Abstract
The highly expressed Id1 (inhibitor of DNA binding/differentiation) protein promotes angiogenesis in HCC and is a well established target for anti-angiogenesis therapeutic strategies. Heparan sulfate (HS) mimetics such as PI-88 can abrogate HS-protein interactions to inhibit angiogenesis. Id1 is the direct downstream effector of bone morphogenetic proteins (BMPs), which are angiogenic and HS-binding proteins. Thus, targeting BMPs by HS mimetics may inhibit angiogenesis via attenuating Id1 expression. We report here that a HS mimetic WSS25 potently inhibited the tube formation of HMEC-1 cells on Matrigel and their migration. Meanwhile, WSS25 (25 μg/ml) nearly completely blocked Id1 expression in the HMEC-1 cells as demonstrated by oligo-angiogenesis microarray analysis and further confirmed by RT-PCR and Western blotting. BMP/Smad/Id1 signaling also was blocked by WSS25 treatment in HMEC-1 cells. Importantly, Id1 knockdown in HMEC-1 cells caused the disruption of their tube formation on Matrigel. By employing quartz crystal microbalance analysis, we found that WSS25 strongly bound to BMP2. Moreover, WSS25 impaired BMP2-induced tube formation of HMEC-1 cells on Matrigel and angiogenesis in Matrigel transplanted into C57BL6 mice. Furthermore, WSS25 (100 mg/kg) abrogated the growth of HCC cells xenografted in male nude mice. Immunohistochemical analysis showed that both the expression of Id1 and the endothelial cell marker CD31 were lower in the WSS25-treated tumor tissue than in the control. Therefore, WSS25 is a potential drug candidate for HCC therapy as a tumor angiogenesis inhibitor.
Keywords: Anticancer Drug, Heparan Sulfate, Microarray, Protein Drug Interactions, Signal Transduction, BMP/Smad/Id1 Signaling, Hepatocellular Cancer, Id1, Angiogenesis, Quartz Crystal Microbalance (QCM)
Introduction
Hepatocellular cancer (HCC)3 is the fourth leading cause of death from cancer worldwide and the second most lethal malignant cancer in China since the 1990s (1). However, there are few treatment options aside from surgery (1). HCC is characterized by hypervascularity, which can distinguish HCC from benign lesions by angiography. The strategy of blocking angiogenesis in HCC to inhibit tumor growth has been used for more than 20 years in the clinic (2). However, there are no ideal antiangiogenesis chemotherapeutic agents developed thus far for HCC therapy.
Id1 is one of the inhibitors of DNA binding proteins (Ids), which belongs to the basic helix loop helix transcriptional factor superfamily (3). There are four members, Id1, Id2, Id3, and Id4, in the Ids family. Accumulating evidence show that Id1 is overexpressed in solid tumors and their supporting vasculatures (4, 5). Although essential during embryo development, Id1 expression is extremely low in adult tissues, including the quiescent endothelial cells (6). Partial loss of Id1 by genetic manipulation in mice effectively inhibits tumor angiogenesis (6). Id1 ablation leads to the impairment of bone marrow endothelial progenitor cell recruitment and mobilization to form tumor vasculature (7, 8). Id1 also has been implicated in the regulation of cell senescence, drug resistance, tumor cell invasion, and apoptosis resistance (9). Interestingly, Id1 has been demonstrated to be highly expressed in malignant HCC and to promote angiogenesis in HCC (10, 11). Thus, Id1 is a rational target for the development of antiangiogenesis therapeutics for HCC.
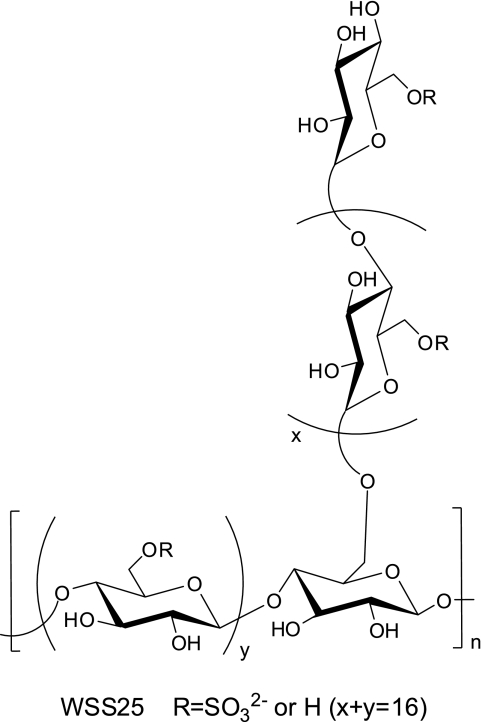
Heparan sulfate proteoglycans are glycoconjugates composed of protein cores to which heparan sulfate (HS) chains are attached. Direct genetic evidence supports that heparan sulfate proteoglycans are necessary for tumor angiogenesis (12). Heparan sulfate proteoglycans, especially those located on the cell surface, act as co-receptors through their HS chains (13). HS chains are negatively charged polymers composed of hexaronic acid and glucosamine disaccharide repeats. Due to their negative charge, they bind multiple functional proteins, including proangiogenic factors such as VEGF and FGF2, and thus mediate angiogenic signaling (14). HS mimetics such as PI-88 can inhibit angiogenesis via disrupting those interactions between HS and growth factors (15). TGF-β family proteins can also bind HS chains. The binding is isoform-specific, and the established examples include BMP2 (16) and BMP4 (17), two angiogenic factors (18), and the upstream molecules of the BMP/Smad signaling pathway (19), whereas Id1 is a canonical and direct downstream effector of this pathway (20). More importantly, stimulation of Id1 expression by BMP proteins is sufficient and necessary for activating endothelial cells (21). Id1 also is the downstream effector of VEGF and FGF2 signaling pathway (3, 22). Moreover, targeting the HS-protein interaction is a new approach for drug design (23). Therefore, we hypothesized that HS mimetics may inhibit angiogenesis through blocking BMP-induced Id1 expression. We previously isolated an α-d-(1–4) glucan with an α-d-(1–4) branch periodically at O-6 from a well known Chinese herb Gastrodia elata Bl. WSS25 (Fig. 1) is a sulfated derivative of the aforementioned glucan and a HS mimetic (24). In this study, we investigated the role of WSS25 on angiogenesis, Id1 expression, BMP2-induced BMP/Smad/Id1 signaling, and growth of HCC.
FIGURE 1.
Structural diagram of WSS25.
