Abstract
Citrus fruits have been used as an edible fruit and a traditional medicine since ancient times. In particular, the peels of immature citrus fruits are used widely in traditional herbal medicine in Korea, as they are believed to contain bioactive components exerting anti-inflammatory activity. This study examined whether the crude methanol extract of Citrus aurantium L. (CME) has a suppressive effect on inducible enzymes and proinflammatory cytokines by inhibiting the NF-κB pathway in LPS-stimulated macrophage RAW 264.7 cells. The cells were pretreated with the indicated concentrations of CME (5, 10, 20, and 50 μg/mL) and then treated with LPS (1 μg/mL). The results showed that CME (10, 20, and 50 μg/mL) inhibited the LPS- (1 μg/mL) induced mRNA and protein expression of iNOS in macrophage Raw 264.7 cells. In addition, the expression of COX-2 was inhibited at the mRNA and protein levels by CME in a dose-dependent manner. The mRNA expression of proinflammatory cytokines, such as TNF-α and IL-6, were markedly reduced by CME (10, 20, and 50 μg/mL). Moreover, CME clearly suppressed the nuclear translocation of the NF-κB p65 subunits, which was correlated with its inhibitory effect on I-κB phosphorylation. These results suggest that CME has anti-inflammatory properties by modulating the expression of COX-2, iNOS, and proinflammatory cytokines, such as TNF-α and IL-6, in macrophage RAW 264.7 cells via the NF-κB pathway.
1. Introduction
Citrus aurantium L. (bitter orange) is a flowering plant that belongs to the Rutaceae family of the order Sapindales, and is distributed widely in tropical and subtropical southeast regions of the world. The dried, entire immature peels of citrus fruit are used as a traditional herbal medicine to treat indigestion and respiratory symptoms, such as bronchial and asthmatic conditions [1, 2]. Citrus fruit including C. aurantium L. is composed of several components, such as limonoid, polyphenols, and flavonoids [3]. The major flavonoids isolated from Citrus aurantium L. are hesperidin, naringenin, and nobiletin [4]. These flavonoids have the ability to regulate the inflammatory response and carcinogenesis at many key regulatory points through a variety of mechanisms [5]. Nobiletin isolated from Citrus spp. has an inhibitory effect on phorbol ester-induced skin inflammation [6, 7]. In addition, it was reported that hesperidin can inhibit the expression of lipopolysaccharine- (LPS-) induced cyclooxygenase (COX-2) and inducible nitric oxide (iNOS) in macrophage RAW 264.7 cells [8]. Therefore, this study examined the anti-inflammatory effect of the dried peel of Citrus aurantium L.
Inflammation initiated by the invasion of pathogens or cell injury is a normal physiological and immune response. The normal inflammatory response involves the activation of several different cellular components, such as macrophages, neutrophils, and lymphocytes. In particular, macrophages play an important role in modulating inflammation and the immune response to maintain a defensive reaction [9, 10]. During the inflammatory response, activated macrophages secrete large amounts of proinflammatory mediators, such as nitric oxide (NO) and prostaglandinE2 (PGE2), via the inducible isoforms of NO synthase (iNOS) and cyclooxygenase-2 (COX-2), respectively, as well as proinflammatory cytokines, such as TNF-α, IL-6, and IL-1β [11, 12].
Most NO is synthesized through the oxidative deamination of L-arginine by a family of nitric oxide synthase (NOS) in mammalian cells [13]. These NOSs are found in three isoforms, neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS). In particular, during the inflammatory response, iNOS is expressed in response to endotoxin, such as LPS and various proinflammatory cytokines including INF-γ and TNF-α [14]. In addition, NO produced by iNOS is regarded as a critical mediator of carcinogenesis and high levels of NO cause inflammatory diseases, such as atherosclerosis, bowel disease, rheumatoid arthritis, and septic shock [15–18].
Similar to iNOS, cyclooxygenase (COX) is a key enzyme catalyzing the formation of prostaglandin from arachidonic acid during the inflammatory reaction [19]. There are two types of cyclooxygenase, COX-1 and COX-2. COX-1 is expressed constitutively in most cells and believed to be involved in homeostatic prostanoid biosynthesis. In contrast, COX-2 is not expressed in most normal tissues but is increased rapidly by oncogenes, growth factors, and cytokines [20]. COX-2 is involved in many biological processes, including inflammation, tumorigenesis, and carcinogenesis [21]. Briefly, the overexpression of proinflammatory mediators, such as iNOS, COX-2, and various cytokines, is involved in the pathogenesis of many disease processes. Therefore, the targeted inhibition of iNOS and COX-2 is a promising approach to inhibiting inflammation and carcinogenesis, as well as preventing cancer.
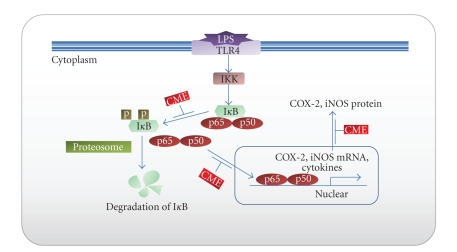
Nuclear transcription factor kappa-B (NF-κB) is a critical key transcription factor that expresses the genes involved in inflammation. NF-κB is composed of a range of homo or heterodimeric combinations of NF-κB/Rel proteins, such as Rel (cRel), RelA (p65), RelB, NF-κB1 (p50), and NF-κB2 (p52) in mammals. The main inducible form is a heterodimeric consisting of the p50/p65 subunit. NF-κB is present in the cytoplasm as an inactive complex associated with an inhibitory protein called IκB. Various external or internal stimuli cause the dissociation of the NF-κB/IκB complex through the phosphorylation and degradation of IκB by cytoplasmic IκB kinase (IKK). After activation, the NF-κB heterodimer (p65/p50) is translocated rapidly from the cytoplasm to the nucleus (Figure 1). In the nucleus, p65/p50 of the NF-κB subunit binds to a specific DNA motif and modulates the transcription of target genes including proinflammatory mediators, such as iNOS, COX-2, various cytokines, chemokines, and adhesion molecules [22–24].
