Abstract
Patients with high risk melanoma and neuroblastoma frequently recur despite surgical resection and appropriate adjuvant therapies. Immunotherapy with the immunocytokine, hu14.18-IL2, was developed via fusion of two molecules of IL2 to the monoclonal antibody, 14.18, that recognizes GD2, expressed on the above malignancies. This article will discuss the results of preclinical work utilizing hu14.18-IL2 therapy, including data suggesting that intratumoral therapy may have enhanced antitumor benefit compared with IV therapy. Initial clinical trials in adult melanoma and pediatric neuroblastoma have demonstrated acceptable toxicity profiles in dosing that induces immune activation. Preclinical and initial clinical data suggest greater efficacy in the setting of minimal residual disease, therefore future clinical testing is planned to test the benefit of hu14.18-IL2 in this setting.
1. Introduction
Immunocytokines (IC) are fusion proteins that genetically fuse immunologically reactive monoclonal antibodies (mAb) to cytokines. The goal is to retain the functions of both the cytokine and the antibody components in a single bifunctional molecule, and to ultimately expand the biologic activities of one component (the antibody) with the biologic function of the other component of the IC (the cytokine). Hu14.18-IL2 was created, linking IL-2 to the 14.18 mAb that recognizes the GD2 disialoganglioside primarily expressed on human melanoma and neuroblastoma. Phase I and Phase II studies have been completed in MEL and NBL patients. Our purpose in this review is to offer background on this IC and to summarize both the preclinical and clinical testing of 14.18-IL2 IC.
2. Background
Melanoma (MEL) and Neuroblastoma (NBL)
In 2007, there were 59,940 new diagnoses and 8000 deaths in the USA due to melanoma. These numbers continue to rise, and MEL is estimated to account for 63,480 new diagnoses and claim approximately 8420 deaths in 2008[1;1;2]. While remission can be accomplished surgically for most newly diagnosed high risk MEL patients (and for most patients with local or regional recurrence), many of these high risk patients will recur. Thus far interferon (IFN) is the only treatment shown to help delay or prevent recurrence in some of these high risk patients[3;4].
Neuroblastoma is the most common extracranial solid tumor of childhood, and high risk features are present in nearly half of all new diagnoses[5]. Treatment has improved, and for children under age 15, the 5-year overall survival rates for all newly diagnosed patients went from 52% in 1975-77, to 69% in 1996–2003[1;6]. With current standard therapy (an aggressive combination of multi-agent chemotherapy, surgery, radiation therapy and ablative chemotherapy followed by autologous hematopoietic stem cell reinfusion), most patients with high-risk disease achieve a complete response, but undetected amounts of minimal residual disease(MRD) remain in many. As a result of the remaining residual NBL, most high-risk patients develop recurrent refractory disease and only ~30% overall are cured[7]. Melanoma, NBL, and some osteosarcoma, small cell lung cancer, and soft tissue sarcomas express the disialoganglioside, GD2. These GD2+ diseases account for approximately 8% of all cancer deaths in the US[8], and therefore, the results from studies looking at the clinical response of patients treated with hu14.18-IL2 for MEL and NBL might potentially be translatable to all GD2+ diseases.
2.1 Development of hu14.18-IL2 IC
GD2 disialoganglioside is expressed on neuroectodermal tumors including MEL and NBL[4;9;10], and on some small-cell lung cancer, osteosarcoma, and soft tissue sarcomas[4;4;11–13]. In normal tissues, GD2 has limited expression on neurons, melanocytes, and peripheral pain fibers, and therefore is an appropriate target for antitumor therapy[9;14;15].
The originally described IgG3 murine anti-GD2 mAbs were 3F8 and 14.18[4;9;10]. Initial clinical testing was performed with 3F8 and 14.G2a(the murine IgG2a class switch variant of 14.18.,and with human/mouse chimeric 14.18 (designated ch14.18) in patients with NBL and MEL[4;16–23]. The ch14.18, was created to decrease the immunogenicity associated with the murine antibody[14;24]. Two trials were performed with ch14.18 mAb as a single agent for patients with stage 4 NBL[14;19;20]. Ch14.18 was well tolerated, with similar toxicities as seen with the murine antibody (pain, fever, hypertension, tachycardia, urticaria and transient neuropathy)[14;19;20]. In the Pediatric Oncology Group study, the chimeric antibody was less immunogenic than the murine antibody, with a longer half-life[14;25]. Antitumor effects of these anti-GD2 mAbs were observed in phase-I and-II trials, and include shrinkage of measurable MEL or NBL[4;17–23;26] and improved microscopic metastatic disease in bone marrows of children with NBL[4;17;19;27].
Interleukin 2(IL-2) is a strong pro-inflammatory agent that activates immune cells to mediate antitumor effects[4;14;28–30], and is approved as a single agent treatment for metastatic MEL as well as renal cell carcinoma. IL-2 activates NK cells in a dose dependent fashion, whereby using a tumor reactive monoclonal antibody(mAb) to better direct the lytic activity of activated NK cells[4;31–33]. Activated NK cells bind the Fc portion of the mAb through their FcγRIII and mediate ADCC[14;34;35]. In murine models, mice receiving IL-2 and tumor specific mAb had improved antitumor effects compared with either agent alone[32;33;36;37]. Because systemic cytokine administration causes toxicity arising from nonspecific inflammatory activation[38], immunocytokines (IC) were created to both limit toxicity associated with systemic cytokine administration, and to augment the antitumor effect of IL-2 and mAb.
2.2 Immunocytokine
IC links cytokine proteins to the Fc portion of the mAb. Preclinical in vitro and in vivo studies demonstrated greater ADCC when mAb is linked to IL-2, versus both therapies administered together as separate agents[39–41]. The IC retains the antigen-binding specificity of the mAb, and delivers cytokine directly to the tumor microenvironment providing both the antibody effector function and the cytokine effect[14;41], allowing NK cell activation via the IL-2 component. IL-2 also stimulates antigen-specific T cells to kill tumor cells[14;35;42]. This allows for a lower dose of cytokine than with systemic administration of the cytokine alone[12;14;43;44].
The 14.G2a, ch14.18 and 3F8 mAbs all demonstrated enhanced ADCC with effector cells activated by IL-2[12;36]. This led to adding anti-GD2 mAbs with vivo IL-2 therapy[45;46]. Blood from patients in these studies demonstrated ADCC could be mediated in vitro[47], and occasional patients receiving these therapies had decrease in measurable tumor.