EXPERIMENTAL PROCEDURES
General Materials
WSS25 was prepared in our lab as described previously (24) and dissolved in normal saline for experimental use after passing through a 0.22-μm filter. Matrigel with growth factors and reduced growth factors were obtained from BD Biosciences. The proteinase inhibitor mixture and actin primary antibody were from Sigma-Aldrich. FBS was from Sijiqing Co., Ltd. (Hangzhou, China). Other antibodies used include the Id1 antibody (c20) (Santa Cruz Biotechnology), phosphorylated Smad1/5/8 primary antibody (Cell Signaling Technology), HRP conjugated anti-rabbit and anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories), anti-CD31 (Boster Biological Technology, Ltd. Wuhan, China), Ki-67 antibody (Abcam, San Francisco, CA), and anti-TUNEL antibody (Trevigen, Inc., Gaithersburg, MD). Other reagents, unless specified, were obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
Cell Lines and Cell Culture
Human microvascular endothelial cells (HMEC-1) (25) were maintained in MCDB131 (Invitrogen) medium containing 15% FBS (v/v), 2 mm l-glutamine, 10 ng/ml EGF (Shanghai PrimeGene Bio-Tech Co., Ltd., Shanghai, China) and antibiotics (100 units/ml penicillin, 100 μg/ml streptomycin, Invitrogen). SMMC7721 and Bel7402 cells (both from the Cell Bank in the Type Culture Collection Center in Chinese Academy of Sciences, Shanghai, China) were cultured in RPMI 1640 medium supplemented with 10% FBS and antibiotics. All cells were cultured in a humidified incubator at 37 °C with 5% CO2.
Tube Formation Assay
The tube formation assay was performed to determine the effect of WSS25 on angiogenesis in vitro. Briefly, a 96-well plate coated with 50 μl Matrigel/well was allowed to solidify at 37 °C for 30 min. HMEC-1 cells (3 × 104 cells/well) were seeded into the plate and cultured in MCDB131 medium containing 6.25, 25, or 50 μg/ml of WSS25 for 10 h. For the BMP2 (Shanghai PrimeGene Bio-Tech Co., Ltd., Shanghai, China) induced tube formation in FBS restricted conditions (0.1% FBS in MCDB-131 medium), BMP2 dissolved in 10 mm HAc (200 ng/ml) with or without WSS25 (25 μg/ml) was added to the cells on the growth factor reduced Matrigel in the 96-well plate, with 10 mm HAc as the control. Noggin (Shanghai PrimeGene Bio-Tech Co., Ltd., Shanghai, China) was also dissolved in 10 mm HAc (1 μg/ml) and added into the cells together with BMP2 (200 ng/ml) as mentioned previously. The enclosed capillary networks of tubes were photographed by a microscope (IX51, Olympus Imaging).
Migration Assay
The migration assay was performed by using a 24-well chamber (Costar, Cambridge, MA) as the outer chamber and polycarbonate filters (8-μm pores) as the inner chambers. HMEC-1 cells (1.5 × 105 cells/well) were seeded into the inner chamber in MCDB-131 medium containing WSS25 or vehicle (control). The outer chamber contained the same medium with 15% FBS. After incubation for 14 h at 37 °C, the cells on the filter were fixed in 90% ethanol for 10 min. Nonmigrated cells on the upper surface of the filter were removed by gentle scraping with a cotton swab. Migrated cells on the lower surface of the filter were stained with 0.1% crystal violet and washed with distilled water until the water was colorless. Images of migrated cells were captured using a microscope (IX51) and measured at 595 nm after extraction with 10% acetic acid.
Wound Healing Assay
HMEC-1 cells (5 × 105 cells per well) were seeded into a 6-well plate. A wound in each well was created by scratching with a yellow tip after incubation for 24 h. After rinsing with PBS three times, the cells were incubated with new medium containing WSS25 (25 μg/ml) or the vehicle. Photos were taken immediately or 48 h later under a microscope (IX51). The distance of the wound was calculated by NIH ImageJ software.
MTT Assay
HMEC-1 cells (4.5 × 103 cells/well) were seeded into the 96-well plate for 24 h before WSS25 was added into the plate. After treatment with WSS25 for 24, 48, or 72 h, thiazolyl blue tetrazolium bromide (5 mg/ml) (MTT, Sigma-Aldrich) was then added to each well and incubated for 4 h. The formazan crystals formed from MTT by the living cells were dissolved in the lysis buffer (10% SDS; 5% isopropanol; 0.1 m HCl) for 12 h, and the purple solution of the formazan was detected using a spectrophotometer at 570 nm. The inhibitory ratio was calculated as ((control − sample)/control) × 100%.
Oligo Angiogenesis Array Analysis
Total cellular RNA was extracted from HMEC-1 cells using TRIzol (Invitrogen). RNA was quantified by using the Nanodrop ND-1000. Using the True-Labeling AMP Linear RNA amplification kit (SuperArray Bioscience Corp., Frederick, MD), the mRNA was reverse transcribed to obtain cDNA and converted into biotin-labeled cRNA using biotin-16-dUTP (Roche Applied Science) by in vitro transcription. Before hybridization, the cRNA probes were purified with an ArrayGrade cRNA cleanup kit (SuperArray Bioscience Corp.). The purified cRNA probes were then hybridized to the pretreated Oligo Human Angiogenesis Arrays (SuperArray Bioscience Corp.), which covers 114 angiogenesis-related genes plus controls (supplemental Fig. 1). After the washing steps, array spots with bound cRNA were detected by the chemiluminescence method according to the manufacturer's procedure. Spots were then analyzed by using the GEArray Expression Suite (SuperArray Bioscience Corp.). All genes covered in the array can be found at the website of SuperArray Bioscience.
RT-PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's manual after WSS25 treatment for 18 h. First-strand cDNA was synthesized from 1 μg total RNA using avian myeloblastosis virus reverse transcriptase (Takara Biotechnology, Dalian, China) according to the manufacturer's instructions. The primers and conditions for Id1 were the same as that used by Peddada et al. (26) or McAllister et al. (27). The PCR primers and conditions for BMP2, BMP receptor IA (BMPRIA), BMP receptor IB (BMPRIB), BMP receptor II (BMPRII), and Smad4 also were published previously (28).
Western Blotting Analysis
HMEC-1 cells were seeded (5 × 105 cells/well) into 6-well plates. Cells treated under different conditions were lysed with an equal volume of RIPA buffer (0.5% Triton-X 100, 0.5% deoxycholic acid sodium salt, 0.1% SDS, and 1% PMSF supplemented with 1% proteinase inhibitor mixture). Protein concentrations were determined by a protein assay according to the manufacturer's instructions (Bio-Rad). The proteins were separated by SDS-PAGE and transferred to a PVDF membrane (Bio-Rad). After blocking with TBST containing 5% nonfat milk for 30 min, the membrane was incubated with antibodies against actin, Id1 and phospho-Smad1/5/8 (Ser463/465) overnight at 4 °C. After incubation in horseradish peroxidase-conjugated secondary antibody for 1 h, Pierce ECL Western blot Substrate (Pierce) was used for detection.
Gene Knockdown Using shRNA
The sequences for the Id1 shRNA and negative control were 5′-cac cgc cca ttt ctg ttt cag cca gtt tca aga gaa ctg gct gaa aca gaa tgg gct ttt ttg-3′, and 5′-cac cgt tct ccg aac gtg tca cgt caa gag att acg tga cac gtt cgg aga att ttt tg-3′, respectively. HMEC-1 cells (2.5 × 105 cells/well) were seeded into a 6-well plate 24 h before transfection. The plasmids (the vector is pGPU6/GFP/Neo) were transfected into the cells three times 24 h apart using the X-fect polymer (Clontech) according to the instructions from the company. After another 24 h of incubation, the cells were used in the tube formation assay as described above. The shRNA plasmids were from Shanghai GenePharma Co., Ltd., Shanghai, China.