Figure 1.
Main molecular targets of CME that lead to inhibitory effects on LPS-induced inflammation action. CME blocking NF-κB signaling pathway via inhibition of (i) IκB phosphorylation, (ii) subunits (p65/p50) of NF-κB trnaslocation in nuclear, and (iii) proinflammatory mediators (COX-2, iNOS, etc.) transcription. Blue arrows indicate the NF-κB signal pathway and target gene that DNA binding site of NF-κB. Red boxes indicate the inhibition effect of CME. CME: Crude methanol extract of Citrus aurantium L.; IKK: IκB kinase; IκB: Inhibitor of κB in cytoplasm; p50/p65: subunits of NF-κB; COX-2: cyclooxygenase-2; iNOS: inducible nitric oxide synthase.
Previous studies suggested that the activation of NF-κB triggers the transcription of iNOS, COX-2, and various proinflammatory cytokines, such as IL-6, IL-1, and TNF-α [25].
Therefore, the objective of this study is to elucidate the anti-inflammatory effects of crude methanol extract of Citrus aurantium L. on the expression of iNOS, COX-2, IL-6, and TNF-α via blocking of NF-κB signal pathway in LPS-stimulated macrophage RAW 264.7 cells. Thus, we investigated the following: (1) the inhibitory effect of CME on the LPS-induced iNOS and COX-2 at the mRNA and protein levels; and (2) the inhibitory effect of CME on the LPS-induced proinflammatory cytokines, such as TNF-α and IL-6.
2. Methods
2.1. Chemicals
The following reagents were acquired commercially: RPMI 1640 from HyClone (Logan, Utah, USA); Fetal bovine serum (FBS) and antibiotics (streptomycin/penicillin) from Gibco (BRL Life technologies, Grand Island, NY,); Lipopolysaccharide (Escherichia coli O111:B4) and methyl thiazolyl tetrazolium (MTT) from Sigma (St Louis, MO 63103, USA).
2.2. Antibodies
Antibodies for iNOS, COX-2, and NF-κB p65 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA,). β-actin antibody was purchased from Chemicon International (Temecula, CA,) and phospho-IκB antibody was purchased from Cell Signaling (Beverly, MA, USA).
2.3. Cell Culture and Treatment
The murine macrophage RAW 264.7 cell line (KCKB 40071) was purchased from the Korean Cell Line Bank (KCLB, Seoul, Korea). Mouse macrophage cell line RAW 264.7 was cultured in RPMI 1640 medium containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/μL streptomycin at 37°C in an atmosphere containing 5% CO2. The cells cultured in 6 well-plates were pretreated with the indicated concentrations of CME for 2 hours in the presence or absence of LPS (1 μg/mL).
2.4. Preparation of Herbal Extracts
Citrus aurantium L. was purchased from ABRB (Animal Bio-Resources Bank). The extract of C. aurantium L. was prepared using a modified method reported by Jung et al. [3]. Briefly, the powdered peel of C. aurantium L. (20 g) was extracted in 200 mL of absolute methanol at room temperature for 48 hours. The methanolic extracts were filtered through filter paper, evaporated in vacuo, and lyophilized to give a powdered extract. The yield of the citrus methanol extract was 10% (w/v). The concentrated CME was dissolved in PBS (pH 7.4) and stored at −20°C until needed.
2.5. MTT Assay
The cell viability was measured using a 3-(4, 5-dimethyethiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. The cells were seeded in a 6-well plate and incubated for 24 hours. The macrophage cells were treated with various concentrations of CME and incubated for 18 hours. 200 μL of a MTT solution (5 mg/mL in a PBS) was added to the wells and incubated for three hours. After media suction, 500 μL of dimethyl sulfoxide (DMSO) was added to each well to dissolve the crystalline deposits on the cells. The optical density of the cells at 540 nm was measured using an ELISA plate reader.
2.6. Western Blot
The cells were cultured in 6-well plates and incubated with CME (various concentrations) in the presence or absence of LPS (1 μg/mL) for 18 hours. After washing with ice-cold PBS, the cells were lysed in a buffer [50 mM Tris-Hcl (pH 8.0), 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, and 1% NP-40] containing the protease inhibitor cocktail, 0.5 M EDTA, and phosphatase inhibitor at a final concentration of 1X. The cell lysates were centrifuged at 13,000 rpm for 30 minutes to remove the debris. The protein concentration was determined using Bradford assay (Bio-rad, Hercules, CA, USA). The entire cell lysates were separated by 10% SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane (Immunobilon-P, 0.45 mm; Millipore, USA) using the TE 77 Semi-Dry Transfer Unit (CE Healthcare Life Sciences). The membranes were blocked with 5% nonfat skim milk in Tris buffered saline containing 0.5% Tween-20 (TBS, pH 7.4) at room temperature for 30 minutes, and incubated overnight at 4°C with the antibodies to iNOS, COX-2, β-actin, p-IκB, and NF-κB p65. The membranes were washed four times with TBS-T for 10 minutes each and incubated with a 1 : 2000 dilution of horseradish peroxidase-conjugated secondary antibody for 1 hour. The membranes were then rewashed five times with TBS-T. The proteins were visualized using an enhanced chemiluminescence kit (ECL), Western Blotting Detection Reagents (GE Healthcare Life Sciences), and exposed to X-ray film (Fuji, Tokyo, Japan). Then each band was quantitatively determined using Image J, and the density ratio represented the relative intensity of each band against those of β-actin as a control in each experiment.
