate PKCη and PKD; and PKD is fully activated through phosphorylation by PKCη. Subsequently, PKD can phosphorylate and activate Golgi-localized PI4KIIIβ, leading to the generation of phosphatidylinositol 4-phosphate and likely the specific recruitment of additional proteins. Further details of the fission pathway and mechanisms remain to be clarified. Our observations showing that inhibition of PKD and PLC blocks Golgi fragmentation by inducibly targeted Golgi-β1γ2 support this model (Fig. 3).
Tubulation of the TGN represents inhibition of the fission of transport carriers and has been used to define critical components of the Golgi-localized signaling pathway. For example, inhibition of PKD not only inhibited protein transport from the TGN to the cell surface but also caused tubulation of the TGN; because fission is blocked, cargo specifically destined for the cell surface accumulates in the elongated tubules (39, 40). Here, we also observed Golgi tubulation when we inhibited the protein transport by sequestering Gβγ at the Golgi membrane using Golgi-targeted GRK2ct (Fig. 6) or when Gβ was knocked down (Fig. 8). This tubulation supports the idea that Gβγ is specifically involved in regulating the signaling pathway leading to transport carrier fission.
Although several components of the Golgi-localized signaling pathway downstream of Gβγ have been defined, the signals upstream of Gβγ remain unknown. Moreover, the mechanisms that allow Gβγ to localize at the Golgi membrane to regulate this signaling pathway need to be addressed. Assembly of the heterotrimeric G protein occurs at endomembranes and is necessary for delivery of the heterotrimer to the PM (1, 21, 26). However, the exact trafficking pathway is controversial in regards to the involvement of the Golgi. Some studies suggest a Golgi-dependent trafficking pathway to the PM of the heterotrimer, whereas others are consistent with heterotrimeric G protein movement from the ER to the PM in a manner independent of the Golgi (26, 53). A further study suggested that the route to the PM of newly synthesized G proteins depends upon complex formation with additional proteins; in this model, if G proteins form a complex with GPCRs at endomembranes, then trafficking to the PM requires the Golgi, but without the GPCR the G protein takes a Golgi-independent pathway (7). As an alternative to Gβγ reaching the Golgi immediately after synthesis, recent evidence has suggested that G proteins can cycle between the PM and the Golgi. Upon activation by a cell-surface GPCR, certain Gβγ can translocate from the PM to the Golgi (54). In addition, there appears to be a constitutive cycle of rapid movement of the G protein heterotrimer between the PM and Golgi (8). Thus, it is possible that a pool of Gβγ resides at the Golgi, or that Gβγ localizes at the Golgi on its way to the PM, or that Gβγ signals at the Golgi during cycles of transport between the PM and Golgi. Our data here provides novel evidence that in fact a pool of Gβ exists at the Golgi membrane (Fig. 8). It will be important to define the cellular pathway and molecular mechanisms that deliver Gβγ to the Golgi so that it can regulate the signaling pathway leading to fission of PM-destined transport carriers.
How is Gβγ itself regulated in this signaling pathway? One model speculates that there are Golgi-localized GPCRs that are activated by specific PM-destined cargo, and this in turn activates Golgi-localized heterotrimeric G proteins thereby releasing free Gβγ (14). Alternatively, a second model proposes that unknown extracellular signals bind to and activate a cell-surface GPCR, which then activates a heterotrimeric G protein at the inner surface of the PM. This would generate free Gβγ at the PM, which would then translocate to the TGN (19). In this regard, during the preparation of this report a study showed that overexpression of certain Gγ subunits that are able to translocate from the PM to the Golgi membrane upon activation of an overexpressed GPCR are able to induce Golgi fragmentation (55). In our experiments, the failure of PM-targeted GRK2ct to inhibit VSV-G transport argues against the second model (Fig. 6). However, the mechanisms of Gβγ activation may differ for regulated versus constitutive transport, as discussed below. The existence of functional GPCRs at endomembrane locations is consistent with the first model (56); however, there is no evidence as of yet for a GPCR that is activated by PM-destined cargo. Another possibility is that Gβγ could be activated by a non-GPCR activator such as a member of the activator of G protein signaling protein family (57). Nevertheless, the relevant upstream regulators of Gβγ in this pathway remain to be identified.
