Abstract
The mechanisms involved in mucosal immune control of cervical human papillomavirus (HPV) infection remain ill-defined. Because Toll-like receptors (TLRs) are key players in innate immune responses, we investigated the association between TLR expression and viral persistence or clearance in young women with incident infections with oncogenic HPV types 16 or 51. Messenger RNA expression of TLR1, TLR2, TLR3, TLR4, TLR6, TLR7, TLR8, and TLR9 was measured by quantitative reverse transcription-PCR using human endocervical specimens, collected before and following viral acquisition, in a cohort well characterized for HPV infections. Wilcoxon rank sum test was used to compare the change seen from pre-infection to incident infection between women who subsequently cleared infection with those who did not. HPV 16 infections that cleared were significantly (P < 0.05) associated with an increase in expression of the four viral nucleic acid-sensing TLRs (TLR3, TLR7, TLR8 and TLR9) as well as TLR2 upon viral acquisition. Similar associations were not observed for HPV 51. In women who subsequently cleared their HPV 16 infection, changes in TLR1, TLR3, TLR7, and TLR8 expression levels between pre-incident and incident visits were significantly correlated with parallel changes in the levels of interferon-α2, measured by immunoassay in cervical lavage specimens. This study suggests that dampened TLR expression in the cervical mucosa is a type-specific mechanism by which HPV 16 interferes with innate immune responses, contributing to viral persistence, and that TLR upregulation and resultant cytokine induction is important in subsequent viral clearance.
Keywords: toll-like receptors, human papillomavirus, persistence
Introduction
Human papillomaviruses (HPV) are ubiquitous viruses that infect skin and mucous membrane of humans. HPV types found predominantly in genital infections are classified into low-risk types that are mainly associated with benign genital warts and high-risk types that are associated with anogenital cancers. 1 Most HPV infections, whether low risk or high risk are transient in nature; a few women, however, are unable to clear the virus, resulting in long term persistence, the key factor in the development of cancers. 2-4 HPV 16 accounts for 50–60% of cervical cancers. 1, 5 Although HPV 16 is common in women without cancer, the proportion of HPV 16 in women with normal cytology is not near that of those with cancer. There is some evidence to suggest that HPV 16 is more likely to persist than other types, helping to explain its higher prevalence in cervical cancer cases. 5 The exact mechanisms involved in viral clearance are not well described; both innate and adaptive immune responses are likely to play critical roles. 6-9 Natural history studies show that most women clear infection within 24 months, with some showing clearance within weeks and others after 1–2 years. 3 Given that adaptive immune responses to HPV infections appear to develop slowly, 10, 11 early clearance, when it occurs, may involve innate immune responses. Several studies have documented cell-mediated immune responses in association with clearance after both long and short intervals of infections.12, 13 Innate immune responses have been more difficult to measure in the setting of HPV infection, however, in vitro studies suggest innate responses such as natural killer cells are often targets of immune evasion by HPV11, although these have been difficult to measure in the setting of HPV infection and are often targets of viral immune evasion. 11, 14
Efficient innate immune responses largely depend on the body's ability to detect an invading pathogen. Toll-like receptors (TLRs) are present on numerous cell types and have the ability to detect and bind distinct pathogen-associated ligands, thus signaling the presence of an invading microbe and consequently initiating and directing an immune response against it. 15 Ligation of TLRs leads to expression of a number of effector molecules, such as proinflammatory and immune-mediating cytokines. 15-18 Ten TLRs have been identified in man and several have been shown to be important for detection of viral pathogens. 19 TLR3, TLR7, TLR8, and TLR9 are localized intracellularly, where they are involved in viral nucleic acid sensing. Also, cell surface-expressed TLR2 (in heterodimer form with TLR1 or TLR6) and TLR4 may interact with viral proteins, although the implications for the host are less clear for these. 19
Although interactions between TLRs and HPV have been studied in vitro and in animal models 20, 21, no studies have addressed the role of TLRs in the in vivo clearance of HPV. It is likely that one or more of the viral-recognizing TLRs, acting as one of the immune system's earliest points of pathogen recognition, are important in shaping the host response to cervical HPV infection. We examined here, by quantitative reverse transcription-PCR, the association between changes in cervical mucosal TLR expression upon incident infection with oncogenic HPV types 16 or 51, and subsequent clearance or persistence of infection, in a cohort of women participating in a prospective study of the natural history of HPV infection. Because 8 TLRs have been demonstrated to be involved in viral recognition (TLR1, TLR2, TLR3, TLR4, TLR6, TLR7, TLR8, and TLR9), these were chosen for study. We also examined, in the same women, local levels of interferon [IFN]-α2 involved in mediating innate and adaptive immunity by a multiplex immunoassay, hypothesizing downstream pathway activation following engagement of TLRs.
