Abstract
The flavivirus methyltransferase (MTase) sequentially methylates the N7 and 2′-O positions of the viral RNA cap (GpppA-RNA → m7GpppA-RNA → m7GpppAm-RNA), using S-adenosyl-l-methionine (AdoMet) as a methyl donor. We report here that sinefungin (SIN), an AdoMet analog, inhibits several flaviviruses through suppression of viral MTase. The crystal structure of West Nile virus MTase in complex with SIN inhibitor at 2.0-Å resolution revealed a flavivirus-conserved hydrophobic pocket located next to the AdoMet-binding site. The pocket is functionally critical in the viral replication and cap methylations. In addition, the N7 methylation efficiency was found to correlate with the viral replication ability. Thus, SIN analogs with modifications that interact with the hydrophobic pocket are potential specific inhibitors of flavivirus MTase.
Keywords: Antiviral Agents, Flaviviruses, RNA, RNA Methylation, RNA Methyltransferase, RNA Modification, RNA-Protein Interaction, S-Adenosylmethionine (AdoMet), Virus, RNA Capping
Introduction
The genus Flavivirus in the family Flaviviridae is composed of more than 70 viruses (1). Many flaviviruses are arthropod-borne and cause significant human disease. The four serotypes of Dengue virus (DENV),5 yellow fever virus (YFV), West Nile virus (WNV), Japanese encephalitis virus, and tick-borne encephalitis virus, are categorized as global emerging pathogens and are also National Institutes of Health NIAID Priority Pathogens (2). The World Health Organization has estimated annual human cases of more than 50 million, 200,000, and 50,000 for DENV (3), YFV (4), and Japanese encephalitis virus (5), respectively. Approximately 2.5 billion people are at risk of DENV infection (3). Since 1999, WNV has spread rapidly throughout the Western Hemisphere (6). Vaccines for humans are currently available only for YFV, Japanese encephalitis virus, and tick-borne encephalitis virus (2). No clinically approved antiviral therapy is available for treatment of flavivirus infections. Therefore, the development of vaccines and antiviral agents for prevention and treatment of flavivirus infections is a clear public health priority (6).
The flavivirus genomic RNA is single-stranded and of positive (i.e. mRNA) polarity. A type I cap (see details below) is present at the 5′ end, followed by the conserved dinucleotide sequence 5′-AG-3′ (m7GpppAmG) (7). The 3′ end of the genome terminates with 5′-CUOH-3′ rather than with a poly(A) tract (8). The viral genome is ∼11 kb in length, consisting of a 5′ UTR, a single long open reading frame (ORF), and a 3′ UTR (9). The single ORF encodes a polyprotein that is co- and post-translationally processed by viral and cellular proteases into three structural proteins (capsid (C), premembrane (prM), or membrane (M), and envelope (E)), and seven nonstructural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5) (10). The nonstructural proteins are assumed to be involved primarily in the replication of viral RNA, as components of a replicase complex. Among the NS proteins, NS3 is a multifunctional protein with activities of a serine protease, an RNA triphosphatase, a nucleoside triphosphatase, and a helicase (11–13); NS5 has the functions of an RNA-dependent RNA polymerase (14–16) and an MTase involved in methylation of the 5′-RNA cap structure (17–20).
Most eukaryotic and viral mRNAs possess a 5′-cap that is important for mRNA stability and efficient translation (21). Based on what is known about cap formation for cellular mRNA (22, 23), four enzymatic modifications are involved in the formation of the type 1 cap structure as follows. (i) The 5′-triphosphate of nascent pre-mRNA is hydrolyzed to a 5′-diphosphate by an RNA triphosphatase. (ii) The diphosphate RNA end is capped with GMP by an RNA guanylyltransferase. (iii) The GpppN cap is methylated at the N7 position of guanine by an RNA guanine methyltransferase (N7 MTase), resulting in type 0 cap (m7GpppN) (24). (iv) The first and second nucleotides of many cellular and viral mRNAs are further methylated at the ribose 2′-OH position by a nucleoside 2′-O-MTase, to form type 1 cap (m7GpppNm) and type 2 cap (m7GpppNmNm) structures, respectively (21). Both N7 and 2′-O-MTases use S-adenosyl-l-methionine (AdoMet) as a methyl donor and generate S-adenosyl-l-homocysteine (AdoHcy) as a by-product.
Because flaviviruses replicate in the host cytoplasm, they are assumed to encode their own capping enzymes, rather than using the capping apparatus of the host that is located in the nucleus. Among the four enzymes required for flavivirus m7GpppAm-cap formation, the RNA triphosphatase has been mapped to NS3 (12, 13). Recent studies suggest that the N-terminal methyltransferase (MTase) domain of NS5 has a guanylyltransferase activity, transferring the GMP moiety of GTP to the 5′ end of ppA-RNA, resulting in GpppA-RNA (25, 26). We and others have shown that recombinant NS5 proteins from various flaviviruses possess both N7 and 2′-O-MTase activities (18–20, 27–29). Compared with cellular and many other viral MTases, flavivirus MTase is unique in that a single MTase domain catalyzes two methylation events, in the order of GpppA → m7GpppA → m7GpppAm (19, 20). Additionally, both cap methylations are dependent on viral RNA sequence (27). Despite these two distinct methylation activities, the crystal structure of flavivirus methyltransferase exhibits only a single AdoMet-binding site as methyl donor (18, 30–34).
Viruses represent an attractive system for the study of RNA capping. The capping mechanisms of some viral mRNAs are different from those seen for the cellular mRNA (21). The differences between host and viral cap formation could potentially be used for development of antiviral therapy. Flavivirus MTase has been shown to be essential for WNV (20, 27), Kunjin virus (35), YFV (36), and DENV replication (37). Furthermore, sinefungin (SIN), an AdoMet analog, was shown to inhibit both N7 and 2′-O-methylations of WNV MTase, as well as to inhibit WNV replication in cell culture (28). Those results suggest that SIN analogs have the potential to be developed for flavivirus therapy. However, because AdoMet is also a methyl donor for host RNA and protein methylations, its analogs would nonspecifically suppress host MTases, resulting in toxicity. Therefore, identification of features unique to the flaviviral MTase is critical for the design of specific inhibitors of viral MTase.
