Abstract
The purpose of this study was to measure the rate dependent changes in the relative motion of subsynovial connective tissue (SSCT) and median nerve in the human carpal tunnel.
Using fluoroscopy, we measured the relative motion of middle finger flexor digitorum superficialis tendon, SSCT and median nerve in eight human cadavers during simulated active finger flexion motions at 2.0mm/s, 5.0mm/s, 7.5mm/s and 10.0mm/s. The shear index was defined as the difference in motion between tendon and SSCT or tendon and nerve, expressed as a percentage of tendon excursion. The motion patterns of the SSCT and median nerve relative to tendon excursion were measured at each 10% increment (decile) of maximum tendon excursion.
The tendon-SSCT shear index was significantly higher at 10.0 mm/s than at 2.0mm/s in the single digit motion. There were corresponding significant decreases in SSCT and median nerve motion for the 10.0 mm/s velocity compared to the 2.0 mm/s velocity.
This study demonstrates that the relative motion of the tissues in the carpal tunnel appears to be dependent on tendon velocity, specifically with less nerve and SSCT motion at higher velocity tendon motion. This suggests that SSCT may be predisposed to shear injury from high velocity tendon motion.
Keywords: Carpal Tunnel, Subsynovial Connective Tissue (SSCT), Median Nerve, Fluoroscopy, Human Cadaver
INTRODUCTION
Carpal tunnel syndrome (CTS) is one of the most common peripheral neuropathies. Despite its frequent occurrence, though, in most cases CTS is idiopathic [1].
The most characteristic non-neurological histopathological finding in idiopathic CTS is fibrosis of the subsynovial connective tissue (SSCT) [2, 3], the tissue that surrounds the tendons within the carpal tunnel. The SSCT loosely connects the finger flexor tendons, median nerve and the synovial membrane. The SSCT provides a unique multilayer gliding environment, in which tendon motion gradually recruits successive layers of the SSCT, until finally the visceral synovium moves [4]. Oh et al. [5], using color Doppler ultrasound, demonstrated that different tendon motion velocities were associated with different velocities of SSCT motion. Based on this previously demonstrated difference in relative velocities, we hypothesized that the relative motions of the SSCT and median nerve might also be different at different finger motion velocities. Larger differences in relative motion would imply larger shear strains in the SSCT. Since a proposed mechanism for the SSCT fibrosis of CTS is shear injury [6] and since it is known that certain tasks of hand motions are implicated in at least some cases of CTS [7–9], it would be helpful to know the normal shear strains and motion pattern between tendon and SSCT and between tendon and median nerve for various clinically relevant velocities of finger flexor motion. In this study we used fluoroscopy to study the rate dependent change in relative motion of the SSCT and median nerve at different velocities of finger flexor motion, to test the hypothesis that tendon velocity and excursion might affect SSCT shear.
MATERIAL AND METHODS
This study protocol was approved by our Institutional Review Board. A review of available premortem medical records was performed on cadavers donated to our institution, to obtain clinical and demographic data. Cadaver specimens were excluded if there was a history of carpal tunnel syndrome or other peripheral nerve disease, as well as conditions potentially associated with peripheral nerve disease or carpal tunnel syndrome, including diabetes or glucose intolerance, thyroid disease, rheumatoid arthritis, osteoarthritis, gout, hemodialysis, sarcoidosis, amyloidosis, or traumatic injuries to the ipsilateral arm. Eight fresh frozen upper extremity specimens without exclusion criteria (three male, five female, aged 73 to 97, mean 86.5 years) were amputated approximately 15 cm proximal to the wrist joint and were thawed at room temperature prior to testing.
