Abstract
Background
Inflammatory proteins including interleukin-6 (IL-6) and C-reactive protein (CRP) have been associated with incident cognitive impairment, but little research has addressed their effects on the rate of cognitive change, and findings are mixed. The purpose of this study was to examine the relationship between serum levels of IL-6 and CRP and the rate of cognitive change across a range of cognitive domains in a sample of healthy older persons.
Methods
Growth curve analysis was performed on data from the MacArthur Study of Successful Aging, a longitudinal cohort study of high-functioning older adults aged 70–79 years at baseline in 1988 and reinterviewed in 1991 and 1995 (N = 851). Individual growth curve parameters were derived from baseline and follow-up performance in abstraction, language, spatial ability, verbal recall, spatial recognition, and global cognitive function based on age, IL-6, CRP, and covariates.
Results
Cross-sectionally, there is a generally linear negative relationship between inflammation and cognition, such that higher levels of inflammation are associated with lower levels of baseline cognitive function. After controlling for potential confounders, there was no effect of inflammation on baseline cognitive function or the rate of longitudinal cognitive change. However, persons in the top tertile on IL-6 were at an increased risk of incident declines on the Short Portable Mental Status Questionnaire (SPMSQ).
Conclusions
Although high levels of inflammation are associated with incident cognitive impairment, these results do not generalize to the full range of cognitive changes, where the role of inflammation appears to be marginal.
Keywords: Cognitive impairment, Interleukin-6, C-reactive protein
Inflammation has been hypothesized to be related to Alzheimer’s disease (AD) and vascular dementia (1), but its relationship to cognitive decline in healthy older persons remains a topic of debate. Serum levels of inflammatory markers have been related cross-sectionally to cognitive function (2–4) and longitudinally to risk of AD and other dementia (5–7). However, less is known about the role of inflammation in the rate of cognitive decline in complex cognitive tasks or in healthy older persons. Several studies have reported that inflammation is related to risk of cognitive decline (8–10), whereas others have found no difference in the rate of decline by inflammatory status (11,12).
Differences in the methods used may explain some of this variation in findings. Studies reporting greater declines among persons with high inflammation have tended to look at the dichotomous risk of decline over some threshold (8–10) or to use measures like the Mini-Mental State Examination (MMSE) (8), designed primarily to detect dementia (13), rather than to observe changes in higher-level cognitive function among healthy individuals. It is possible that findings using these methods reflect a relationship between inflammation and cognitive impairment or dementia, rather than the impact of inflammation on early, subclinical cognitive change among healthy older persons. Evidence from Dik and colleagues (11) supports this view; although interleukin-6 (IL-6) and C-reactive protein (CRP) were not associated with the rate of cognitive decline in their full sample, they were strongly associated with cognitive decline among persons impaired at baseline.
The MacArthur study measured cognition in multiple domains using a battery of tests among participants selected for high cognitive functioning at baseline, providing the opportunity to study the effect of inflammation across a range of cognitive scores. Previous findings from the MacArthur Studies of Successful Aging have documented an association between high baseline IL-6 levels and risk of large incident cognitive declines (9) but have not explored the nature of the relationship between levels of inflammation and rate of cognitive change across their full ranges. Because the MacArthur sample includes a group of healthy older adults nondemented at baseline, who were tested on a range of cognitively complex tasks, it is well-suited to an examination of the effects of inflammation on decline in higher-order cognitive tasks. Identifying the particular cognitive domains most associated with inflammation may shed light on the ways in which inflammation affects the rate of cognitive decline. The purpose of this study was to determine whether inflammation, as measured by serum IL-6 and CRP levels, is related to baseline cognitive function and the rate of cognitive change on a set of complex cognitive tasks in a sample of high-functioning older adults over 7 years.
Methods
Data
Data came from three waves of the MacArthur Study of Successful Aging, a population-based prospective study of high-functioning men and women 70–79 years old at baseline. As described in detail elsewhere (14), participants were subsampled from the Established Populations for Epidemiologic Studies of the Elderly (EPESE) on the basis of age and physical and cognitive functioning, with the goal of identifying the top third of the population 70–79 years old. Data were collected through in-person interviews conducted in 1988, 1991, and 1995. Interviews assessed physical and cognitive performance, health status, health behaviors, and social and psychological characteristics; participants were also asked to provide blood samples, which were processed and frozen at −80°C within 4 hours.