2.7. Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
The total RNA was isolated using a TRIzol reagent (GeneALL Biotechnology CO., LTD, KOREA), according to the manufacturer`s instructions. The total RNA (1 μg) was reverse-transcribed into cDNA using commercially available cDNA synthesis kits (iScript cDNA Synthesis Kit; BIO-RAD). The tubes were incubated at 25°C for 5 minutes and then at 42°C for 30 minutes followed by heating at 85°C for 5 minutes. The sample was then stored at −70°C until further analysis. The PCR primers used with mouse macrophage RAW 264.7 cell cDNA are listed in Table 1. The PCR primers used to amplify the mouse COX-2, NOS2, TNF-α, IL-6, and GAPDH cDNA were purchased from Bioneer Corporation Inc. After initial heat denaturation at 95°C for 4 minutes., the PCR cycles were repeated 30~35 times under the following conditions: denaturation at 95°C for 30 seconds, annealing at 52~55°C for 1 minute, and extension at 72°C for 1 minute. The amplified PCR products were stored at −20°C until analysis. The PCR products were analyzed on ethidium bromide-stained 1.5% agarose gels. The amount of mRNA was evaluated by densitometry. The signal intensity of the specific mRNAs were normalized by a comparison with that of GAPDH and calculated as the relative amounts.
Table 1.
Primer design for RT-PCR.
| Gene | Direction | Sequence (5′-3′) |
|---|---|---|
| TNF-α | Sense | AGCACAGAAAGCATGATCCG |
| Antisense | GTTTGCTACGACGTGGGCTA | |
| IL-6 | Sense | CGATGATGCACTTGCAGAAA |
| Antisense | TGGAAATTGGGGTAGGAAGG | |
| COX-2 | Sense | CCCAGAGCTCCTTTTCAACC |
| Antisense | ATTTGGCACATTTCTTCCCC | |
| iNOS | Sense | CTCCCCTCTCTCCCTTTCCT |
| Antisense | TGGAAATTGGGGTAGGAAGG | |
| GAPDH | Sense | AAGGGTCATCATCTCTGCCC |
| Antisense | GTGATGGCATGGACTGTGGT |
2.8. Statistical Analysis
The data is presented as the mean ± SD of the results of at least three experiments. One-way analysis of variance (ANOVA) was used for multiple comparison. A P-value <.05 was considered significant.
3. Results
3.1. Cell Cytotoxicity and Morphology of Macrophage RAW 264.7 Cells
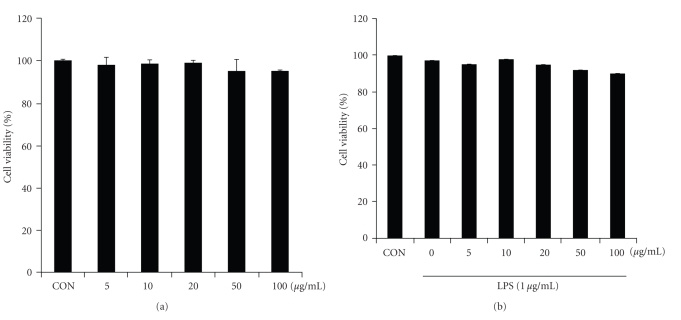
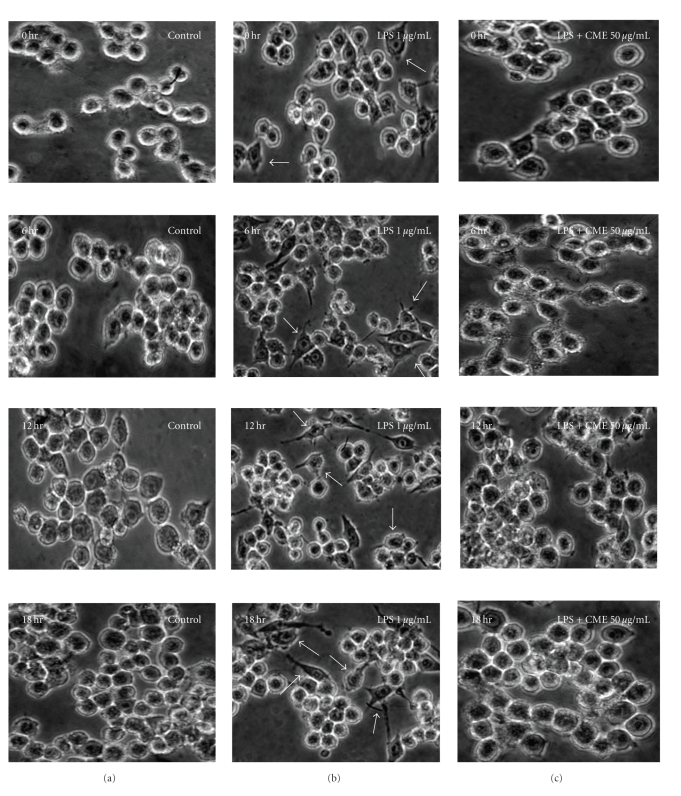
The cytotoxicity of CME was examined by a MTT assay in the presence or absence of LPS (1 μg/mL) to determine the effective concentration for treatment. Figure 2 shows the cell viability at various CME concentrations (5, 10, 20, 50, and 100 μg/mL) and cotreated LPS (1 μg/mL) for 18 hours. The results showed that LPS (1 μg/mL) and CME had no cytotoxicity at any of the concentrations examined. Figure 3 presents the cell morphology of the macrophage RAW 264.7 cells under CME treatment in the presence or absence of LPS (1 μg/mL). The cells were monitored under optical microscopy (400×) for 18 hours; see Figure 3. In the LPS-unstimulated cells, the cell morphology generally showed a round form the in whereas LPS-activated RAW 264.7 cells had changed to an irregular form with accelerated spreading and forming pseudopodia. The cotreatment of LPS with CME reduced the level of cell spreading and pseudopodia formation by suppressing cell differentiation.
Figure 2.
Effect of CME on the cell viability via an MTT assay. The cells were incubated with CME in the (a) absence or (b) presence of LPS for 18 hours. The results are reported as a percentage compared to the untreated controls. The data is reported as the mean ± SD of triplicate experiments.
Figure 3.
Morphological change in macrophage RAW 264.7 cells. Morphology of macrophage RAW 264.7 cell visualized by optical microscopy (×400). The cells were pretreated with CME before incubation with LPS for 18 hours. (a) Control, (b) LPS- (1 μg/mL) treated only, and (c) LPS-treated with CME (50 μg/mL).
