Abstract
Adipocyte dysfunction is strongly associated with the development of obesity, which is a major risk factor for many disorders, including diabetes, hypertension, and heart disease. This study shows that ultraviolet A (UVA) inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells and its action mechanisms. The mRNA levels of peroxidase proliferator-activated receptor (PPAR) γ and CCAAT/enhancer-binding protein α (C/EBPα), but not CCAAT/enhancer-binding protein ((C/EBP) β and δ, were reduced by UVA. Moreover, the mRNA levels of PPAR γ target genes (lipoprotein lipase (LPL), CD36, adipocyte protein (aP2), and liver X receptor α (LXR)) were down-regulated by UVA. Additionally, attempts to elucidate a possible mechanism underlying the UVA-mediated effects revealed that UVA induced migration inhibitory factor (MIF) gene expression, and this was mediated through activation of AP-1 (especially JNK and p42/44 MAPK) and nuclear factor-κB. In addition, reduced adipogenesis by UVA was recovered upon the treatment with anti-MIF antibodies. AMP-activated protein kinase phosphorylation and up-regulation of Kruppel-like factor 2 (KLF2) were induced by UVA. Taken together, these findings suggest that the inhibition of adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells by UVA occurs primarily through the reduced expression of PPAR γ, which is mediated by up-regulation of KLF2 via the activation of MIF-AMP-activated protein kinase signaling.
Keywords: Adipocyte, Adipose Tissue, AMP-activated Kinase (AMPK), AP-1 Transcription Factor, C/EBP Transcription Factor, Cell Differentiation, Gene Expression, Gene Regulation, Obesity, PPAR
Introduction
Obesity is associated with a number of pathological disorders, including non-insulin-dependent diabetes, hypertension, hyperlipidemia, and cardiovascular diseases (1). Adipose tissue biology studies have led to improved understanding of the mechanisms that link obesity to metabolic syndrome and other complications. In addition, recent advances in adipocyte biology have established that adipose tissue serves as a means of energy storage in the form of triglycerides as well as exerts secretory/endocrine gland functions, producing various secretory molecules such as hormones and cytokines (2). In view of the preventive and therapeutic aspects of the diseases mentioned above, most intensive clinical interventions have primarily been directed at decreasing excessive amounts of adipose tissue by changing the balance between intake and the expenditure of energy (3). Such changes are typically induced via daily exercise and diet control (4). Mechanical stimuli such as stretching and rubbing of fat and skeletal muscle have also been found to decrease obesity. Indeed, Tanabe et al. (5) reported that mechanical stretching inhibits adipocyte differentiation through extracellular signal-regulated kinase (ERK)-mediated down-regulation of proliferator-activated receptor (PPAR)2 γ2.
Adipocytes are the major cellular component in adipose tissue, and their excessive growth, differentiation, and hypertrophy are fundamental processes involved in obesity. Maturation of adipocytes can occur among cells from a pre-existing pool of adipocyte progenitor cells that are present, irrespective of age (6). Therefore, from a pathophysiological point of view, both the proliferation and differentiation of preadipocytes into mature adipocytes remain important issues.
Ultraviolet (UV) irradiation is a major environmental factor responsible for a high incidence of skin aging, which is referred to as photoaging, as well as skin cancer and melanoma. UVA (320–380 nm) irradiation represents 90% of the solar UV light that reaches the surface of the earth; therefore, its contribution to human life may be significant. The UVA component of sunlight has oxidizing properties that may be deleterious to skin cells and tissue but that can also lead to strong up-regulation of the heme-catabolizing enzyme, heme oxygenase-1. This enzyme has well established antioxidant actions in cells as well as anti-inflammatory properties in mammals. There is also evidence from rodent models that this enzyme is responsible for the UVA-mediated protection against UVB-induced immunosuppression that occurs in skin. The relevance of these findings to the acute and chronic effects of sunlight, including skin carcinogenesis, is currently under investigation as are the potential implications for sunlight protection in humans (7, 8). A range of mammalian cells such as fibroblasts (9), keratinocytes (10), melanocytes (11), cardiomyocytes (12), vascular endothelial cells (13), smooth muscle cells (14), and osteoblasts (15) can respond to UVA irradiation. To the best of our knowledge, no studies have been conducted to evaluate the direct effects of UVA irradiation on adipocyte differentiation. Here, we demonstrate that UVA irradiation inhibited the differentiation of human adipose tissue-derived mesenchymal stem cells (hAMSCs) into adipocytes. This effect was mostly due to the reduced expression of PPAR γ, an adipocyte-specific nuclear hormone receptor/adipogenic transcription factor (16). This reduced expression was mediated by the activation of migration inhibitory factor (MIF)-AMP-activated protein kinase (AMPK)-Kruppel-like factor (KLF) 2 signaling. The modulation of adipocyte differentiation in response to UVA irradiation might provide further insight into the physiological significance of the local application of UVA irradiation to adipose tissues with respect to the inhibition and renewal of adipocytes.
EXPERIMENTAL PROCEDURES
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), anti-peroxisome proliferator-activated receptor γ2 (PPAR γ2 (N-19)), and anti-MIF were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin monoclonal antibody, isobutylmethylxanthine, dexamethasone, and insulin were purchased from Sigma. TRIzol reagent, random primers, and Moloney murine leukemia virus reverse transcriptase were obtained from Invitrogen. AMPK inhibitor (compound C) was purchased from Calbiochem. Anti-phospho-AMPKα antibody (Thr-172) and anti-AMPKα antibody were purchased from Cell Signaling Technology, Inc. (Beverly, MA).
3T3-L1 Cell Culture and Stimulation
3T3-L1 preadipocytes (ATCC, Manassas, VA) were seeded in 6-cm diameter dishes at a density of 15 × 104 cells/well. Cells were grown in phenol red-free DMEM supplemented with 10% charcoal-stripped FBS at 37 °C under 5% CO2. To induce differentiation, 2-day post-confluent 3T3-L1 preadipocytes (day 0) were incubated for 3 days with differentiation medium (0.5 mm isobutylmethylxanthine, 0.25 μm dexamethasone, and 1 μg/ml insulin in phenol red-free DMEM supplemented with 10% charcoal-stripped FBS). The preadipocytes were then maintained in and re-fed every 3 days with maintenance medium (phenol red-free DMEM supplemented with 10% charcoal-stripped FBS and 1 μg/ml insulin). To examine the effects of UVA irradiation on adipocyte differentiation, 2-day post-confluent 3T3-L1 preadipocytes were irradiated with the indicated doses of UVA and then stimulated with differentiation medium for 3 days. The medium was then replaced with maintenance media every 3 days until the end of the experiment on day 9.