The ch14.18-IL2, is an IC that is formed by linking the gene sequence of IL-2 to DNA encoding the carboxyl end of the constant region of the chimeric mouse-human IgG1 molecule that composes the ch14.18 mAb[14;43;43;48]. ADCC depends on the number and function of FcRs on effector cells including NK cells, and when NK cells are activated and expanded with IL-2 in vivo, they mediate augmented ADCC[4;12;36;49]. These activated NK cells have augmented IL-2Rα expression[50] and have a dramatic in vitro response to IL-2[51]. This should result in effector cells binding tumor, followed by activation of effector cell mediated lysis for T-cells with IL-2Rs and NK cells via Fc and IL- receptors.[48;52]. Anti-MEL activity is induced by ch14.18-IL-2 in a murine SCID human-tumor-xenograft model[53], and in mice bearing syngeneic tumors transfected to express GD2[54;55].
The IL2 component of this IC can activate NK cells without Fc receptors, through their IL2 receptors[56]. Up to 50% of NK cells from cancer patients do not have FcRs after IL-2 treatment, and these FcR(−) cells are more lytic to tumor cells in direct assays not dependent on mAb and FcRs[4;56]. The antitumor effect against B78MEL is predominantly T-cell mediated after ch14.18-IL-2[57]. Thus, ch14.18-IL-2 can function as both a T-cell inducing vaccine, as well as an activator of NK mediated ADCC. These data provided the basis for initiating clinical testing of this 14.18 based IC molecule as therapy for NBL and MEL[4].
A problem with mouse mAb therapy has been the development of blocking antibodies to the mAb itself, called a HAMA(human anti-mouse antibody) response[14;24]. The development of a HAMA response has been detected within 7 days of treatment and can neutralize any further treatments with the mouse anti-GD2 antibody[4;14]. This phenomenon has led to the progressive development of increasingly human antibodies for therapy. Chimeric human-mouse antibodies that retain the Fab fragment of the mouse antibody with its antigen binding specificity, were bound to the Fc portion of human antibodies in order to create mAbs which were less immunogenic. However, HACA(human anti-chimeric antibody) response was still detected[14;58]. Therefore, the current humanized mAb, hu14.18, was developed retaining only the complementarity-determining regions of the original mouse antibody so that it is otherwise ~ 98% human amino acid sequence(Fig 1) [4;14;59]. This humanized form of IC, hu14.18-IL-2, was made with hopes of reducing immunogenicity of the IC in patients and has been studied in a recently completed Phase I trial[14;60].
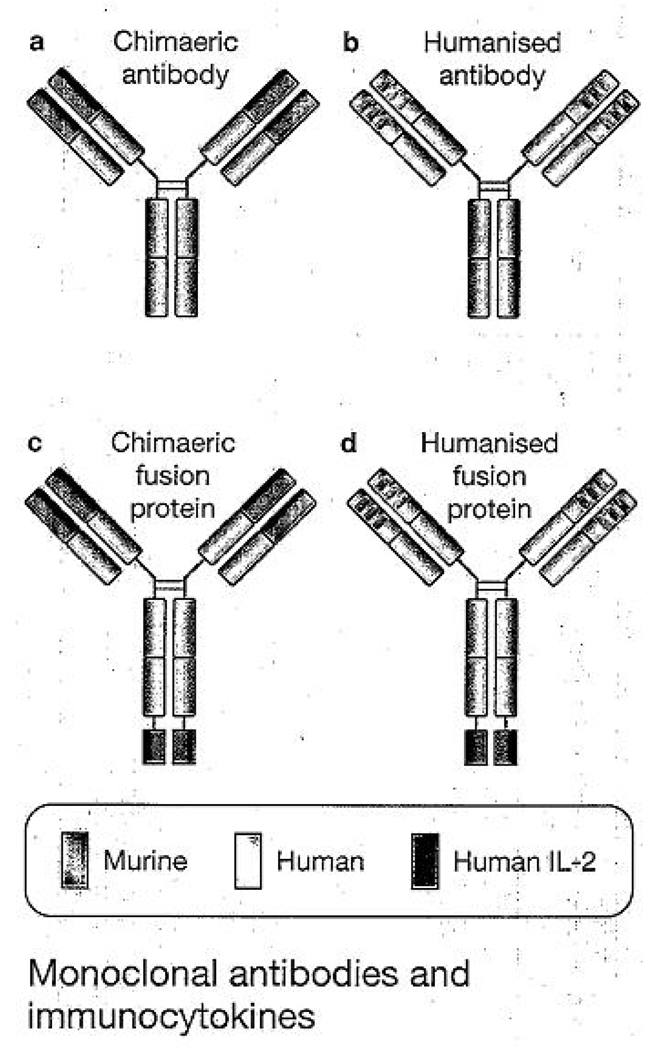
Fig 1. Monoclonal antibodies and immunocytokines.
(a) Chimeric monoclonal antibody (mAb) combining the constant region of a human antibody with the variable domain of a murine antibody. Antigen specificity is conferred by the murine variable domain. (b) In humanised mAb, human framework determinants, of both the heavy and light chains replace the murine framework determinants, but the antigen specificity of the original murine mAb is retained. (c,d) immunocytokines combine the mAb with covalently linked cytokines, such as molecules of interleukin 2 (lL-2), to the C-terminus of each of the heavy chains. Adapted, with permission, from Ref 59 (© 1999 Elsevier).
3. Clinical Efficacy (Testing of hu14.18-IL-2 in Melanoma)
3.1 Preclinical Development and Study Design
When murine or chimeric anti-GD2 IgG mAbs were injected IV, the half-life was 2–5 days[19;45]. In contrast, the half-life of the ch14.18-IL-2 IC in mice was only approximately 4 hours[61]. Both the ch14.18-IL-2 and hu14.18-IL-2 were sensitive to enzymatic cleavage by proteases in mouse serum, where the IL-2 component was cleaved from the mAb, leaving hu14.18 and IL-2 as separate moieties. When intact IC is injected IV into mice, the half-life is about 4 hours as is the half-life of IL-2 bound to IC, whereas the half-life of the hu14.18 component of the IC is 27 hours. This all suggests that the IC circulates intact for approximately 4 hours and then is cleaved. After the IL-2 is cleaved and cleared after 4 hours, the hu14.18 IgG circulates for a longer half-life. These data led to hu14.18-IL2 IC being given frequently (daily) to maintain both IL-2 and hu14.18 in vivo activity[4].
3.2 Phase I Testing in MEL
3.2.1 Dosage
In this phase I study, 33 adults with refractory, metastatic MEL were treated with hu14.18-IL2 with the purpose of evaluating this IC for safety, toxicity, maximal tolerated dose(MTD), and immune activation after treatment[62]. The IC was administered for 3 days as 4-hour IV infusions during week 1, because slower infusions have demonstrated less neuropathic pain than faster infusions of anti-GD2 mAb[63]. If patients demonstrated stable or regressed disease, they could receive a second course at week 5. In nonhuman primate studies[4] an MTD of 16 mg/m2/d demonstrated toxicity primarily due to IL-2 (hypotension and capillary leak). To minimize unexpected toxicity in human studies, the starting dose in the phase I trial was 1/20 of the daily MTD in primates (0.8 mg/m2/d) [4;46;62]. Therefore, dose levels evaluated included 0.8, 1.6.3.2, 4.8, 6.0, and 7.5 mg/m2/day.