Quartz Crystal Microbalance (QCM) Analysis
Biotinylated WSS25 was synthesized by adding biotin-PEG4-hydrazide (50 μl, 50 mm) in a water-miscible solvent (H2O/DMSO, 4:1) to WSS25 (450 μl, 31 μm) in the coupling buffer (0.1 m sodium phosphate, 0.15 m NaCl, pH 7.2), and the mixture was stirred at room temperature for 2 h. The biotinylated WSS25 was purified from nonreacted biotin reagent by using a desalting column. To measure carbohydrate-protein interactions, biosensor experiments were carried out on an Attana A100 QCM instrument (Attana AB, Stockholm, Sweden). The Attana biotin sensor surfaces were mounted in the QCM system and equilibrated with buffer solution (10 mm Hepes, 150 mm NaCl, 0.005%, Tween 20, pH 7.4). Subsequently, the streptavidin solution (100 μg/ml) was injected, and the biotinylated WSS25 was immobilized on the streptavidin surface to produce a WSS25 biosensor surface. The interaction between WSS25 and BMP2 were then measured by injecting BMP2 (50 μg/ml, 50 μl) in running buffer (10 mm Hepes, 150 mm NaCl, 0.005% Tween 20, pH 7.4) onto the WSS25 biosensor surface, and frequency data were collected. A continuous flow of running buffer at a flow rate of 25 μl/min was used throughout, and the samples were prepared in the same buffer. The frequency responses produced from the interactions were monitored by frequency logging with Attester 1.1 (Attana), where the mass changes from the bound or released ligands were recorded as the resulting frequency shifts (Δf).
Animals
All mice were housed in sterile cages within laminar air flow hoods under specific pathogen-free conditions with sterile food and water ad libitum. All animal experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committee.
Matrigel Plug Assay
Human recombinant BMP2 protein was mixed with growth factor reduced Matrigel via vortexing, at the ratio of 4 μg of protein per 1 ml of Matrigel. The mixture was injected subcutaneously into the ventral region of the female C57BL6 mice ages 4–6 weeks, and 0.1% BSA served as a control. The mice were randomly grouped, five mice for each group. WSS25 (25 and 100 mg/kg) and normal saline (vehicle) were administered subcutaneously every other day for 10 times from the second day after injecting the Matrigel. The mice were then sacrificed and followed by excision of the Matrigel plug, and photographs were taken.
Tumor Xenograft Experiment
Bel7402 cells (1 × 106 cells/mouse) or SMMC7721 (1 × 107 cells/mouse) cells were subcutaneously injected into BALB/cA nu/nu male mice ages 4 to 6 weeks. For the Bel7402 tumor xenografts, as the tumor volume reached ∼100 mm3, the mice were randomly assigned into control and treatment groups (five mice/group). For the SMMC7721 tumor xenografts, well developed tumors were cut into 1–3 mm3 fragments and transplanted subcutaneously into the right flank of nude mice using a trocar under sterile conditions. When the tumor volume reached ∼100 mm3, the mice were randomly assigned into control and treatment groups (six mice/group). The vehicle (normal saline) or 100 mg/kg body weight of WSS25 was administered via tail vein injection every other day.
Measurement of Tumor Volume
The tumor volume (V) was calculated as follows: V = (length × width2)/2. The individual relative tumor volume (RTV) was calculated as follows: RTV = Vt/V0, where Vt is the volume on each day, and V0 is the volume at the beginning of the treatment. The therapeutic effect of the compounds was expressed as the volume ratio of treatment to control (T/C). T/C (%) = 100% × (mean RTV of the treated group/mean RTV of the control group).
Immunohistochemistry
At the end of the experiment, the mice were sacrificed, and the tumor tissues were excised, fixed in 4% neutral paraformaldehyde, and embedded in paraffin. After sectioning, the 5-μm tissues were deparaffinized by xylene, ethanol, 95% ethanol, and 80% ethanol sequentially. The endogenous peroxidase was removed by incubating with 3% H2O2 at room temperature for 10 min. The retrieval of antigen was performed by boiling the tissue in citrate buffer at pH 6.0 three times, each with a 10-min interval. The tissue was sequentially probed with primary antibodies against CD31 (1:10), phosphorylated Smad1/5/8 (1:100) or Id1 (1:100) overnight at 4 °C. After application of the anti-rabbit/mouse secondary antibody and appropriate washes, the signals were detected by staining the sections with 3,3′-diaminobenzidine and counterstaining with hematoxylin.
Statistical Analysis
Results were expressed as the mean ± S.E. The data were analyzed by the Student's t test; p values of < 0.05 indicated significant differences (*, p < 0.05; **, p < 0.01).
RESULTS
WSS25 Inhibits Tube Formation of HMEC-1 Cells on Matrigel and Migration
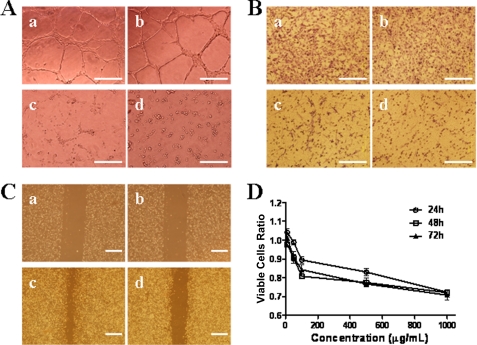
As HS mimetics have the potential to inhibit angiogenesis, we first tested whether WSS25 in different concentrations also has this impact on angiogenesis in vitro. Therefore, the tube formation of HMEC-1 cells on Matrigel, a typical in vitro angiogenesis model, was employed (29). The results showed that WSS25 at 25 or 50 μg/ml almost completely disrupted the enclosed capillary networks (Fig. 2A, panels c and d, respectively) formed by the HMEC-1 cells (Fig. 2A, panel a), whereas little effect was observed at 6.25 μg/ml (Fig. 2A, panel b). WSS25 is the sulfated derivative of WGEW (24). However, WGEW showed no inhibitory effect on the tube formation of HMEC-1 cells on Matrigel even at 1 mg/ml (supplemental Fig. 2). These results indicated that the sulfation indeed contributed to antiangiogenic effects of WSS25.
FIGURE 2.