Human Adipose Tissue-derived Mesenchymal Stem Cell Culture and Stimulation
hAMSCs (Invitrogen) were seeded in 6-cm diameter dishes at a density of 15 × 104 cells/well. Cells were grown in MesenPro RSTM media (Invitrogen) at 37 °C under 5% CO2. To induce differentiation, 2-day post-confluent hAMSCs (day 0) were incubated for 14 days with STEM PRO® adipocyte differentiation media (Invitrogen). To examine the effects of UVA irradiation on adipocyte differentiation of hAMSCs, 2-day post-confluent hAMSCs were irradiated with the indicated doses of UVA and then stimulated with STEM PRO® adipocyte differentiation media (Invitrogen) or STEM PRO® osteogenesis differentiation media (Invitrogen) for 3 days. The medium was then replaced with STEM PRO® adipocyte differentiation media (Invitrogen) or STEM PRO® osteogenesis differentiation media (Invitrogen) every 3 days until the end of the experiment on day 14.
UVA Irradiation
For UVA exposure, the medium was removed, and the cells were washed with PBS and gently overlaid with PBS. The cells were then irradiated with various fluencies. UVA irradiation was conducted in DMEM devoid of phenol red with a Vilber-Lourmat UVA table centered on 365 nm (TF-20L) with a dose rate of 3 milliwatt/cm2. A piece of glass with a thickness of 4 mm was placed above the table to absorb residual UVB radiation.
Oil Red O Staining
3T3-L1 adipocytes or hAMSCs that were treated as described above were washed with PBS and then fixed with 10% formalin for 30 min. Next, the cells were washed twice with distilled water, after which they were stained for at least 1 h at room temperature in freshly diluted Oil Red O solution (6 parts Oil Red O stock solution and 4 parts H2O; Oil Red O stock solution is 0.5% Oil Red O in isopropyl alcohol). The results were confirmed by three independent experiments.
Triglyceride Assay
hAMSCs that were treated as described above were washed with PBS and then harvested into 25 mm Tris buffer, pH 7.5, containing 1 mm EDTA. The samples were then sonicated three times for 15 s each using UP50H with MS7 (Hielscher Ultrasonics GmbH, Germany) to homogenize the cell suspension, after which the total triglyceride content was evaluated using a triglyceride assay kit (Cayman Chemical, Ann Arbor, MI). The protein content of an aliquot of the homogenate was also determined using a protein assay kit (Pierce). The results were confirmed by three independent experiments.
Glycerol-3-phosphate Dehydrogenase (GPDH) Activity
hAMSCs that were treated as described above were washed twice with PBS and then harvested into 25 mm Tris buffer, pH 7.5, containing 1 mm EDTA and 1 mm DTT. The cells were then disrupted by sonication, after which they were centrifuged at 12,000 × g for 20 min at 4 °C. Next, the supernatants were assayed for GPDH activity using GPDH activity assay kits (Takara Bio Inc., Japan) following the method described by Kozak and Jensen (17). The results were confirmed by four independent experiments.
Cell Viability Assay
The general viability of cultured cells that were treated as described above was determined by the reduction of WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) (Dojindo Laboratories, Japan) to a highly water-soluble formazan dye (18). This assay was performed following the incubation of hAMSCs or 3T3-L1 cells under the conditions described above. Next, 10 μl of WST-8 solution was added to each well, after which the samples were incubated at 37 °C for 3 h and the absorbance was measured at 450 nm using a spectrophotometer (PowerWave, BioTek Inc., Winooski, VT). The results were confirmed by four independent experiments.
DNA Damage Assay
The cell-damaging effects of UVA irradiation were determined using a DNA Damage ELISA kit (Assay Designs, Inc., Ann Arbor, MI). This kit detects 8-hydroxy-2′-deoxyguanosine, which is a by-product of DNA damage. The assay was conducted after incubation of hAMSCs or 3T3-L1 cells under the conditions described above. The cell lysates were added to 96-well plates, and then diluted biotinylated hydroxy-2′-deoxyguanosine was added to each sample well. Next, the samples were incubated at room temperature for 1 h, after which the sample wells were washed. Streptavidin-HRP was then distributed to the sample wells and the plate was incubated for 1 h at room temperature. The wells were then washed and 3,3′,5,5′-tetramethylbenzidine substrate solution was added. Finally, the samples were incubated in the dark, after which the absorbance was read at 450 nm according to the manufacturer's instructions.
Assessment of the Percentage of Apoptotic Cells
To detect apoptotic cells, cells were stained by DNA binding with Hoechst 33342 (Sigma). This assay was conducted after the incubation of hAMSCs or 3T3-L1 cells under the conditions described above. The cells were then fixed with 4% formaldehyde in phosphate-buffered saline (PBS) for 10 min at 4 °C, after which they were washed with PBS. To stain the nuclei, the cells were incubated for 20 min with 20 μg/ml Hoechst 33342. After washing with PBS, the cells were observed under a fluorescence microscope (Zeiss Axiophot 2, Carl Zeiss, Germany). Cells exhibiting condensed chromatin and fragmented nuclei were scored as apoptotic cells. A minimum of 500 cells were scored from each sample.
RNA Preparation and Real Time RT-PCR (TaqManTM) Analysis
Total cellular RNA was extracted from the hAMSCs 1, 7, or 14 days after inducing differentiation with TRIzol reagent according to the manufacturer's instructions (Invitrogen). The cDNA was synthesized from 1 μg of total RNA in a 20-μl reaction mix using random primers and Moloney murine leukemia virus reverse transcriptase. Real time RT-PCR analysis was performed with an ABI7900HT machine (Applied Biosystems). All TaqMan RT-PCR reagents, including primers and probes, were purchased from Applied Biosystems. TaqMan analysis was performed with predesigned and optimized Assays on Demand (Applied Biosystems). The following assays were used: CD36 (Hs00169627_m1), PPAR γ (Hs01115729_m1), C/EBP α (Hs00269972_s1), C/EBP β (Hs00942496_s1), C/EBP δ (Hs00997180_s1), LPL (Hs00173425_m1), aP2 (Hs02559763_s1), KLF2 (Hs00360439_g1), 18 S ribosomal RNA (Hs99999901_s1), LXR α (Hs00172885_m1). The reaction parameters were as follows: 2 min 50 °C hold, 30 min 60 °C hold, and 5 min 95 °C hold, followed by 45 cycles of 20 s 94 °C melting and 1 min 60 °C annealing/extension. All measurements were performed in duplicate or triplicate, and the results were analyzed using the ABI sequence detector software version 2.0 (Applied Biosystems). Relative quantitation was performed as described earlier (34), and 18 S ribosomal RNA (rRNA) was used as a reference gene. Because all assays used were optimized for PCR efficiency by the manufacturer (Applied Biosystems), mRNA expression levels were estimated by the ΔCt values.
Plasmids
PPRE×3 luciferase reporter was provided by Dr. A. Fukuhara (Osaka, Japan). Renilla luciferase expression vector driven by a thymidine kinase promoter was purchased from Promega (Madison, WI).