3.2.2 Toxicity/Safety
In 33 patients, 19 patients completed their first course with stable disease and received a second course of IC, and of these 8 patients had stable disease after completion of course 2. All patients had grade 2 fever. Most patients in the three highest dose levels had pain (pelvic, abdominal, chest, or extremity) that resolved with IV opioids. Grade 3 adverse events included asymptomatic hypophosphatemia (11 patients), hyperglycemia (three patients), hypotension (two patients), thrombocytopenia (one patient), hypoxia (three patients), elevated hepatic transaminases (two patients), and hyperbilirubinemia (one patient). There were no grade 4 adverse events. Dose-limiting toxicities at the MTD included reversible hypoxia, transient hypotension, and transiently elevated ALT/AST. Two of six patients developing DLT at 7.5 mg/m2, this dose level met the study criteria for MTD[4]. The grade 3 toxicities were similar to what has been previously reported for IL-2 and anti-GD2 mAb treatments[30;45;46;58;64]. Dose limiting toxicities (DLT) caused by anti-GD2 mAbs include fever, chills, nausea, and anaphylactoid reactions due to the cytokine activation. The most characteristic DLT for anti-GD2 mAbs is transient neuropathic pain[4;65;66].
3.2.3 Tumor Response
In terms of tumor response, no patient demonstrated either a complete or partial response(CR or PR). Eight patients had stable disease following two courses of treatment. One subject had an objective decrease in a lung nodule following two courses of therapy, but the overall disease response was scored as disease progression due to growth in a distant node. The node was resected following hu14.18-IL2 therapy, and the subject remained free from disease progression for 3 years, after which disease progression was noted and alternate therapy begun.
Five of the 33 subjects entered the study with no measurable disease following surgical resection of recurrences or metastases. Two of these 5 subjects (treated at 0.8 and 6.0 mg/m2/day) continue with no evidence of disease (69–102 months). The other 3 showed recurrence at 2, 6 and 92 months after starting hu14.18-IL2 treatment. One additional subject entered the study following resection of proven pulmonary and nodal metastases, with residual small lung abnormalities that were potentially residual melanoma. This subject had no evidence of progressive disease for 59 months after treatment (4.8 mg/m2/day), when recurrent disease was noted. The findings are consistent with the hypothesis that clinical benefit from an immunotherapeutic intervention is more likely in subjects with a low tumor burden, as has been shown for tumor bearing mice treated with this agent[67].
3.2.4 Pharmacokinetics and immune activation
Serum analyses for all 33 patients was performed in order to evaluate pharmacokinetics and immune activation as measured by lymphocytosis, peripheral-blood NK activity, and serum levels of the soluble alpha chain of the IL-2R complex.
In order to evaluate pharmacokinetics, serum samples were obtained from all patients on days 1 and 3 at each of the following times: before the 4-hr infusion, 0.5 h into and at the completion of the 4h infusion, and then 0.5h, 1h, 2h, 4h, 12h, and 20h after completion of the 4hr infusion. All specimens were tested in an ELISA assay using an anti-id mAb(1A7) to capture and an anti-IL2 mAb to measure intact hu14.18-IL-2 IC[44;68;69]. The peak serum levels of IC show a progressive, nearly linear, increase with the first dose of IC. After Course-1,day-1 (C1D1), the half-life of IC was 3.7±0.9hrs, which was between the half-life of 45 minutes for IL-2 and 2–3 days for ch14.18mAb, and comparable to the half-life of ch14.18-IL-2 in mice[61]. ELISAs were performed for hu14.18mAb and had similar PK curves as for the intact IC, differing from mice data. In mice, once cleaved from intact IC the residual ch14.18mAb circulates with a half-life of 27 hours[61]. These data confirmed that the hu14.18-IL2 molecule did not undergo cleavage of its IL2 component, either in vivo when administered IV to patients or in human serum.
For both courses, there was an initial peripheral blood lymphopenia on days 2–4 that was followed by a rebound lymphocytosis on days 5–22 in course 1. Both changes were dose-dependent, and similarly occurred during course 2. Soluble IL-2Rα (sIL-2Rα) levels[70;71] were obtained and were significantly increased over baseline at each time point from days 2–8 in course 1 and 2. SIL-2Rα levels peaked at day 4–5 then declined, and the increase in levels was dose-dependent(p<0.0014).
3.3 Phase I Testing in NBL
The Children’s Oncology Group has completed a trial using hu14.18-IL-2 in 27 pediatric patients with recurrent NBL and MEL, using the same administration schedule of IC as the previous adult MEL study except that 4 courses could be administered for those with stable disease[60]. The clinical toxicities were similar to those in the adult MEL trial. The MTD was 12 mg/m2/d, and DLTs of hypotension(one patient requiring dopamine), allergic reaction, blurred vision, neutropenia, thrombocytopenia, and leucopenia. All toxicities were reversible, and there were no treatment-related deaths. Immune activation was noted with elevated sIL2Rα and lymphocytosis. Again, no CR or PR was noted, however, three patients had clinical changes suggestive of antitumor activity with radiographic and bone marrow response.
3.4 Phase II Testing in MEL and NBL
Based on the results from the phase I trials in MEL and NBL, phase II trials have recently been completed in these settings as well. Both studies have been reported in abstract form[72;73]. The MEL trial treated 14 patients at 6 mg/m2/d for three days, repeated every 28 days and the Children’s Oncology Group administered hu14.18-IL2 at 12 mg/m2/d for three days, repeated every 28 days in 37 pediatric patients with recurrent or refractory stage 4 NBL. The toxicities seen were similar to that seen in the Phase I study, there was 1 PR in the MEL study, and 5 CRs in the NBL study, particularly in children without bulky disease. Immunological monitoring in the phase II MEL study had similar findings as reported in the phase I study including a peripheral blood lymphocytosis on day 8 and increased C-reactive protein. Since greater efficacy was seen in the MRD setting in mice and in patients with NBL, subsequent testing in MEL is planned to evaluate patients in MRD. A recently initiated pilot MEL study will determine the histological response to hu14.18-IL2 administered IV to patients with resectable metastatic MEL. We will collect pilot data on the time to recurrence and survival for patients with MEL in complete remission following surgery and treatment with hu14.18-IL2. Subsequent studies to further explore IV administration of hu14.18-IL2 for patients with non-bulky, MRD NBL or MEL are also being planned.