WSS25 impaired the tube formation of HMEC-1 cells on Matrigel and migration. A, WSS25 inhibited the tube formation of HMEC-1 cells on Matrigel. HMEC-1 cells (90 μl) treated with WSS25 (10 μl) at different final concentrations (panel b, 6.25 μg/ml; panel c, 25 μg/ml; panel d, 50 μg/ml); or vehicle (panel a) were seeded into the 96-well plate precoated with 50 μl Matrigel for 10 h. B, WSS25 impaired the migration of HMEC-1 cells in a trans-well migration assay. HMEC-1 cells were seeded into the inner chamber with MCDB131 medium containing 10 μg/ml (panel b), 100 μg/ml (panel c), 1 mg/ml WSS25 (panel d) or vehicle (panel a). C, WSS25 inhibited the migration of HMEC-1 cells in a wound healing assay (panels a and c, control; panels b and d, 25 μg/ml). D, HMEC-1 cells were seeded into the 96-well plate. After 24 h of incubation, WSS25 was added to the final concentrations of 1 μg/ml, 10 μg/ml, 100 μg/ml, 500 μg/ml, or 1 mg/ml. The cell viabilities were determined by the MTT assay 24 h (○), 48 h (□), or 72 h ([tric]) later. The results are representative of triplicate experiments.
Angiogenesis involves multiple steps including migration, proliferation, and capillary tube formation of endothelial cells. Hence, the effects of WSS25 on these phenotypes of HMEC-1 cells were examined. There are two methods to evaluate the migration of endothelial cells, the trans-well migration assay and the wound healing assay. We used both of these methods to investigate the effect of WSS25 on the migration of HMEC-1 cells. In the trans-well model, WSS25 impaired the migration of HMEC-1 cells (Fig. 2B, panels b, c, and d) in a dose-dependent manner compared with the control (Fig. 2B, panel a; supplemental Fig. 3A). In the wound healing assay (Fig. 2C), the migration of the HMEC-1 cells also was substantially inhibited (supplemental Fig. 3B) after treatment with 25 μg/ml WSS25 for 48 h (Fig. 2C, panel c), compared with the control (Fig. 2C, panel d). However, there was no significant effect of WSS25 on the proliferation of HMEC-1 cells at concentrations <100 μg/ml (Fig. 2D).
Id1 Expression Is Down-regulated in HMEC-1 Cells after WSS25 Treatment
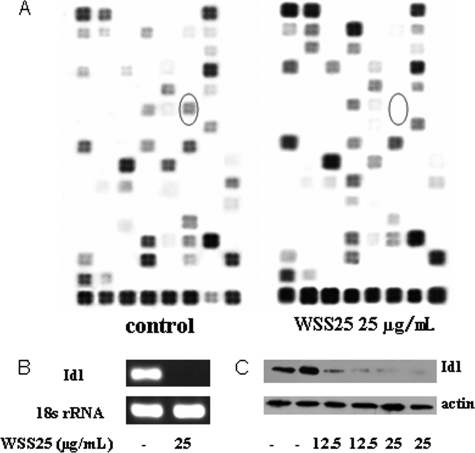
As we hypothesized that HS mimetics may inhibit angiogenesis via abrogating BMP induced Id1 expression, an oligo-angiogenesis microarray assay was performed to explore the changes in expression of angiogenic genes including Id1 in HMEC-1 cells after WSS25 treatment. Because WSS25 at 25 μg/ml could nearly completely inhibit the tube formation of HMEC-1 cells on Matrigel, this concentration of WSS25 was used to treat the cells for the microarray analysis. Among the differentially expressed genes (supplemental Fig. 1), Id1 was the most down-regulated by WSS25 (25 μg/ml) (red circle in Fig. 3A). This result was further confirmed by RT-PCR (Fig. 3B) and Western blotting analysis (Fig. 3C).
FIGURE 3.
WSS25 potently inhibited the expression of Id1. A, HMEC-1 cells were treated with WSS25 (25 μg/ml) for 18 h. RNA was then extracted for the oligo-angiogenesis microarray analysis. Circles indicate Id1. B, WSS25 at 25 μg/ml nearly completely inhibited the Id1 mRNA expression in HMEC-1 cells with 18 S rRNA as the internal control. C, WSS25 down-regulated the Id1 protein expression in HMEC-1 cells in a dose-dependent manner. β-Actin was used as a control for protein loading. Except for the microarray analysis, all experiments were repeated three times.
Id1 Expression Knockdown in HMEC-1 Cells Leads to Disruption of Tube Formation on Matrigel
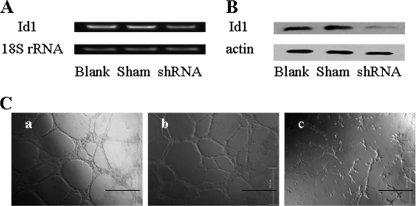
Because the HS mimetic WSS25 not only inhibited angiogenesis in vitro but also blocked Id1 expression in HMEC-1 cells, we wondered whether Id1 is truly responsible for the angiogenesis that is inhibited by WSS25. To validate the proangiogenic effect of Id1 in HMEC-1 cells, we knocked down expression of Id1 by specific shRNA, which indeed led to the disruption of tube formation by the HMEC-1 cells on Matrigel (Fig. 4C, panel c) compared with the blank (Fig. 4C, panel a) and the sham control (Fig. 4C, panel b).
FIGURE 4.
Id1 knockdown in HMEC-1 cells caused disruption of tube formation on Matrigel. A and B, HMEC-1 cells were plated into six-well plates for 24 h before transfection with Id1 shRNA for 30 h. Both RT-PCR and Western blotting were then used to detect Id1 expression. Id1 shRNA could down-regulate Id1 expression at mRNA level (A) and protein level (B). C, the tube formation of HMEC-1 cells on Matrigel was disrupted (panel c) after Id1 shRNA transfection, compared with the blank (panel a) and sham control (panel b). HMEC-1 cells (2.5 × 105 cells/well) were seeded into a six-well plate for 24 h before transfection. The shRNA plasmids were transfected into the cells three times 24 h apart using X-fect polymer (Clontech). The cells were used in the tube formation assay as described under “Experimental Procedures” 24 h after the last transfection. Photos were taken after another 12 h of incubation. The results are representative of three experiments.
WSS25 Blocks BMP/Smad/Id1 Signaling in HMEC-1 Cells
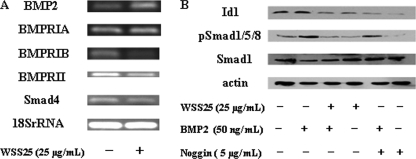
Id1 is the canonical and direct downstream effector of the BMP/Smad pathway (20). Additionally, Id1 expression stimulated by BMP is necessary and sufficient for activation of endothelial cells by BMPs (21). To determine whether the inhibition of Id1 expression by WSS25 in HMEC-1 cells was due to the blocking of BMP/Smad signaling, we investigated the impact of WSS25 on the components of this pathway, including BMP2, BMPRIA, BMPRIB, BMPRII, and Smad4 in HMEC-1 cells. Interestingly, WSS25 increased the expressions of BMP2 but decreased the expression of BMPRIB and BMPRII (Fig. 5A). However, WSS25 had little effect on the expressions of BMPRIA and Smad4 (Fig. 5A).
FIGURE 5.