Here, we extend our previous studies to show that SIN not only inhibits WNV replication but can also inhibit the replications of several other flaviviruses, i.e. DENV and YFV. We have also determined the co-crystal structure of the WNV MTase·SIN complex at 2.0-Å resolution. The crystal structure of the WNV MTase·SIN complex reveals the presence of a hydrophobic pocket extension of the AdoMet-binding pocket; this feature is conserved among various flaviviruses. Point mutations of conserved residues within the pocket impair, to various extents, either one or both of the N7 and 2′-O-methylation activities. We further show that residues within the pocket are important in viral replication and that the N7 methylation efficiency correlates with the viral replication ability. Thus, SIN analogs with modifications that interact with the hydrophobic pocket are potential specific inhibitors of flavivirus methyltransferase.
EXPERIMENTAL PROCEDURES
Cloning, Expression, and Purification of the WNV, WNV Mutant, YFV, and DENV-2 MTase
The WNV MTase domain containing the N-terminal 300 amino acids of NS5 was prepared for co-crystallization and enzyme assays as described previously (19, 20). A QuikChange II XL site-directed mutagenesis kit (Stratagene) was used to engineer the MTase mutants, a standard overlapping PCR-based method that we developed previously (38), and was used for construction of mutant WNV replicons.
DNA fragments representing the N-terminal 272 amino acids of DENV-2 were RT-PCR-amplified from the DENV-2 genomic RNA (NGC strain) and cloned into plasmid pET26b(+) (Novagen) at the NdeI and XhoI sites. The DENV-2 MTase containing a C-terminal His6 tag was expressed and purified through a nickel-nitrilotriacetic acid column followed by a gel filtration 16/60 Superdex column (Amersham Biosciences).
Crystallization, X-ray Data Collection, Structure Determination, and Refinement
Co-crystals of the MTase·SIN complex were grown by methods described previously (20), except in the presence of 5 mm SIN. The co-crystals belong to space group P1 and are isomorphous to the native crystals that we grew previously (Table 1) (20). Prior to data collection, all crystals were transferred to a reservoir solution containing 25% glycerol, then flashed-cooled under a nitrogen stream at 100 K, and stored in liquid nitrogen. Diffraction data were collected to 2.0 Å resolution at 100 K at beamline X4A of the National Synchrotron Light Source (Brookhaven National Laboratory). All of the data were processed and scaled using HKL2000 (Table 1) (39).
TABLE 1.
Diffraction data collection and structure refinement statistics
| Data collection | |
| Space group | P1 |
| Cell parameters | a = 39.36, b = 65.74, c = 77.12 Å α = 112.01°, β = 102.65°, γ = 90.19° |
| Resolution | 39.1-2.0 Å |
| Redundancy | 1.8 (1.5)a |
| Completeness | 81.2% (37.6%) |
| Average I/σ(I) | 5.3 (2.2) |
| Rsym | 10.4% (33.3%) |
| Refinement | |
| Resolution limits | 39.1-2.0 Å |
| No. reflections | 47,068 |
| Rwork | 23.0% |
| Rfree | 26.9% |
| Non-H atoms | |
| Protein | 4202 |
| Water | 505 |
| Glycerol | 2 |
| Sinefungin | 2 |
| Average B | 19.5 Å2 |
| Geometry | |
| r.m.s.d.b bond length | 0.007 Å |
| r.m.s.d. bond angle | 1.2° |
a Values in parentheses are those for the highest resolution shell.
b r.m.s.d., root mean square deviation.
The structure of the MTase·SIN complex was determined by the difference Fourier method, with the native structure of the WNV MTase (PDB code 2OY0) used as a starting model (20). Structural refinement was carried out using CNS (Table 1) (40). At 2.8 Å resolution, the final Rcryst was 23%, with an Rfree of 26.9%. The final refinement statistics are summarized in Table 1.
In Vitro MTase Inhibition Assay
The N7 and 2′-O-methylation inhibition assays were performed as described previously for WNV (28, 41), except that the 5′-end-labeled substrates G*pppA-RNA and m7G*pppA-RNA, representing the first 211 nucleotides of the DENV-2 genome (the asterisk indicates that the following phosphate was 32P-labeled), were used. The N7 methylation was measured by conversion of G*pppA-RNA → m7G*pppA-RNA. The 2′-O-methylation was monitored by conversion of m7G*pppA-RNA → m7G*pppAm-RNA. Both methylation assays were performed with 0.25 μg of DENV-2 MTase, 25 μm AdoMet, and various concentrations of SIN. The methylation reactions were digested with nuclease P1 to release cap moieties (m7G*pppAm, m7G*pppA, and G*pppA). The cap molecules were separated on a TLC and quantified by a PhosphorImager (28, 41).
Inhibition of Virus Replication by SIN
The assay for inhibition of DENV-2 and YFV by SIN was performed as described previously for WNV (28). Baby hamster kidney cells were infected with DENV-2 (NGC strain) or YFV (17D strain) at a multiplicity of infection of 0.3 for 1 h at 37 °C. The viral inocula were washed and replaced with DMEM plus 10% FBS containing various concentrations of SIN. Culture supernatants were collected at 48 h post-infection, and viral titers were determined by plaque assay (42). For the cytotoxicity assay, baby hamster kidney cells were incubated with various concentrations of SIN and assayed for cell viability at 48 h post-treatment using an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay kit (American Type Culture Collection).
Replicon Assay
Transient replication of wild-type (WT) and mutant WNV was performed using a replicon assay as described previously (43). Briefly, mutant replicons (G148A, R160A, R163A, V164A, and L184A) were prepared using a standard PCR-mediated mutagenesis method. Equal amounts of RNA (2 μg) for each replicon were electroporated into baby hamster kidney cells. The transfected cells were resuspended in 25 ml of DMEM with 10% FBS; 1 ml of cell suspension was seeded into each well of a 12-well plate. Luciferase activity was measured at various time points. The protocols for in vitro RNA transcription, RNA transfection, and luciferase assays were reported previously (43).