4. Future Direction
Intratumoral hu14.18-IL-2 IC
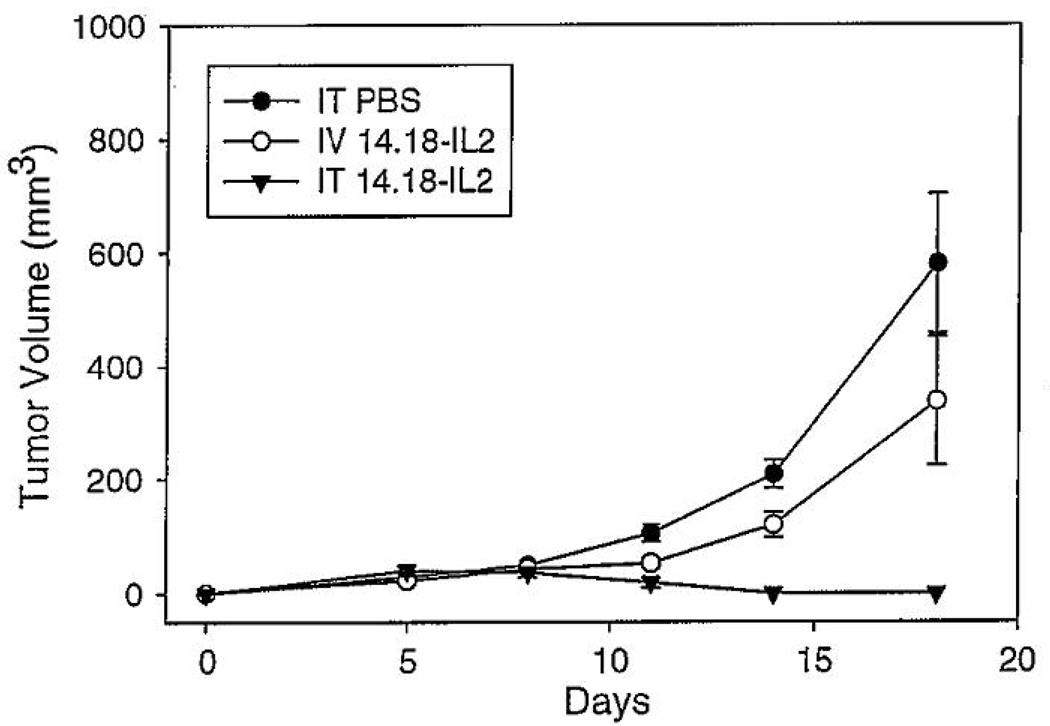
Recent preclinical work demonstrated that intratumorally (IT) administered hu14.18-IL-2 IC results in enhanced antitumor effects against murine NXS2 NBL and human M21 MEL xenografts compared to intravenous (IV) IC[74]. Mice were injected subcutaneously with NXS2 NBL cells, and treated with either IT or IV IC for five days, for a total dose of 75ug of IC. Mice receiving IT IC demonstrated a significantly decreased tumor volume compared to mice receiving comparable IV doses (p<0.02, days 11–18). Tumor regressions were seen in a majority of mice (12 of 17) receiving IT IC(Fig 2)[74]. Rechallenges with NXS2 and YAC-1 were performed in tumor-free mice to test for specific anti-NXS2 memory development. Two of the three re-challenged mice remained NXS2 tumor-free for over 30 days while the YAC-1 tumor challenges were not rejected.
Fig. 2. Enhanced antitumor effect of IT ICs compared to IV IC.
A/J mice (five per group) were implanted with 2 × 10^6 NXS2 cells on day 0, and then treated with either intratumoral (IT) PBS, IT immunocytokine (IC) at 15 ug/50 uL, or intravenous (IV) IC (15 ug/200uL) on days 7–11 after tumor implantation. (p<0.0015 for IT hu14.18-IL2 vs IT PBS, days 11–18; p<0.02 for IT IC vs IV IC on days 11–18.) Adapted from Ref. 73 (With Kind permission of Springer Science+Business Media, ©2007).
To test for which cells are involved in the antitumor repsonse, GD2+ human M21 melanoma tumors were xenografted into nude mice and treated with IT IC. Even in the absence of mature T cells, IT IC resulted in significant antitumor effects, mimicking what has been demonstrated in previous studies[40;74;75]. Clinical application of this concept is now being planned.
5. Mechanisms of Antitumor Efficacy and Tumor Escape in Tumor-Bearing Mice
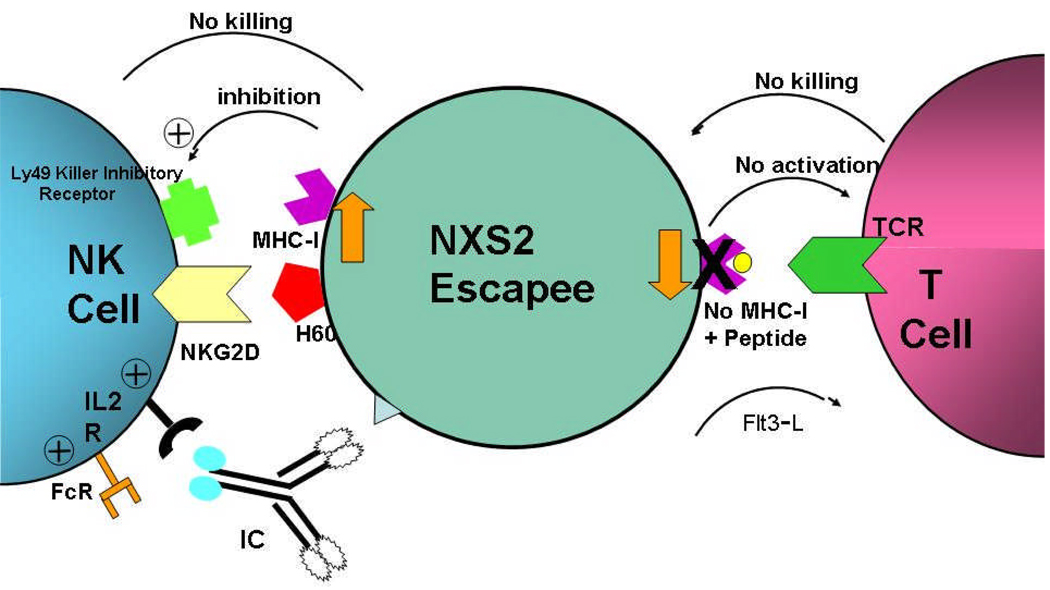
Mechanisms involved with tumor recurrence have been studied[76;77]. Administration of a suboptimal IC dose of immunotherapy results in either NK- based antitumor responses in mice where the NXS2 NBL tumors transiently regress and subsequently recurs. This model was used to evaluate recurrent NXS2 tumors. In mice with recurrence after suboptimal hu14.18-IL-2 therapy, tumors were noted to have enhanced MHC class I antigen expression compared with tumors in PBS-treated mice. This contrasts with mice treated with Flt3-ligand (produces T-cell-dependent antitumor memory), demonstrating recurrent tumor with decreased MHC class I antigen expression. This suggests that tumors may have a selective advantage in resisting the antitumor activity of therapy depending on in vivo immunoediting; NXS2 tumors appear to modulate their phenotype to express higher or lower levels of MHC class I antigen in order to resist either NK- or T-cell-mediated antitumor responses, respectively(Fig 3)[76]. When a combined regimen was administered to recurrent NXS2 tumor-bearing mice, IC plus Flt3-L therapy provided greater antitumor benefit and demonstrated protection from NXS2 rechallenge[77].