Effects of WSS25 on Id1, BMP2, and Smad signaling pathway components. A, WSS25 displayed different effects on the expressions of BMP2, BMPRIA, BMPRIB, BMPRII, and Smad4. RT-PCR analysis was performed as described under “Experimental Procedures.” B, WSS25 or Noggin inhibited both Smad1/5/8 phosphorylation and Id1 expression in HMEC-1 cells induced by BMP2. The cells were pretreated with 25 μg/ml of WSS25, 5 μg/ml noggin or vehicle for 23 h. The cells were then treated with 50 ng/ml of BMP2 or vehicle for another hour. The extracted proteins were analyzed by Western blotting using Id1, Smad1, and pSmad1/5/8 antibodies. β-Actin was used as a control for protein loading. The experiments were repeated twice.
BMPs mediate Id1 gene expression via modulating the phosphorylation of Smad1/5/8, which forms a complex with Smad4 after the phosphorylation and then translocates into the nucleus to modulate Id1 expression. Therefore, we next examined the effect of WSS25 on the phosphorylation of Smad1/5/8 and Id1 expression, both of which are induced by BMP2. Indeed, WSS25 effectively blocked the BMP2 induced phosphorylation of Smad1/5/8 and Id1 expression, similar to the effect of the endogenous BMP2 antagonist noggin used as a control (Fig. 5B). These results indicated that WSS25 down-regulates Id1 expression via inhibition of BMP/Smad signaling in HMEC-1 cells.
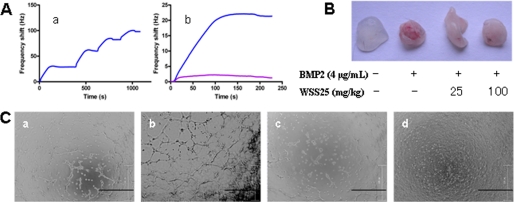
WSS25 Binds BMP2 and Inhibits BMP2-induced Angiogenesis in Vitro and in Vivo
Although WSS25 may induce BMP2 expression, the observations above suggested that the blocking of BMP/Smad signaling is sufficient for inhibiting Id1 expression. We then asked whether WSS25 could target BMP2 to block BMP/Smad signaling and inhibit BMP2 induced angiogenesis. Heparin was reported to disrupt BMP/Smad signaling via binding BMPs (30), suggesting that HS mimetics may also bind BMPs and influence their function. Thus, we analyzed the interaction between WSS25 and BMP2 using QCM analysis and found that WSS25 strongly bound to BMP2 (Fig. 6A). This result demonstrated that BMP2 was at least one of the targets of WSS25. Next, we examined the effect of WSS25 on angiogenesis. Indeed, BMP2 could promote angiogenesis in the Matrigel plugged into C57/BL6 mice (Fig. 6B) and enhance the tube formation of HMEC-1 cells on Matrigel in the FBS restricted condition (b, in Fig. 6C). However, WSS25 could abrogate both the in vitro (Fig. 6C, panel d) and in vivo angiogenic effects of BMP2 (Fig. 6B). Again, as a control and also a BMP2 binding molecule, the endogenous BMP antagonist Noggin impaired the tube formation of HMEC-1 cells on Matrigel in this experiment (Fig. 6C, panel c). The previously mentioned data also suggested that binding to BMP2 conferred WSS25 function as antagonist as noggin did to inhibit the tube formation of HMEC-1 cells.
FIGURE 6.
Interaction of WSS25 with BMP2 and its effects on BMP2-induced angiogenesis. A, WSS25 strongly bound to BMP2 by QCM analysis. The WSS25 biosensor surface was prepared for measuring carbohydrate-protein interactions, where the frequency shift produced from biotinylated WSS25 binding to the streptavidin surface is shown in (panel a). The WSS25-BMP2 interaction was tested by injecting BMP2 (50 μg/ml, 50 μl) in running buffer onto the WSS25 biosensor surface, and the frequency response is displayed in panel b (blue curve). The large shift produced indicated that WSS25 bound to BMP2 strongly. As a control, the frequency responses by the BMP2 interaction with the streptavidin surface were measured, yielding a small response in panel b (pink curve). These results indicate the BMP2-WSS25 interaction was specific. B, WSS25 inhibited BMP2-induced angiogenesis in the Matrigel plug assay. Matrigel (500 μl) with or without BMP2 (4 μg/ml) was subcutaneously injected into the ventral region of C57/BL6 mice. WSS25 (25 mg/kg or 100 mg/kg body weight) was administered to the mice every other day from the second day after the Matrigel was plugged into the mice. Normal saline was used as the control. C, WSS25 and Noggin inhibited BMP2-induced tube formation of HMEC-1 cells on Matrigel. Growth factor reduced Matrigel (50 μl/well) was added to a 96-well plate to be solidified in 37 °C for 30 min. HMEC-1 cells (3 × 104 cells in 98 μl MCDB131 medium supplemented with 0.1% FBS per well) were seeded into the 96-well plate after the Matrigel solidification. BMP2 (200 ng/ml) was added together with the cells only (panel b), or in the presence of Noggin (1 μg/ml) (panel c) or WSS25 (25 μg/ml) (panel d). Photos were taken after incubation for 24 h at 37 °C.
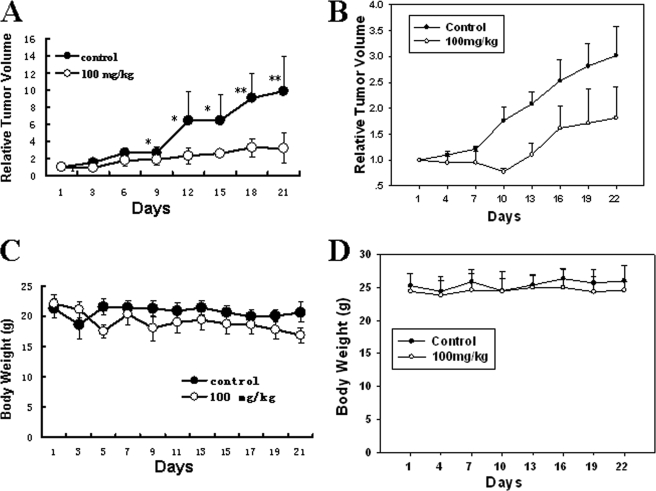
WSS25 Suppresses the Growth of HCC Xenografted in Nude Mice
Angiogenesis is important for tumor growth, and agents that prevent it can efficiently inhibit tumor growth. As mentioned above, we found that WSS25 could potently disrupt the migration and tube formation of HMEC-1 cells. Based on these observations, WSS25 would theoretically have the potential to inhibit tumor growth via disruption of angiogenesis in vivo. Therefore, two types of HCC cells, Bel7402 and SMMC7721, were subcutaneously inoculated into the front pads of male nude mice (ages ranged from 4 to 6 weeks) to evaluate the effect of WSS25 on tumor growth in vivo. WSS25 (100 mg/kg) was administered to the mice via tail vein injection as described under “Experimental Procedures.” The results showed that WSS25 significantly inhibited the growth of both Bel7402 (Fig. 7A) and SMMC7721 (Fig. 7B) cells xenografted into the nude mice. The T/C (%) were 32.2 and 56%, respectively. The result was confirmed by a cell proliferation-associated marker, a ubiquitous nuclear protein Ki-67 staining because the expression of Ki-67 in the WSS25-treated Bel7402 xenografts was lower than the control (supplemental Fig. 4, A and B). During the experiments, the body weight of the mice tested showed no significant change in both groups with the xenografts (Fig. 7, C and D).