3.2. CME Decreases LPS-Induced mRNA Expression of Proinflammatory Cytokines in RAW 264.7 Cells
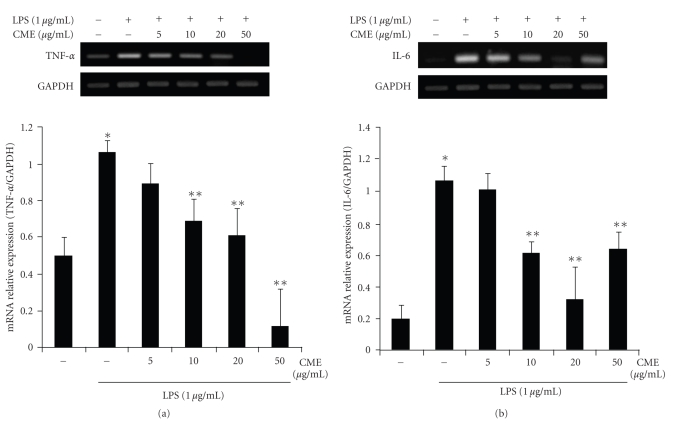
To examine the effect of CME on LPS-induced IL-6 and TNF-α gene expression, the RAW 264.7 cells were pretreated with various doses (5, 10, 20, and 50 μg/mL) of CME for 1 hour followed by a LPS (1 μg/mL) treatment for 6 hours. The level of IL-6 and TNF-α gene expression in the RAW 264.7 cells was increased markedly by the LPS treatment (1 μg/mL). The level of LPS-induced IL-6 and TNF-α gene expression decreased significantly on pretreatment with CME in the dose-dependent manner (Figure 4). This suggests that CME inhibited the mRNA expression of IL-6 and TNF-α in LPS-stimulated RAW 264.7 cells at the transcription levels.
Figure 4.
Suppressive effect of CME on the production of LPS-induced proinflammatory cytokines in RAW 264.7 cells. The RAW 264.7 cells were pretreated with CME (5, 10, 20, and 50 μg/mL) for 1 hour, and then stimulated with LPS (1 μg/mL). After 6 hours, the total mRNA was isolated, and the mRNA levels of (a) TNF-α and (b) IL-6 were examined by RT-PCR. * indicates an increase in mRNA expression relative to the control (P < .05), ** indicates a decrease in mRNA expression relative to the LPS group (P < .05).
3.3. CME Decreases LPS-Induced mRNA Expression of COX-2 and iNOS in RAW 264.7 Cells
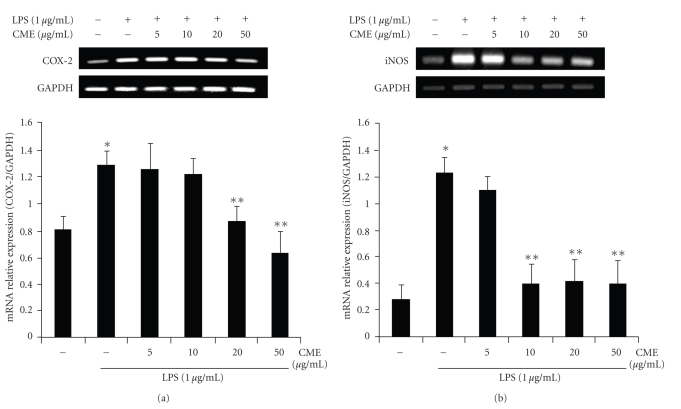
The expression of iNOS and COX-2 mRNA was examined by RT-PCR to determine if CME has a suppressive effect on the proinflammatory mediators, such as iNOS and COX-2, in the LPS-stimulated RAW 264.7 cells. As shown in Figure 5, upon LPS treatment for 6 h, the mRNA expression of COX-2 increased considerably in RAW 264.7 cells. However, the mRNA expression of COX-2 induced in cells treated with LPS was decreased significantly by 20 μg/mL and 50 μg/mL CME compared to cells treated with LPS alone. The mRNA expression of iNOS was also found to increase in the stimulated LPS macrophage RAW 264.7 cells. However, the LPS-induced iNOS mRNA expression was decreased by 10 μg/mL, 20 μg/mL, and 50 μg/mL CME (Figure 5). This suggests that CME may inhibit the mRNA expression of iNOS and COX-2 at the transcription level in LPS-stimulated RAW 264.7 cells.
Figure 5.
Suppressive effects of CME on the LPS-induced mRNA expression of COX-2 and iNOS in RAW 264.7 cells. The RAW 264.7 cells were pretreated with CME for 1 hour, and then incubated with LPS for 6 hours. The total mRNA was isolated, and the mRNA levels of (a) COX-2 and (b) iNOS were performed by RT-PCR. * indicates an increase in mRNA expression relative to the control (P < .05), ** indicates a decrease in mRNA expression relative to the LPS group (P < .05).
3.4. CME Decreases LPS-Induced Protein Expression of iNOS and COX-2 in RAW 264.7 Cells
The effect of CME on the expression of iNOS and COX-2 at the protein levels was examined by western blot analysis. Figure 6 shows a representative western blot of COX-2 and iNOS in the treated cells. After the LPS treatment for 18 hours, the levels of iNOS and COX-2 protein expression was obviously increased, but a cotreatment with LPS and CME (various concentrations) significantly attenuated the expression of the iNOS and COX-2 proteins in a dose-dependent manner (Figure 6). This suggests that the expression of the iNOS and COX-2 proteins in LPS-induced macrophage RAW 264.7 cells was inhibited by CME.
Figure 6.
Suppressive effects of CME on the LPS-induced protein expression of COX-2 and iNOS in RAW 264.7 cells. RAW 264.7 cells were pretreated with CME for 1 hour, and then incubated with LPS for 18 hours. The cells were lysed, and the lysates were examined by immunoblotting for the protein of (a) COX-2 and (b) iNOS. * indicates an increase in protein expression relative to the control (P < .05), ** indicates a decrease in protein expression relative to the LPS group (P < .05).