Fig 3. NXS2 Tumors modulate class I, up or down, to escape T or NK mediated immunotherapy.
In mice with recurrence after suboptimal hu14.18-IL-2 therapy(response is mediated by NK cells), tumors had enhanced MHC class I antigen expression compared with PBS-treated tumors. In contrast mice treated with Flt3-ligand (produces T-cell-dependent antitumor memory), demonstrates recurrent tumor with decreased MHC class I expression. Therefore tumors may have a selective advantage in resisting the antitumor activity of therapy depending on in vivo immunoediting; NXS2 tumors appear to express higher or lower levels of MHC class I in order to resist either NK- or T-cell-mediated antitumor responses, respectively. Adapted from data in Refs 74 and 76.
These preclinical studies have further demonstrated the mechanism of the antitumor effects of this IC. Further studies will need to address how intratumoral therapy and T-cell-mediated therapies can be incorporated into clinical trials for MEL and NBL.
6. Conclusion
Hu14.18-IL-2 is an immunocytokine linking IL-2 to the anti-GD2 mAb, 14.18, targeting GD2+ tumors including melanoma and neuroblastoma. Preclinical data demonstrate hu14.18-IL2’s ability to induce potent antitumor activity via NK-mediated ADCC and T-cell mediated cytotoxicity. Phase I and II clinical trials in both adult MEL and pediatric NBL patients have been performed demonstrating its safety in patients in doses that are able to induce immune activation. The Phase II studies in advanced melanoma and recurrent/refractory neuroblastoma are demonstrating some clinical antitumor effects of hu14.18-IL-2. Preclinical data in MEL, and initial clinical data in neuroblastoma, suggest greater efficacy in the setting of MRD. Current clinical testing is planned to test the clinical benefit of hu14.18-IL2 for patients with MEL and neuroblastoma in MRD.
7. Expert Opinion
Distinct immune therapies for high-risk NBL and MEL are in clinical testing. Some are technologically complex and difficult to deliver to the many patients potentially needing them each year, while others have limited applicability. Large phase III trials are needed to determine which can improve disease free and overall survival in MEL and NBL patients, particularly those put into remission with standard therapy, but with a high likelihood of relapse. Hu14.18-IL2 offers the advantages of long-term storage, ease of ordering and administration, broad patient applicability (without limitations due to HLA-phenotype of patient, as for certain vaccines), and the ability to induce both NK and T-cell mediated antitumor effects. With toxicity studies completed demonstrating acceptable profiles, and phase II studies showing some antitumor activity, subsequent studies are being planned to evaluate anti-tumor activity in patients with minimal disease, the setting where IC has been noted in preclinical animal studies to be the most useful. Also, preclinical studies are investigating mechanisms of IC in terms of antitumor effect where intratumoral administration of IC has better therapeutic effect on both primary and distant tumors. Further studies will need to address optimal administration, given the difficulty with multiple intratumoral injections if translated to the clinical setting for patients with tumors that are not readily accessable for injection. Also, regimens adding IC therapy to various chemotherapeutics or other immune therapies are being explored in preclinical settings. Given the dramatic antitumor effects seen in preclinical studies and the initial results seen in phase I and II clinical trials, continued clinical testing and development of hu14.18-IL2 should continue.
Acknowledgements
Research support is received from NIH Grants CA032685, CA87025, CA14520, GM067386, T32-CA090217, The Midwest Athletes for Childhood Cancer Fund, The Crawdaddy Foundation, The UW- Cure Kids Cancer Coalition, The Evan Dunbar Foundation, CureSearch and The Super Jake Foundation. In addition, a recent clinical trial, not mentioned in this review, receives per patient support from Merck Serono as well as research support from the NCI.
Abbreviations
- IC
immunocytokine
- mAb
monoclonal antibody
- MEL
melanoma
- NBL
neuroblastoma
- IL2R
IL2 receptors
- FcR
Fc receptor
Reference List
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Kirkwood JM, Ibrahim JG, Sosman JA, Sondak VK, Agarwala SS, Ernstoff MS, Rao U. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 4.Sondel PMHJAMGS. Novel Strategies for cytokine administration via targetting. In: Caligiuri MA, Lotze MT, editors. Cancer Drug Discovery and Development, Cytokines in the Genesis and Treatment of Cancer. Totowa, NJ: Humana Press Inc; 2008. [Google Scholar]
- 5.Brodeur GM. Significance of intratumoral genetic heterogeneity in neuroblastomas. Med.Pediatr.Oncol. 2002;38:112–113. doi: 10.1002/mpo.1282. [DOI] [PubMed] [Google Scholar]
- 6.Ries LAG MDKMeale. SEER Cancer Statistics Review, 1975–2004. Bethesda, MD: National Cancer Institute; 2007 http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER Web site, 2007. Ref Type: Internet Communication.
- 7.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N.Engl.J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 8.American Cancer Society. Atlanta, GA: ACS publications; Cancer Facts and Figures. 2004. 2004 Ref Type: Pamphlet.