Materials and Methods
Study subjects
Subjects were selected from an ongoing prospective study of the natural history of HPV in an adolescent/young woman cohort. The purpose of the parent study was to identify and study immune responses to incident HPV infections and thus enrolled young women aged 13–21 years with sexual experience of less than 5 years. Details of recruitment have been previously described. 22 Informed consent was obtained from all study participants according to the guidelines of the Committee for Human Research, at The University of California, San Francisco. Study visits, conducted at four month intervals, include face-to-face interviews and collection of samples for cytology and monitoring sexually transmitted infections, cervicovaginal lavage samples for HPV and cytokine testing and endocervical brush samples for mRNA. 22, 23 HPV detection and typing was performed as previously described 22, using PCR amplification with PGMY09/11 primers and a reverse line blot assay. We identified from the parent study women who had an incident infection with one of two high-risk HPV types (HPV 16 and HPV 51). HPV 16 was selected because of its high prevalence in cervical cancers and because HPV 16 is capable of downregulating TLR9 in vitro. We also examined samples from women with incident HPV 51 infection, which represents a high-risk HPV type of lower oncogenic potential than HPV 16 and is the second most common high-risk type represented in our cohort after HPV 16. For this substudy, incident HPV infection was defined as having at least two preceding visits in which HPV DNA was not detected (illustrated as − − +, where “+” indicates the incident infection and “−” indicates each visit where HPV was not detected). For classification of women by follow-up, HPV clearance was defined as having at least two consecutive follow-up visits with negative HPV tests immediately following the incident visit (− − + − −), whereas persistence was defined as having at least one immediate follow-up visit with a positive test for the same HPV type (− − + +). As no information is available regarding constitutive expression of TLR mRNA in the cervical mucosa, our approach was to allow each woman to serve as her own control by testing endocervical samples from the earlier of two consecutive HPV-negative visits preceding incidence ([−]− +) and from the incident-infection visit (− − [+]) and determining change in TLR expression between these two time points. The earlier of two pre-infection visits was used as the “baseline” or pre-incident visit to help guard against false negative HPV tests. Hypothesizing that change in TLR expression level in an individual woman from the baseline visit to incident infection would be important in predicting subsequent course of infection, we then compared change from the baseline visit to the HPV-incident visit between women who showed HPV type-specific persistence and those who showed HPV clearance. In particular, as HPV 16 has been previously noted to inhibit transcription of TLR920, we hypothesized that a dampening of expression of one or more viral-associated TLRs upon incident HPV 16 infection would be associated with persistence at follow-up. As detailed above, persistence was defined narrowly here to mean having at least one subsequent four-month visit with a positive HPV test for the same HPV type. Women were excluded if they had, at either the baseline or HPV incident visit, abnormal cytology, yeast, Chlamydia trachomatis, Neisseria gonorrhoeae, or Trichomonas vaginalis infection, or were pregnant.
TLR testing by quantitative reverse transcription-PCR
Specimen collection by cytology brush and RNA extraction using TRI Reagent (Molecular Research Center) have been previously described. 24 Multiple sets of primer sequences for the TLR genes were selected from online real-time PCR primer databases 25, 26 and also from the literature. 27 Selection criteria, validation steps, and optimization of primer concentrations have been described previously. 24, 28 Primer sequences are shown in Table I.
Table I. TLR and reference gene primer sequences.
| Symbol | Accession a | Gene name | Sequences (5′ - 3′) b | L c |
|---|---|---|---|---|
| GAPDH | NM_002046 | Glyceraldehyde-3-phosphate dehydrogenase | F: AAGGTCGGAGTCAACGGATTT R: ACCAGAGTTAAAAGCAGCCCTG |
66 |
| RPLP0 | NM_001002 | Large ribosomal protein P0 | F: CCTCATATCCGGGGGAATGTG R: GCAGCAGCTGGCACCTTATTG |
95 |
| TLR1 | NM_003263.3 | Human toll-like receptor 1 | F: AGGCCTTGTCTATACACCAAGTTGT R: CGTGTACCAGACACTGTGAAATTTT |
112 |
| TLR2 | NM_003264.3 | Human toll-like receptor 2 | F: AACCGGAGAGACTTTGCTCA R: CCACTGACAAGTTTCAGG CA |
91 |
| TLR3 | NM_003265.2 | Human toll-like receptor 3 | F: CCTGGTTTGTTAATTGGATTAACGA R: TGAGGTGGAGTGTTGCAAAGG |
82 |
| TLR4 | NM_138554.2 | Human toll-like receptor 4 | F: GGACTGGGTAAGGAATGAGCTAGTA R: CACACCGGGAATAAAGTCTCTGT |
94 |
| TLR6 | NM_006068.2 | Human toll-like receptor 6 | F: TGTACACCGTGTTTTCTGAGATGA R: GAAAACGTTCTGGGTAAAGTTCAAA |
125 |
| TLR7 | NM_016562.3 | Human toll-like receptor 7 | F: AAGCCCTTTCAGAAGTCCAAGTT R: GGTGAGCTTGCGGGTTTGT |
91 |
| TLR8 | NM_016610.2 | Human toll-like receptor 8 | F: GCTGCTGCA AGTTAC GGAAT R: CGC ATAACTCACAGGAACCA |
118 |
| TLR9 | NM_017442.2 | Human toll-like receptor 9 | F: TGAAGACTTCAGGCCCAACTG R: TGCACGGTCACCAGGTTGT |
75 |
GenBank accession number.