Selection criteria for cognitive performance included a score of at least six correct responses on the nine-item Short Portable Mental Status Questionnaire (SPMSQ) (15) and the ability to remember three or more of six elements on delayed recall of a short story. Selection criteria for physical performance included reporting no disability on a seven-item scale of activities of daily living, no more than one disability on eight Nagi items tapping gross mobility, the ability to hold a semitandem balance for at least 10 seconds, and the ability to stand from a seated position five times within 20 seconds without using arms.
Of the 4030 age-eligible men and women, 1313 (32.6%) met all screening criteria, and 1189 of those (90.6) consented to participate. Each participant completed an interview; 880 (74.0%) provided sufficient blood to have plasma stored. Compared to participants with complete baseline data, those excluded because of missing IL-6 or CRP data had lower baseline cognitive function and were more likely to be women and non-white, with lower income and lower alcohol consumption. An additional 29 participants were missing data on one or more covariates, for a final sample of N=851. All surviving participants were reinterviewed at 2.5-year (1991) and 7-year (1995–1996) periods.
Measures
Levels of IL-6 and CRP were determined from stored baseline plasma samples (n = 880) measured by enzyme-linked immunosorbent assay (ELISA) (High Sensitivity Quantikine Kit; R&D Systems, Minneapolis, MN). Performance-based assessments of cognitive function were obtained at baseline and at 3- and 7-year follow-ups, allowing for examination of longitudinal change. Cognitive function was assessed in multiple domains, including abstraction [based on four items from the Similarities Subtest of the Wechsler Adult Intelligence Scale–Revised (16); range = 0–16], spatial ability [copying geometric figures (17); range = 0–20], delayed spatial recognition [delayed recognition Span Test (18); range 0–17], language [confrontation naming using a modified 18-item version of the Boston Naming Test (19); range =0–18], and delayed verbal memory (incidental recall of naming items, range = 0–18). A summary measure of global cognitive function was created from all subtest scores, where 89 represents the highest cognitive function possible (20). Additionally, a nine-item version of the SPMSQ tested orientation and working memory (15).
Potential confounders included sociodemographic and health characteristics. Sociodemographic predictors included age, gender, race, education, and income. Age was measured by the respondent’s age at the time of each interview (range 70–79 years at baseline, 77–86 years in 1995), and centered on the mean age at baseline, 74.3 years. Gender and race were represented by binary variables. Education was measured as years completed (range 0–17 years), and was centered on 12 years. Income was coded as < $10,000 versus ≥$10,000.
A history of diabetes, myocardial infarction, stroke, and hip fracture was obtained by self-report. Measured systolic and diastolic blood pressure, glycated hemoglobin, high-density lipoprotein (HDL) cholesterol, and waist circumference were coded continuously. Nonsteroidal antiinflammatory drug (NSAID) use was based on reported use of aspirin or ibuprofen. Alcohol use in last month (any vs none) and smoking history (ever vs none) were assessed by self-report. Physical activity was assessed using a summary measure adapted from the Yale Physical Activity Survey, focusing on frequency and intensity levels of leisure- and work-related activity (21).
Analysis
Previous research on inflammation and cognition has generally dichotomized inflammation into low and high risk based on sample-specific cut points. Here, we used generalized additive models (GAM) to empirically determine the appropriate functional specification for the relationship between IL-6, CRP, and a cognitive summary score. GAM is a technique to explore departures in linearity when there is limited theoretical justification for making a priori assumptions about the appropriate specification for a relationship (22). Inflammatory markers and age were included as predictors using a cubic spline smoothing term with 3 degrees of freedom (df). Little departure from linearity was detected, and subsequent analyses used linear models to capture the relationship between inflammation and cognition.
Growth curve analysis was then used to model the effects of inflammation on baseline cognitive function (modeled at the intercept, or the first testing occasion) and change over time (linear slope of cognitive function) over the 7-year period under study. Growth curve analysis using hierarchical linear modeling has three distinct advantages: (i) it accounts for correlations within individuals using a multilevel approach, avoiding the underestimation of error that occurs in traditional regression approaches using repeated measures; (ii) it allows us to look separately at predictors of baseline performance and cognitive change (or slope), an issue particularly important in cognitive research; and (iii) it incorporates all available data, including the observations of persons with only one or two observations.