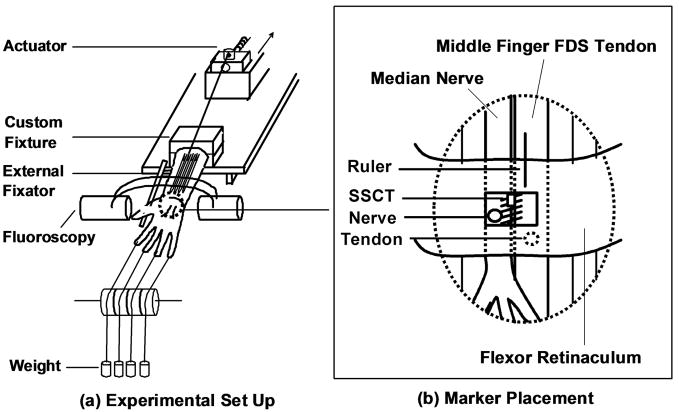
The experimental set up has been described previously [10]. In brief, a custom designed external fixator was used to position the wrist. Each specimen was mounted in the fixator by clamping the proximal ends of the radius and ulna. Each hand was mounted palmar side up (Figure 1). A skin incision was made longitudinally to expose the middle finger FDS tendon from the muscle tendon junction to the proximal end of the finger flexor sheath, leaving the flexor retinaculum and bursa intact. A 5 mm diameter window was made in the flexor retinaculum to expose the median nerve and SSCT. Two metal markers with a diameter of 1.4–1.6 mm (9291K12, McMaster-Carr, Chicago, IL) were inserted into the middle finger FDS tendon and median nerve, respectively. A third marker was glued on the surface of the visceral synovium between the median nerve and middle finger FDS tendon, representing the surface of the SSCT. The carpal tunnel was otherwise left undisturbed. The proximal end of the middle finger FDS tendon was fixed with sutures to a Dacron cord and connected to a mechanical actuator. The proximal FDP tendons were left without tension. A 1 N weight was attached to each fingertip to maintain tension in the system.
Figure 1.
Schematic drawing of the experimental setting. (A) Experimental Set Up (B) Marker Placement
Before testing, the normal FDS tendon excursion of the middle finger was determined in the wrist neutral position by passive full metacarpophalangeal joint (MCP), proximal interphalangeal (PIP) and distal interphalangeal (DIP) joint flexion and extension, with a 5 N load attached to the proximal tendon end. This tendon excursion measurement was used to pre set the mechanical actuator excursion.
The middle finger FDS tendon was pulled in a proximal direction by the actuator against the 5N load until the normal tendon excursion was achieved. This movement of the tendon toward the actuator was regarded as isolated middle finger flexion. The four FDS tendons (index, middle, ring and little fingers) were also pulled together proximally to simulate fist motion. Testing was randomly performed at four different speeds of simulated flexor tendon motion, namely, 2.0mm/s, 5.0mm/s, 7.5mm/s and 10.0mm/s. All testing was done at 20 degrees C.
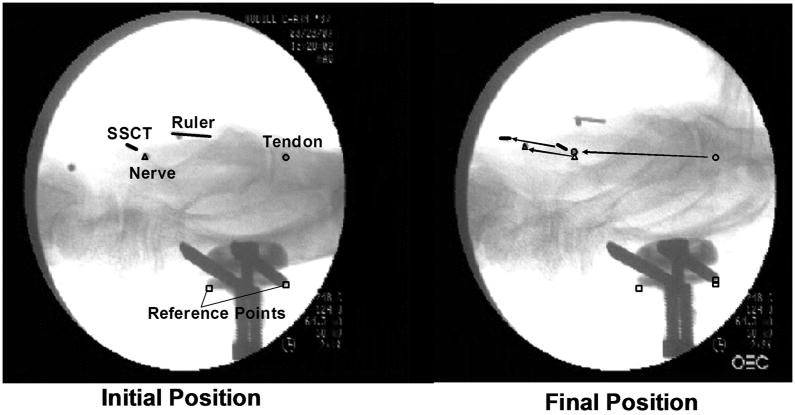
The motion of the three metal markers was recorded by lateral view fluoroscopy (BV 25, Scopofix MDPM, Philips) using a digital video camera (DCR-TRV350, Sony, Japan) (Figure 2). A ten millimeter ruler was included in the camera field, in the same plane as the specimen, to scale the distance measurement obtained from the image acquisition. Each of the marker movements (tendon, SSCT, median nerve) was digitized with Analyze Software (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). In addition, the proximal and distal edges of the fixator were digitized as fixed reference points.
Figure 2.
Fluoroscopic Image
Digitized coordinate data (X, Y) for the metal markers on the tendon, SSCT and median nerve were processed using a custom Matlab program (The Mathworks, Inc, Natick, MA). All coordinate data were converted from pixels to millimeters using the scale factor conversion obtained from the imaged ruler. The coordinates for the tendon, SSCT and median nerve were normalized relative to the external fixator to correct for any translational or rotational motion of the image or specimen during data collection. Finally, the coordinates of the tendon, SSCT and median nerve were transformed to a new coordinate system such that the transformed X axis was aligned with the motion direction of the tendon, as defined by a linear polynomial fit to the tendon time series data.