Luciferase Reporter Assay
To assay for PPRE promoter activities, hAMSCs were transfected with PPRE luciferase reporter along with 1 μg of Renilla luciferase expression vector driven by a thymidine kinase promoter (Promega, Madison, WI) (internal standard) using Superfect transfection reagent (Invitrogen) according to the manufacturer's protocols. Four hours later, the cells were incubated with DMEM in the presence or absence of the indicated doses of UVA irradiation for 20 h, after which the luciferase activities were assayed using a luciferase assay system (Promega, Madison, WI). The cells were then harvested, lysed, and centrifuged. Next, the supernatants were assayed for luciferase activity using a Dual-Luciferase assay system (Promega, WI) and an LB953 luminometer (Berthold, Germany), after which the activity was expressed as a ratio of the PPRE-1-dependent firefly luciferase activity to the control thymidine kinase Renilla luciferase activity (% control). Results were confirmed by eight independent transfections.
Immunoblotting
Two-day post-confluent hAMSCs were irradiated with the indicated doses of UVA and then stimulated with STEM PRO® adipocyte differentiation media (Invitrogen) for 3 days. The medium was then replaced with STEM PRO® adipocyte differentiation media (Invitrogen) every 3 days until the end of the experiment on day 14. On day 14, the cells were washed twice with cold PBS, after which they were lysed in 150 μl of sample buffer (100 mm Tris-HCl, pH 6.8, 10% glycerol, 4% SDS, 1% bromphenol blue, 10% β-mercaptoethanol). Next, the samples were resolved by SDS-PAGE and transferred to Immobilon-P PVDF membranes (Millipore Corp., Bedford, MA). The membranes were then incubated overnight at 4 °C with anti-PPAR γ 2 (N-19) antibody, anti-phospho-AMPKα antibody (Thr-172), anti-AMPK α antibody, and anti-β-actin antibody. The membranes were then washed three times with Tris-buffered saline containing Tween 20 (Sigma) (TBST), probed with horseradish peroxidase-conjugated secondary antibody, and developed using an ECL Western blotting detection system (Amersham Biosciences).
ELISA
The concentrations of TNF-α, IL-1β, TGF-β1, VEGF, or MIF in the culture supernatant were measured using ELISA kits (Genzyme, Minneapolis, MN). Culture supernatants were added to 96-well plates, and diluted biotinylated TNF-α, IL-1β, TGF-β1, VEGF, or MIF was then added to the sample wells. Next, the samples were incubated at room temperature for 3 h, after which the sample wells were washed. Streptavidin-HRP was distributed to the sample wells, and the plate was then incubated for 30 min at room temperature. The wells were then washed, and 3,3′,5,5′-tetramethylbenzidine substrate solution was added. Finally, the samples were incubated in the dark, after which the absorbance was read at 450 nm for 12–15 min according to the manufacturer's instructions.
AMPK Activity Assay
AMPK activity was determined using the SAMS peptide assay as described previously (19). Briefly, proteins were extracted using appropriate lysis buffer, and the protein content was determined using bicinchoninic acid assay (Pierce). For measurement of isoform-specific AMPK activity, 20 μg of tissue lysates were immunoprecipitated by incubation with specific antibodies against the α1-AMPK catalytic subunits (Cell Signaling Technology, Beverly, MA) and 15 μl of 25% (w/v) protein G/A-Sepharose beads (Santa Cruz Biotechnology) overnight at 4 °C. The immunoprecipitates were incubated in kinase assay buffer (62.5 mm HEPES, pH 7.0, 62.5 mm NaCl, 62.5 mm NaF, 6.25 mm sodium pyrophosphate, 1.25 mm EDTA, 1.25 mm EGTA, and 1 mm dithiothreitol) containing 200 μm AMP, ATP mixture (200 μm ATP and 1.5 μCi of [γ-32P]ATP), with or without 200 μm SAMS peptide (HMRSAMSGLHLVKRR) at 30 °C for 10 min. The reaction was terminated by spotting the reaction mixture on phosphocellulose paper, and the paper was extensively washed with 150 mm phosphoric acid. The radioactivity was measured using a scintillation counter.
Measurement of Intracellular ATP
ATP contents were determined by the luciferin-luciferase method using an ENLITEN ATP assay system bioluminescence detection kit (Promega Corp., Madison, WI) according to the manufacturer's instructions. Briefly, cultured cells were rinsed with cold phosphate-buffered saline (PBS, pH 7.4), incubated in cold somatic cell ATP releasing reagent for 5 min on ice, harvested with a cell scraper, and then lysated. A 10-μl aliquot was added to the luciferase ATP assay mixture, and the initial light emission was determined with a luminometer. Data were compared with ATP standards to calculate the total ATP content of each sample. The result was normalized to the protein content of the cell lysate determined using the bicinchoninic acid method.
Small Interference RNA (siRNA)
siRNAs against AMPKa1 (5′-UGCCUACCAUCUCAUAAUAdTdT),and nontargeting scrambled siRNAs were synthesized by Sigma-Proligo. Cells were then transfected with the indicated siRNAs at 50 nm during 48 h using DharmaFECT transfection agent (Dharmacon Research) according to the manufacturer's instructions.
Alkaline Phosphatase Staining
Alkaline phosphatase staining was conducted using a StemTAGTM alkaline phosphatase staining kit (Cell Biolabs, Inc., San Diego). Briefly, hAMSCs that were treated as described above were washed with PBS containing 0.05% Tween 20 and then fixed with fixing solution for 2 min. Next, the cells were washed twice with PBS containing 0.05% Tween 20, after which they were stained for 15–30 min at room temperature in freshly prepared StemTAGTM solution. The results were confirmed by three independent experiments.
Alkaline Phosphatase Activity Assay
hAMSCs were treated as described above, and the alkaline phosphatase activity was evaluated as a marker for preosteoblasts. Alkaline phosphatase activity was measured in cell lysates, using a colorimetric assay (Sigma) and normalized to the level of total cellular protein (Pierce). The results were confirmed by four independent experiments.
Statistical Analysis
All data are expressed as the means ± S.D. Differences between the control and the treated group were evaluated by a Student's t test using the Statview software (Abacus Concepts, Cary, NC). For all analyses, a p < 0.05 was considered to be statistically significant.