Sources for primer sequences were RTPrimerDB (http://medgen.ugent.be/rtprimerdb/, GAPDH and RPLP0) 21, qPrimerDepot (http://primerdepot.nci.nih.gov/, TLR2 and TLR8) 20, and Rebbapragada et al. (TLR1, TLR3, TLR4, TLR6, TLR7, and TLR9) 22.
Amplicon length (bp)
One μg total RNA was DNase-treated with TURBO DNA-free (Ambion), following the manufacturer's instructions. Reverse transcription was performed using 0.5 μg DNase-treated RNA in a 20-μl reaction volume with random hexamer (37 ng/μl final concentration, Promega) and random nonamer (25 μM, Gene Link) priming, RNasin® RNase inhibitor (10 U/reaction, Promega), and OmniScript RT (4 U/reaction, Qiagen) at 37°C for 60 min, following the manufacturer's instructions.
Quantitative PCR was performed in duplicate in a 384-well plate format on an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems) using Power SYBR Green Master Mix (Applied Biosystems), forward and reverse primers (Integrated DNA Technologies) at 150 nM final concentration, and either cervical unknown cDNA template (12.5 ng/reaction), standard, or water for the no-template controls. Standard curves for each plate were prepared from Stratagene QPCR Human Reference Total RNA (Stratagene), reverse transcribed identically to the cervical RNA samples, and diluted in 4-fold steps from 80 ng/reaction (final concentration) to 0.078 ng/reaction. The amplification program has been previously described. 24 Quantitative (threshold cycle) PCR values, were obtained during exponential amplification. Threshold cycle differences between duplicate reactions smaller than 0.5 cycles were considered acceptable. TLR expression, in relative units obtained by interpolation from the standard curve, was normalized to the geometric mean 29 of two reference genes previously validated for cervical samples in HPV studies, GAPDH and RPLP0. 24
Cytokine protein measurement in cervical lavage specimens
For measurement of IFN-α2, cervical lavage specimens were collected in 5 ml of normal saline and frozen at −80°C until testing. Specimens were thawed and briefly centrifuged to pellet cellular material and debris and cell-free supernatants were analyzed in duplicate on a Luminex 100 instrument using the Milliplex Human Cytokine Kit (Millipore) per the manufacturer's instructions. Data analysis was performed using MiraiBio MasterPlex QT software (Hitachi Software Engineering America), with five-parameter logistic curve fitting.
Data analysis
Normalized TLR expression data are presented in arbitrary units relative to the human reference standard. Non-parametric tests were used as the data were not normally distributed. Percent change from a baseline visit, which is HPV negative, to HPV-incident visit was compared between women who subsequently cleared infection and those with persistence on follow-up, by Wilcoxon rank sum test. The Hochberg Step-up Bonferroni method was used to adjust for multiple comparisons. 30 To examine change in TLR expression between baseline and incident visits, separately in women who subsequently cleared HPV 16 and those who did not, Wilcoxon signed rank test was used since the data were paired data with perturbed distribution. Inter-TLR correlations were assessed using Cohen's Kappa procedure. Pearson correlation was used to examine correlation between changes in IFN-α2 and changes in TLR expression. However, change was measured as the ratio of cytokine or TLR expression between visits rather than percent change since logarithmic transformation was used to stabilize variance.