3.5. CME Inhibits IκB Phosphorylation in Cytoplasm
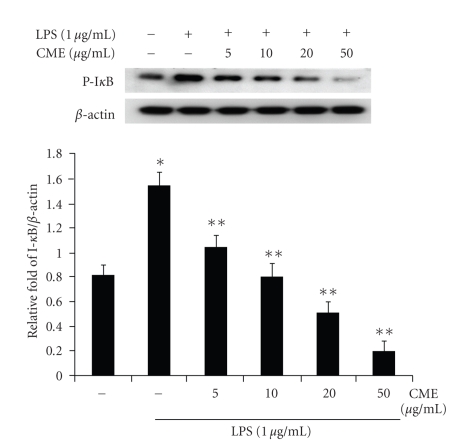
In the NF-κB signal pathway, the phosphorylation and degradation of IκB is a crucial step for NF-κB activation in activated macrophages. This study investigated whether CME inhibits the LPS-induced phosphorylation of IκB and degradation of IκB in RAW 264.7 macrophages by Western blot analysis. As shown in Figure 7, the phosphorylated forms of IκB were barely detectable in the unstimulated RAW 264.7 cells. However, level of IκB phosphorylation was increased significantly after the LPS (1 μg/mL) treatment for 30 minutes. A pretreatment with CME moderately inhibited the LPS-mediated IκB phosphorylation at all CME concentrations. However, CME had no effect on the level of IκB degradation (data not shown).
Figure 7.
CME suppressed the LPS-induced phosphorylation of I-κB in RAW 264.7 cells. The cells were pretreated with CME at various concentrations for 1 hour and then with LPS for 30 minutes. The cell lysates were analyzed by immunoblotting for the detection of the phosphorylated form of I-κB. * indicates an increase in protein relative to the control (P < .05), ** indicates a decrease in protein relative to the LPS group (P < .05).
3.6. CME Inhibits NF-κB Activation and Its Nuclear Translocation
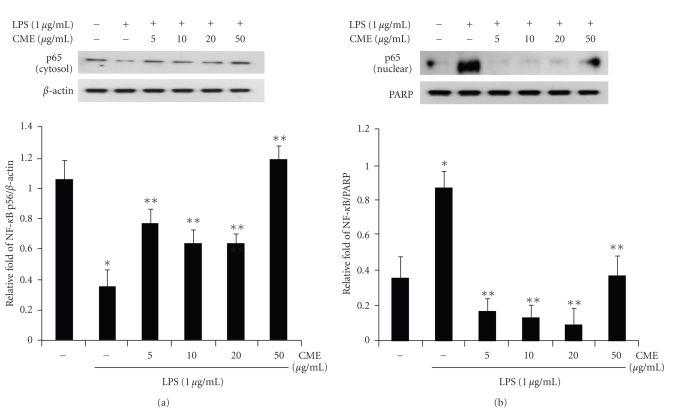
p65 of the NF-κB subunit is a major component of NF-κB activated by LPS in macrophages, and the activation of NF-κB is initiated by IκB degradation after IκB-α phosphorylation. In this study, Western blot analysis was used to determine if CME inhibited the activation of NF-κB p65 and nuclear translocation of p65 as a result of the inhibition of IκB-α phosphorylation. As shown in Figure 8, p65 was less expressed in the cytosol fraction and strongly expressed in the nuclear fraction after exposure to LPS. However, p65 of the cytosol fraction and nuclear fraction were increased and decreased by CME, respectively. Hence, it is hypothesized that CME may block the LPS-induced translocation of p65 from the cytosol to the nucleus by inhibiting IκB phosphorylation.
Figure 8.
CME suppressed the LPS-induced activation and translocation of NF-κB in RAW 264.7 cells. The cells were pretreated with CME at concentration of 5, 10, 20, and 50 μg/mL for 1 hour and treated with LPS (1 μg/mL). After 30 minutes incubation, the (a) cytosol and (b) nuclear protein fractions were prepared by cell lysis. The fraction proteins were analyzed by immunoblotting for the detection of p65. * indicates an increase in protein relative to the control (P < .05), ** indicates a decrease in protein relative to the LPS group (P < .05).
4. Discussion
Herbal medicinal plants or extracts are used as an alternative therapy. Citrus aurantium L. has been used as a traditional medicine to treat the inflammatory response. Many studies have reported the pharmaceutical effects of natural herbal products, such as anti-inflammatory, anticancer, and antioxidative effects, and so forth [26–28]. Recent reports suggest that natural herbs have an anti-inflammatory effect by regulating the activation of transcription factors including NF-κB or activator protein-1 (AP-1). In particular, flavonoids, such as epigallocatechin gallate (EGCG), curcumin, and quercetin, have been reported to be potential inhibitors via the NF-κB pathway [29, 30]. Flavonoids are one of the most critical phytochemical compounds in many plants, particularly in the genus Citrus [31]. Citrus-derived flavonoids such as naringenin, nobiletin, and hesperidin have considerable biological activity, including anti-inflammatory effects [32]. Naringenin inhibited several cytokines induced by LPS-stimulated macrophages [33]. Nobiletin suppressed the production of PGE2 by downregulating the COX-2 gene in human synovial fibroblasts as well as decreasing the expression of IL-1α, IL-1β, TNF-α, and IL-6 mRNAs in mouse macrophages [34]. Zielińska-Przyjemska and Ignatowicz reported that citrus fruit flavonoids have protective effects against oxidative stress related to inflammation [35].
The present study examined the suppressive effect of CME on the proinflammatory mediators in macrophage RAW 264.7 cells. Therefore, an MTT assay was carried out to examine the cytotoxicity of CME. CME did not exhibit cytotoxicity at any of the concentrations examined. The morphological change in macrophage RAW 264.7 cells induced by the CME treatment in the presence or absence of LPS was also examined. The cells were round without the LPS treatment but became irregularly shaped showing increased spreading and pseudopodia formation after LPS stimulation. The cotreatment with 50 μg/mL CME decreased the degree of cell spreading and pseudopodia formation. Interestingly, this amount of CME also decreased the levels of iNOS and COX-2.