An interesting question is whether Gβγ plays a different role or is differently regulated in extracellular stimulus-regulated Golgi-to-PM transport versus constitutive Golgi-to-PM transport. The recent report of Saini et al., showed that expression of non-translocating Gγ subunit, presumably acting in a dominant negative manner to prevent PM-to-Golgi movement of endogenous Gβγ, was able to inhibit M3 receptor stimulation of insulin secretion (55). However, no inhibition of basal levels of insulin secretion was observed. Thus, translocation of Gβγ from the PM may not account for the stimulation of constitutive, basal protein transport. In this regard, our studies here focused on the constitutive Golgi-to-PM transport of VSV-G and ss-HRP (Figs. 5–7). Further, our work suggests that it is unlikely that Gβγ regulation of constitutive protein transport requires that Gβγ be translocated from the PM to the Golgi in response to extracellular activation of a PM-localized GPCR. First, as discussed above, PM-GRK2ct did not inhibit VSV-G and ss-HRP transport. Second, the VSV-G and ss-HRP transport assays were performed under serum-free media conditions, making it unlikely that extracellular activation of GPCRs was occurring and necessary for constitutive transport.
Another important question pertains to the specificity of Gβγ isoforms. A recent study overexpressed each of β1-β5 together with each of γ1-γ5 and found that only β1γ2 and β3γ2 out of the 25 different combinations were able to cause Golgi vesiculation (13). In agreement with that study, our results show that β5γ2 does not cause Golgi vesiculation, even when constitutively or inducibly targeted to the Golgi (Figs. 1 and 2). Thus, β5γ2 served as a negative control in our studies for the dramatic Golgi fragmentation caused by β1γ2. The inability of β5γ2 and other Gβγ combinations besides β1γ2 and β3γ2 to cause Golgi vesiculation and regulate the PKD-dependent Golgi signaling pathway needs to be addressed to understand the molecular basis of this specificity. Interestingly, β5γ2 is unable to activate PLCβ3, although β5γ2 can activate other PLCβ isoforms (58–60).
In addition to addressing Golgi-localized Gβγ, these studies are the first to take advantage of the rapamycin-regulated heterodimerization system to control heterotrimeric G protein localization and function. The recruitment to an appropriate cellular membrane is often sufficient to activate a signaling pathway, as shown here for rapid Gβγ translocation to the Golgi in response to the addition of rapamycin (Figs. 2–4). It is anticipated that this system will be useful for future studies using Gβγ and Gα subunits as a method of rapidly and inducibly activating G protein signaling pathways in the absence of stimulation of a GPCR.
In summary, the results presented here have clearly demonstrated a role for Golgi-localized Gβγ in regulating a PKD-dependent signaling pathway that controls the fission at the TGN of PM-destined transport carriers. Interestingly, a number of reports have demonstrated a requirement for PKD in diverse processes that rely on TGN-to-PM protein transport. PKD has been implicated in regulating the secretion of several important molecules, including insulin (61–64). In neurons, PKD has been shown to regulate Golgi-dependent trafficking of dendritic membrane proteins (65), neuronal polarity (66), and dendritic arborization (67). PKD has been also shown to be involved in regulating cell motility (68), due to its role in controlling the delivery of proteins to the PM. It will be important to determine whether Gβγ is also a key component of these various processes, and thus establish Gβγ as a novel therapeutic target for controlling protein transport.
Supplementary Material
Acknowledgments
We thank Matthew Martz and Satoshi Takida for generating the γ2-FRB and PM-γ2 plasmids, respectively, and Jeffrey Benovic for providing the GRK2 antibody. We also thank Breann Barker, Rocío Díaz-Benjumea, and Matthew Martz for critically reading the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant GM56444 (to P. B. W.). This work was also supported by an award from the American Heart Association (to R. I.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- GPCR
- G protein-coupled receptor
- GRK
- GPCR kinase
- TGN
- trans-Golgi network
- FKBP
- FK506-binding protein
- FRB
- FKBP-rapamycin binding domain of FRAP
- PKD
- protein kinase D
- PM
- plasma membrane
- VSV-G
- vesicular stomatitis virus-G
- DAG
- diacylglycerol
- PLC
- phospholipase C
- ER
- endoplasmic reticulum
- KDELr
- KDEL receptor
- ss-HRP
- horseradish peroxidase containing a signal sequence from human growth hormone
- CFP
- cyan fluorescent protein.