Individual cognition score trajectories were modeled as a linear function of time, parameterized by an intercept (for the score at baseline testing) and a slope (for the rate of decline per year since baseline testing), and controlled for age at study entry, a practice effect (zero at baseline testing, and one at all follow-up testing), and a mortality selection effect (zero for those who survived, one for those who died over the study period). These covariates were included to address potential issues of measurement and estimation error; participants who died had significantly lower baseline scores in language and verbal recall. Both the intercept and slope were allowed to vary with inflammation (measured at baseline) and covariates. To account for intra-individual correlation between repeated measurements of cognition, we included random effects for both the slope and intercept. All analyses were performed using SAS PROC MIXED (23).
Results
The median values of IL-6 and CRP were 2.77 pg/mL (interquartile ratio [IQR]: 1.86–4.64) and 1.79 mg/L (IQR: 0.95–3.13), respectively. Table 1 provides sample characteristics of the population by inflammatory tertile at baseline. Participants with higher levels of IL-6 and CRP had lower scores in abstraction and language abilities and lower summary scores. Higher levels of IL-6 were also associated with lower spatial abilities, whereas higher levels of CRP were associated with lower SPMSQ scores.
Table 1.
Baseline Characteristics of Study Population by Tertiles of Inflammatory Markers: Mean or % (N = 851)
| Variable | IL-6 (pg/mL) |
CRP (mg/L) |
||||||
|---|---|---|---|---|---|---|---|---|
| Low < 2.15 | Middle 2.15–3.80 | High > 3.80 | p for Trend | Low < 1.24 | Middle 1.24–2.65 | High > 2.64 | p for Trend | |
| Demographicng | ||||||||
| Age | 74.1 | 74.5 | 74.4 | 74.1 | 74.4 | 74.3 | ||
| Female | 58.9 | 49.5 | 51.8 | 52.5 | 54.0 | 53.5 | ||
| Black | 15.6 | 16.8 | 18.7 | 15.5 | 15.2 | 20.4 | ||
| Education, y | 11.3 | 10.8 | 9.9 | * | 11.5 | 10.4 | 10.1 | * |
| Income > $10,000 | 61.7 | 52.6 | 49.3 | † | 60.8 | 51.9 | 51.7 | ‡ |
| Disease | ||||||||
| Diabetes | 11.7 | 14.7 | 15.5 | 11.9 | 13.8 | 16.2 | ||
| Myocardial infarction | 8.9 | 9.5 | 16.2 | ‡ | 10.8 | 9.0 | 14.8 | |
| Stroke | 1.1 | 2.5 | 3.5 | 2.2 | 2.4 | 2.5 | ||
| Hip fracture | 1.1 | 2.5 | 1.4 | 1.8 | 1.0 | 2.1 | ||
| Biological | ||||||||
| Systolic blood pressure, mmHg | 135.0 | 138.0 | 139.6 | ‡ | 136.4 | 137.7 | 138.6 | |
| Diastolic blood pressure, mmHg | 76.1 | 76.9 | 77.1 | † | 76.5 | 76.9 | 76.6 | |
| Glycated hemoglobin, % | 6.6 | 6.8 | 7.1 | 6.5 | 6.8 | 7.2 | * | |
| HDL cholesterol, mg/dL | 50.8 | 46.6 | 44.8 | * | 51.2 | 47.2 | 43.9 | * |
| Waist circumference, in | 35.1 | 37.0 | 37.4 | * | 35.1 | 36.4 | 37.9 | * |
| Behavioral | ||||||||
| NSAID use | 19.2 | 19.0 | 16.6 | 16.9 | 16.6 | 21.1 | ||
| Alcohol in last month | 44.0 | 50.5 | 44.0 | 48.6 | 43.6 | 46.5 | ||
| Physical activity scale, 0–160 | 28.4 | 22.4 | 14.3 | * | 28.4 | 20.5 | 16.2 | * |
| Ever smoked | 47.2 | 58.6 | 52.5 | ‡ | 47.5 | 53.6 | 57.0 | |
| Cognitive | ||||||||
| Abstraction (0–16) | 7.6 | 6.8 | 5.9 | ‡ | 7.4 | 7.2 | 5.8 | ‡ |
| Language (0–18) | 16.9 | 16.8 | 16.6 | ‡ | 16.8 | 16.9 | 16.6 | ‡ |
| Spatial ability (0–20) | 15.3 | 15.1 | 14.5 | † | 15.2 | 15.1 | 14.6 | |
| Verbal recall (0–18) | 5.6 | 5.5 | 5.5 | 5.7 | 5.5 | 5.4 | ||
| Spatial recognition (0–17) | 9.6 | 9.2 | 9.2 | 9.5 | 9.4 | 9.1 | ||
| Summary score (0–89) | 55.2 | 53.4 | 51.7 | * | 54.4 | 54.4 | 51.5 | * |
| SPMSQ (0–9) | 8.3 | 8.2 | 8.2 | 8.3 | 8.3 | 8.1 | † | |
Notes:
p <.001,
p <.01,
p <.05, obtained from chi-square test or analysis of variance.