- 9.Mujoo K, Cheresh DA, Yang HM, Reisfeld RA. Disialoganglioside GD2 on human neuroblastoma cells: target antigen for monoclonal antibody-mediated cytolysis and suppression of tumor growth. Cancer Res. 1987;47:1098–1104. [PubMed] [Google Scholar]
- 10.Cheung NK, Saarinen UM, Neely JE, Landmeier B, Donovan D, Coccia PF. Monoclonal antibodies to a glycolipid antigen on human neuroblastoma cells. Cancer Res. 1985;45:2642–2649. [PubMed] [Google Scholar]
- 11.Chang HR, Cordon-Cardo C, Houghton AN, Cheung NK, Brennan MF. Expression of disialogangliosides GD2 and GD3 on human soft tissue sarcomas. Cancer. 1992;70:633–638. doi: 10.1002/1097-0142(19920801)70:3<633::aid-cncr2820700315>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Sondel PM, Hank JA. Antibody-directed, effector cell-mediated tumor destruction. Hematol.Oncol Clin North Am. 2001;15:703–721. doi: 10.1016/s0889-8588(05)70243-4. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida S, Kawaguchi H, Sato S, Ueda R, Furukawa K. An anti-GD2 monoclonal antibody enhances apoptotic effects of anti-cancer drugs against small cell lung cancer cells via JNK (c-Jun terminal kinase) activation. Jpn.J Cancer Res. 2002;93:816–824. doi: 10.1111/j.1349-7006.2002.tb01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson E, Dean SM, Sondel PM. Antibody-based immunotherapy in high-risk neuroblastoma. Expert.Rev.Mol.Med. 2007;9:1–21. doi: 10.1017/S1462399407000518. [DOI] [PubMed] [Google Scholar]
- 15.Svennerholm LBKFPJBLAMJRB. Gangliosides and allied glycosphingolipids in human peripheral nerve and spinal cord. Biochim Biophys Acta. 1994;1214:115–123. doi: 10.1016/0005-2760(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 16.Cheung NK, Lazarus H, Miraldi FD, Abramowsky CR, Kallick S, Saarinen UM, Spitzer T, Strandjord SE, Coccia PF, Berger NA. Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. J Clin Oncol. 1987;5:1430–1440. doi: 10.1200/JCO.1987.5.9.1430. [DOI] [PubMed] [Google Scholar]
- 17.Cheung NK, Kushner BH, Cheung IY, Kramer K, Canete A, Gerald W, Bonilla MA, Finn R, Yeh SJ, Larson SM. Anti-G(D2) antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. J Clin Oncol. 1998;16:3053–3060. doi: 10.1200/JCO.1998.16.9.3053. [DOI] [PubMed] [Google Scholar]
- 18.Handgretinger R, Baader P, Dopfer R, Klingebiel T, Reuland P, Treuner J, Reisfeld RA, Niethammer D. A phase I study of neuroblastoma with the anti-ganglioside GD2 antibody 14.G2a. Cancer Immunol Immunother. 1992;35:199–204. doi: 10.1007/BF01756188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu AL, Uttenreuther-Fischer MM, Huang CS, Tsui CC, Gillies SD, Reisfeld RA, Kung FH. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol. 1998;16:2169–2180. doi: 10.1200/JCO.1998.16.6.2169. [DOI] [PubMed] [Google Scholar]
- 20.Handgretinger R, Anderson K, Lang P, Dopfer R, Klingebiel T, Schrappe M, Reuland P, Gillies SD, Reisfeld RA, Neithammer D. A phase I study of human/mouse chimeric antiganglioside GD2 antibody ch14.18 in patients with neuroblastoma. Eur.J Cancer. 1995;31A:261–267. doi: 10.1016/0959-8049(94)00413-y. [DOI] [PubMed] [Google Scholar]
- 21.Murray JL, Cunningham JE, Brewer H, Mujoo K, Zukiwski AA, Podoloff DA, Kasi LP, Bhadkamkar V, Fritsche HA, Benjamin RS. Phase I trial of murine monoclonal antibody 14G2a administered by prolonged intravenous infusion in patients with neuroectodermal tumors. J Clin Oncol. 1994;12:184–193. doi: 10.1200/JCO.1994.12.1.184. [DOI] [PubMed] [Google Scholar]
- 22.Saleh MN, Khazaeli MB, Wheeler RH, Allen L, Tilden AB, Grizzle W, Reisfeld RA, Yu AL, Gillies SD, LoBuglio AF. Phase I trial of the chimeric anti-GD2 monoclonal antibody ch14.18 in patients with malignant melanoma. Hum.Antibodies Hybridomas. 1992;3:19–24. [PubMed] [Google Scholar]
- 23.Saleh MN, Khazaeli MB, Wheeler RH, Dropcho E, Liu T, Urist M, Miller DM, Lawson S, Dixon P, Russell CH. Phase I trial of the murine monoclonal anti-GD2 antibody 14G2a in metastatic melanoma. Cancer Res. 1992;52:4342–4347. [PubMed] [Google Scholar]
- 24.Barker E, Mueller BM, Handgretinger R, Herter M, Yu AL, Reisfeld RA. Effect of a chimeric anti-ganglioside GD2 antibody on cell-mediated lysis of human neuroblastoma cells. Cancer Res. 1991;51:144–149. [PubMed] [Google Scholar]
- 25.Uttenreuther-Fischer MM, Huang CS, Yu AL. Pharmacokinetics of human-mouse chimeric anti-GD2 mAb ch14.18 in a phase I trial in neuroblastoma patients. Cancer Immunol Immunother. 1995;41:331–338. doi: 10.1007/BF01526552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung NK, Lazarus H, Miraldi FD, Abramowsky CR, Kallick S, Saarinen UM, Spitzer T, Strandjord SE, Coccia PF, Berger NA. Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. J Clin Oncol. 1987;5:1430–1440. doi: 10.1200/JCO.1987.5.9.1430. [DOI] [PubMed] [Google Scholar]
- 27.Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Niethammer D, Berthold F. Consolidation treatment with chimeric anti-GD2-antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. J Clin Oncol. 2004;22:3549–3557. doi: 10.1200/JCO.2004.08.143. [DOI] [PubMed] [Google Scholar]
- 28.Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton KE, White DE, Liewehr DJ, Merino MJ, Rosenberg SA. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinreich DM, Rosenberg SA. Response rates of patients with metastatic melanoma to high-dose intravenous interleukin-2 after prior exposure to alpha-interferon or low-dose interleukin-2. J Immunother. 2002;25:185–187. doi: 10.1097/00002371-200203000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark JI, Atkins MB, Urba WJ, Creech S, Figlin RA, Dutcher JP, Flaherty L, Sosman JA, Logan TF, White R, Weiss GR, Redman BG, Tretter CP, McDermott D, Smith JW, Gordon MS, Margolin KA. Adjuvant high-dose bolus interleukin-2 for patients with high-risk renal cell carcinoma: a cytokine working group randomized trial. J Clin Oncol. 2003;21:3133–3140. doi: 10.1200/JCO.2003.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Weil-Hillman G, Voss SD, Fisch P, Schell K, Hank JA, Sosman JA, Sugamura K, Sondel PM. Natural killer cells activated by interleukin 2 treatment in vivo respond to interleukin 2 primarily through the p75 receptor and maintain the p55 (TAC) negative phenotype. Cancer Res. 1990;50:2683–2691. [PubMed] [Google Scholar]
- 32.Shiloni E, Eisenthal A, Sachs D, Rosenberg SA. Antibody-dependent cellular cytotoxicity mediated by murine lymphocytes activated in recombinant interleukin 2. J Immunol. 1987;138:1992–1998. [PubMed] [Google Scholar]
- 33.Bernstein NSCLR. Specific enhancement of the therapeutic effect of anti-idiotype antibodies on a murine B cell lymphoma by IL-2. J Immunol. 1998;140:2839–2845. [PubMed] [Google Scholar]
- 34.Sondel PM, Hank JA, Kohler PC, Chen BP, Minkoff DZ, Molenda JA. Destruction of autologous human lymphocytes by interleukin 2-activated cytotoxic cells. J Immunol. 1986;137:502–511. [PubMed] [Google Scholar]
- 35.Mule JJ, Yang JC, Afreniere RL, Shu SY, Rosenberg SA. Identification of cellular mechanisms operational in vivo during the regression of established pulmonary metastases by the systemic administration of high-dose recombinant interleukin 2. J Immunol. 1987;139:285–294. [PubMed] [Google Scholar]
- 36.Hank JA, Robinson RR, Surfus J, Mueller BM, Reisfeld RA, Cheung NK, Sondel PM. Augmentation of antibody dependent cell mediated cytotoxicity following in vivo therapy with recombinant interleukin 2. Cancer Res. 1990;50:5234–5239. [PubMed] [Google Scholar]