REFERENCES
- 1.Marrari Y., Crouthamel M., Irannejad R., Wedegaertner P. B. (2007) Biochemistry 46, 7665–7677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smrcka A. V. (2008) Cell Mol. Life Sci. 65, 2191–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boivin B., Vaniotis G., Allen B. G., Hébert T. E. (2008) J. Recept. Signal Transduct. Res. 28, 15–28 [DOI] [PubMed] [Google Scholar]
- 4.Dupré D. J., Robitaille M., Rebois R. V., Hébert T. E. (2009) Annu. Rev. Pharmacol. Toxicol. 49, 31–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denker S. P., McCaffery J. M., Palade G. E., Insel P. A., Farquhar M. G. (1996) J. Cell Biol. 133, 1027–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stow J. L., de Almeida J. B., Narula N., Holtzman E. J., Ercolani L., Ausiello D. A. (1991) J. Cell Biol. 114, 1113–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dupré D. J., Robitaille M., Ethier N., Villeneuve L. R., Mamarbachi A. M., Hébert T. E. (2006) J. Biol. Chem. 281, 34561–34573 [DOI] [PubMed] [Google Scholar]
- 8.Chisari M., Saini D. K., Kalyanaraman V., Gautam N. (2007) J. Biol. Chem. 282, 24092–24098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slessareva J. E., Routt S. M., Temple B., Bankaitis V. A., Dohlman H. G. (2006) Cell 126, 191–203 [DOI] [PubMed] [Google Scholar]
- 10.García-Regalado A., Guzmán-Hernández M. L., Ramírez-Rangel I., Robles-Molina E., Balla T., Vázquez-Prado J., Reyes-Cruz G. (2008) Mol. Biol. Cell 19, 4188–4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jamora C., Takizawa P. A., Zaarour R. F., Denesvre C., Faulkner D. J., Malhotra V. (1997) Cell 91, 617–626 [DOI] [PubMed] [Google Scholar]
- 12.Jamora C., Yamanouye N., Van Lint J., Laudenslager J., Vandenheede J. R., Faulkner D. J., Malhotra V. (1999) Cell 98, 59–68 [DOI] [PubMed] [Google Scholar]
- 13.Díaz Añel A. M., Malhotra V. (2005) J. Cell Biol. 169, 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bard F., Malhotra V. (2006) Annu. Rev. Cell Dev. Biol. 22, 439–455 [DOI] [PubMed] [Google Scholar]
- 15.Fugmann T., Hausser A., Schöffler P., Schmid S., Pfizenmaier K., Olayioye M. A. (2007) J. Cell Biol. 178, 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baron C. L., Malhotra V. (2002) Science 295, 325–328 [DOI] [PubMed] [Google Scholar]
- 17.Hausser A., Storz P., Märtens S., Link G., Toker A., Pfizenmaier K. (2005) Nat. Cell Biol. 7, 880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghanekar Y., Lowe M. (2005) Trends Cell Biol. 15, 511–514 [DOI] [PubMed] [Google Scholar]
- 19.Díaz Añel A. M. (2007) Biochem. J. 406, 157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evanko D. S., Thiyagarajan M. M., Siderovski D. P., Wedegaertner P. B. (2001) J. Biol. Chem. 276, 23945–23953 [DOI] [PubMed] [Google Scholar]
- 21.Takida S., Wedegaertner P. B. (2003) J. Biol. Chem. 278, 17284–17290 [DOI] [PubMed] [Google Scholar]
- 22.Inoue T., Heo W. D., Grimley J. S., Wandless T. J., Meyer T. (2005) Nat. Methods 2, 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ausubel F. M., Brent R. E., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. (1992) Short Protocols in Molecular Biology, 2nd. Ed., pp. 8-23–8-25, John Wiley & Sons, New York [Google Scholar]
- 24.Carman C. V., Barak L. S., Chen C., Liu-Chen L. Y., Onorato J. J., Kennedy S. P., Caron M. G., Benovic J. L. (2000) J. Biol. Chem. 275, 10443–10452 [DOI] [PubMed] [Google Scholar]