RESULTS
Inhibition of Flavivirus Replication by SIN
SIN differs from AdoMet in that the S-CH3 sulfonium moiety is replaced with a C-NH2 amine (Fig. 1A). To examine whether SIN inhibits flaviviruses other than WNV, we performed viral titer reduction assays using DENV-2 and YFV. We found that SIN inhibits DENV-2 and YFV in a dose-responsive manner, with values for the EC50 (the concentration at which 50% of virus is inhibited) of 77 and 250 μm, respectively (Fig. 1B). Interestingly, DENV-2 was more sensitive to SIN inhibition than was YFV; at 250 μm, SIN reduced DENV-2 and YFV titers by about 6-fold and 50%, respectively. A cell proliferation-based MTT assay (42) showed no cytotoxicity when uninfected cells were treated with SIN at concentrations up to 300 μm, indicating that the observed antiviral activity was not due to compound-mediated cytotoxicity (Fig. 1C).
FIGURE 1.
Inhibition of flavivirus replication through suppression of viral MTase by SIN. A, chemical structures of AdoMet (SAM) and SIN. B, inhibition of DENV-2 and YFV by SIN. Data represent means ± S.D. (n ≥ 3). PFU, plaque-forming units. C, cytotoxicity of SIN. D, inhibition of N7 and 2′-O-methylations of DENV-2 MTase by SIN. Left panel, SDS-PAGE analysis of recombinant DENV-2 MTase. Molecular masses of protein markers are labeled. Right panel, SIN-mediated inhibitions of N7 and 2′-O-methylations of DENV-2 MTase were analyzed on TLC plates. The N7 methylation was measured by conversion of G*pppA-RNA → m7G*pppA-RNA (the asterisk indicates that the following phosphate is 32P-labeled; the RNA represents the first 211 nucleotides of the DENV-2 genome). The 2′-O-methylation was monitored by conversion of m7G*pppA-RNA →m7G*pppAm-RNA. The spots representing different cap structures on TLC plates were quantified by a PhosphorImager. The remaining enzymatic activities are presented below the TLC plates. The methylation activities without SIN were set at 100%. The position of the origin and the migration positions of the G*pppA, m7G*pppA, and m7G*pppAm molecules are labeled on the side of the TLC images.
SIN Directly Inhibits Flavivirus MTase Activities
To demonstrate that the observed antiviral activity of SIN was due to direct inhibition of the viral MTase, we expressed and purified the DENV-2 MTase domain (N-terminal 272 amino acids of NS5; Fig. 1D, left panel). The recombinant MTase was assayed for methylations in the presence or absence of SIN. The conversion of G*pppA-RNA → m7G*pppA-RNA (the asterisk indicates that the following phosphate was 32P-labeled; the RNA represents the first 211 nucleotides of the DENV-2 genomic RNA) was measured for N7 methylation. The conversion of m7G*pppA-RNA → m7G*pppAm-RNA was measured for 2′-O-methylation. As shown in Fig. 1D (middle and right panels), SIN inhibited both N7 and 2′-O-methylations of DENV-2 MTase in a dose-responsive manner, with values for IC50 (the concentration at which 50% methylation activity is inhibited) of 0.7 and 19 μm, respectively. The difference in IC50 values suggests that, during the two methylation reactions, AdoMet is bound to MTase in two distinct conformations. Alternatively, it may suggest that the inhibitor affects how the distinct moieties (the base and the ribose) of the 5′ end of the RNA substrates interact with the active site during the two methylation reactions. Nevertheless, the above results, together with our previous WNV data (28), demonstrate that SIN inhibits multiple members of flaviviruses through suppression of the viral MTase.
Crystal Structure of WNV MTase in Complex with SIN
Because SIN inhibits flavivirus replication, structural information about the MTase·SIN complex will be valuable to guide rational design of new inhibitors of flavivirus MTase. We determined the crystal structure of the WNV NS5 MTase domain in complex with SIN at 2.0 Å resolution (Table 1 and Fig. 2), with a final Rcryst of 23% and an Rfree of 26.9%.
FIGURE 2.
Crystal structure of the WNV MTase·SIN complex. A, representative omit (Fo – Fc) electron density map (magenta) showing the bound SIN molecule and its interactions with the surrounding MTase residues. The MTase is shown in a ribbon representation (green). The bound SIN is shown in ball-and-stick representation, with atom colors as follows: carbon (yellow), oxygen (red), nitrogen (blue), and sulfur (green). Hydrogen bonds are shown as orange dashed lines. B, superposition of the crystal structures of the MTase·SIN complex (pink) and the MTase·AdoHcy complex (cyan) (20). AdoHcy and SIN are shown in stick representation. C, surface representation of the WNV MTase, showing the additional pocket adjacent to the adenine of SIN (stick representation with green and blue bond colors, partially buried and see below for the close-up view (Fig. 2E)). The surface of the pocket is colored according to atomic colors: carbon, yellow; oxygen, red; nitrogen, blue. The rest of the MTase surface is colored white. SIN is shown as stick representation with carbon colored green and nitrogen blue. D, amino acids involved in the formation of the additional pocket. Residues are labeled and shown in a ball-and-stick representation, with atom colors as follows: carbon, yellow; oxygen, red; and nitrogen, blue. Hydrogen bonds are shown as orange dashed lines. E, close-up view of the flavivirus-conserved pocket continuous with the AdoMet-binding pocket in the WNV MTase·SIN complex (this study), with surface and atom colors the same as used in C (left panel). Six water molecules (red spheres) binds to the additional pocket of the WNV MTase (left panel). Similar pockets exist in the DENV-2 MTase (PDB code 1L9K (18); (middle panel)) and YFV MTase (PDB code 3EVA (33); (right panel)); in each frame the AdoHcy (SAH)/AdoMet/SIN molecule is depicted in stick representation. F, close-up view of the AdoMet-binding region of representative human AdoMet-utilizing proteins (PDB codes are labeled), including SET-like histone lysine MTase (PDB code 3FPD) (45), RNA MTase domain of human TARBP1 (PDB code 2HA8) (46), catechol-O-MTase domain containing 1 (PDB code 2AVD, Structural Genomics Consortium), and dimethyladenosine transferase (PDB code 1ZQ9, Structural Genomics Consortium). The images were produced using PyMOL (48).