Results
TLR change on HPV incidence as a predictor of HPV persistence
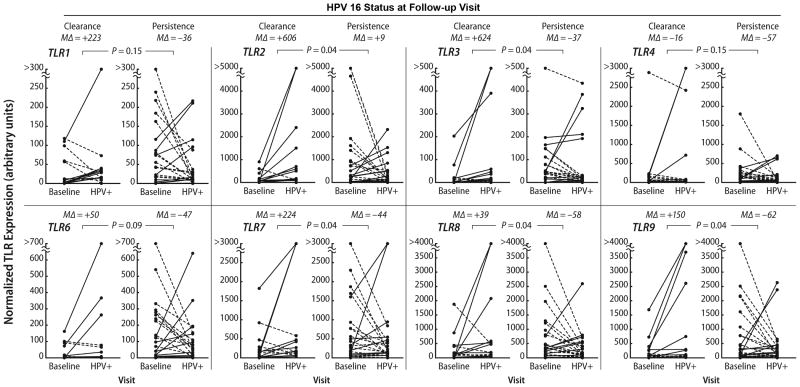
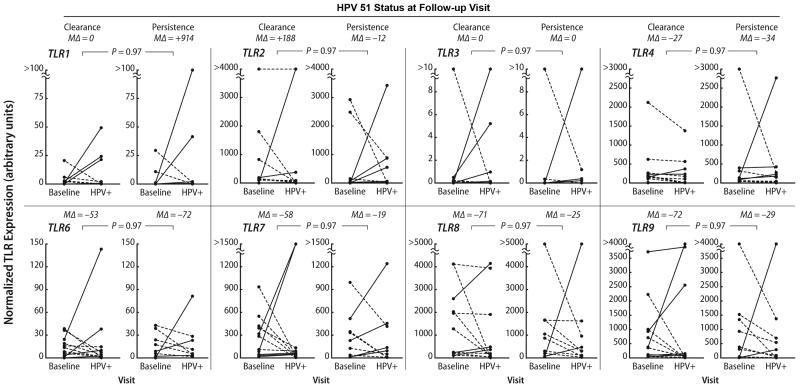
Endocervical samples were collected from 65 women who fulfilled the criteria for this study with a mean age of 20.6 (± 4.0, SD) years. Thirty-seven women had an incident HPV 16 infection and 20 had an incident HPV 51 infection. There were significant (P ≤ 0.05) differences in change in expression from baseline to the HPV incident visit for TLR2, TLR3, TLR7, TLR8, and TLR9, in women with subsequent persistence compared to those who cleared their HPV 16 infection on follow-up (Fig. 1). The median percent change shows that the women with clearance had a greater increase in TLR expression (range of medians was from 30% to 624%) than those who persisted where the median percent change was negative (range of medians was from -36% to -62%). Although the median change for TLR2 was in the positive direction among the persistors, the median positive change for TLR2 in women who cleared (median = 606%) was 80-fold greater than of women who persisted (median = 7%). No significant associations were found for HPV 51 (Fig. 2). To investigate this further we compared the levels at incident with baseline visits separately for women who cleared and women who persisted. In the women who cleared, all the TRLs except for TLR4 showed significant increases in expression on incidence (TLR1; p=.04, TLR2; p=0.001; TLR3 p=0.002; TLR4;p=0.84; TLR6; p=0.04, TLR7; p=.01; TLR8; p=0.07; TLR9; p=0.04). None of the TLRs, in contrast, showed significant change in the women with subsequent clearance.
Figure 1. TLR expression and incident HPV 16 by follow-up status.
The graphs show change in TLR mRNA expression between a baseline (i.e., preceding HPV incidence) visit and the HPV 16 incident (“HPV+”) visit. Upward change within a subject is shown by solid lines and downward change by dashed lines. P values represent a comparison, by Wicoxon rank sum test, with adjustment for multiple comparisons30, of relative change from baseline to HPV incidence between women with HPV clearance on the visit immediately following incidence and with those with HPV 16 persistence. MΔ = median percent change and + denotes an increase; - denotes a decrease.
Figure 2. TLR expression and incident HPV 51 by follow-up status.
The graphs show change in TLR mRNA expression between a baseline (i.e., preceding HPV incidence) visit and the HPV 51 incident (“HPV+”) visit. Upward change within a subject is shown by solid lines and downward change by dashed lines. P values represent a comparison, by Wicoxon rank sum test, with correction for multiple comparisons30, of relative change from baseline to HPV incidence between women with HPV clearance on the visit immediately following incidence and with those with HPV 51 persistence. MΔ = median percent change and + denotes an increase; - denotes a decrease.
Although our primary approach was to evaluate relative, within-subject change in TLR expression at the time of HPV acquisition and its association with persistence, we noted a pattern of higher baseline (i.e., pre-HPV-incidence) TLR mRNA expression in women who would subsequently acquire a persistent HPV 16 infection compared with those who would acquire an HPV 16 infection that was followed by clearance (Fig. 1). We therefore compared, by Wilcoxon rank sum test, the baseline expression levels between these two groups. For all TLRs studied except TLR4, the baseline TLR expression was significantly (P < 0.05) higher in women who subsequently acquired an HPV 16 infection that persisted on follow-up compared with those who acquired an infection that cleared on follow-up (data not shown). This pattern was not noted for HPV type 51. To investigate whether women with HPV 16 and 51 differed in other ways to explain these baseline differences, we examined differences in condom use, number of sexual partners, hormonal birth control use, and a history of C. trachomatis or N. gonorrhoeae. No differences were found for any of these factors. We also examined if multiple type infections may have influenced this observation since infections with multiple HPV types were not excluded. Multiple type infections were common in both groups with 47% of HPV 16 infections and 40% of HPV 51 infections having multiple types (data not shown). However, multiple type infection was not associated with clearance in either group.