Proximal and distal motions were defined as positive and negative motions, respectively. The coordinates at the initial positions for each marker were defined as zero excursion. For the tendon, SSCT and nerve, the distances along the motion direction of the tendon excursion were calculated. To estimate the shear strain between tendon, SSCT and nerve, the relative motions of those structures were compared using a shear index, defined as the ratio of the difference in motion along the direction of tendon excursion between two tissues divided by tendon excursion, expressed as a percentage. For example, the tendon-SSCT shear index would be defined as:
The motion patterns of SSCT and median nerve relative to the tendon excursion were calculated. The maximum tendon excursion was defined as 100% and the percentage of SSCT and median nerve motion relative to tendon excursion were measured at each 10% increment (decile) of tendon excursion.
STATISTICAL ANALYSIS
The different speed motions were compared for each marker motion and shear index.
A generalized linear model was used to analyze the variables. All results were expressed as mean (standard deviation, SD). The tendon motion speed factors were considered significantly different when the p-value was less than 0.05 in a full model setting. A post hoc pairwise comparison was adapted using Scheffe’s test criteria for 6 combinations for the tendon motion speed factor (2.0mm/s vs. 5.0mm/s vs. 7.5mm/s vs. 10mm/s). Thus, p-values <0.008 (0.05/6) were considered significant for each velocity combination. All analyses were conducted using SAS version 9.1 STAT software GLM procedure (SAS Institute, Cary, NC).
RESULTS
Summary results of displacements for each tissue and the shear indices are shown in Tables 1 and 2. Displacement of the SSCT was significantly lower for the 10.0mm/s velocity than for the 2.0mm/s velocity in the single digit motion (P < 0.008). The tendon-SSCT shear indices was also significantly higher for the 10.0mm/s velocity than for the 2.0mm/s velocity in the single digit motion (P < 0.008). For the fist motion, even it showed p-value less than 0.05 in the full model setting, it did not reach the significance level of P<0.008 for the pairwise comparison.
Table 1.
Results of Displacement
| Tendon | SSCT | Nerve | |
|---|---|---|---|
| Fist motion | |||
| 2.0 mm/s | 34.1 (1.5) | 10.8 (2.7) | 8.9 (2.1) |
| 5.0 mm/s | 34.3 (1.6) | 10.0 (2.5) | 8.1 (1.4) |
| 7.5 mm/s | 34.3 (1.6) | 10.1 (2.6) | 8.3 (1.8) |
| 10.0 mm/s | 34.3 (1.3) | 9.6 (2.6) | 7.7 (1.7) |
| Overall P-value | 0.8337 | 0.0357 | 0.0507 |
| Single digit motion | |||
| 2.0 mm/s | 32.9 (1.9) | 6.4 (2.6) * | 5.1 (2.0) |
| 5.0 mm/s | 33.0 (1.8) | 5.8 (2.6) | 4.6 (2.0) |
| 7.5 mm/s | 33.1 (1.6) | 6.0 (2.4) | 4.6 (2.0) |
| 10.0 mm/s | 33.0 (1.3) | 5.7 (2.5) * | 4.6 (2.2) |
| Overall P-value | 0.9160 | 0.0272 | 0.0372 |
Values are mean ± SD.
Significant difference between 2.0 mm/s motion and 10.0 mm/s motion (P<0.008).
Table 2.
Results of Shear Index
| Tendon-SSCT | Tendon-Nerve | SSCT-Nerve | |
|---|---|---|---|
| Fist motion | |||
| 2.0 mm/s | 68.3 (8.3) | 73.7 (6.6) | 5.5 (3.3) |
| 5.0 mm/s | 71.0 (7.2) | 76.2 (4.1) | 5.3 (3.8) |
| 7.5 mm/s | 70.6 (8.0) | 75.7 (5.3) | 5.1 (4.4) |
| 10.0 mm/s | 71.9 (7.8) | 77.4 (5.1) | 5.5 (4.6) |
| Overall P-value | 0.0273 | 0.0387 | 0.8987 |
| Single digit motion | |||
| 2.0 mm/s | 80.4 (8.5) * | 84.3 (6.3) | 4.0 (3.8) |
| 5.0 mm/s | 82.2 (8.4) | 86.1 (6.4) | 3.9 (3.8) |
| 7.5 mm/s | 81.9 (7.7) | 86.2 (6.0) | 4.2 (4.0) |
| 10.0 mm/s | 82.7 (7.9) * | 86.1 (6.7) | 3.5 (3.5) |
| Overall P-value | 0.0145 | 0.0239 | 0.9347 |
Values are mean ± SD.