Inducible enzymes, such as iNOS and COX-2, are critical factors in the inflammatory response, cell proliferation, and skin tumor promotion [21, 36]. In the inflammatory response, iNOS and COX-2 expression are involved in the production of NO and prostaglandin, respectively. The overexpression of iNOS and COX-2 is related to the pathogenesis of a range of disease processes [11, 37]. Previous studies reported that Zedoarondiol isolated from the rhizome of Curcuma heyneana inhibits the LPS-induced expression of iNOS and COX-2 with the concomitant reduction of PGE2 and NO production in RAW 264.7 cells [38]. Y.-L. Lin and J.-K. Lin showed that (-)-epigallocatechin-3-gallate (EGCG), one of the flavonoids in green tea, decreased the protein expression of iNOS by reducing the mRNA levels of iNOS through the inhibition of NF-κB binding [39]. Chrysin, a flavonoid contained in propolis and honey, inhibited LPS-induced expression of COX-2 in macrophages by blocking the DNA-binding activity of C/EBP, which plays a major role in inducing the expression of COX-2 [40]. Moreover, the methanol extract of Citrus reticulate decreased the production of NO by suppressing the expression of iNOS at the mRNA and protein levels [3]. Therefore, the regulation of iNOS and COX-2 is important in the inflammation response. This study examined the effect of CME on the expression of iNOS and COX-2 at the protein and mRNA levels. The results showed that CME dose dependently suppressed the expression of COX-2 at both the protein and mRNA levels. In addition, the expression of iNOS protein and mRNA in LPS-stimulated macrophage RAW 264.7 cells was suppressed by various concentrations of CME (10, 20, and 50 μg/mL). This shows that CME inhibited the expression of iNOS and COX-2 in LPS-stimulated RAW 264.7 cells at both the protein and mRNA levels.
Proinflammatory cytokines, such as IL-1β, IL-2, IL-6, IL-10, and TNF-α, are important mediators in a range of acute and chronic responses to inflammatory diseases [29, 41, 42]. The production of TNF-α is essential for the synergistic release of NO in IFN-γ and/or LPS-stimulated macrophages. In addition, TNF-α is involved in many physiological effects, such as septic shock, inflammation, cachexia, and cytotoxicity. IL-6 is believed to be an endogenous pyrogen that causes fever by triggering metabolic changes in the hypothalamic thermoregulatory center for the duration of the inflammation response [11, 25]. This study investigated the suppressive effect of CME on LPS-induced gene expression of IL-6 and TNF-α in macrophage RAW 264.7 cells. The results showed that the mRNA expression of TNF-α and IL-6 in LPS-stimulated RAW 264.7 cells was decreased by various concentrations of CME (10, 20, and 50 μg/mL). These results suggest that CME successfully suppressed the expression of TNF-α and IL-6 in LPS-stimulated macrophages.
NF-κB is a major factor regulating the expression of inflammation-induced enzymes and cytokines, such as iNOS, COX-2, TNF-α, and IL-6, which include the NF-κB binding sites in their promoters, and has attracted attention as a new target for treating inflammatory diseases [43–46]. Therefore, the suitable regulation of NF-κB may be beneficial in treating many inflammatory disorders. Recently, Jung et al. reported that the root of Panax notoginseng (PN) inhibited the LPS-induced inflammatory mediators, including iNOS and COX-2 by blocking IκB degradation in the cytosol and the nuclear translocation of the NF-κB p65 subunit [12]. Choi et al. showed that nobiletin isolated from the fruit peel of Citrus sunki inhibits the expression of the genes involved in inflammation by blocking the DNA-binding activity of NF-κB. However, nobiletin did not affect the LPS-induced phosphorylation and degradation of the IκB-α protein or the nuclear translocation of NF-κB [47]. The present study examined whether CME inhibits the LPS-induced degradation and phosphorylation of IκB or the activation and translocation of NF-κB p65. These results showed that CME inhibits significantly the LPS-induced activation and nuclear translocation of NF-κB p65 at various concentrations (5, 10, 20, and 50 μg/mL). Moreover, in RAW 264.7 cells pretreated with CME, the phosphorylation of IκB was inhibited in a dose-dependent manner. However, there were no significant differences in the level of IκB degradation (date not shown). These findings suggest that CME suppresses NF-κB activation and nuclear translocation in LPS-stimulated RAW 264.7 cells by blocking the phosphorylation of IκB.
In summary, the anti-inflammatory activity of CME was characterized by the suppression of iNOS, COX-2, and various cytokines, such as TNF-α and IL-6, through the NF-κB signal pathway. The results showed that CME inhibits the LPS-induced expression of iNOS and COX-2 at the mRNA and protein levels as well as the expression of TNF-α, IL-6 transcripts in macrophage RAW 264.7 cells. These suppressive effects are mediated by inhibiting the activation of NF-κB and phosphorylation of IκB. The major transcriptional factors of the target gene, NF-κB, are inactivated by CME. This is important because NF-κB plays a key role in modulating many genes related to the inflammatory response. These results suggest that crude methanol extract of Citrus aurantium L. has anti-inflammatory properties, and may be effective as a medicine for preventing inflammation.
Acknowledgments
This work was supported by the National Research Foundation (NRF) of Korea grant funded by the Korean government (MOST) (no. 2009-0084454) and the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family affairs, Republic of Korea (no. 0820050).