FIGURE 7.
WSS25 represses the growth of HCC xenografted in nude mice. Bel7402 (A) and SMMC7721 (B) cells were subcutaneously injected into the front pad of male nude mice. For the Bel7402 xengografts (five mice/group), after the tumor volume grew to ∼100 mm3, 100 mg/kg of WSS25 in normal saline or the vehicle were injected via tail vein every other day. For the SMMC7721 xenografts, well developed tumors were cut into 1–3 mm3 fragments and transplanted subcutaneously into the right flank of the nude mice using a trocar under sterile conditions. When the tumor volume reached ∼100 mm3, the mice were randomly assigned into control and treatment groups (six mice/group), and the vehicle or 100 mg/kg WSS25 was administered via tail vein every other day. Tumor volume was measured using a caliber on the indicated days. Mice were sacrificed 22 days after treatment. The therapeutic effect of the compounds was expressed as the volume ratio of treatment to control (T/C). T/C (%) = 100% × (mean RTV of the treated group/mean RTV of the control group). The tumor growth was significantly inhibited in both tumor models. *, p < 0.05; **, p < 0.01. The T/C (%) for Bel7402 and SMMC7721 was 32.2 and 56%, respectively. The body weights of the mice showed no significant changes during the experiments.
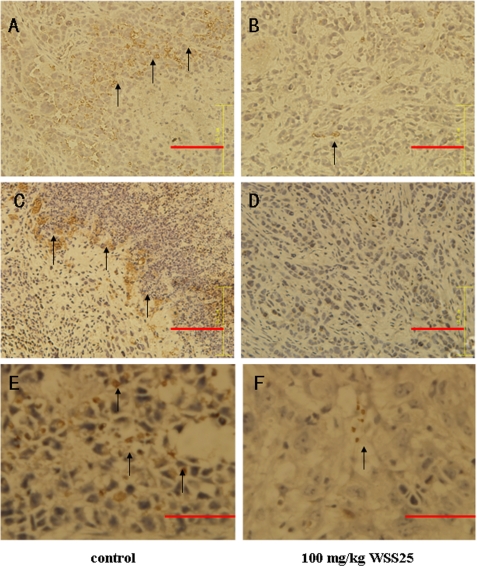
WSS25 Inhibits Angiogenesis and Blocks BMP/Smad/Id1 Signaling in HCC Xenografted in Nude Mice
Because we showed that WSS25 inhibited the growth of HCC xenografted in nude mice, the next question was whether the inhibitory effect of WSS25 on the growth of tumor xenografts could be attributed to attenuation of angiogenesis via inhibition of Id1 expression. To address this question, immunohistochemical analysis was performed to detect the expression of CD31 and Id1 expression in the tumor tissues. CD31 is a well established biomarker in endothelial cells (31), as the microvessel density is positively related to its expression. Compared with the control (Fig. 8A), the CD31 expression was significantly lower in the tissue from the WSS25 treated mice (Fig. 8B), suggesting that the angiogenesis was also inhibited in vivo by WSS25. Importantly, the expression of Id1 was also down-regulated in the tumor tissue from the mice treated with WSS25 (Fig. 8D). Meanwhile, compared with the control (Fig. 8E), the expression of phosphorylated Smad1/5/8 in tumor tissues (Fig. 8F) after WSS25 treatment was also reduced. These results suggested that the inhibition of tumor growth could be indeed attributed to the inhibition of Id1 expression and subsequent impairment of angiogenesis. Additionally, the Id1 expression inhibition, at least partly, was attributed to the blockade of BMP/Smad signaling.
FIGURE 8.
Id1, phosphorylated Smad1/5/8, and CD31 expression levels were lower in WSS25-treated tumor tissue than in the control. Immunohistochemical analysis was performed on tumors from mice treated with 100 mg/kg of WSS25 via tail vein injection or the vehicle as described above. Compared with the control (A), there was also a reduction in the number of blood vessels (shown by reduced expression of the CD31 endothelial marker) in tumors treated with 100 mg/kg WSS25 (B) as indicated by the arrow. Representative images showed that treatment with WSS25 down-regulated Id1 expression in the tumor tissue (C) compared with the control (D) as indicated by the arrow. Phosphorylated Smad1/5/8 expression was also reduced in WSS25-treated tissues (F) compared with the control (E). All images are at 400× magnification.
DISCUSSION
In this study, we demonstrated that the sulfated glucan WSS25 potently inhibited the tube formation of HMEC-1 cells on Matrigel and migration. However, WSS25 only slightly inhibited the proliferation of the cells at concentrations <100 μg/ml. The inhibition may be due to the ablation of Id1 expression which could be attributed, at least in part, to the blockade of BMP/Smad signaling. More importantly, WSS25 inhibited angiogenesis and the growth of HCC xenografted in nude mice, whereas the Id1 expression in the tumor tissue also was substantially down-regulated. Furthermore, WSS25 also blocked the Id1 expression (supplemental Fig. 5, B and C) and abrogated the BMP/Smad signaling (supplemental Fig. 6) in Bel7402 cells.
Id1 is well established as an anti-angiogenesis target. This also was confirmed by our results (Fig. 4). However, it is extremely difficult to directly inhibit Id1 due to its nuclear localization and structural homology to the basic helix loop helix transcriptional factors. Although Id1 expression was demonstrated to be effectively inhibited by antisense nucleic acids (32, 33), the use of this technology is still not routine in the clinic. Thus, alternative ways to inhibit Id1 are necessary. Id1 is the downstream effecter of VEGF (22) and FGF (3). However, both resistance and adverse effect were reported in the U.S. Food and Drug Administration approved anti-angiogenesis agents, including sunitinib, sorafenib, and bevacizumab, all of which exert their effects via targeting VEGF signaling (34). Id1 was reported to be a common downstream effector of oncogenic tyrosine kinase in leukemic cells (35). Src inhibitor could also impair the expression of Id1 in cancer cells from different sources (36). The Epac/Rap1-related pathway also appears to be the upstream modulator of Id1 (37). However, Id1 is the canonical and direct downstream effector of the BMP/Smad signaling pathway. More importantly, Id1 stimulated by BMPs is sufficient and necessary for the activation of endothelial cells by BMPs (21). Thus, blocking BMP/Smad signaling is an ideal choice for inhibiting Id1 expression.