In the final model, each chain of the MTase homodimer contains 262 amino acids, and each molecule binds one SIN molecule (Fig. 2A). Although the completeness of diffraction data is less than ideal, the high resolution, 2.0 Å, conferred a good accuracy to the structural model of the WNV MTase, allowing the correct tracing of a fragment (amino acids 172–177) that had been poorly defined in the MTase·AdoHcy complex structure at 2.8 Å resolution (Fig. 2B) (20). It should be noted that this loop is spatially distant from the active site of the MTase, as discussed previously (20). Therefore, the conformational discrepancy of this loop does not affect our original interpretation of the MTase function (20, 28, 29).
Structural Comparison between the MTase·SIN and MTase·AdoHcy Complexes
The overall structure of the WNV MTase·SIN complex (Fig. 2, B and C) is nearly identical to that of the MTase·AdoHcy complex that was determined previously (20). The comparison yields an overall Cα root mean square deviation (r.m.s.d.) of 0.34 Å. No significant conformational change occurs upon replacement of AdoHcy with SIN. In the AdoHcy/AdoMet/SIN-binding pocket, all of the residues superimpose very well (data not shown). The only significant conformational difference occurs for the side chain of a nearby residue Arg-163; both conformations are confirmed by clear electron densities in their respective structures. Arg-163 is one of the important residues forming the extended flavivirus-conserved pocket (see below); mutation of Arg-163 resulted in 50% reduction of the N7 methylation activity (see below). Nevertheless, the conformational differences seen for this residue may not be related to the AdoHcy/SIN occupancy, because Arg-163 is over 9 Å from the adenine base of AdoHcy/SIN (Fig. 2D). Instead, given that Arg-163 is surface-exposed, the difference may simply reflect a degree of conformational flexibility of surface residues.
SIN binds to the AdoMet pocket of the MTase in a conformation similar to that of AdoHcy in the MTase·AdoHcy complex. The overall r.m.s.d. for all SIN and AdoHcy atoms is about 0.47 Å, which is slightly higher than the overall Cα r.m.s.d. of the MTase molecules in the two structures. In general, SIN binds more deeply in the pocket than AdoHcy does (Fig. 2B). In the MTase·SIN complex, the free amine Nϵ of the C-NH2 group, i.e. the group that replaces the S-CH3 group of AdoMet, points outward from the pocket. Interestingly, the free amine Nϵ of SIN is only 3.7–3.9 Å away from the carboxyl side chain atom OD1 of Asp-146 (Fig. 2D). Asp-146 was previously shown to be essential for both N7 and 2′-O-methylation activities of the WNV MTase (20). It is reasonable to postulate that in an MTase·AdoMet complex, the CH3 donor group of AdoMet will be at a position similar to that of SIN Nϵ. The proximity of the donor methyl group of AdoMet and the Asp-146 side chain provides structural evidence for a potential catalytic role of Asp-146 in the N7 MTase function. Nevertheless, the conformational similarity between the MTase·AdoHcy and MTase·SIN complexes is consistent with the fact that SIN and AdoMet/AdoHcy do not have significant structural differences.
Additional Pocket Near the Adenosine Base of SIN
Upon examination of the crystal structures of the WNV MTase·SIN and MTase·AdoHcy complexes (20), we observed an additional pocket that is an extension of the cavity holding the adenine base of AdoMet or the analogs (AdoHcy or SIN) (Fig. 2C). The extended pocket is nearly square shaped with approximate dimensions of 8 × 9 Å and ∼5 Å deep. The additional pocket and the AdoMet-binding pocket together form a large continuous pocket.
The nature of the extended pocket is mainly hydrophobic, with a deep hole in the bottom along the wall away from the adenine base (Fig. 2E, left panel). That side of the pocket is occupied with six ordered water molecules (only five are visible and the sixth is deeply trapped inside the hole) that form hydrogen bonds with the main chain and side chain atoms of residues forming the pocket. Four of the water molecules are located above the pocket (i.e. out in the solvent), forming hydrogen bonds with Arg-163 side chain atom NH1, and with Glu-149(N) and Arg-160(O). Interestingly, these four water molecules, which form hydrogen bonds with one another, are organized in such a way that they could be connected to form a ring structure as shown by a continuous electron density in the map (map not shown). The other two water molecules are trapped inside the deep hole (one visible and one deeply buried), forming hydrogen bonds with main chain atoms (Ile-147(O), Ile-147(N), Lys-182(O), and Leu-184(N)) (data not shown).
Further analysis indicated that a similar pocket exists in all known structures of flavivirus MTases (18, 20, 30–34, 44). Fig. 2E shows the surface of the additional pocket of MTases of representative flaviviruses DENV-2 (18) and YFV (33) (middle and right panels), although the deep hole is not seen in the latter two structures. These results indicate that the additional pocket is a general feature for flavivirus MTases. In addition, upon examination of known structures of AdoMet-utilizing proteins (over 100 structures available), we found that the extended pocket is specific to the flaviviruses, as it has not been seen in structures of other AdoMet-utilizing proteins. Fig. 2F shows the surface representation of the AdoMet-binding region of several representative human AdoMet-utilizing proteins, including SET-like histone lysine MTase (PDB code 3FPD) (45), RNA MTase domain of human TARBP1 (PDB code 2HA8) (46), small molecule catechol-O-MTase domain containing 1 (PDB code 2AVD, Structural Genomics Consortium), and dimethyladenosine transferase (PDB code 1ZQ9, Structural Genomics Consortium). Clearly, none of these AdoMet-utilizing proteins present the extended pocket as seen for the flavivirus MTases.
The extended pocket is formed by residues Phe-133, Ile-147, Gly-148, Glu-149, Arg-160, Arg-163, Val-164, and Leu-184 of the WNV MTase (Fig. 2D). The long side chain of Arg-163 and part of Phe-133 forms the steep wall of one side of the pocket, whereas the opposite side is lined up by the long side chains of Arg-160 and Glu-149. Residues Ile-147 and Val-164 together with part of Phe-133 form the floor of the pocket. Leu-184 is located inside the deep hole of the pocket. Gly-148 stabilizes the conformation of Glu-149, a residue critical for the N7 methylation (19, 28).