References
- 1.Nogata Y, Sakamoto K, Shiratsuchi H, Ishii T, Yano M, Ohta H. Flavonoid composition of fruit tissues of citrus species. Bioscience, Biotechnology and Biochemistry. 2006;70(1):178–192. doi: 10.1271/bbb.70.178. [DOI] [PubMed] [Google Scholar]
- 2.Fugh-Berman A, Myers A. Citrus aurantium, an ingredient of dietary supplements marketed for weight loss: current status of clinical and basic research. Experimental Biology and Medicine. 2004;229(8):698–704. doi: 10.1177/153537020422900802. [DOI] [PubMed] [Google Scholar]
- 3.Jung KH, Ha E, Kim MJ, et al. Suppressive effects of nitric oxide (NO) production and inducible nitric oxide synthase (iNOS) expression by Citrus reticulata extract in RAW 264.7 macrophage cells. Food and Chemical Toxicology. 2007;45(8):1545–1550. doi: 10.1016/j.fct.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Nogata Y, Sakamoto K, Shiratsuchi H, Ishii T, Yano M, Ohta H. Flavonoid composition of fruit tissues of citrus species. Bioscience, Biotechnology and Biochemistry. 2006;70(1):178–192. doi: 10.1271/bbb.70.178. [DOI] [PubMed] [Google Scholar]
- 5.Manthey JA, Grohmann K, Guthrie N. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Current Medicinal Chemistry. 2001;8(2):135–153. doi: 10.2174/0929867013373723. [DOI] [PubMed] [Google Scholar]
- 6.Manthey JA, Grohmann K, Montanari A, Ash K, Manthey CL. Polymethoxylated flavones derived from citrus suppress tumor necrosis factor-α expression by human monocytes. Journal of Natural Products. 1999;62(3):441–444. doi: 10.1021/np980431j. [DOI] [PubMed] [Google Scholar]
- 7.Murakami A, Nakamura Y, Torikai K, et al. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion mice. Cancer Research. 2000;60(18):5059–5066. [PubMed] [Google Scholar]
- 8.Sakata K, Hirose Y, Qiao Z, Tanaka T, Mori H. Inhibition of inducible isoforms of cyclooxygenase and nitric oxide synthase by flavonoid hesperidin in mouse macrophage cell line. Cancer Letters. 2003;199(2):139–145. doi: 10.1016/s0304-3835(03)00386-0. [DOI] [PubMed] [Google Scholar]
- 9.Wadleigh DJ, Reddy ST, Kopp E, Ghosh S, Herschman HR. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. Journal of Biological Chemistry. 2000;275(9):6259–6266. doi: 10.1074/jbc.275.9.6259. [DOI] [PubMed] [Google Scholar]
- 10.Erwig L-P, Rees AJ. Macrophage activation and programming and its role for macrophage function in glomerular inflammation. Kidney and Blood Pressure Research. 1999;22(1-2):21–25. doi: 10.1159/000025905. [DOI] [PubMed] [Google Scholar]
- 11.Kim J-B, Han A-R, Park E-Y, et al. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-κB inactivation in RAW 264.7 macrophage cells. Biological and Pharmaceutical Bulletin. 2007;30(12):2345–2351. doi: 10.1248/bpb.30.2345. [DOI] [PubMed] [Google Scholar]
- 12.Jung H-W, Seo U-K, Kim J-H, Leem K-H, Park Y-K. Flower extract of Panax notoginseng attenuates lipopolysaccharide-induced inflammatory response via blocking of NF-κB signaling pathway in murine macrophages. Journal of Ethnopharmacology. 2009;122(2):313–319. doi: 10.1016/j.jep.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Tayeh MA, Marletta MA. Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate. Tetrahydrobiopterin is required as a cofactor. Journal of Biological Chemistry. 1989;264(33):19654–19658. [PubMed] [Google Scholar]
- 14.Kleinert H, Pautz A, Linker K, Schwarz PM. Regulation of the expression of inducible nitric oxide synthase. European Journal of Pharmacology. 2004;500(1–3):255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Bingham CO., III The pathogenesis of rheumatoid arthritis: pivotal cytokines involved in bone degradation and imflammation. Journal of Rheumatology. 2002;29(65):3–9. [PubMed] [Google Scholar]
- 16.Blantz RC, Munger K. Role of nitric oxide in inflammatory conditions. Nephron. 2002;90(4):373–378. doi: 10.1159/000054723. [DOI] [PubMed] [Google Scholar]
- 17.Perner A, Rask-Madsen J. Review article: the potential role of nitric oxide in chronic inflammatory bowel disorders. Alimentary Pharmacology and Therapeutics. 1999;13(2):135–144. doi: 10.1046/j.1365-2036.1999.00453.x. [DOI] [PubMed] [Google Scholar]
- 18.Tracey KJ. Tumor necrosis factor (cachectin) in the biology of septic shock syndrome. Circulatory Shock. 1991;35(2):123–128. [PubMed] [Google Scholar]
- 19.DuBois RN, Abramson SB, Crofford L, et al. Cyclooxygenase in biology and disease. FASEB Journal. 1998;12(12):1063–1073. [PubMed] [Google Scholar]
- 20.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annual Review of Biochemistry. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 21.Lai C-S, Li S, Chai C-Y, et al. Inhibitory effect of citrus 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone on 12-O-tetradecanoylphorbol 13-acetate-induced skin inflammation and tumor promotion in mice. Carcinogenesis. 2007;28(12):2581–2588. doi: 10.1093/carcin/bgm231. [DOI] [PubMed] [Google Scholar]
- 22.Pahl HL. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 1999;18(49):6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 23.Baeuerle PA, Baltimore D. Nf-κB: ten years after. Cell. 1996;87(1):13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 24.Chao W-W, Hong Y-H, Chen M-L, Lin B-F. Inhibitory effects of Angelica sinensis ethyl acetate extract and major compounds on NF-κB trans-activation activity and LPS-induced inflammation. Journal of Ethnopharmacology. 2010;129(2):244–249. doi: 10.1016/j.jep.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Wu L-C, Fan N-C, Lin M-H, et al. Anti-inflammatory effect of spilanthol from Spilanthes acmella on murine macrophage by down-regulating LPS-induced inflammatory mediators. Journal of Agricultural and Food Chemistry. 2008;56(7):2341–2349. doi: 10.1021/jf073057e. [DOI] [PubMed] [Google Scholar]