HDL = high-density lipoprotein; NSAID = nonsteroidal antiinflammatory drug; SPMSQ = Short Portable Mental Status Questionnaire.
Figure 1 displays descriptive results and GAM results relating levels of IL-6 and CRP to baseline performance on the cognitive summary score. Results suggested a generally linear inverse association between both IL-6 and CRP and baseline cognitive function. Based on this finding, subsequent analysis used continuous coding of IL-6 and CRP and cognition scores to fit linear models.
Figure 1.
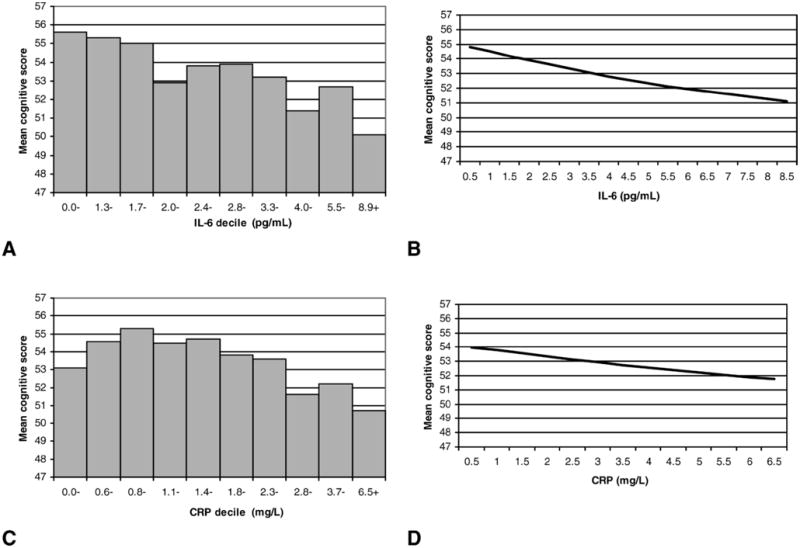
Descriptive and generalized additive model (GAM) results for relationships between interleukin-6 (IL-6), C-reactive protein (CRP), and baseline cognitive function. A, Average cognitive summary score by IL-6 decile; B, GAM estimation of cognitive function by IL-6 controlling for age; C, average cognitive summary score by CRP decile; D, GAM estimation of cognitive function by CRP controlling for age.
Table 2 details estimates and parameter variance for intercepts and slopes for each cognitive measure in models controlling only for age at entry, practice effect, and the selection effect for death. These models indicate significant variation in the intercept for all measures except the SPMSQ, which is not surprising given that a score > 6 on the SPMSQ was required for study entry. Models also indicate significant variation in the slope for all measures except spatial recognition and abstraction, suggesting that decline in these areas was fairly uniform across participants. The largest domain-specific declines occurred on measures testing memory, specifically verbal recall and delayed spatial recognition, which declined approximately 1 point every 3–5 years. On average, participants’ scores declined on the summary score by 1.09 points per year.
Table 2.