It should be noted that the AdoMet-binding pocket and the additional pocket together form one continuous pocket. Several residues such as Phe-133, Ile-147, and Glu-149 are shared by the two portions of the pocket and they participate in AdoMet binding, whereas others are associated only with either the extension portion or the AdoMet-binding portion. Among these residues, Lys-105, His-110, and Asp-131 form the steep wall side of the AdoMet-binding pocket to hold the adenine base of the AdoMet/AdoHcy/SIN cofactor/by-product/inhibitor; these three residues are not part of the additional pocket. Within this additional pocket, residues Gly-148, Arg-160, Arg-163, Val-164, and Leu-184 do not interact directly with the inhibitor SIN or co-factor/by-product AdoMet/AdoHcy (Fig. 2D).
Residues That Form the Additional Pocket Are Conserved
Sequence alignment of flavivirus MTases indicated that residues at positions 133, 147, 148, 149, 160, 163, 164, and 184 of the WNV MTase are conserved among flaviviruses (Table 2 and supplemental Figs. 1 and 2) (20, 34). Among these residues, Gly-148 and Glu-149 are completely conserved. Of 61 flaviviruses with known sequences, residues Ile-147, Arg-160, and Leu-184 are nearly completely conserved, with only one or two conservative (one exception) variations for each residue (Meaban virus (Val-147), Rio Bravo virus (Lys-160), Tamana bat virus (Gly-160, Met-184), and Kedougou virus (Phe-184)). Val-164 is conserved in more than 92% of flavivirus MTases, with remaining ones (5 of 61) showing a conserved substitution of Ile at this position. Arg/Lys-163 is present in over 95% of flavivirus MTases, with three viruses (Rio Bravo virus, Modoc virus, and Apoi virus) showing a Glu, Thr, or Gln at this position, respectively. The majority (37 of 56, 61%) of flavivirus MTase sequences show a Phe at position 133; other bulky residues such as His and Tyr are seen for a few flaviviruses (about 23%) at this position.
TABLE 2.
Amino acid sequence alignment of residues that form the conserved additional pocket of flavivirus MTases
AA indicates amino acid.
1 All virus abbreviations used in the alignment are those of the International Committee on Taxonomy of Viruses. Abbreviations for mosquito-borne flaviviruses are as follows: West Nile virus (WNV); Chaoyang virus (CYV); Dengue virus serotype 1–4 (DENV1–4); Ilheus virus (ILHV); Japanese encephalitis virus (JEV); Lammi virus (LAMV); St. Louis encephalitis virus (SLEV); Zika virus (ZIKV); Aroa virus (AROAV); Bagaza virus (BAGV); Edge Hill virus (EHV); Kokobera virus (KOKV); Sepik virus (SEPV), Wesselsbron virus (WESSV); Banzi virus (BANV); Barkedji virus (BKV), Juga virus (JUGV); Yellow fever virus (YFV); Murray Valley encephalitis virus (MVEV); Usutu virus (USUV); Bouboui virus (BOUV); Nounané virus (NOUV); Potiskum virus (POTV); Saboya virus (SABV); Uganda virus (UGSV); Kedougou virus (KEDV). Abbreviations for tick-borne flaviviruses are as follows: Western, Siberian, and Far Eastern subtypes of the tick-borne encephalitis virus (WTBEV, STBEV, FETBEV); Loupig ill virus (LIV); Omsk hemorrhagic fever virus (OHFV); Greek goat encephalitis virus (GGEV); Gadgets Gully virus (GGYV); Karshi virus (KSIV); Langat virus (LGTV); Powassan virus (POWV); Royal Farm virus (RFV); Saumarez Reef virus (SREV); Spanish sheep encephalitis virus (SSEV); Turkish sheep encephalitis virus (TSEV); Tyuleniy virus (TYUV); Alkhurma hemorrhagic fever virus (AHFV); Kyasanur Forest disease virus (KFDV); Kadam virus (KADV); Meaban virus (MEAV). Abbreviations for tick-borne flaviviruses with no known arthropod vector are as follows: Montana myotis leukoencephalitis virus (MMLV); Aedes flavivirus (AEFV); Entebbe bat virus (ENTV); Nakiwogo virus (NAKV); Yokose virus (YOKV); Culex flavivirus (CFV); Kamiti River virus (KRV); Quang Binh virus (QBV); Cell fusing agent virus (CFAV); Rio Bravo virus (RBV); Modoc virus (MODV); Apoi virus (APOIV); and Tamana bat virus (TABV).
Crystal contact analysis of the WNV MTase structure indicated that as many as 20 residues are within 4 Å distance to contact the bound AdoHcy molecule (supplemental Fig. 1). These residues are Ser-56, Gly-58, Gly-81, Cys-82, Gly-83, Gly-85, Gly-86, Trp-87, Thr-104, Lys-105, Gly-106, His-110, Glu-111, Val-130, Asp-131, Val-132, Phe-133, Asp-146, Ile-147, and Glu-149. With the exception of three residues, Lys-105 (30% conserved), Val-130 (40%), and Phe-133 (61%), all of the residues are well conserved (including eight invariant ones) among flavivirus MTases (supplemental Fig. 1). Therefore, the AdoMet/AdoHcy-binding pocket, together with the pocket extension described above, is highly conserved (supplemental Figs. 1 and 2).
We next investigated whether the sequence conservation described above exists for other AdoMet-utilizing proteins. Structure-based sequence alignment indicated that the sequence conservation of these residues only occurs among flavivirus MTases but not among other AdoMet-dependent MTases nor, on a broader scale, among other AdoMet-utilizing proteins (data not shown and see Ref. 18). Consistently, as we discussed above, the additional pocket adjacent to the AdoMet-binding pocket is not observed in the structures of other AdoMet-utilizing proteins. Therefore, the flavivirus-conserved additional pocket is a unique feature of flavivirus MTases.