RESULTS
UVA Irradiation Inhibits Adipogenic Differentiation
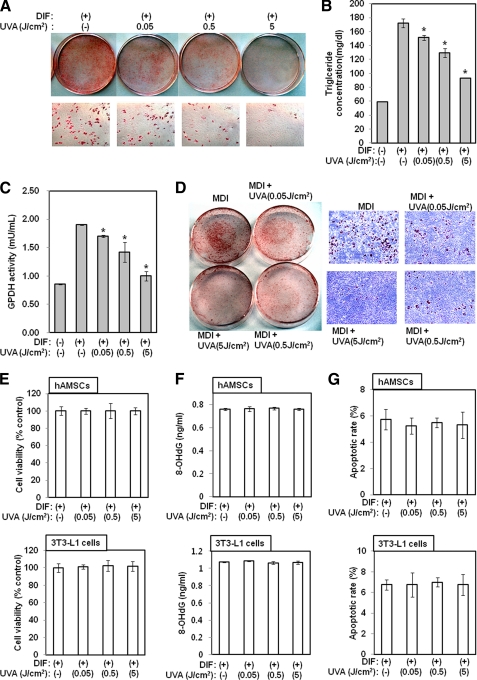
Two-day post-confluent hAMSCs (day 0) were treated with the indicated doses of UVA irradiation and then stimulated with adipocyte differentiation media for 3 days. The medium was then replaced with adipocyte differentiation media every 3 days until the end of the experiment at day 14. After the preadipocytes differentiated into adipocytes, morphological alterations were observed due to the accumulation of lipid droplets in the cytoplasm. Specifically, Oil Red O staining revealed that the lipid accumulation in UVA-irradiated cells was significantly lower than in control cells (Fig. 1A). To further characterize the effects of UVA irradiation on differentiation, the cellular triglyceride content and GPDH enzyme activity were measured. UVA irradiation inhibited triglyceride accumulation on day 21 after full differentiation had occurred (p < 0.05) (Fig. 1B). Consistent with the observed reduction in triglyceride accumulation, GPDH activity was also reduced by UVA irradiation (p < 0.05) (Fig. 1C). The aforementioned results demonstrated that UVA irradiation inhibits adipogenesis in hAMSCs. To further investigate the role of UVA, we studied its effects on the differentiation of 3T3-L1 preadipocytes into adipocytes. Two-day post-confluent 3T3-L1 cells (day 0) were treated with the indicated doses of UVA irradiation every 3 days for 9 days. Similar to the results observed in the hAMSCs, Oil Red O staining revealed that the level of lipid accumulation in UVA-irradiated cells was significantly lower than the level of lipid accumulation in the control cells (Fig. 1D). Taken together, these results suggest that UVA irradiation exerts an anti-adipogenic effect on hAMSCs and 3T3-L1 preadipocytes. To exclude the possibility that these effects of UVA irradiation are simply a consequence of UVA cytotoxicity, cell viability, DNA damage, and apoptosis assays were conducted. As shown in Fig. 1, the tested doses of UVA irradiation induced no cytotoxic, DNA-damaging, or apoptotic effects in hAMSCs or 3T3-L1 cells.
FIGURE 1.
UVA irradiation inhibits adipocyte differentiation. Two-day post-confluent hAMSCs (day 0) were treated with the indicated doses of UVA irradiation and then stimulated with STEM PRO® adipocyte differentiation (DIF) media for 3 days. The medium was then replaced with STEM PRO® adipocyte differentiation media every 3 days until the end of the experiment at day 14. The assays were performed on fully differentiated adipocytes (day 14). A, intracellular lipids were stained with Oil Red O. The results were confirmed by three independent experiments, which were each conducted in duplicate. B, triglyceride content was measured using a triglyceride assay kit (Cayman Chemical, Ann Arbor, MI). Data are expressed as the means ± S.D. *, p < 0.05 versus controls. The results were verified by three repetitions of the experiments, each of which was conducted in triplicate. C, GPDH activity was measured using GPDH activity assay kits (Takara Bio Inc., Japan). Data are expressed as the means ± S.D. *, p < 0.05 versus controls. The results were verified by four repetitions of the experiments. D, 2-day post-confluent 3T3-L1 preadipocytes (day 0) were treated with the indicated concentrations of UVA irradiation and then stimulated with differentiation medium for 3 days. The medium was then replaced with maintenance media every 3 days until the end of the experiment on day 9. The assays were performed on fully differentiated adipocytes (day 9). Intracellular lipids were stained with Oil Red O. The results were confirmed by three independent experiments, which were each conducted in duplicate. E, general viability of cultured cells was determined by the reduction of WST-8 to a highly water-soluble formazan dye. The results were verified by four repetitions of the experiments. F, DNA-damaging effects of UVA irradiation were determined using a DNA Damage ELISA kit. The results were verified by four repetitions of the experiments. G, apoptotic effects of UVA irradiation were determined by Hoechst 33332 staining. The results were verified by four repetitions of the experiments. DIF/MDI, differentiation media.
Effects of UVA Irradiation on the Expression of the Adipogenic Transcription Factors C/EBPα, -β, and -δ, PPAR γ, and Its Target Genes
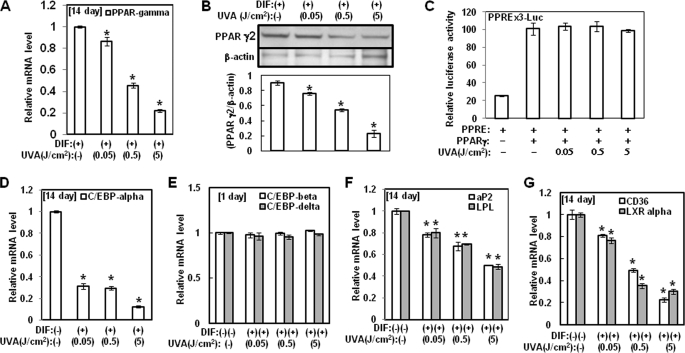
As mentioned previously, we found that UVA irradiation has anti-adipogenesis activity. At the molecular level, adipogenesis is driven by a complex transcriptional cascade that involves the sequential activation of C/EBPs and PPAR γ (1), which are rapidly and transiently expressed after the hormonal induction of differentiation. These factors act synergistically to induce the expression of C/EBP α and PPAR γ, the master adipogenic transcription regulators, which in turn promote terminal differentiation by activating the transcription of genes involved in the creation and maintenance of the adipocyte phenotype. Loss-of-function studies have shown that PPAR γ is necessary as well as sufficient to promote adipogenesis and that C/EBP α is influential in the maintenance of the expression of PPAR γ. Therefore, we examined its effect on the expression of adipokines, including PPAR γ. As a first step to accomplish this, the effects of UVA irradiation on the expression and transactivation of PPAR γ, one of major adipogenic transcription factors, were investigated. As shown in Fig. 2A, the mRNA level of PPAR γ was significantly lower in cells that were treated with UVA irradiation during adipocyte differentiation than in the control cells. These results were confirmed by Western blot analysis for PPAR γ, which revealed that UVA irradiation inhibited PPAR γ2 protein expression in a concentration-dependent manner at day 14 of differentiation (Fig. 2B). Furthermore, we evaluated UVA irradiation to determine whether it had an effect on the transactivation activity of PPAR γ in mature adipocytes. However, as shown in Fig. 2C, cotransfection assays in 3T3-L1 cells that used PPAR γ cDNA and PPRE×3-Luc reporter revealed that UVA irradiation did not induce an inhibitory effect on PPAR γ-mediated transactivation. These results suggest that UVA inhibits adipogenesis through the down-regulation of PPAR γ expression. Taken together, these findings indicate that PPAR γ is involved in the anti-adipogenesis effect of UVA irradiation. The mRNA levels of C/EBPα were also significantly lower in cells that were irradiated with UVA during adipocyte differentiation when compared with the control cells (Fig. 2D). However, the mRNA levels of C/EBP β and δ, the upstream regulators of PPAR γ and C/EBP α, were not reduced by UVA irradiation (Fig. 2E).
FIGURE 2.