Significant difference between 2.0mm/s motion and 10.0 mm/s motion (P<0.008).
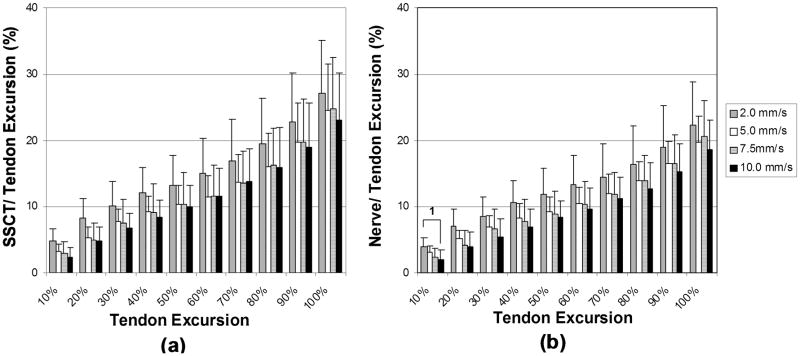
Summary results of the motion pattern analysis are shown in Figures 3 and 4. In simulated fist motion, there were significant decreases in nerve motion at the 1st deciles of tendon excursion for the 10.0mm/s velocity compared to the 2.0mm/s velocity (P < 0.008). Although there was a trend which showed decrease in the SSCT motion for the 10.0mm/s velocity compared to the 2.0mm/s (P<0.05), the SSCT motion did not reach the significance level of P<0.008.
Figure 3.
Simulated Fist Motion Pattern (a) SSCT motion (b) Median nerve motion. Error bar indicates 1 SD. 1: Significant difference in comparison between 2.0mm/s and 10mm/s velocity motions (p<0.008).
Figure 4.
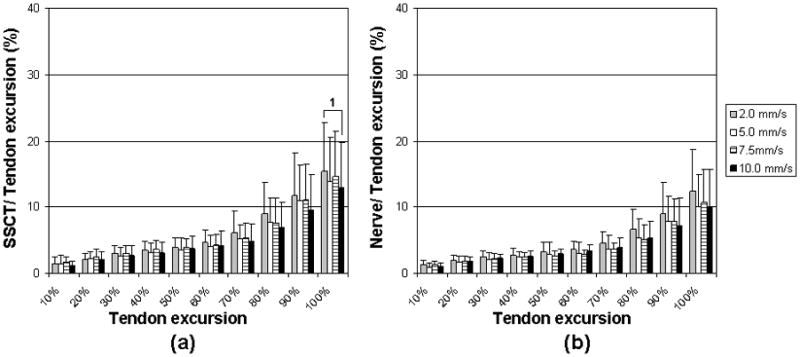
Simulated Single Digit Motion Pattern (a) SSCT motion. (b) Median nerve motion. Error bar indicates 1 SD.
1: Significant difference in comparison between 2.0mm/s and 10mm/s velocity motions. (p<0.008).
DISCUSSION
In this study, we demonstrated that the relative motions of SSCT and median nerve were affected by both the type (single digit vs fist) and the velocity of tendon motion. The velocities we chose, ranging from 2–10mm/s, are, we believe, clinically relevant. With the wrist in the neutral position, full FDS excursion is roughly 20–30 mm [11, 12], so 2mm/sec represents roughly 2–3 flexion/extension cycles per minute, while 10 mm/sec is consistent with 10–15 cycles per minute, which fits the definition of high repetition for work activity [13]. While the maximum velocity that we used is about an order of magnitude less than can be achieved by rapidly opening and closing the fist, such rapid motions are not sustainable for more than a few minutes. We have been unable to find any reference for the velocity of tendon motion or finger joint angle (as a proxy for tendon excursion) for everyday activities. We plan to collect such data in the future. Despite this limitation, we have shown that the SSCT motion relative to flexor tendon motion was significantly less at 10.0mm/s of tendon motion compared to 2.0mm/s of tendon motion and that this effect was greatest in the final few deciles of full tendon excursion. This data also suggests that rapid, full finger motion, such as that associated with repetitive gripping of a small object, creates a small but significantly greater shear in the SSCT than slower and less full motion, as would occur with slower gripping of a larger object. We hope to obtain better resolution and to study faster speeds, with a new ultrasound method, which will be the subject of a subsequent paper. Even with the limitations as noted, our data suggest that velocity can affect the shear forces in the SSCT and that higher velocities may result in higher shear strains and therefore a higher risk of injury. This is relevant to our underlying hypothesis, that SSCT injury is a cause of carpal tunnel syndrome (CTS), since this physiological evidence supports epidemiological data that high velocity activities, especially when combined with high force, are associated with an increased risk of CTS.