Distinct Effects of the Additional Pocket on the WNV N7 and 2′-O-Methylations
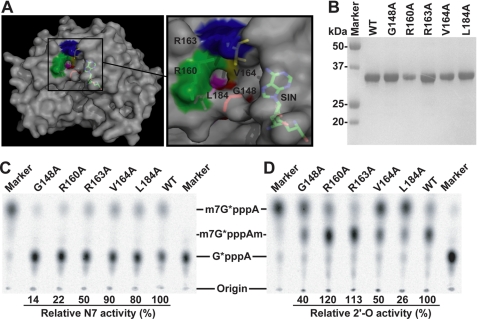
We performed structure-based mutagenesis to explore the function of the additional pocket. A panel of five mutants, each with an Ala substitution for one of the conserved residues in the pocket, was prepared and assayed for cap methylations, as described previously (19). Fig. 3A depicts the locations of the mutated residues: Gly-148, Arg-160, Arg-163, Val-164, and Leu-184. SDS-PAGE analysis showed that the recombinant wild-type (WT) and mutant MTases (N-terminal 300 amino acids of NS5) had similar purities of >90% (Fig. 4B). Different mutant MTases exhibited distinct effects on the N7 (Fig. 3C) and 2′-O-methylation activities (Fig. 3D). Based on the methylation results, the five mutants could be categorized into three groups: the group I mutant (G148A) was defective in both N7 and 2′-O-methylation; the group II mutants (R160A and R163A) were defective in N7 methylation but not in 2′-O-methylation; and the group III mutants (V164A and L184A) were selectively defective in 2′-O-methylation but not N7 methylation.
FIGURE 3.
Structure-based mutagenesis of the additional pocket in WNV MTase. A, locations of five conserved amino acids within the additional pocket of the WNV MTase on the surface. To better view the locations of each mutated residue, the molecule is shown in an orientation slightly different from that shown in Fig. 2, C and E. Left panel, surface representation of the WNV MTase in complex with SIN. Right panel, close-up view of the additional pocket showing the five amino acids selected for mutagenesis analysis (Gly-148, red; Arg-160, green; Arg-163, blue; Val-164, yellow; and Leu-184, magenta). The MTase protein was rendered with partial transparency to permit the complete molecule of SIN to be viewed, with atom colors as following: carbon, green; oxygen, red; and nitrogen, blue. The images were produced with PyMOL (48). B, SDS-PAGE analysis of recombinant WT and mutant MTases of WNV. Molecular masses of protein markers are labeled. C, effects of mutations of the additional pocket on WNV N7 cap methylation. The assay measured the G*pppA-RNA → m7G*pppA-RNA conversion. D, effects of mutations of residues of the additional pocket on WNV 2′-O cap methylation. The assay quantified the m7G*pppA-RNA → m7G*pppAm-RNA conversion. The methylation efficiencies of mutant MTases were compared against that of the WT MTase (set at 100%). The positions of the origin, G*pppA, m7G*pppA, and m7G*pppAm are indicated to the side of the TLC images. The assays were performed as reported previously (20).
FIGURE 4.
Effects of mutations of residues of the additional pocket in WNV replication. A, schematic of a luciferase reporting replicon of WNV (38). B, transient replication of WT and mutant replicons of WNV. An average of triplicate results is shown. C, sequencing of the replicating mutant replicons. Total cellular RNA was extracted using RNeasy (Qiagen) and subjected to RT-PCR, using primers targeting the WNV NS5 gene. The RT-PCR products were directly sequenced. The sequencing results of mutated regions are presented. D, summary of N7 methylation, 2′-O-methylation, and viral replication. Effects of a panel of mutations, engineered in various regions of the WNV MTase sequence, on cap methylations and viral replication are shown. In this study, viral replication was monitored by replicon, with wild-type replication level set as +++ (strong), mutants replication levels as ++ (medium), + (weak), and − (nonreplicative). In previous studies (20, 28), viral replication was monitored by plaque assay, with wild-type replication level set as +; symbols “+” and “−” indicate that genome-length RNAs containing the MTase mutations are replicative and nonreplicative, respectively.
Previous studies have established functional significance of conserved residues within the AdoMet-binding pocket (19, 20, 28, 37). Those studies showed that mutations of residues predicted to be involved in AdoMet binding (such as Ser-56, Gly-81, Gly-83, Gly-85, Trp-87, Lys-105, His-110, Glu-111, Asp-131, Ile-147, Asp-146, and Glu-149) reduced, and in some cases abolished, either the N7 or the 2′-O-methylation, or both. Except Lys-105, all of these residues are well conserved among flavivirus MTases (supplemental Fig. 1). Together with our results in this study, these results indicated the functional significance of residues within the AdoMet-binding pocket and the pocket extension described above.
Effects of the Additional Pocket on WNV Replication
To validate the biological relevance of the identified pocket, we performed mutagenesis using a luciferase-reporting replicon of WNV (38). The replicon contained a luciferase reporter in-frame fused with the viral ORF, in the position where structural genes were deleted (Fig. 4A) (38). Five mutant replicons were prepared, each with an Ala substitution within the MTase hydrophobic pocket (Fig. 4B). For every mutant, two of the three nucleosides within the codon were changed to avoid potential reversion (Fig. 4B). The transfection experiments showed comparable luciferase activities at 2 h post-transfection (indicative of input replicon translation) for all replicons, suggesting that transfection efficiencies were similar. In contrast, luciferase activities at ≥24 h post-transfection (indicative of RNA synthesis) were dramatically different among the various replicons (Fig. 4B). Based on the luciferase profile, the replication kinetics of replicons could be ranked as WT ≥ V164A > L184A > R163A > G148A > R160A. The luciferase curve for replicon R160A was identical to that of a nonreplicative replicon (containing mutations in the polymerase GDD active site; data not shown). However, at 96 h post-transfection, the luciferase levels from mutants V164A, L184A, and R163A were comparable with that from the WT replicon (Fig. 4B). To exclude the possibility that the late luciferase signals were due to reversion of the engineered mutations to the WT sequence, we sequenced the replicative RNAs recovered from the transfected cells at 96 h post-transfection The sequencing results showed that the engineered mutations were indeed retained in the replicative RNAs (Fig. 4C), and no second site mutation was present in the NS5 gene (data not shown). The replication delay of mutant replicons could be due to the low methylation activity of MTase, and an accumulated level of mutant NS5 protein is required to efficiently methylate the viral RNA cap to assume viral replication. Overall, the results have demonstrated that the hydrophobic pocket is important for WNV replication.