To investigate whether TLRs changed in the HPV 16 group in coordinated or compensatory fashion, Kappa coefficients were calculated for each pairing of the TLRs found significantly associated with clearance. Among women with persistence on follow-up (Table II), Kappa coefficients ranged from 0.32 to 0.85 for all pairings, showing moderate or better agreement, except the pairing TLR2:TLR9, which showed fair agreement (K = 0.32). Among women with clearance on follow-up (Table III), Kappa coefficients showed considerably less agreement among pairs, with only the pairings TLR3:TLR8, TLR3:TLR9, and TLR7:TLR9 showing moderate or better agreement (K ≥ 0.4).
Table II. Kappa co-efficients for comparison of change in expression between TLRs 2, 3, 7, 8, and 9 in HPV 16 persistent women.
| 2 | 3 | 7 | 8 | 9 | |
|---|---|---|---|---|---|
| 2 |  |
.7 | .46 | .55 | .32 |
| 3 |  |
 |
.46 | .53 | .46 |
| 7 |  |
 |
 |
.62 | .85 |
| 8 |  |
 |
 |
 |
.6 |
Table III. Kappa co-efficients for comparison of change in expression between TLRs 2, 3, 7, 8, and 9 in HPV 16 women who cleared.
| 2 | 3 | 7 | 8 | 9 | |
|---|---|---|---|---|---|
| 2 |  |
.13 | .40 | .07 | .3 |
| 3 |  |
 |
.55 | .67 | .45 |
| 7 |  |
 |
 |
.23 | .67 |
| 8 |  |
 |
 |
 |
.35 |
TLR expression and cytokine production
A key result of TLR activation is release of proinflammatory and immune-mediating cytokines, of which IFN-α2 is considered the most immediate product of TLR activation.15-18 To investigate possible downstream immunologic consequences of the HPV 16 persistence-associated dampening of TLR expression, we examined the association between change in TLR expression and change in levels of IFN-α2 measured in cervical lavage specimens. Using Pearson correlation, we found that a change in IFN-α2 was positively correlated with a change in TLR8 expression (r =.40, P = 0.01). Trend associations were also shown between IFN-α2 and TLR1 (r=.28; P=0.1), TLR3 (r=.28; P=0.1) and TLR7 (r=0.3; P=0.07) expression. When we looked among only those who cleared, the correlations were much stronger. Changes in IFN-α2 were associated with changes in TLR1 (r=0.6; P=0.02), TLR3 (r=0.75 P=0.001), TLR7 (r=0.53; P=0.04), and TLR8 (r=0.75; P=0.001). When we looked among the women who persisted, no correlation was significant.
Discussion
To our knowledge, this is the first in vivo study showing an increase in TLR expression in cervical samples with incident HPV 16 infection that is subsequently associated with viral clearance and lack of an observed increase was associated with persistence. TLRs whose change in expression was significantly different between women with HPV clearance and those with persistence on follow-up included TLR2, TLR3, TLR7, TLR8, and TLR9. Notably, with the exception of TLR2, these are exclusively localized in the endosomal membrane and are known to recognize viral nucleic acids. The difference in expression of multiple nucleic acid-sensing TLRs is particularly interesting in light of synergistic co-activation which has been described for TLR3 and TLR7 31 as well as TLR3 and TLR9.32 The difference in expression of TLR2 is interesting in that this is a cell surface-expressed receptor; TLR2 has been found to be activated by various viral protein components. 33, 34 However, human macrophage recognition of hepatitis C virus through herterodimers of TLR2 with TLR1 or TLR6 has also been recently demonstrated. 35 The true role of surface-expressed TLRs in anti-viral host responses, however, is not clear. 19 For example, activation of TLR2 by measles virus or herpes simplex virus proteins may be detrimental to the host through upregulation of viral receptors or induction of proinflammatory cytokines, respectively. 33, 36 In contrast, TLR2-mediated activation of natural killer cells has been shown to be important in early control of murine cytomegalovirus. 37 In some cases, particularly viruses with low structural complexity, the viral protein responsible for TLR engagement has been identified, such as measles virus hemagglutinin protein and TLR2 33 and respiratory syncytial virus fusion protein and TLR4. 38 In other cases, the protein ligand is unknown. The association shown here between TLR2 and clearance of HPV16 suggests that it may play a beneficial role in the host in infection with this virus. Further work, particularly in vitro studies, will be needed to identify HPV proteins that engage TLRs.