Accumulating evidence has demonstrated that BMP proteins are involved in angiogenesis regulation. The well established examples include BMP2 and BMP4. BMP2 was found to promote the tumor angiogenesis of non-small lung cancer cell in nude mice (38, 39). Both BMP2 and BMP4 were reported to enhance angiogenesis in melanoma tumors (40). The angiogenic effect was exerted through the signaling triggered through the BMP type I receptor (BMPRIA, BMPRIB) and BMPRII. Their co-receptor betaglycan (type III TGF-β receptor), which is a proteoglycan, also plays an important role in the signal transduction process (18). Additionally, Id1 expression stimulated by BMP proteins was sufficient and necessary for activation of endothelial cells (21). More importantly, BMPRII was demonstrated to be required for maintenance of vascular integrity (41). In our study, BMP2, BMPRIA, BMPRIB, and BMPRII were all expressed in the HMEC-1 cells (Fig. 5A). Both the expression of Id1 and the phosphorylation of Smad1/5/8 induced by BMP2 were inhibited by WSS25 (Fig. 5B). Although the expression of BMP2 was up-regulated after 18 h of treatment with WSS25, the overall effects of WSS25 treatment was the blockade of BMP/Smad signaling. Furthermore, the expression of BMP2 was increased only by WSS25 after 18 h of treatment (supplemental Fig. 7). In addition to the BMP blocking effect, the overall effects of WSS25 could be partly due to the down-regulation of BMPRIB and BMPRII, as BMPRIB overexpressed by plasmid transfection into the HMEC-1 cells could partially rescue the inhibition of tube formation by WSS25 treatment (supplemental Fig. 8).
HS mimetics are widely reported to inhibit angiogenesis through blocking the interaction between the angiogenic factors and their receptors. The most frequently reported angiogenic factors are FGF2 and VEGF. For example, the HS mimetic PI-88, which is in phase II clinical trials for several types of cancer (42), inhibits angiogenesis by strongly binding to FGF1, FGF2, and VEGF (15, 43). HS was also reported to bind to the BMP proteins to interfere with BMP/Smad signaling. For example, heparin could bind with BMP2 proteins as demonstrated by QCM (30) and surface plasmon resonance (16) analysis. This interaction would lead to the blocking of BMP/Smad signaling. WSS25, as a sulfated derivative of an α-d-glucan and a HS mimetic, could strongly bind with the BMP2 protein (Fig. 6A) and block BMP/Smad signaling as heparin did. Furthermore, WSS25 inhibited BMP2 induced angiogenesis in vitro and in vivo. Thus, these findings could reasonably explain the down-regulation of Id1 and inhibition of angiogenesis and HCC growth by WSS25. Moreover, heparin was reported to bind to the BMP receptors, BMPRIA, BMPRIB, and BMPRII. Because WSS25 is structurally similar to heparin and glycosaminoglycan chains attached to the core protein of betaglycan, we therefore speculated that WSS25 also may bind with the BMP receptors to block the activation of the BMP/Smad signaling. Recently, BMP2 was reported to induce angiogenesis through noncanonical pathways such as the Wnt/β-catenin and Wnt/RhoA/Rac1 pathways (44). As mentioned earlier, WSS25 can tightly bind with BMP2, and this interaction may also lead to inhibition of the noncanonical angiogenic signals by blocking the activation by BMP2. Although WSS25 was found to strongly bind with BMP2, the exact structural domain required for the binding is still not clear and remains to be determined in future work.
Id1 is the downstream effector of VEGF (22) and FGF (3). HS could bind with VEGF and FGF to modulate angiogenesis. Thus, WSS25 may inhibit Id1 expression through disrupting the interaction between them, although this hypothesis needs to be confirmed by further work. Overexpression of Id1 in HCC cells induces cell proliferation (10). Accordingly, the inhibitory effect of WSS25 on the growth of HCC xenografted in nude mice could also be attributed to the direct inhibition of the HCC cell growth, whereas it only slightly inhibited the proliferation of HCC cells (supplemental Fig. 2A).
In summary, we reported here that WSS25, a HS mimetic, inhibited the growth of HCC xenografted in nude mice via disruption of angiogenesis by abrogating Id1 expression. This inhibition of Id1 expression was due, at least partly, to the blocking of BMP/Smad signaling. Thus, WSS25 is a potential drug candidate for HCC therapy that would function via blockade of Id1 expression.
Supplementary Material
Acknowledgments
We thank Shengguang Fu in Shanghai University of Traditional Chinese Medicine for great assistance in animal study. We also thank other members in the lab for critical comments on the manuscript.
This work was supported by grants from the National Natural Science Foundation of China (30670470); the National High Technology Research and Development Program (863) of China (2006AA022102); the New Drug Research Program of the Shanghai Institute of Materia Medica, Chinese Academy of Sciences (07G604F036); the “100 Talents Project” of Chinese Academy of Sciences, China (to K. D.); the Knowledge Innovation Program of Chinese Academy of Sciences (KSCX1-YW-R-18); and the National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program” (2009ZX09301-001 and 2009ZX09103-071).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1 and 2 and Figs. 1–9.
- HCC
- hepatocellular cancer
- HS
- heparan sulfate
- BMP
- bone morphogenetic protein
- BMPRIA
- BMP receptor IA
- BMPRIB
- BMP receptor IB
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- QCM
- quartz crystal microbalance
- HMEC
- human microvascular endothelial cells.
REFERENCES
- 1.Tang Z. Y. (2001) World J. Gastroenterol. 7, 445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun H. C., Tang Z. Y. (2004) J. Cancer Res. Clin. Oncol. 130, 307–319 [DOI] [PubMed] [Google Scholar]
- 3.Perk J., Iavarone A., Benezra R. (2005) Nat. Rev. Cancer. 5, 603–614 [DOI] [PubMed] [Google Scholar]
- 4.Ouyang X. S., Wang X., Lee D. T., Tsao S. W., Wong Y. C. (2002) J. Urol. 167, 2598–2602 [PubMed] [Google Scholar]