DISCUSSION
Many flaviviruses cause significant human disease. However, currently no clinically approved antiviral therapy is available for treatment of flavivirus-associated diseases. Given that we previously found that the N7 methylation activity is essential for the WNV life cycle, the viral methyltransferase represents a novel target for flavivirus therapy (19, 20, 28, 29, 47).
SIN was previously shown to inhibit WNV in cell culture with EC50 and CC50 values of 23 μm and 4.5 mm, respectively (28). In this study, we have demonstrated that SIN has antiviral activity in two other flaviviruses (Fig. 1). Because of the high similarity between AdoMet and SIN, toxicity is a major concern for the development of SIN as a therapeutic agent, because SIN could inhibit many AdoMet-utilizing enzymes of the host. To explore the potential for modification of the inhibitor, we determined the crystal structure of the WNV MTase in complex with SIN. Analysis of the SIN-MTase structure revealed an additional pocket that is continuous with the AdoMet-binding pocket. Re-visiting the structure of the WNV MTase·AdoHcy complex as determined previously (20) and examinations of the structures of MTases from several other flaviviruses indicated that this additional pocket is conserved among flavivirus MTases with known structures. In addition, sequence alignment indicated that the residues forming the additional (extended) pocket are conserved among flavivirus MTases. Furthermore, the sequence and structure conservations of this additional pocket are not seen among other AdoMet-utilizing proteins. The recognition that the AdoMet-binding pocket has an extended and flavivirus-conserved pocket beyond the AdoMet adenosine base has provided us with a unique opportunity to use SIN as a lead compound for drug development. We noticed that within the extended pocket, four ordered water molecules form a ring-like structure (Fig. 2E). Therefore, it may be possible to modify the adenine base of SIN with a covalently bound ring structure. Indeed, a docking analysis indicated that the additional pocket can easily accommodate a cyclopentanediol ring next to the adenine base of SIN (data not shown). SIN analogs with added moieties that interact with the additional pocket are expected to show enhanced binding affinities, leading to more potent and specific inhibitors of flavivirus MTase.
Using both enzymatic and replicon-based approaches, we performed mutagenesis to examine the function of the additional pocket. Five residues within the pocket were individually mutated to Ala; the effects of the mutations on N7 and 2′-O-methylations, as well as on viral replication, were examined. As summarized in Fig. 4D, mutations with dramatic negative effect on N7 methylation (e.g. G148A and R160A) significantly impaired viral replication. We further note that residues Gly-148, Glu-149, and Arg-160, all of which are critical for N7 methylation, form a hydrogen-bonding network (Fig. 2D). The Gly-148 O hydrogen bonds to Ser-150 N, whereas Arg-160 NH1 atom forms two hydrogen bonds with the carboxyl oxygen atoms of residues Glu-149 and Ser-150. Therefore, the maintenance of the local conformation around Glu-149 is critical for the viral MTase function.
In contrast to the above-noted mutations that indicated an essential role of N7 methylation activity in viral replication, mutations that have a dramatic effect on 2′-O-methylation did not significantly affect replicon replication. The correlation between the N7 methylation activity and viral replication is supported by previous mutagenesis results, for other regions of the WNV MTase (Fig. 4D). The overall results strongly suggest that, when MTase inhibitors are being developed, emphasis should be placed on compounds that can achieve inhibition of N7 methylation activity. However, we note that such a correlation between N7 methylation and viral replication has so far only been observed in WNV; experiments are needed to test whether this correlation can be extended to other flaviviruses.
Supplementary Material
Acknowledgments
We thank A. Verschoor for a critical reading of the manuscript and the Wadsworth Macromolecular Crystallography Core for facility usage. Diffraction data for this study were measured at beamline X4A of National Synchrotron Light Source, which is supported by the Department of Energy, by grants from the National Institutes of Health, and by the New York Structural Biology Center.
This work was supported, in whole or in part, by National Institutes of Health Grants AI07079201A1 (to H. L.) and AI061193 (to P.-Y. S. and H. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
The atomic coordinates and structure factors (code 3LKZ) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- DENV
- Dengue virus
- PDB
- Protein Data Bank
- YFV
- yellow fever virus
- WNV
- West Nile virus
- SIN
- sinefungin
- MTase
- methyltransferase
- AdoHcy
- S-adenosyl-l-homocysteine
- AdoMet
- S-adenosyl-l-methionine
- r.m.s.d.
- root mean square deviation.
REFERENCES
- 1.Lindenbach B., Rice C. M. (2001) in Fields Virology (Knipe D. M., Howley P. M. eds) 4th Ed., pp. 991–1042, Lippincott/William & Wilkins, Baltimore [Google Scholar]
- 2.Burke D. S., Monath T. P. (2001) in Fields Virology (Knipe D. M., Howley P. M. eds) 4th Ed., pp. 1043–1126, Lippincott/William & Wilkins, Baltimore [Google Scholar]
- 3.World Health Organization (2009) ( www.who.int/mediacentre/factsheets/fs117/en/)
- 4.World Health Organization (2009) ( www.who.int/mediacentre/factsheets/fs100/en/)
- 5.World Health Organization (2009) ( www.who.int/nuvi/je/en/)
- 6.Kramer L. D., Li J., Shi P. Y. (2007) Lancet Neurol. 6, 171–181 [DOI] [PubMed] [Google Scholar]
- 7.Cleaves G. R., Dubin D. T. (1979) Virology 96, 159–165 [DOI] [PubMed] [Google Scholar]