Examination of changes within groups revealed that the difference between women exhibiting clearance on follow-up and those with persistence was primarily due to significant increases in TLR expression upon HPV 16 incidence in the former group, rather than decreases in expression in the latter group. These results suggest that increases in TLR expression are necessary for clearance. This observation is in line with studies that show that activation of TLRs is necessary for a successful innate immune response. In addition, the increase in IFN-α2 associated with the TLR increase in women who cleared HPV 16 gives further evidence that this TLR response assisted in HPV clearance. 16-18
Our findings are consistent, however, with in vitro and animal studies that have shown that HPV 16 is able to evade immune recognition and this evasion results in its persistence, 20, 21 as indicated by the lack of TLR expression increase in the persistence group and even trends, albeit non-significant, in the downward direction. Interestingly, IFN-α2 was not significantly associated with this decrease. Likely, in the absence of TLR activation there is no correlation between TLR expression and cytokine levels. Taken together, we believe these data suggest that HPV may evade the immune system by avoiding TLR recognition. Certainly, studies have shown that HPV is associated with several mechanisms that may assist in avoiding immune recognition including diminishing Langerhans cell populations, dampening natural killer cell activity and MHC expression. 11, 39
We also investigated whether the changes in TLR expression in the women with incident HPV 16 infections occurred in parallel or opposite directions. Generally, there was good agreement in the direction of change in the women with persistence on follow-up, except between TLR2 and TLR9. In contrast, there was less agreement among women with clearance on follow-up. In this group, pairings involving the nucleic acid-sensing TLRs tended to be those showing moderate or better agreement, while TLR2 showed poor agreement in all pairings. The cases of moderate or better agreement may suggest either coordinated regulation or migration of cells bearing multiple TLRs in or out of the mucosa. That TLR2 showed relatively poor agreement with other TLRs in both groups is consistent with its presumed less central role in response to viruses.
Our findings appear to be specific for HPV 16 and were not paralleled by findings for the other high-risk type, HPV 51. Hasan et al. reported that, in vitro, HPV 16 is much more efficient than HPV 18 in downregulating TLR9, 20 suggesting that modulation of TLR expression may be part of a mechanism by which HPV 16 evades innate immunity, and that this is not common to all high-risk HPV types. Unfortunately, we were unable to identify enough cases of HPV 18 in our cohort to study. However, HPV 51, which was the second most prevalent HPV type in our cohort, did not show similar differences between women with clearance on followup and those without. The reason for this remains unclear and underscore the difficulties of interpreting in vivo studies which are potentially affected by numerous environmental factors. Certainly, HPV 16 is 50 times more common in cervical cancers than HPV 51. 40 Our different findings for types 16 and 51 support the hypothesis that immune mechanisms involved in clearance, as well as in viral immune evasion, may differ by HPV types.
The approach presented here, evaluating change in TLR expression within each subject, recognizes the fact that there is no information regarding what constitutes a “normal” level of expression, and that this may be different in each woman owing to the myriad immunogenic factors that make the cervical mucosa such a complex environment. While our findings support our hypothesis that HPV 16 uniquely affects TLR expression in a way that helps predict the subsequent course of infection, we were surprised that baseline TLR expression appeared to be markedly different between the persistence and clearance groups. This observation may suggest some level of pre-existing non-specific immune activation in the former group. Because women were excluded from this study if they had, at baseline (or incident), abnormal cytology, yeast, C. trachomatis, N. gonorrhoeae, or T. vaginalis infection, or were pregnant, none of these covariates appears to explain the difference. However, the cervical vaginal microbiologic and immunologic milieu is extremely complicated and is affected by a host of environmental and genetic factors. 41 We speculate that there may be some genetic component that affected baseline TLR expression and resulted in increasing these women's vulnerability to HPV persistence.
For the purposes of this study, follow-up was limited to one four-month visit after the incident HPV infection and clearance or persistence defined based on that follow-up interval. As HPV, and in particular, HPV 16, may sometimes persist for extended periods with eventual clearance occurring months to years later or not at all 3, much longer prospective follow-up will be needed to determine the role of TLR expression in regression of these more prolonged infections. Extended follow-up, while beyond the scope of this substudy, remains ongoing in our cohort.
In summary, we have shown that incident HPV 16 infection that results in clearance is associated with increased expression of several TLRs compared to women who do not clear, including the 4 TLRs known to recognize viral nucleic acids. Imiquimod, a TLR7 agonist, is approved for treatment of genital warts and has been suggested for treatment of other HPV-associated disease. 42, 43 If TLR agonists are capable of mitigating the diminished TLR expression shown here, their use in the treatment of cervical HPV persistence may be promising. Understanding the mechanisms involved might lay the foundation for new therapies for HPV-associated diseases.
Acknowledgments
Grant Support: This work was supported by grants from the National Cancer Institute (NIH/NCI R37 CA051323: M.E.S., Y.M., S.S., S.F., and A.B.M.) and the Kenya Medical Research Institute-University of California San Francisco Infectious Disease Research Training Program (NIH FIC D43 TW007388: I.I.D.), and was carried out in part in the Pediatric Clinical Research Center, Clinical and Translational Science Institute at the University of California, San Francisco (NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131).