Effects of UVA irradiation on the expression of the adipogenic transcription factors. hAMSCs (day 0) were treated with the indicated doses of UVA irradiation and then stimulated with STEM PRO® adipocyte differentiation media for 3 days. The medium was then replaced with STEM PRO® adipocyte differentiation media every 3 days until the end of the experiment on day 14. A and D--G, at 1 day or 14 days after the induction of differentiation, the total RNA was isolated, and the mRNA levels of the PPAR γ (A), C/EBP α (D), C/EBP β and C/EBP δ (E), aP2 and LPL (F), and CD36 and LXR α and (G) genes were measured by real time quantitative RT-PCR. The results are expressed relative to untreated cells after normalization against the 18 S rRNA. Data are expressed as the means ± S.D. *, p < 0.05 versus controls. The results were verified by four repetitions of the experiments, each of which was conducted in triplicate. B, at day 14 after the induction of differentiation, the cell lysates were analyzed by Western blot analysis using the indicated antibodies. The results were verified by three repetitions of the experiments, each of which was conducted in duplicate. C, hAMSCs were cotransfected with luciferase constructs that contained PPRE×3-Luc reporter and PPAR γ cDNA plasmid. After 16 h, the transfected cells were incubated for 20 h with the indicated doses of UVA irradiation. The cells were then harvested and lysed. Luciferase activity is expressed as the ratio of PPRE×3 promoter-dependent firefly luciferase activity divided by control thymidine kinase Renilla luciferase activity (relative luciferase units). Data are expressed as the means ± S.D. *, p < 0.05 versus controls. The experiments were repeated eight times, with each experiment being conducted in triplicate. DIF, differentiation media.
Activation of PPAR γ induces the expression of genes that control adipocyte fatty acid metabolism, including lipoprotein lipase, fatty acid translocase (CD36), and liver X receptor α (LXR α). In this study, we demonstrated that UVA irradiation reduces the expression of PPAR γ target genes during adipogenesis. Therefore, we evaluated this down-regulation to determine whether it was related to the observed decrease in the level of PPAR γ mRNA. We found that the mRNA levels of aP2, LPL, CD36, and LXR α were significantly lower during adipocyte differentiation following UVA irradiation (Fig. 2, F and G). Collectively, these results indicate that PPAR γ expression plays an important role in the regulation of adipocyte differentiation by UVA irradiation.
UVA Irradiation Up-regulates MIF Expression during Adipocyte Differentiation
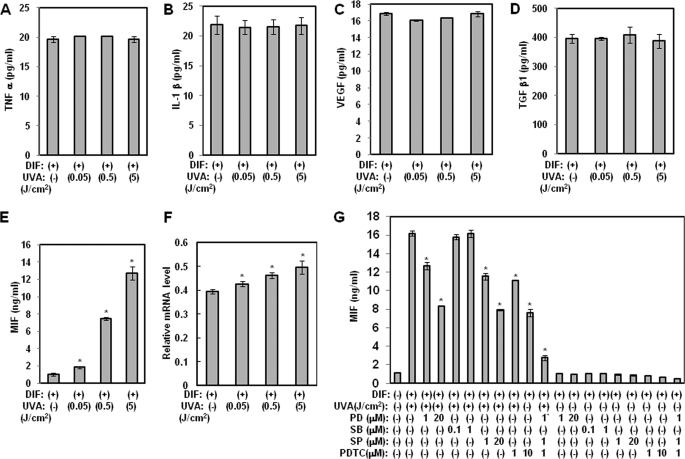
Several molecular targets for anti-adipogenesis have been reported, namely TNF-α, IL-1β, VEGF, TGF-β1, and MIF (5, 20, 21). Therefore, we evaluated UVA irradiation to determine whether it exerted a stimulatory effect on production of the aforementioned cytokines. To accomplish this, we performed an ELISA in hAMSCs. As shown in Fig. 3, A–D, we found that although UVA irradiation did not have any effects on the production of TNF-α, IL-1β, VEGF, and TGF-β1, MIF production was induced by UVA irradiation in a dose-dependent manner (Fig. 3E). Consistent with these findings, hAMSCs that were irradiated with UVA had significantly higher levels of MIF mRNA during adipocyte differentiation than control cells (Fig. 3F). These findings revealed that UVA irradiation increased MIF protein expression in a dose-dependent manner.
FIGURE 3.
Effects of UVA irradiation on the production of anti-adipogenic cytokines. hAMSCs (day 0) were treated with the indicated doses of UVA irradiation and then stimulated with STEM PRO® adipocyte differentiation media for 3 days. The medium was then replaced with STEM PRO® adipocyte differentiation media every 3 days until the end of the experiment on day 14. At day 14 after the induction of differentiation, the supernatants were harvested for TNF-α (A), IL-1β (B), VEGF (C), TGF-β1 (D), and MIF (E and G) measurements. Data are expressed as the means ± S.D. *, p < 0.05 versus controls. The results were verified by three repetitions of the experiments, each of which was conducted in duplicate. F, at 14 days after the induction of differentiation, the total RNA was isolated, and the mRNA levels of the PPAR γ gene were measured by real time quantitative RT-PCR. The results are expressed relative to untreated cells after normalization against 18 S rRNA. Data are expressed as the means ± S.D. *, p < 0.05 versus controls. The results were verified by four repetitions of the experiments, each of which was conducted in triplicate. DMI, differentiation media; PD, PD98059 (a p42/44 MAPK inhibitor); SB, SB203580 (a p38 MAPK inhibitor); SP, SP600125 (JNK inhibitor); PDTC, pyrrolidine dithiocarbamate.
MIF Expression by UVA Irradiation Is Mediated through AP-1 (p42/44 MAPK and JNK) and NF-κB Activation
To determine whether the stimulatory effect of UVA irradiation on MIF gene expression is mediated by the activation of nuclear factor-κB (NF-κB) or activator protein (AP)-1, ELISA for MIF was conducted using inhibitors of NF-κB and AP-1 in confluent hAMSCs. As shown in Fig. 3G, UVA-induced production of MIF was reduced by treatment with PD98059 (a p42/44 MAPK inhibitor), SP600125 (a JNK inhibitor), and pyrrolidine dithiocarbamate (a NF-κB inhibitor) but not SB203580 (a p38 MAPK inhibitor). In addition, combined treatment with PD98059, SP600125, and pyrrolidine dithiocarbamate synergistically inhibited UVA irradiation-induced production of MIF. These findings suggest that the increase in MIF expression in response to UVA irradiation may be dependent on activation of JNK, p42/44 MAPK, and NF-κB.