Using an in vitro model, Osamura et al have shown that the normal SSCT fails in shear at a strain of roughly 30% [14], which, while not entirely comparable due to the very different experimental conditions, is within the range shown here (SSCT moving 25% of a normal 25–30 mm FDS motion or an SSCT-FDS shear of over 15–20 mm. These shear strains are apt to be approached at the higher speeds and excursions in our model, suggesting that such strains might pose a risk of shear injury to the SSCT. Thus at higher velocities the SSCT could be stretched in a range making it liable to injury, especially with high force. Thus we consider these findings highly relevant clinically. We have presented anatomical evidence that activity within the physiological range might be sufficient to induce strain damage the SSCT and that this risk may be greater with higher velocity activity.
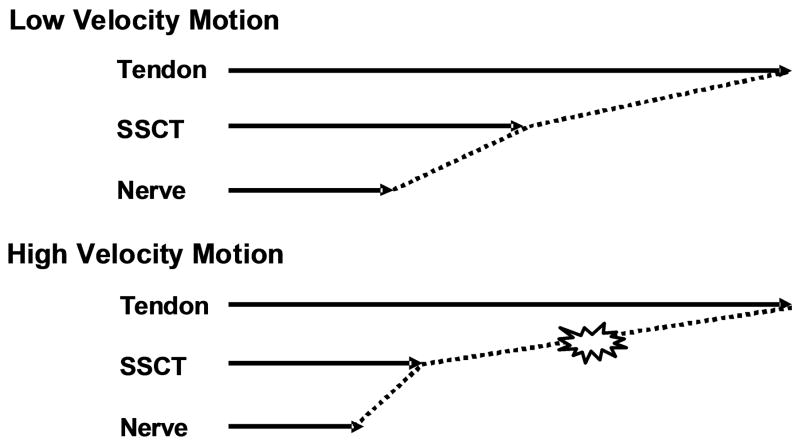
Osamura et al. [15] measured the permeability of the SSCT and found it to be quite low. This suggests that fluid flows within the SSCT occur with difficulty. While we did not measure the viscoelastic behavior of the SSCT, we believe that this finding by Osamura et al suggest that any viscoelastic behavior, which is also dependent on fluid flows, would likely occur over longer time frames than might be the case in a more permeable tissue. Thus one might expect that the SSCT would be stiffer at faster velocities and thus more susceptible to injury as well. (Figure 5)
Figure 5.
Image of the shear conditions in high and low velocity motions.
There are several limitations in this study. First, we did not measure friction within the SSCT or between tissues during tendon motion. Second, this was a cadaver study. Fluid conditions may be different in living tissues. Third, there was no proximal constraint on any of the carpal tunnel structures, except for the FDS tendon, pulled by the actuator. However, we found that the nerve marker always came back to its original position when the tendon was placed in its initial position during testing. This suggests that the nerve has some connection to the tendon (based on our dissections, via the SSCT) and maintained tension in the proximal direction. We plan to repeat these studies in living volunteers using ultrasonography, as recently described by Oh, et al [5], to address some of these issues, which are inherent in cadaver models. Fourth, while there was a trend which showed the decrease of the SSCT and median nerve motion pattern in the 10.0mm/s velocity compared to the 2.0mm/s velocity, it did not reach statistical significance. This may be addressed by using larger sample sizes in any future studies. Lastly, we have performed a two dimensional analysis of a three dimensional motion and three dimensional structures. Future studies should address three dimensional shear conditions.