- 25.Krumins A. M., Gilman A. G. (2006) J. Biol. Chem. 281, 10250–10262 [DOI] [PubMed] [Google Scholar]
- 26.Michaelson D., Ahearn I., Bergo M., Young S., Philips M. (2002) Mol. Biol. Cell 13, 3294–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu V. K., Bivona T., Hach A., Sajous J. B., Silletti J., Wiener H., Johnson R. L., 2nd, Cox A. D., Philips M. R. (2002) Nat. Cell Biol. 4, 343–350 [DOI] [PubMed] [Google Scholar]
- 28.Cole N. B., Smith C. L., Sciaky N., Terasaki M., Edidin M., Lippincott-Schwartz J. (1996) Science 273, 797–801 [DOI] [PubMed] [Google Scholar]
- 29.Arozarena I., Matallanas D., Berciano M. T., Sanz-Moreno V., Calvo F., Muñoz M. T., Egea G., Lafarga M., Crespo P. (2004) Mol. Cell Biol. 24, 1516–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orci L., Stamnes M., Ravazzola M., Amherdt M., Perrelet A., Söllner T. H., Rothman J. E. (1997) Cell 90, 335–349 [DOI] [PubMed] [Google Scholar]
- 31.Pitcher J. A., Inglese J., Higgins J. B., Arriza J. L., Casey P. J., Kim C., Benovic J. L., Kwatra M. M., Caron M. G., Lefkowitz R. J. (1992) Science 257, 1264–1267 [DOI] [PubMed] [Google Scholar]
- 32.Bayle J. H., Grimley J. S., Stankunas K., Gestwicki J. E., Wandless T. J., Crabtree G. R. (2006) Chem Biol. 13, 99–107 [DOI] [PubMed] [Google Scholar]
- 33.Banaszynski L. A., Liu C. W., Wandless T. J. (2005) J. Am. Chem. Soc. 127, 4715–4721 [DOI] [PubMed] [Google Scholar]
- 34.Chen J., Zheng X. F., Brown E. J., Schreiber S. L. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 4947–4951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi J., Chen J., Schreiber S. L., Clardy J. (1996) Science 273, 239–242 [DOI] [PubMed] [Google Scholar]
- 36.Bonacci T. M., Mathews J. L., Yuan C., Lehmann D. M., Malik S., Wu D., Font J. L., Bidlack J. M., Smrcka A. V. (2006) Science 312, 443–446 [DOI] [PubMed] [Google Scholar]
- 37.Lehmann D. M., Seneviratne A. M., Smrcka A. V. (2008) Mol. Pharmacol. 73, 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onken B., Wiener H., Philips M. R., Chang E. C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 9045–9050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeaman C., Ayala M. I., Wright J. R., Bard F., Bossard C., Ang A., Maeda Y., Seufferlein T., Mellman I., Nelson W. J., Malhotra V. (2004) Nat. Cell Biol. 6, 106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liljedahl M., Maeda Y., Colanzi A., Ayala I., Van Lint J., Malhotra V. (2001) Cell 104, 409–420 [DOI] [PubMed] [Google Scholar]
- 41.De Matteis M. A., Luini A. (2008) Nat. Rev. Mol. Cell Biol. 9, 273–284 [DOI] [PubMed] [Google Scholar]
- 42.Bard F., Casano L., Mallabiabarrena A., Wallace E., Saito K., Kitayama H., Guizzunti G., Hu Y., Wendler F., Dasgupta R., Perrimon N., Malhotra V. (2006) Nature 439, 604–607 [DOI] [PubMed] [Google Scholar]
- 43.Connolly C. N., Futter C. E., Gibson A., Hopkins C. R., Cutler D. F. (1994) J. Cell Biol. 127, 641–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sehgal P. B., Mukhopadhyay S., Xu F., Patel K., Shah M. (2007) Am. J. Physiol. Lung Cell Mol. Physiol. 292, L1526–1542 [DOI] [PubMed] [Google Scholar]
- 45.Bossard C., Bresson D., Polishchuk R. S., Malhotra V. (2007) J. Cell Biol. 179, 1123–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stow J. L., Heimann K. (1998) Biochim. Biophys. Acta 1404, 161–171 [DOI] [PubMed] [Google Scholar]