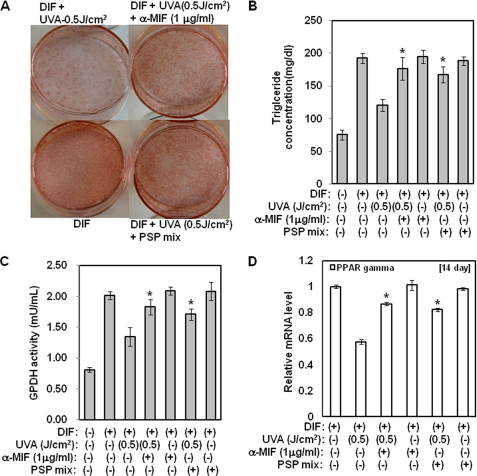
Anti-MIF Antibodies Attenuate Inhibition of Adipocyte Differentiation by UVA Irradiation
The results described above demonstrated that MIF production plays an important role in the anti-adipogenesis effect of UVA irradiation. To characterize the effects of anti-MIF antibodies or mixtures of inhibitors (a p42/44 MAPK inhibitor, JNK inhibitor, and NF-κB inhibitor) on the reduction in differentiation by UVA irradiation, the cellular triglyceride content and GPDH enzyme activity were measured, and Oil Red O staining assays were performed. Treatment with anti-MIF antibodies or the mixture of inhibitors attenuated the reduction in both triglyceride accumulation and GPDH activity that occurred in response to treatment with UVA irradiation (p < 0.05) (Fig. 4, B and C). Consistent with these findings, the reduction in lipid accumulation that was induced by UVA irradiation was also significantly recovered by anti-MIF antibodies (p < 0.05) (Fig. 4A). Moreover, the mRNA level of PPAR γ was significantly higher in cells that were treated with anti-MIF antibodies or the mixture of inhibitors in the presence of UVA irradiation during adipocyte differentiation than in cells that were treated with UVA irradiation (Fig. 4D). Taken together, these findings suggest that MIF production by UVA irradiation inhibits adipocyte differentiation.
FIGURE 4.
Inhibition of adipocyte differentiation by UVA irradiation is attenuated by anti-MIF antibodies or a mixture of inhibitors that suppress expression of the MIF gene. Two-day post-confluent hAMSCs (day 0) were pretreated with the indicated concentrations of the mixture of inhibitors or anti-MIF antibodies and then irradiated with UVA (500 mJ/cm2). After 14 days of incubation, the assays were performed. A, intracellular lipids were stained with Oil Red O using anti-MIF antibodies or a mixture of inhibitors. The results were confirmed by three independent experiments, which were each conducted in duplicate. B, triglyceride content was measured using a triglyceride assay kit (Cayman Chemical, Ann Arbor, MI). Data are expressed as the means ± S.D. *, p < 0.05 versus UVA-treated controls. The results were verified by three repetitions of the experiments, which were each conducted in triplicate. C, GPDH activity was measured using GPDH activity assay kits (Takara Bio Inc., Japan). Data are expressed as the means ± S.D. *, p < 0.05 versus UVA-irradiated controls. The results were verified by four repetitions of the experiments, each of which was conducted in triplicate. D, at day 14 after the induction of differentiation, the total RNA was isolated, and the mRNA level of the PPAR γ gene was measured by real time quantitative RT-PCR. The results are expressed relative to untreated cells after normalization against 18 S rRNA. Data are expressed as the means ± S.D. *, p < 0.05 versus UVA-irradiated controls. The results were verified by four repetitions of the experiments, each of which was conducted in triplicate. DIF, differentiation media; PSP mix, PD98059 (1 μm) + SP600125 (1 μm) + pyrrolidine dithiocarbamate (1 μm).
Effect of UVA Irradiation on AMPK Activation and KLF2 Expression
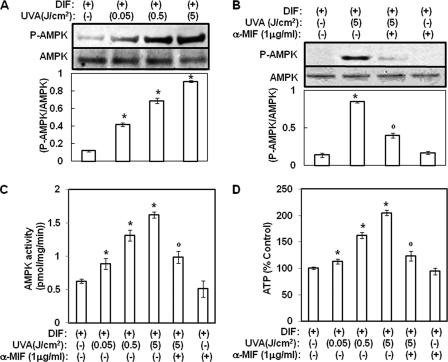
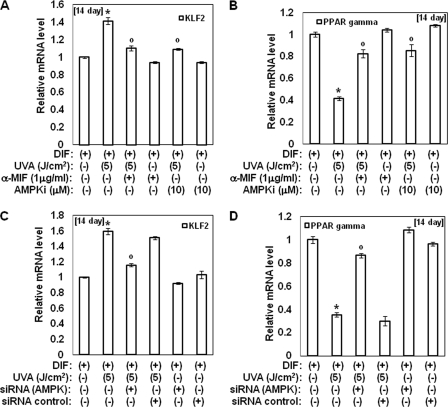
Several groups reported that MIF activates AMPK and that AMPK sequentially induces up-regulation of the KLF2 gene (22, 23). Therefore, we investigated the effects of UVA irradiation on the phosphorylation of AMPK and KLF2 expression. In this study, irradiation of hAMSCs with UVA was found to lead to a significant increase in AMPK phosphorylation during adipocyte differentiation (Fig. 5A). AMPK activity and ATP contents were also increased by UVA irradiation during adipocyte differentiation (Fig. 5, C and D). Moreover, UVA irradiation-induced activation of AMPK was attenuated by anti-MIF antibodies (Fig. 5, B and C). Treatment with anti-MIF antibodies also reduced ATP contents induced by UVA irradiation. These results suggest that UVA irradiation activates AMPK through the production of MIF. Moreover, the expression of KLF2 was induced by UVA irradiation, and its effect was reduced upon treatment with anti-MIF antibodies (Fig. 6, A and B). Similar to anti-MIF antibodies, AMPK inhibitor compound c and siRNA for AMPK also attenuated the changes in the expression of KLF2 and PPAR γ that were induced by UVA irradiation, respectively (Fig. 6, C and D). Collectively, these results suggest that UVA-induced production of MIF is an upstream step in AMPK-KLF2 signaling and that UVA irradiation also exerts an anti-adipogenic effect via the AMPK-KLF2 signaling pathway through MIF.
FIGURE 5.
UVA irradiation activates AMPK through the MIF. Two-day post-confluent hAMSCs (day 0) were irradiated with the indicated doses of UVA in the presence or absence of anti-MIF antibodies and then incubated for 14 days. A and B, total lysates were analyzed by Western blot using the indicated antibodies. The results were verified by repeating the experiments three times. Data are expressed as the means ± S.D. *, p < 0.05 versus controls; °, p < 0.05 versus UVA-irradiated controls. C, 14 days after the induction of differentiation, the AMPK activity in cell lysates was determined by SAMS peptide assays. AMPK activity was calculated as the number of picomoles/min/mg of protein. Data are expressed as the means ± S.D. *, p < 0.05 versus controls; °, p < 0.05 versus UVA-irradiated controls. The results were verified by repeating the experiments three times. D, ATP concentrations were measured by a luciferase-based assay. Data were normalized to the total amount of protein in each sample and expressed as a percentage of that under control conditions. *, p < 0.05 versus controls; °, p < 0.05 versus UVA-irradiated controls. The results were verified by repeating the experiments three times. DIF, differentiation media.
FIGURE 6.