- 5.Perk J., Gil-Bazo I., Chin Y., de Candia P., Chen J. J., Zhao Y., Chao S., Cheong W., Ke Y., Al-Ahmadie H., Gerald W. L., Brogi E., Benezra R. (2006) Cancer Res. 66, 10870–10877 [DOI] [PubMed] [Google Scholar]
- 6.Lyden D., Young A. Z., Zagzag D., Yan W., Gerald W., O'Reilly R., Bader B. L., Hynes R. O., Zhuang Y., Manova K., Benezra R. (1999) Nature 401, 670–677 [DOI] [PubMed] [Google Scholar]
- 7.Ruzinova M. B., Schoer R. A., Gerald W., Egan J. E., Pandolfi P. P., Rafii S., Manova K., Mittal V., Benezra R. (2003) Cancer Cell. 4, 277–289 [DOI] [PubMed] [Google Scholar]
- 8.Lyden D., Hattori K., Dias S., Costa C., Blaikie P., Butros L., Chadburn A., Heissig B., Marks W., Witte L., Wu Y., Hicklin D., Zhu Z., Hackett N. R., Crystal R. G., Moore M. A., Hajjar K. A., Manova K., Benezra R., Rafii S. (2001) Nat. Med. 7, 1194–1201 [DOI] [PubMed] [Google Scholar]
- 9.Ling M. T., Wang X., Zhang X., Wong Y. C. (2006) Differentiation 74, 481–487 [DOI] [PubMed] [Google Scholar]
- 10.Lee T. K., Man K., Ling M. T., Wang X. H., Wong Y. C., Lo C. M., Poon R. T., Ng I. O., Fan S. T. (2003) Carcinogenesis 24, 1729–1736 [DOI] [PubMed] [Google Scholar]
- 11.Lee T. K., Poon R. T., Yuen A. P., Ling M. T., Wang X. H., Wong Y. C., Guan X. Y., Man K., Tang Z. Y., Fan S. T. (2006) Clin. Cancer. Res. 12, 6910–6919 [DOI] [PubMed] [Google Scholar]
- 12.Iozzo R. V., San Antonio J. D. (2001) J. Clin. Invest. 108, 349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding K., Lopez-Burks M., Sánchez-Duran J. A., Korc M., Lander A. D. (2005) J. Cell. Biol. 171, 729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esko J. D., Selleck S. B. (2002) Annu. Rev. Biochem. 71, 435–471 [DOI] [PubMed] [Google Scholar]
- 15.Karoli T., Liu L., Fairweather J. K., Hammond E., Li C. P., Cochran S., Bergefall K., Trybala E., Addison R. S., Ferro V. (2005) J. Med. Chem. 48, 8229–8236 [DOI] [PubMed] [Google Scholar]
- 16.Ruppert R., Hoffmann E., Sebald W. (1996) Eur. J. Biochem. 237, 295–302 [DOI] [PubMed] [Google Scholar]
- 17.Ohkawara B., Iemura S., ten Dijke P., Ueno N. (2002) Curr. Biol. 12, 205–209 [DOI] [PubMed] [Google Scholar]
- 18.David L., Feige J. J., Bailly S. (2009) Cytokine Growth Factor Rev. 20, 203–212 [DOI] [PubMed] [Google Scholar]
- 19.Rider C. C. (2006) Biochem. Soc Trans. 34, 458–460 [DOI] [PubMed] [Google Scholar]
- 20.Miyazono K., Miyazawa K. (2002) Sci. STKE 2002, pe40. [DOI] [PubMed] [Google Scholar]
- 21.Valdimarsdottir G., Goumans M. J., Rosendahl A., Brugman M., Itoh S., Lebrin F., Sideras P., ten Dijke P. (2002) Circulation 106, 2263–2270 [DOI] [PubMed] [Google Scholar]
- 22.Sakurai D., Tsuchiya N., Yamaguchi A., Okaji Y., Tsuno N. H., Kobata T., Takahashi K., Tokunaga K. (2004) J. Immunol. 173, 5801–5809 [DOI] [PubMed] [Google Scholar]
- 23.Lindahl U. (2007) Thromb. Haemost. 98, 109–115 [PubMed] [Google Scholar]
- 24.Qiu H., Tang W., Tong X., Ding K., Zuo J. (2007) Carbohydr. Res. 342, 2230–2236 [DOI] [PubMed] [Google Scholar]
- 25.Xu Y., Swerlick R. A., Sepp N., Bosse D., Ades E. W., Lawley T. J. (1994) J. Invest. Dermatol. 102, 833–837 [DOI] [PubMed] [Google Scholar]
- 26.Peddada S., Yasui D. H., LaSalle J. M. (2006) Hum. Mol. Genet. 15, 2003–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McAllister S. D., Christian R. T., Horowitz M. P., Garcia A., Desprez P. Y. (2007) Mol. Cancer. Ther. 6, 2921–2927 [DOI] [PubMed] [Google Scholar]
- 28.Cejalvo T., Sacedón R., Hernández-López C., Diez B., Gutierrez-Frías C., Valencia J., Zapata A. G., Varas A., Vicente A. (2007) Immunology 121, 94–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folkman J., Haudenschild C. (1980) Nature 288, 551–556 [DOI] [PubMed] [Google Scholar]
- 30.Kanzaki S., Takahashi T., Kanno T., Ariyoshi W., Shinmyouzu K., Tujisawa T., Nishihara T. (2008) J. Cell. Physiol. 216, 844–850 [DOI] [PubMed] [Google Scholar]
- 31.Jia J., Wang J., Teh M., Sun W., Zhang J., Kee I., Chow P. K., Liang R. C., Chung M. C., Ge R. (2010) Proteomics 10, 224–234 [DOI] [PubMed] [Google Scholar]
- 32.Fong S., Itahana Y., Sumida T., Singh J., Coppe J. P., Liu Y., Richards P. C., Bennington J. L., Lee N. M., Debs R. J., Desprez P. Y. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13543–13548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henke E., Perk J., Vider J., de Candia P., Chin Y., Solit D. B., Ponomarev V., Cartegni L., Manova K., Rosen N., Benezra R. (2008) Nat. Biotechnol. 26, 91–100 [DOI] [PubMed] [Google Scholar]
- 34.Bergers G., Hanahan D. (2008) Nat. Rev. Cancer. 8, 592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tam W. F., Gu T. L., Chen J., Lee B. H., Bullinger L., Fröhling S., Wang A., Monti S., Golub T. R., Gilliland D. G. (2008) Blood 112, 1981–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gautschi O., Tepper C. G., Purnell P. R., Izumiya Y., Evans C. P., Green T. P., Desprez P. Y., Lara P. N., Gandara D. R., Mack P. C., Kung H. J. (2008) Cancer Res. 68, 2250–2258 [DOI] [PubMed] [Google Scholar]
- 37.Doebele R. C., Schulze-Hoepfner F. T., Hong J., Chlenski A., Zeitlin B. D., Goel K., Gomes S., Liu Y., Abe M. K., Nor J. E., Lingen M. W., Rosner M. R. (2009) Blood 114, 4592–4600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langenfeld E. M., Langenfeld J. (2004) Mol. Cancer. Res. 2, 141–149 [PubMed] [Google Scholar]
- 39.Raida M., Clement J. H., Leek R. D., Ameri K., Bicknell R., Niederwieser D., Harris A. L. (2005) J. Cancer Res. Clin. Oncol. 131, 741–750 [DOI] [PubMed] [Google Scholar]
- 40.Rothhammer T., Bataille F., Spruss T., Eissner G., Bosserhoff A. K. (2007) Oncogene 26, 4158–4170 [DOI] [PubMed] [Google Scholar]
- 41.Liu D., Wang J., Kinzel B., Müeller M., Mao X., Valdez R., Liu Y., Li E. (2007) Blood 110, 1502–1510 [DOI] [PubMed] [Google Scholar]
- 42.Ferro V., Dredge K., Liu L., Hammond E., Bytheway I., Li C., Johnstone K., Karoli T., Davis K., Copeman E., Gautam A. (2007) Semin. Thromb. Hemost. 33, 557–568 [DOI] [PubMed] [Google Scholar]
- 43.Parish C. R., Freeman C., Brown K. J., Francis D. J., Cowden W. B. (1999) Cancer Res. 59, 3433–3441 [PubMed] [Google Scholar]
- 44.de Jesus Perez V. A., Alastalo T. P., Wu J. C., Axelrod J. D., Cooke J. P., Amieva M., Rabinovitch M. (2009) J. Cell Biol. 184, 83–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.