We thank Dorothy Thai for reading slides for bacterial vaginosis, the clinical staff of the Natural History of HPV: Infection to Neoplasia Study, and Anthony Kung for assistance with manuscript preparation.
Abbreviations used
- TLR
toll-like receptors
- HPV
human papillomavirus
- IL
interleukin
- TH
helper t cell type
Footnotes
Novelty and Impact of Paper: This is the first study to demonstrate an association between HPV 16 and mucosal innate immune response. HPV 16 persistence was associated with a dampened TLR 3, 7, 8, and 9 expression.
References
- 1.Muñoz N, Bosch FX, De Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. Epidemiological Classification of Human Papillomavirus Types Associated with Cervical Cancer. NEJM. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 3.Moscicki AB, Shiboski S, Broering J, Powell K, Clayton L, Jay N, Darragh TM, Brescia R, Kanowitz S, Miller SB, Stone J, Hanson E, Palefsky J. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132:277–84. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 4.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman M, Herrero R, Desalle R, Hildesheim A, Wacholder S, Rodriguez AC, Bratti MC, Sherman ME, Morales J, Guillen D, Alfaro M, Hutchinson M, Wright TC, Solomon D, Chen Z, Schussler J, Castle PE, Burk RD. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337:76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa M, Stites D, Patel S, Scott M, Hills N, Palefsky J, Moscicki AB. Persistence of Human Papillomavirus 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigen. J Infect Dis. 2000;182:595–8. doi: 10.1086/315706. [DOI] [PubMed] [Google Scholar]
- 7.Scott M, Stites DP, Moscicki AB. Th1 cytokine patterns in cervical human papillomavirus infection. Clinical and Diagnostic Laboratory Immunology. 1999;6:751–5. doi: 10.1128/cdli.6.5.751-755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol. 2008;109:S15–21. doi: 10.1016/j.ygyno.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Woodworth CD. HPV Innate Immunity. Front Biosci. 2002;7:d2058–71. doi: 10.2741/A898. [DOI] [PubMed] [Google Scholar]
- 10.Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, Galloway DA. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181:1911–9. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 11.Frazer IH. Interaction of human papillomaviruses with the host immune system: a well evolved relationship. Virology. 2009;384:410–4. doi: 10.1016/j.virol.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Moscicki AB, Tsang L, Brockman A, Nakagawa M. Memory T cells specific for novel human papillomavirus type 16 (HPV16) E6 epitopes in women whose HPV16 infection has become undetectable. Clin Vaccine Immunol. 2008;15:937–45. doi: 10.1128/CVI.00404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farhat S, Nakagawa M, Moscicki AB. Cell-mediated immune responses to human papillomavirus 16 E6 and E7 antigens as measured by interferon gamma enzyme-linked immunospot in women with cleared or persistent human papillomavirus infection. Int J Gynecol Cancer. 2009;19:508–12. doi: 10.1111/IGC.0b013e3181a388c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;24 1:S16–22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 16.Brugnolo F, Sampognaro S, Liotta F, Cosmi L, Annunziato F, Manuelli C, Campi P, Maggi E, Romagnani S, Parronchi P. The novel synthetic immune response modifier R-848 (Resiquimod) shifts human allergen-specific CD4+ TH2 lymphocytes into IFN-gamma-producing cells. J Allergy Clin Immunol. 2003;111:380–8. doi: 10.1067/mai.2003.102. [DOI] [PubMed] [Google Scholar]
- 17.Kaisho T, Akira S. Pleiotropic function of Toll-like receptors. Microbes Infect. 2004;6:1388–94. doi: 10.1016/j.micinf.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4:249–58. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 19.Barton GM. Viral recognition by Toll-like receptors. Semin Immunol. 2007;19:33–40. doi: 10.1016/j.smim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Hasan UA, Bates E, Takeshita F, Biliato A, Accardi R, Bouvard V, Mansour M, Vincent I, Gissmann L, Iftner T, Sideri M, Stubenrauch F, Tommasino M. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J Immunol. 2007;178:3186–97. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- 21.Yang R, Murillo FM, Delannoy MJ, Blosser RL, Yutzy WH, Uematsu S, Takeda K, Akira S, Viscidi RP, Roden RBS. B lymphocyte activation by human papillomavirus-like particles directly induces Ig class switch recombination via TLR4-MyD88. J Immunol. 2005;174:7912–9. doi: 10.4049/jimmunol.174.12.7912. [DOI] [PubMed] [Google Scholar]