UVA irradiation activates the MIF-AMPK-KLF2 signaling pathway. A and B, 2-day post-confluent hAMSCs (day 0) were irradiated with the indicated doses of UVA in the presence or absence of anti-MIF antibodies or AMPK inhibitor (compound C) and then incubated for 14 days. At 14 days after the induction of differentiation, the total RNA was isolated, and the mRNA levels of the KLF2 (A) and PPAR γ (B) genes were measured by real time quantitative RT-PCR. The results are expressed relative to untreated cells after normalization against 18 S rRNA. Data are expressed as the means ± S.D. *, p < 0.05 versus controls; °, p < 0.05 versus UVA-irradiated controls. The results were verified by four repetitions of the experiments, each of which was conducted in triplicate. C and D, knockdown of AMPKα1 reduces the expression of KLF2 induced by UVA irradiation. The cells were transfected with siRNA for AMPK or siRNA control. After transfection, the cells were induced to differentiate for 14 days, after which the total RNA was isolated, and the mRNA levels of the KLF2 (C) and PPAR γ (D) gene were measured by real time quantitative RT-PCR. The results are expressed relative to untreated cells after normalization against 18 S rRNA. Data are expressed as the means ± S.D. *, p < 0.05 versus controls; °, p < 0.05 versus UVA-irradiated controls. The results were verified by four repetitions of the experiments, each of which was conducted in triplicate. DIF, differentiation media.
Effect of UVA Irradiation on Osteogenesis
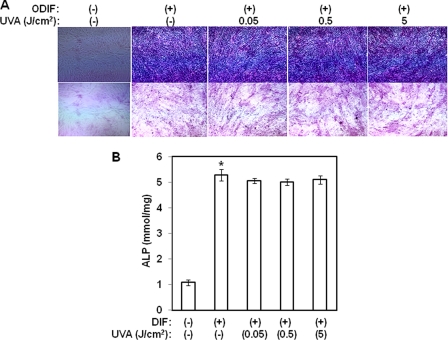
The aforementioned results indicate that UVA irradiation inhibits adipogenic differentiation. To determine whether this effect of UVA irradiation is specific to adipogenic differentiation, we next investigated the effects of UVA irradiation on osteogenesis of hAMSCs. Two-day post-confluent hAMSCs (day 0) were treated with the indicated doses of UVA irradiation and then stimulated with osteogenic differentiation media for 3 days. The medium was then replaced with osteogenic differentiation media every 3 days until the end of the experiment at day 14. After 14 days of treatment, the cells were subjected to alkaline phosphatase staining. As shown in Fig. 7A, UVA irradiation had no effect on alkaline phosphatase activity when compared with cells that were treated only with osteogenic differentiation media. These results were further confirmed by an alkaline phosphatase activity assay (Fig. 7B). UVA irradiation had no inhibitory effects on alkaline phosphatase activity induced by osteogenic differentiation media. These results suggest that unlike adipogenic differentiation, UVA irradiation has no effect on osteoblast differentiation.
FIGURE 7.
UVA irradiation has no effects on osteoblast differentiation in hAMSCs. Two-day post-confluent hAMSCs (day 0) were treated with the indicated doses of UVA irradiation and then stimulated with STEM PRO® osteogenesis differentiation media for 3 days. The medium was then replaced with STEM PRO® osteogenesis differentiation media every 3 days until the end of the experiment at day 14. A, alkaline phosphatase staining. Osteogenesis was confirmed with alkaline phosphatase staining. The results were confirmed by three independent experiments, which were each conducted in duplicate. B, alkaline phosphatase activity was measured in cell lysates for 14 days, using a colorimetric assay, and then normalized to the level of total cellular protein. Data are expressed as the means ± S.D. *, p < 0.05 versus controls. The results were confirmed by three independent experiments, which were each conducted in duplicate. ODIF, osteogenic differentiation media.
DISCUSSION
The results of this study provide direct evidence of the effects of UVA irradiation on adipocyte differentiation, as well as its inhibitory mechanisms on adipogenesis in hAMSCs. Specifically, the findings presented here demonstrated that UVA irradiation up-regulates MIF gene expression through activation of JNK, p42/44 MAPK, and NF-κB, after which it activates AMPK-KLF2 signaling and reduces adipogenic gene expression, consequently inhibiting adipocyte differentiation. The cytosolic enzyme, GPDH, plays a central role in the pathway of triglyceride synthesis and is linked to the characteristic changes that occur during adipose conversion (17). Here, we demonstrated that UVA irradiation led to significant reductions in GPDH activity and the triglyceride content of adipocytes.
Members of the Kruppel-like family of transcription factors have been shown to play important roles in cellular differentiation and growth (24). KLF2 was initially identified through a homology screening strategy using the zinc finger domain of KLF1 as a probe (25). Studies targeting KLF2 in mice have shown that it plays an essential role in embryonic development as these animals die during mid-gestation because of abnormal blood vessel development (26). In addition, T-cell maturation (27) and lung development (28) are impaired. It was also recently reported that KLF2 operates as an anti-adipogenic factor that binds to and represses the PPAR γ promoter (29). Consistent with these findings, the expression of the KLF2 gene was induced upon UVA irradiation in this study. Moreover, although the mRNA levels of PPAR γ and C/EBP α were reduced by UVA irradiation, C/EBP β and δ, the upstream regulators of PPAR γ and C/EBP α, were not. The results of this study may have important implications for adipocyte biology, as well as for the role of KLF2 in UVA-induced anti-adipogenesis.
Many attempts have been made to correct the metabolic disparity that occurs in obesity using reagents such as Sibutramine (appetite suppressor), Orlistat (gastrointestinal lipid inhibitor), and Fibrates (PPAR α agonists) (30, 31). However, the administration of these drugs frequently causes undesirable side effects such as a dry mouth, anorexia, constipation, insomnia, dizziness, and nausea (32). Therefore, there is a high demand for therapeutically potent, yet safe anti-obesity reagents. Our in vitro experimental data indicate that UVA irradiation inhibits adipogenesis and the expression of adipokines through the PPAR γ pathway via activation of MIF-AMPK-KLF2 signaling, implying that UVA irradiation may be beneficial for reducing diet-induced obesity via its ability to regulate adipocyte differentiation. Nevertheless, the physiological significance of UVA-induced anti-adipogenesis should be briefly addressed. It was reported that, although UVB and UVC reach the epidermis and dermis, respectively, UVA penetrates into the hypodermis where adipose tissue-derived mesenchymal stem cells, preadipocytes, and adipocytes exist (33). Therefore, the UVA sensitivity of hAMSCs suggests that tanning using UVA and/or UVA treatment acts directly on hAMSCs as a stimulus. This stimulation might activate the MIF-AMPK-KLF2 system, which in turn could lead to the prevention of adipocyte differentiation and renewal. This line of reasoning further implies that local UV treatment (UVA exposure to the body) might ameliorate obesity-associated physical conditions such as abdominal subcutaneous and visceral fat stores in terms of the prevention of adipocyte differentiation. However, the safety and efficacy profiles of UVA irradiation in vivo remain to be determined. Until the safety and efficacy are determined, it is not clear whether the UVA can be used for the treatment of obesity-associated physical conditions.