- 37.Schultz KR, Klarnet JP, Peace DJ, Cheever MA, Badger CC, Bernstein ID, Greenberg PD. Monoclonal antibody therapy of murine lymphoma: enhanced efficacy by concurrent administration of interleukin 2 or lymphokine-activated killer cells. Cancer Res. 1990;50:5421–5425. [PubMed] [Google Scholar]
- 38.Sondel PM, Hank JA, Gan J, Neal Z, Albertini MR. Preclinical and clinical development of immunocytokines. Curr.Opin.Investig.Drugs. 2003;4:696–700. [PubMed] [Google Scholar]
- 39.Reisfeld RA, Gillies SD. Recombinant antibody fusion proteins for cancer immunotherapy. Curr.Top.Microbiol.Immunol. 1996;213(Pt 3):27–53. doi: 10.1007/978-3-642-80071-9_3. [DOI] [PubMed] [Google Scholar]
- 40.Lode HN, Xiang R, Varki NM, Dolman CS, Gillies SD, Reisfeld RA. Targeted interleukin-2 therapy for spontaneous neuroblastoma metastases to bone marrow. J Natl Cancer Inst. 1997;89:1586–1594. doi: 10.1093/jnci/89.21.1586. [DOI] [PubMed] [Google Scholar]
- 41.Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;91:1706–1715. [PubMed] [Google Scholar]
- 42.Sondel PM, Hank JA. Combination therapy with interleukin-2 and antitumor monoclonal antibodies. Cancer J Sci Am. 1997;3 Suppl 1:S121–S127. [PubMed] [Google Scholar]
- 43.Gillies SD, Young D, Lo KM, Roberts S. Biological activity and in vivo clearance of antitumor antibody/cytokine fusion proteins. Bioconjug.Chem. 1993;4:230–235. doi: 10.1021/bc00021a008. [DOI] [PubMed] [Google Scholar]
- 44.Gan J, Kendra K, Ricci M, Hank JA, Gillies SD, Sondel PM. Specific enzyme-linked immunosorbent assays for quantitation of antibody-cytokine fusion proteins. Clin Diagn.Lab Immunol. 1999;6:236–242. doi: 10.1128/cdli.6.2.236-242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frost JD, Hank JA, Reaman GH, Frierdich S, Seeger RC, Gan J, Anderson PM, Ettinger LJ, Cairo MS, Blazar BR, Matthay KK, Reisfeld RA, Sondel PM. A phase I/IB trial of murine monoclonal anti-GD2 antibody 14.G2a plus interleukin-2 in children with refractory neuroblastoma: a report of the Children's Cancer Group. Cancer. 1997;80:317–333. doi: 10.1002/(sici)1097-0142(19970715)80:2<317::aid-cncr21>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 46.Albertini MR, Hank JA, Schiller JH, Khorsand M, Borchert AA, Gan J, Bechhofer R, Storer B, Reisfeld RA, Sondel PM. Phase IB trial of chimeric antidisialoganglioside antibody plus interleukin 2 for melanoma patients. Clin Cancer Res. 1997;3:1277–1288. [PubMed] [Google Scholar]
- 47.Hank JA, Surfus J, Gan J, Chew TL, Hong R, Tans K, Reisfeld R, Seeger RC, Reynolds CP, Bauer M. Treatment of neuroblastoma patients with antiganglioside GD2 antibody plus interleukin-2 induces antibody-dependent cellular cytotoxicity against neuroblastoma detected in vitro. J Immunother.Emphasis.Tumor Immunol. 1994;15:29–37. doi: 10.1097/00002371-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Gillies SD, Reilly EB, Lo KM, Reisfeld RA. Antibody-targeted interleukin 2 stimulates T-cell killing of autologous tumor cells. Proc Natl Acad Sci U S A. 1992;89:1428–1432. doi: 10.1073/pnas.89.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 50.Voss SD, Robb RJ, Weil-Hillman G, Hank JA, Sugamura K, Tsudo M, Sondel PM. Increased expression of the interleukin 2 (IL-2) receptor beta chain (p70) on CD56+ natural killer cells after in vivo IL-2 therapy: p70 expression does not alone predict the level of intermediate affinity IL-2 binding. J Exp.Med. 1990;172:1101–1114. doi: 10.1084/jem.172.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sondel PM, Kohler PC, Hank JA, Moore KH, Rosenthal NS, Rosenthal NS, Sosman JA, Bechhofer R, Storer B. Clinical and immunological effects of recombinant interleukin 2 given by repetitive weekly cycles to patients with cancer. Cancer Res. 1988;48:2561–2567. [PubMed] [Google Scholar]
- 52.Hank JA, Surfus JE, Gan J, Jaeger P, Gillies SD, Reisfeld RA, Sondel PM. Activation of human effector cells by a tumor reactive recombinant anti-ganglioside GD2 interleukin-2 fusion protein (ch14.18-IL2) Clin Cancer Res. 1996;2:1951–1959. [PubMed] [Google Scholar]
- 53.Sabzevari H, Gillies SD, Mueller BM, Pancook JD, Reisfeld RA. A recombinant antibody-interleukin 2 fusion protein suppresses growth of hepatic human neuroblastoma metastases in severe combined immunodeficiency mice. Proc Natl Acad Sci U S A. 1994;91:9626–9630. doi: 10.1073/pnas.91.20.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Becker JC, Pancook JD, Gillies SD, Furukawa K, Reisfeld RA. T cell-mediated eradication of murine metastatic melanoma induced by targeted interleukin 2 therapy. J Exp.Med. 1996;183:2361–2366. doi: 10.1084/jem.183.5.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Becker JC, Varki N, Gillies SD, Furukawa K, Reisfeld RA. Long-lived and transferable tumor immunity in mice after targeted interleukin-2 therapy. J Clin Invest. 1996;98:2801–2804. doi: 10.1172/JCI119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weil-Hillman G, Fisch P, Prieve AF, Sosman JA, Hank JA, Sondel PM. Lymphokine-activated killer activity induced by in vivo interleukin 2 therapy: predominant role for lymphocytes with increased expression of CD2 and leu19 antigens but negative expression of CD16 antigens. Cancer Res. 1989;49:3680–3688. [PubMed] [Google Scholar]
- 57.Becker JC, Varki N, Gillies SD, Furukawa K, Reisfeld RA. An antibody-interleukin 2 fusion protein overcomes tumor heterogeneity by induction of a cellular immune response. Proc Natl Acad Sci U S A. 1996;93:7826–7831. doi: 10.1073/pnas.93.15.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ozkaynak MF, Sondel PM, Krailo MD, Gan J, Javorsky B, Reisfeld RA, Matthay KK, Reaman GH, Seeger RC. Phase I study of chimeric human/murine anti-ganglioside G(D2) monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: a Children's Cancer Group Study. J Clin Oncol. 2000;18:4077–4085. doi: 10.1200/JCO.2000.18.24.4077. [DOI] [PubMed] [Google Scholar]
- 59.Hank JAMaSP. Monoclonal antibodies, cytokines and fusion proteins in the treatment of malignant disease. Cancer Chemother Biol Response Modif. 1999;18:210–222. [PubMed] [Google Scholar]
- 60.Osenga KL, Hank JA, Albertini MR, Gan J, Sternberg AG, Eickhoff J, Seeger RC, Matthay KK, Reynolds CP, Twist C, Krailo M, Adamson PC, Reisfeld RA, Gillies SD, Sondel PM. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children's Oncology Group. Clin Cancer Res. 2006;12:1750–1759. doi: 10.1158/1078-0432.CCR-05-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kendra K, Gan J, Ricci M, Surfus J, Shaker A, Super M, Frost JD, Rakhmilevich A, Hank JA, Gillies SD, Sondel PM. Pharmacokinetics and stability of the ch14.18-interleukin-2 fusion protein in mice. Cancer Immunol Immunother. 1999;48:219–229. doi: 10.1007/s002620050569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.King DM, Albertini MR, Schalch H, Hank JA, Gan J, Surfus J, Mahvi D, Schiller JH, Warner T, Kim K, Eickhoff J, Kendra K, Reisfeld R, Gillies SD, Sondel P. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J Clin Oncol. 2004;22:4463–4473. doi: 10.1200/JCO.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Albertini MR KKKDea. Phase I/Ib trial of the hu14.18-IL-2 fusion protein in patients with GD2+ tumors. CTEP-UWCCC protocol #CO98901. Amendment 7, approved 1/17/02. 2002 Available on NCI-PDQ website. Ref Type: Electronic Citation.