- 8.Wengler G., Wengler G. (1981) Virology 113, 544–555 [DOI] [PubMed] [Google Scholar]
- 9.Rice C. M., Lenches E. M., Eddy S. R., Shin S. J., Sheets R. L., Strauss J. H. (1985) Science 229, 726–733 [DOI] [PubMed] [Google Scholar]
- 10.Chambers T. J., Hahn C. S., Galler R., Rice C. M. (1990) Annu. Rev. Microbiol. 44, 649–688 [DOI] [PubMed] [Google Scholar]
- 11.Warrener P., Tamura J. K., Collett M. S. (1993) J. Virol. 67, 989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wengler G., Wengler G. (1991) Virology 184, 707–715 [DOI] [PubMed] [Google Scholar]
- 13.Bartelma G., Padmanabhan R. (2002) Virology 299, 122–132 [DOI] [PubMed] [Google Scholar]
- 14.Tan B. H., Fu J., Sugrue R. J., Yap E. H., Chan Y. C., Tan Y. H. (1996) Virology 216, 317–325 [DOI] [PubMed] [Google Scholar]
- 15.Ackermann M., Padmanabhan R. (2001) J. Biol. Chem. 276, 39926–39937 [DOI] [PubMed] [Google Scholar]
- 16.Guyatt K. J., Westaway E. G., Khromykh A. A. (2001) J. Virol. Methods 92, 37–44 [DOI] [PubMed] [Google Scholar]
- 17.Koonin E. V. (1993) J. Gen. Virol. 74, 733–740 [DOI] [PubMed] [Google Scholar]
- 18.Egloff M. P., Benarroch D., Selisko B., Romette J. L., Canard B. (2002) EMBO J. 21, 2757–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray D., Shah A., Tilgner M., Guo Y., Zhao Y., Dong H., Deas T. S., Zhou Y., Li H., Shi P. Y. (2006) J. Virol. 80, 8362–8370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y., Ray D., Zhao Y., Dong H., Ren S., Li Z., Guo Y., Bernard K. A., Shi P. Y., Li H. (2007) J. Virol. 81, 3891–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuichi Y., Shatkin A. J. (2000) Adv. Virus Res. 55, 135–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furuichi Y., Muthukrishnan S., Shatkin A. J. (1975) Proc. Natl. Acad. Sci. U.S.A. 72, 742–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muthukrishnan S., Both G. W., Furuichi Y., Shatkin A. J. (1975) Nature 255, 33–37 [DOI] [PubMed] [Google Scholar]
- 24.Shuman S. (2001) Prog. Nucleic Acids Res. Mol. Biol. 66, 1–40 [DOI] [PubMed] [Google Scholar]
- 25.Egloff M. P., Decroly E., Malet H., Selisko B., Benarroch D., Ferron F., Canard B. (2007) J. Mol. Biol. 372, 723–736 [DOI] [PubMed] [Google Scholar]
- 26.Issur M., Geiss B. J., Bougie I., Picard-Jean F., Despins S., Mayette J., Hobdey S. E., Bisaillon M. (2009) RNA 15, 2340–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong H., Ray D., Ren S., Zhang B., Puig-Basagoiti F., Takagi Y., Ho C. K., Li H., Shi P. Y. (2007) J. Virol. 81, 4412–4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong H., Ren S., Zhang B., Zhou Y., Puig-Basagoiti F., Li H., Shi P. Y. (2008) J. Virol. 82, 4295–4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong H., Ren S., Li H., Shi P. Y. (2008) Virology 377, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assenberg R., Ren J., Verma A., Walter T. S., Alderton D., Hurrelbrink R. J., Fuller S. D., Bressanelli S., Owens R. J., Stuart D. I., Grimes J. M. (2007) J. Gen. Virol. 88, 2228–2236 [DOI] [PubMed] [Google Scholar]
- 31.Bollati M., Milani M., Mastrangelo E., de Lamballerie X., Canard B., Bolognesi M. (2009) Biochem. Biophys. Res. Commun. 382, 200–204 [DOI] [PubMed] [Google Scholar]
- 32.Bollati M., Milani M., Mastrangelo E., Ricagno S., Tedeschi G., Nonnis S., Decroly E., Selisko B., de Lamballerie X., Coutard B., Canard B., Bolognesi M. (2009) J. Mol. Biol. 385, 140–152 [DOI] [PubMed] [Google Scholar]
- 33.Geiss B. J., Thompson A. A., Andrews A. J., Sons R. L., Gari H. H., Keenan S. M., Peersen O. B. (2009) J. Mol. Biol. 385, 1643–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mastrangelo E., Bollati M., Milani M., Selisko B., Peyrane F., Canard B., Grard G., de Lamballerie X., Bolognesi M. (2007) Protein Sci. 16, 1133–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khromykh A. A., Kenney M. T., Westaway E. G. (1998) J. Virol. 72, 7270–7279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattacharya D., Hoover S., Falk S. P., Weisblum B., Vestling M., Striker R. (2008) Virology 380, 276–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kroschewski H., Lim S. P., Butcher R. E., Yap T. L., Lescar J., Wright P. J., Vasudevan S. G., Davidson A. D. (2008) J. Biol. Chem. 283, 19410–19421 [DOI] [PubMed] [Google Scholar]
- 38.Lo M. K., Tilgner M., Bernard K. A., Shi P. Y. (2003) J. Virol. 77, 10004–10014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–325 [DOI] [PubMed] [Google Scholar]
- 40.Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 41.Ray B. K., Lawson T. G., Kramer J. C., Cladaras M. H., Grifo J. A., Abramson R. D., Merrick W. C., Thach R. E. (1985) J. Biol. Chem. 260, 7651–7658 [PubMed] [Google Scholar]
- 42.Puig-Basagoiti F., Tilgner M., Forshey B. M., Philpott S. M., Espina N. G., Wentworth D. E., Goebel S. J., Masters P. S., Falgout B., Ren P., Ferguson D. M., Shi P. Y. (2006) Antimicrob. Agents Chemother. 50, 1320–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tilgner M., Shi P. Y. (2004) J. Virol. 78, 8159–8171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jansson A. M., Jakobsson E., Johansson P., Lantez V., Coutard B., de Lamballerie X., Unge T., Jones T. A. (2009) Acta Crystallogr. D. Biol. Crystallogr. 65, 796–803 [DOI] [PubMed] [Google Scholar]
- 45.Chang Y., Zhang X., Horton J. R., Upadhyay A. K., Spannhoff A., Liu J., Snyder J. P., Bedford M. T., Cheng X. (2009) Nat. Struct. Mol. Biol. 16, 312–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu H., Min J., Zeng H., Plotnikov A. N. (2008) Proteins 72, 519–525 [DOI] [PubMed] [Google Scholar]
- 47.Dong H., Zhang B., Shi P. Y. (2008) Antiviral Res. 80, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeLano W. (2002) The PyMOL Molecular Graphics System, DeLano Scientific LLC, San Carlos, CA [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.