- 47.Leyte A., Barr F. A., Kehlenbach R. H., Huttner W. B. (1992) EMBO J. 11, 4795–4804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barr F. A., Leyte A., Mollner S., Pfeuffer T., Tooze S. A., Huttner W. B. (1991) FEBS Lett. 294, 239–243 [DOI] [PubMed] [Google Scholar]
- 49.Gkantiragas I., Brügger B., Stüven E., Kaloyanova D., Li X. Y., Löhr K., Lottspeich F., Wieland F. T., Helms J. B. (2001) Mol. Biol. Cell 12, 1819–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Helms J. B., Helms-Brons D., Brügger B., Gkantiragas I., Eberle H., Nickel W., Nürnberg B., Gerdes H. H., Wieland F. T. (1998) J. Biol. Chem. 273, 15203–15208 [DOI] [PubMed] [Google Scholar]
- 51.Takizawa P. A., Yucel J. K., Veit B., Faulkner D. J., Deerinck T., Soto G., Ellisman M., Malhotra V. (1993) Cell 73, 1079–1090 [DOI] [PubMed] [Google Scholar]
- 52.Maeda Y., Beznoussenko G. V., Van Lint J., Mironov A. A., Malhotra V. (2001) EMBO J. 20, 5982–5990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takida S., Wedegaertner P. B. (2004) FEBS Lett. 567, 209–213 [DOI] [PubMed] [Google Scholar]
- 54.Akgoz M., Kalyanaraman V., Gautam N. (2004) J. Biol. Chem. 279, 51541–51544 [DOI] [PubMed] [Google Scholar]
- 55.Saini D. K., Karunarathne W. K., Angaswamy N., Saini D., Cho J. H., Kalyanaraman V., Gautam N. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 11417–11422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dupré D. J., Hébert T. E. (2006) Cell Signal 18, 1549–1559 [DOI] [PubMed] [Google Scholar]
- 57.Blumer J. B., Smrcka A. V., Lanier S. M. (2007) Pharmacol. Ther. 113, 488–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshikawa D. M., Hatwar M., Smrcka A. V. (2000) Biochemistry 39, 11340–11347 [DOI] [PubMed] [Google Scholar]
- 59.Yost E. A., Mervine S. M., Sabo J. L., Hynes T. R., Berlot C. H. (2007) Mol. Pharmacol. 72, 812–825 [DOI] [PubMed] [Google Scholar]
- 60.Watson A. J., Katz A., Simon M. I. (1994) J. Biol. Chem. 269, 22150–22156 [PubMed] [Google Scholar]
- 61.Sumara G., Formentini I., Collins S., Sumara I., Windak R., Bodenmiller B., Ramracheya R., Caille D., Jiang H., Platt K. A., Meda P., Aebersold R., Rorsman P., Ricci R. (2009) Cell 136, 235–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim M. S., Wang F., Puthanveetil P., Kewalramani G., Hosseini-Beheshti E., Ng N., Wang Y., Kumar U., Innis S., Proud C. G., Abrahani A., Rodrigues B. (2008) Circ. Res. 103, 252–260 [DOI] [PubMed] [Google Scholar]
- 63.Li J., Chen L. A., Townsend C. M., Jr., Evers B. M. (2008) J. Biol. Chem. 283, 2614–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.von Wichert G., Edenfeld T., von Blume J., Krisp H., Krndija D., Schmid H., Oswald F., Lother U., Walther P., Adler G., Seufferlein T. (2008) Cell Signal 20, 925–934 [DOI] [PubMed] [Google Scholar]
- 65.Bisbal M., Conde C., Donoso M., Bollati F., Sesma J., Quiroga S., Díaz Añel A., Malhotra V., Marzolo M. P., Cáceres A. (2008) J. Neurosci. 28, 9297–9308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yin D. M., Huang Y. H., Zhu Y. B., Wang Y. (2008) J. Neurosci. 28, 8832–8843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Czöndör K., Ellwanger K., Fuchs Y. F., Lutz S., Gulyás M., Mansuy I. M., Hausser A., Pfizenmaier K., Schlett K. (2009) Mol. Biol. Cell 20, 2108–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prigozhina N. L., Waterman-Storer C. M. (2004) Curr. Biol. 14, 88–98 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.