Taken together, the results of this study demonstrate that UVA irradiation inhibits adipogenic differentiation of hAMSCs by reducing the PPAR γ mRNA levels. Additionally, these results show that the anti-adipogenesis induced by UVA irradiation is mediated by the reduced expression of PPAR γ, which is mediated by up-regulation of KLF2 via the activation of MIF-AMPK signaling.
Footnotes
- PPAR
- peroxidase proliferator-activated receptor γ
- GPDH
- glycerol 3-phosphate dehydrogenase
- C/EBP
- CCAAT/enhancer-binding protein
- LXR
- liver X receptor
- MIF
- migration inhibitory factor
- AMPK
- AMP-activated protein kinase
- KLF2
- Kruppel-like factor
- hAMSCs
- human adipose tissue-derived mesenchymal stem cells
- AP-1
- activator protein-1
- NF-κB
- nuclear factor-κB.
REFERENCES
- 1.Alessi M. C., Lijnen H. R., Bastelica D., Juhan-Vague I. (2003) Pathophysiol. Haemost. Thromb. 33, 290–297 [DOI] [PubMed] [Google Scholar]
- 2.Kahn B. B., Flier J. S. (2000) J. Clin. Invest. 106, 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamauchi T., Kuno T., Takada H., Nagura Y., Kanmatsuse K., Takahashi S. (2003) Nephrol. Dial. Transplant. 18, 1842–1847 [DOI] [PubMed] [Google Scholar]
- 4.Spiegelman B. M., Flier J. S. (2001) Cell 104, 531–543 [DOI] [PubMed] [Google Scholar]
- 5.Tanabe Y., Koga M., Saito M., Matsunaga Y., Nakayama K. (2004) J. Cell Sci. 117, 3605–3614 [DOI] [PubMed] [Google Scholar]
- 6.Gregoire F. M., Smas C. M., Sul H. S. (1998) Physiol. Rev. 78, 783–809 [DOI] [PubMed] [Google Scholar]
- 7.Halliday G. M., Rana S. (2008) Photochem. Photobiol. 84, 35–46 [DOI] [PubMed] [Google Scholar]
- 8.Tyrrell R. M., Reeve V. E. (2006) Prog. Biophys. Mol. Biol. 92, 86–91 [DOI] [PubMed] [Google Scholar]
- 9.Zhong J. L., Edwards G. P., Raval C., Li H., Tyrrell R. M. (2010) Photochem. Photobiol. Sci. 9, 18–24 [DOI] [PubMed] [Google Scholar]
- 10.Potapovich A. I., Pastore S., Kostyuk V. A., Lulli D., Mariani V., De Luca C., Dudich E. I., Korkina L. G. (2009) Br. J. Pharmacol. 158, 1236–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noonan F. P., De Fabo E. C. (2009) J. Invest. Dermatol. 129, 1608–1610 [DOI] [PubMed] [Google Scholar]
- 12.La C., You Y., Zhabyeyev P., Pelzer D. J., McDonald T. F. (2006) J. Membr. Biol. 210, 43–50 [DOI] [PubMed] [Google Scholar]
- 13.Cabrijan L., Lipozencić J., Batinac T., Lenković M., Stanic Zgombić Z. (2008) Coll. Antropol. 32, 53–56 [PubMed] [Google Scholar]
- 14.March K. L., Patton B. L., Wilensky R. L., Hathaway D. R. (1993) Circulation 87, 184–191 [DOI] [PubMed] [Google Scholar]
- 15.Malinin G. I., Hornicek F. J., Banovac K., Malinin T. I. (1995) Biochem. Biophys. Res. Commun. 207, 877–881 [DOI] [PubMed] [Google Scholar]
- 16.Tontonoz P., Hu E., Spiegelman B. M. (1994) Cell 79, 1147–1156 [DOI] [PubMed] [Google Scholar]
- 17.Kozak L. P., Jensen J. T. (1974) J. Biol. Chem. 249, 7775–7781 [PubMed] [Google Scholar]
- 18.Lee J., Jung E., Lee J., Huh S., Boo Y. C., Hyun C. G., Kim Y. S., Park D. (2007) Br. J. Dermatol. 157, 242–248 [DOI] [PubMed] [Google Scholar]
- 19.Davies S. P., Carling D., Hardie D. G. (1989) Eur. J. Biochem. 186, 123–128 [DOI] [PubMed] [Google Scholar]
- 20.Moldes M., Zuo Y., Morrison R. F., Silva D., Park B. H., Liu J., Farmer S. R. (2003) Biochem. J. 376, 607–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martini C. N., Plaza M. V., Vila Mdel C. (2009) Mol. Cell. Endocrinol. 298, 42–47 [DOI] [PubMed] [Google Scholar]
- 22.Miller E. J., Li J., Leng L., McDonald C., Atsumi T., Bucala R., Young L. H. (2008) Nature 451, 578–582 [DOI] [PubMed] [Google Scholar]
- 23.Young A., Wu W., Sun W., Larman H. B., Wang N., Li Y. S., Shyy J. Y., Chien S., García-Cardeña G. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 1902–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bieker J. J. (2001) J. Biol. Chem. 276, 34355–34358 [DOI] [PubMed] [Google Scholar]
- 25.Anderson K. P., Kern C. B., Crable S. C., Lingrel J. B. (1995) Mol. Cell. Biol. 15, 5957–5965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo C. T., Veselits M. L., Barton K. P., Lu M. M., Clendenin C., Leiden J. M. (1997) Genes Dev. 11, 2996–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo C. T., Veselits M. L., Leiden J. M. (1997) Science 277, 1986–1990 [DOI] [PubMed] [Google Scholar]
- 28.Wani M. A., Wert S. E., Lingrel J. B. (1999) J. Biol. Chem. 274, 21180–21185 [DOI] [PubMed] [Google Scholar]
- 29.Banerjee S. S., Feinberg M. W., Watanabe M., Gray S., Haspel R. L., Denkinger D. J., Kawahara R., Hauner H., Jain M. K. (2003) J. Biol. Chem. 278, 2581–2584 [DOI] [PubMed] [Google Scholar]
- 30.Padwal R. S., Majumdar S. R. (2007) Lancet 369, 71–77 [DOI] [PubMed] [Google Scholar]
- 31.Chapman M. J. (2003) Atherosclerosis 171, 1–13 [DOI] [PubMed] [Google Scholar]
- 32.Bray G. A. (2001) Rev. Endocr. Metab. Disord. 2, 403–418 [DOI] [PubMed] [Google Scholar]
- 33.Bruls W. A., Slaper H., van der Leun J. C., Berrens L. (1984) Photochem. Photobiol. 40, 485–494 [DOI] [PubMed] [Google Scholar]
- 34.Langmann T., Mauerer R., Zahn A., Moehle C., Probst M., Stremmel W., Schmitz G. (2003) Clin. Chem. 49, 230–238 [DOI] [PubMed] [Google Scholar]