- 64.Gilman AL, Ozkaynak MF, Matthay KK, Krailo M, Yu AL, Gan J, Sternberg A, Hank JA, Seeger R, Reaman GH, Sondel PM. Phase I Study of ch14.18 With Granulocyte-Macrophage Colony-Stimulating Factor and Interleukin-2 in Children With Neuroblastoma After Autologous Bone Marrow Transplantation or Stem-Cell Rescue: A Report From the Children's Oncology Group. J Clin Oncol. 2008 doi: 10.1200/JCO.2006.10.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Electrophysiological characteristics of primary afferent fibers after systemic administration of anti-GD2 ganglioside antibody. Pain. 1997;69:145–151. doi: 10.1016/s0304-3959(96)03280-0. [DOI] [PubMed] [Google Scholar]
- 66.Ganglioside GD2 expression in the human nervous system and in neuroblastomas-an immunohistochemical study. Int.J Oncol. 1993;3:909–915. doi: 10.3892/ijo.3.5.909. [DOI] [PubMed] [Google Scholar]
- 67.Neal ZC, Yang JC, Rakhmilevich AL, Buhtoiarov IN, Lum HE, Imboden M, Hank JA, Lode HN, Reisfeld RA, Gillies SD, Sondel PM. Enhanced activity of hu14.18-IL2 immunocytokine against murine NXS2 neuroblastoma when combined with interleukin 2 therapy. Clin Cancer Res. 2004;10:4839–4847. doi: 10.1158/1078-0432.CCR-03-0799. [DOI] [PubMed] [Google Scholar]
- 68.Foon KA, Sen G, Hutchins L, Kashala OL, Baral R, Banerjee M, Chakraborty M, Garrison J, Reisfeld RA, Bhattacharya-Chatterjee M. Antibody responses in melanoma patients immunized with an anti-idiotype antibody mimicking disialoganglioside GD2. Clin Cancer Res. 1998;4:1117–1124. [PubMed] [Google Scholar]
- 69.Batova A, Kamps A, Gillies SD, Reisfeld RA, Yu AL. The Ch14.18-GM-CSF fusion protein is effective at mediating antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity in vitro. Clin Cancer Res. 1999;5:4259–4263. [PubMed] [Google Scholar]
- 70.Bogner MP, Voss SD, Bechhofer R, Hank JA, Roper M, Poplack D, Hammond D, Sondel PM. Serum CD25 levels during interleukin-2 therapy: dose dependence and correlations with clinical toxicity and lymphocyte surface sCD25 expression. J Immunother.(1991.) 1992;11:111–118. [PubMed] [Google Scholar]
- 71.Voss SD, Hank JA, Nobis CA, Fisch P, Sosman JA, Sondel PM. Serum levels of the low-affinity interleukin-2 receptor molecule (TAC) during IL-2 therapy reflect systemic lymphoid mass activation. Cancer Immunol Immunother. 1989;29:261–269. doi: 10.1007/BF00199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shusterman SLWGSHJVSSRHTRRMJSP. Anti-neuroblastoma activity of hu14.18-IL2 against minimal residual disease in a Children's Oncology Group (COG) phase II study. J Clin Oncol. 2008;26 doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Albertini MRHJSHKJCRGJKKCBGSSP. Phase II trial of hu14.18-IL2 (EMD 273063) for patients with metastatic melanoma. J Clin Oncol. 2008;26 [Google Scholar]
- 74.Johnson EE, Lum HD, Rakhmilevich AL, Schmidt BE, Furlong M, Buhtoiarov IN, Hank JA, Raubitschek A, Colcher D, Reisfeld RA, Gillies SD, Sondel PM. Intratumoral immunocytokine treatment results in enhanced antitumor effects. Cancer Immunol Immunother. 2008;57:1891–1902. doi: 10.1007/s00262-008-0519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Imboden M, Murphy KR, Rakhmilevich AL, Neal ZC, Xiang R, Reisfeld RA, Gillies SD, Sondel PM. The level of MHC class I expression on murine adenocarcinoma can change the antitumor effector mechanism of immunocytokine therapy. Cancer Res. 2001;61:1500–1507. [PubMed] [Google Scholar]
- 76.Neal ZC, Imboden M, Rakhmilevich AL, Kim KM, Hank JA, Surfus J, Dixon JR, Lode HN, Reisfeld RA, Gillies SD, Sondel PM. NXS2 murine neuroblastomas express increased levels of MHC class I antigens upon recurrence following NK-dependent immunotherapy. Cancer Immunol Immunother. 2004;53:41–52. doi: 10.1007/s00262-003-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Neal ZC, Sondel PM, Bates MK, Gillies SD, Herweijer H. Flt3-L gene therapy enhances immunocytokine-mediated antitumor effects and induces long-term memory. Cancer Immunol Immunother. 2007;56:1765–1774. doi: 10.1007/s00262-007-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]