In conclusion, this study assessed the relative motion of SSCT, tendon and nerve during different velocities of finger motion. We demonstrated that higher velocity finger motion results in less SSCT and nerve motion relative to tendon motion, which should result in higher shear strain in the SSCT. Future studies will be directed toward assessing the effect of SSCT shear in vivo, as well as analyzing shear in three dimensions. When compared to data on SSCT stress in vivo, these results may be useful in estimating the risk of SSCT injury due to shear with various finger arcs of motion and velocities.
Acknowledgments
The project described was supported by NIH/NIAMS Grant AR49823. The authors would like to thank Mr. Stephen Cha for help with the statistical analysis.
References
- 1.Cranford CS, et al. Carpal tunnel syndrome. J Am Acad Orthop Surg. 2007;15:537–48. doi: 10.5435/00124635-200709000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Ettema AM, et al. A Histological and Immunohistochemical Study of the Subsynovial Connective Tissue in Idiopathic Carpal Tunnel Syndrome. J Bone Joint Surg Am. 2004;86(7):1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Oh Jinrok CZ, Amadio Peter C, An Kai-Nan, Zobitz Mark E, Wold Lester E. Vascular pathologic changes in the flexor tenosynovium (subsynovial connective tissue) in idiopathic carpal tunnel syndrome. Journal of Orthopaedic Research. 2004;22(6):1310–1315. doi: 10.1016/j.orthres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Guimberteau JC. New ideas in hand flexor tendon surgery. Institut Aquitain De La Main; 2001. The sliding system. Vascularized flexor tendon transfers. [Google Scholar]
- 5.Oh S, et al. Detection of differential gliding characteristics of the flexor digitorum superficialis tendon and subsynovial connective tissue using color Doppler sonographic imaging. J Ultrasound Med. 2007;26:149–155. doi: 10.7863/jum.2007.26.2.149. [DOI] [PubMed] [Google Scholar]
- 6.Moore A, Wells R, Ranney D. Quantifying exposure in occupational manual tasks with cumulative trauma disorder potential. Ergonomics. 1991;34:1433–53. doi: 10.1080/00140139108964888. [DOI] [PubMed] [Google Scholar]
- 7.Stetson DS, Silverstein BA, Keyserling WM. Median sensory distal amplitude and latency: comparison between nonexposed managerial/professional employees and industrial workers. Am J Ind Med. 1993;24:175–89. doi: 10.1002/ajim.4700240205. [DOI] [PubMed] [Google Scholar]
- 8.Werner R, et al. Intracarpal canal pressures: the role of finger, hand, wrist and forearm position. Clin Biomech. 1997;12:44–51. doi: 10.1016/s0268-0033(96)00044-7. [DOI] [PubMed] [Google Scholar]
- 9.Wieslander G, et al. Carpal tunnel syndrome (CTS) and exposure to vibration, repetitive movements, and heavy manual work: a case-referent study. British J Industrial Medicine. 1989;46(1):43–7. doi: 10.1136/oem.46.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshii Y, et al. The effect of wrist position on the relative motion of tendon, nerve, and subsynovial connective tissue within the carpal tunnel in a human cadaver model. J Orthop Res. 2008;26:1153–8. doi: 10.1002/jor.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An KN, et al. Tendon excursion and moment arm of index finger muscles. J Biomech. 1983;16:419–25. doi: 10.1016/0021-9290(83)90074-x. [DOI] [PubMed] [Google Scholar]
- 12.Szabo RM, et al. Median nerve displacement through the carpal canal. J Hand Surg Am. 1994;19:901–6. doi: 10.1016/0363-5023(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 13.Silverstein BA, Fine LJ, Armstrong TJ. Occupational factors and carpal tunnel syndrome. American Journal of Industrial Medicine. 1987;11(3):343–58. doi: 10.1002/ajim.4700110310. [DOI] [PubMed] [Google Scholar]
- 14.Osamura N, et al. Evaluation of the material properties of the subsynovial connective tissue in carpal tunnel syndrome. Clin Biomech. 2007;22:999–1003. doi: 10.1016/j.clinbiomech.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osamura N, et al. Permeability of the subsynovial connective tissue in the human carpal tunnel: a cadaver study. Clin Biomech. 2007;22:524–8. doi: 10.1016/j.clinbiomech.2007.01.004. [DOI] [PubMed] [Google Scholar]