- 26.Lee WS, Baek Y-I, Kim J-R, Cho K-H, Sok D-E, Jeong T-S. Antioxidant activities of a new lignan and a neolignan from Saururus chinensis. Bioorganic and Medicinal Chemistry Letters. 2004;14(22):5623–5628. doi: 10.1016/j.bmcl.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 27.Sang S, Hong J, Wu H, et al. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. Journal of Agricultural and Food Chemistry. 2009;57(22):10645–10650. doi: 10.1021/jf9027443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim EH, Shim B, Kang S, et al. Anti-inflammatory effects of Scutellaria baicalensis extract via suppression of immune modulators and MAP kinase signaling molecules. Journal of Ethnopharmacology. 2009;126(2):320–331. doi: 10.1016/j.jep.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Nam N-H. Naturally occurring NF-κB inhibitors. Mini-Reviews in Medicinal Chemistry. 2006;6(8):945–951. doi: 10.2174/138955706777934937. [DOI] [PubMed] [Google Scholar]
- 30.Kim HK, Cheon BS, Kim YH, Kim SY, Kim HP. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochemical Pharmacology. 1999;58(5):759–765. doi: 10.1016/s0006-2952(99)00160-4. [DOI] [PubMed] [Google Scholar]
- 31.Benavente-García O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. Journal of Agricultural and Food Chemistry. 2008;56(15):6185–6205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- 32.Choi S-Y, Ko H-C, Ko S-Y, et al. Correlation between flavonoid content and the NO production inhibitory activity of peel extracts from various citrus fruits. Biological and Pharmaceutical Bulletin. 2007;30(4):772–778. doi: 10.1248/bpb.30.772. [DOI] [PubMed] [Google Scholar]
- 33.Bodet C, La VD, Epifano F, Grenier D. Naringenin has anti-inflammatory properties in macrophage and ex vivo human whole-blood models. Journal of Periodontal Research. 2008;43(4):400–407. doi: 10.1111/j.1600-0765.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 34.Lin N, Sato T, Takayama Y, et al. Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochemical Pharmacology. 2003;65(12):2065–2071. doi: 10.1016/s0006-2952(03)00203-x. [DOI] [PubMed] [Google Scholar]
- 35.Zielińska-Przyjemska M, Ignatowicz E. Citrus fruit flavonoids influence on neutrophil apoptosis and oxidative metabolism. Phytotherapy Research. 2008;22(12):1557–1562. doi: 10.1002/ptr.2449. [DOI] [PubMed] [Google Scholar]
- 36.Saleem M, Afaq F, Adhami VM, Mukhtar H. Lupeol modulates NF-κB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene. 2004;23(30):5203–5214. doi: 10.1038/sj.onc.1207641. [DOI] [PubMed] [Google Scholar]
- 37.Chen C-H, Sheu M-T, Chen T-F, et al. Suppression of endotoxin-induced proinflammatory responses by citrus pectin through blocking LPS signaling pathways. Biochemical Pharmacology. 2006;72(8):1001–1009. doi: 10.1016/j.bcp.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Cho W, Nam J-W, Kang H-J, Windono T, Seo E-K, Lee K-T. Zedoarondiol isolated from the rhizoma of Curcuma heyneana is involved in the inhibition of iNOS, COX-2 and pro-inflammatory cytokines via the downregulation of NF-κB pathway in LPS-stimulated murine macrophages. International Immunopharmacology. 2009;9(9):1049–1057. doi: 10.1016/j.intimp.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Lin Y-L, Lin J-K. (-)-epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-κB. Molecular Pharmacology. 1997;52(3):465–472. [PubMed] [Google Scholar]
- 40.Woo KJ, Jeong Y-J, Inoue H, Park J-W, Kwon TK. Chrysin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression through the inhibition of nuclear factor for IL-6 (NF-IL6) DNA-binding activity. FEBS Letters. 2005;579(3):705–711. doi: 10.1016/j.febslet.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 41.Blancke F, Claeys MJ, Jorens P, et al. Systemic inflammation and reperfusion injury in patients with acute myocardial infarction. Mediators of Inflammation. 2005;2005(6):385–389. doi: 10.1155/MI.2005.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laskin DL, Laskin JD. Role of macrophages and inflammatory mediators in chemically induced toxicity. Toxicology. 2001;160(1-3):111–118. doi: 10.1016/s0300-483x(00)00437-6. [DOI] [PubMed] [Google Scholar]
- 43.Isomura I, Morita A. Regulation of NF-κB signaling by decoy oligodeoxynucleotides. Microbiology and Immunology. 2006;50(8):559–563. doi: 10.1111/j.1348-0421.2006.tb03827.x. [DOI] [PubMed] [Google Scholar]
- 44.Kim YH, Kim DH, Lim H, Baek D-Y, Shin H-K, Kim J-K. The anti-inflammatory effects of methylsulfonylmethane on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biological and Pharmaceutical Bulletin. 2009;32(4):651–656. doi: 10.1248/bpb.32.651. [DOI] [PubMed] [Google Scholar]
- 45.Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-κB in the resolution of inflammation. Nature Medicine. 2001;7(12):1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- 46.Zhao L, Tao J-Y, Zhang S-L, Jin F, Pang R, Dong J-H. N-butanol extract from melilotus suaveolens ledeb affects pro- and anti-inflammatory cytokines and mediators. Evidence-Based Complementary and Alternative Medicine. 2010;7(1):97–106. doi: 10.1093/ecam/nem165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi S-Y, Hwang J-H, Ko H-C, Park J-G, Kim S-J. Nobiletin from citrus fruit peel inhibits the DNA-binding activity of NF-κB and ROS production in LPS-activated RAW 264.7 cells. Journal of Ethnopharmacology. 2007;113(1):149–155. doi: 10.1016/j.jep.2007.05.021. [DOI] [PubMed] [Google Scholar]