- 22.Scott ME, Ma Y, Farhat S, Shiboski S, Moscicki AB. Covariates of cervical cytokine mRNA expression by real-time PCR in adolescents and young women: effects of Chlamydia trachomatis infection, hormonal contraception, and smoking. J Clin Immunol. 2006;26:222–32. doi: 10.1007/s10875-006-9010-x. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman JA, Moscicki AB, Sumerel JL, Ma Y, Scott ME. Determination of cytokine protein levels in cervical mucus samples from young women by a multiplex immunoassay method and assessment of correlates. Clin Vaccine Immunol. 2008;15:49–54. doi: 10.1128/CVI.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daud II, Scott ME. Validation of reference genes in cervical cell samples from human papillomavirus-infected and -uninfected women for quantitative reverse transcription-PCR assays. Clin Vaccine Immunol. 2008;15:1369–73. doi: 10.1128/CVI.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui W, Taub DD, Gardner K. qPrimerDepot: a primer database for quantitative real time PCR. Nucleic Acids Res. 2007;35:D805–9. doi: 10.1093/nar/gkl767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pattyn F, Robbrecht P, De Paepe A, Speleman F, Vandesompele J. RTPrimerDB: the real-time PCR primer and probe database, major update 2006. Nucleic Acids Res. 2006;34:D684–8. doi: 10.1093/nar/gkj155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rebbapragada A, Wachihi C, Pettengell C, Sunderji S, Huibner S, Jaoko W, Ball B, Fowke K, Mazzulli T, Plummer FA, Kaul R. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. AIDS. 2007;21:589–98. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 28.Bookout AL, Cummins CL, Mangelsdorf DJ, Pesola JM, Kramer MF. High-throughput real-time quantitative reverse transcription PCR. Curr Protoc Mol Biol. 2006;Chapter 15:Unit 15 8. doi: 10.1002/0471142727.mb1508s73. [DOI] [PubMed] [Google Scholar]
- 29.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hochberg YA. A sharper Bonferroni Procedure for multiple tests of significance. Biometrika. 1988;75:800–2. [Google Scholar]
- 31.Warger T, Osterloh P, Rechtsteiner G, Fassbender M, Heib V, Schmid B, Schmitt E, Schild H, Radsak MP. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood. 2006;108:544–50. doi: 10.1182/blood-2005-10-4015. [DOI] [PubMed] [Google Scholar]
- 32.Whitmore MM, DeVeer MJ, Edling A, Oates RK, Simons B, Lindner D, Williams BRG. Synergistic activation of innate immunity by double-stranded RNA and CpG DNA promotes enhanced antitumor activity. Cancer Res. 2004;64:5850–60. doi: 10.1158/0008-5472.CAN-04-0063. [DOI] [PubMed] [Google Scholar]
- 33.Bieback K, Lien E, Klagge IM, Avota E, Schneider-Schaulies J, Duprex WP, Wagner H, Kirschning CJ, Ter Meulen V, Schneider-Schaulies S. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol. 2002;76:8729–36. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77:4588–96. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J Leukoc Biol. 2007;82:479–87. doi: 10.1189/jlb.0207128. [DOI] [PubMed] [Google Scholar]
- 36.Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A. 2004;101:1315–20. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szomolanyi-Tsuda E, Liang X, Welsh RM, Kurt-Jones EA, Finberg RW. Role for TLR2 in NK cell-mediated control of murine cytomegalovirus in vivo. J Virol. 2006;80:4286–91. doi: 10.1128/JVI.80.9.4286-4291.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 39.Jimenez-Flores R, Mendez-Cruz R, Ojeda-Ortiz J, Muñoz-Molina R, Balderas-Carrillo O, de la Luz Diaz-Soberanes M, Lebecque S, Saeland S, Daneri-Navarro A, Garcia-Carranca A, Ullrich SE, Flores-Romo L. High-risk human papilloma virus infection decreases the frequency of dendritic Langerhans' cells in the human female genital tract. Immunology. 2006;117:220–8. doi: 10.1111/j.1365-2567.2005.02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clifford G, Franceschi S, Diaz M, Munoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24:S26–34. doi: 10.1016/j.vaccine.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 41.Vitali B, Pugliese C, Biagi E, Candela M, Turroni S, Bellen G, Donders GG, Brigidi P. Dynamics of vaginal bacterial communities in women developing bacterial vaginosis, candidiasis, or no infection, analyzed by PCR-denaturing gradient gel electrophoresis and real-time PCR. Appl Environ Microbiol. 2007;73:5731–41. doi: 10.1128/AEM.01251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin Exp Dermatol. 2002;27:571–7. doi: 10.1046/j.1365-2230.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 43.van Poelgeest MIE, van Seters M, van Beurden M, Kwappenberg KMC, Heijmans-Antonissen C, Drijfhout JW, Melief CJM, Kenter GG, Helmerhorst TJM, Offringa R, van der Burg SH. Detection of human papillomavirus (HPV) 16-specific CD4+ T-cell immunity in patients with persistent HPV16-induced vulvar intraepithelial neoplasia in relation to clinical impact of imiquimod treatment. Clin Cancer Res. 2005;11:5273–80. doi: 10.1158/1078-0432.CCR-05-0616. [DOI] [PubMed] [Google Scholar]