Results of Growth Curve Models for Selected Cognitive Measures Controlling for Age, Practice Effect, and Death
| Measure and Parameter | Estimate (SE) | Variance |
|---|---|---|
| Abstraction (0–16) | ||
| Baseline score | 6.94 (0.18) | 15.12* |
| Change/y | −0.16 (0.04) | 0.03 |
| Language (0–18) | ||
| Baseline score | 16.86 (0.06) | 1.62* |
| Change/y | −0.10 (0.02) | 0.04* |
| Spatial ability (0–20) | ||
| Baseline score | 15.08 (0.12) | 5.82* |
| Change/y | −0.22 (0.03) | 0.09* |
| Verbal recall (0–18) | ||
| Baseline score | 5.77 (0.09) | 3.07* |
| Change/y | −0.26 (0.02) | 0.04* |
| Spatial recognition (0–17) | ||
| Baseline score | 9.47 (0.13) | 4.14* |
| Change/y | −0.34 (0.04) | 0.02 |
| Summary score (0–89) | ||
| Baseline score | 54.11 (0.38) | 71.77* |
| Change/y | −1.09 (0.08) | 0.76* |
| SPMSQ (0–9) | ||
| Baseline score | 8.29 (0.03) | 0.02 |
| Change/y | −0.18 (0.02) | 0.03* |
Notes:
p <.001.
SE = standard error; SPMSQ = Short Portable Mental Status Questionnaire.
Table 3 presents the results of conditional models used to test the effects of IL-6 and CRP levels on cognitive function over time. The inflammation model includes both baseline IL-6 and CRP in addition to time, age, practice, and mortality selection. Higher levels of IL-6 and CRP were negatively related to abstraction scores, and higher levels of IL-6 were negatively related to language and summary scores. No slope effects were observed. Neither IL-6 nor CRP was related to any measure of cognitive function after controlling for potential confounders in the full model, suggesting that covariates accounted for variation in cognitive function previously observed to be due to inflammation.
Table 3.
Results of Conditional Growth Curve Models: Effects of Inflammatory Markers on Estimates of Cognitive Function at Mean Age and Cognitive Decline
| Measure and Inflammatory Marker | Inflammation Model* |
Full Model† |
||
|---|---|---|---|---|
| Intercept Effect | Slope Effect | Intercept Effect | Slope Effect | |
| Abstraction | ||||
| IL-6, pg/mL | −0.063‡ | −0.002 | 0.003 | 0.000 |
| CRP, mg/dL | −0.068‡ | 0.005 | −0.050 | 0.004 |
| Language | ||||
| IL-6, pg/mL | −0.026‡ | 0.002 | −0.009 | 0.001 |
| CRP, mg/dL | −0.015 | −0.001 | −0.016 | −0.001 |
| Spatial ability | ||||
| IL-6, pg/mL | −0.018 | −0.001 | 0.016 | 0.001 |
| CRP, mg/dL | −0.006 | −0.006 | −0.001 | −0.006 |
| Verbal recall | ||||
| IL-6, pg/mL | −0.010 | 0.002 | −0.001 | 0.001 |
| CRP, mg/dL | −0.008 | −0.006 | −0.014 | −0.005 |
| Spatial recognition | ||||
| IL-6, pg/mL | −0.004 | −0.004 | 0.007 | −0.001 |
| CRP, mg/dL | −0.022 | 0.005 | −0.019 | 0.008 |
| Summary score | ||||
| IL-6, pg/mL | −0.123‡ | −0.001 | 0.016 | 0.004 |
| CRP, mg/dL | −0.120 | 0.001 | −0.102 | 0.003 |
| SPMSQ | ||||
| IL-6, pg/mL | 0.001 | −0.004 | 0.006 | −0.003 |
| CRP, mg/dL | −0.010 | −0.002 | −0.008 | −0.001 |
Notes:
Inflammation model includes time, age, mortality indicator, practice effect, IL-6, and CRP.
Full model includes time, age, mortality indicator, practice effect, IL-6, CRP, female, black, education (y), income (> $10,000), diabetes, myocardial infarction, stroke, hip fracture, systolic and diastolic blood pressure, glycated hemoglobin, high-density lipoprotein cholesterol, waist circumference, non-steroidal antiinflammatory drug use, alcohol use in last month, physical activity, and smoking history.
p <.05.
IL-6 = interleukin-6; CRP = C-reactive protein; SPMSQ = Short Portable Mental Status Questionnaire.
In an effort to provide comparisons with previous studies, we conducted three additional analyses, testing different specifications of the independent and dependent variables. First, we performed additional GAM analysis assessing the relationship between IL-6 and CRP and SPMSQ score and between IL-6 and CRP and longitudinal change in the cognitive summary score and SPMSQ. We found an inverse linear relationship between inflammation and baseline SPMSQ score similar to that described above, and we found no significant relationship between inflammation and longitudinal change in cognitive summary score or SPMSQ. Second, we dichotomized IL-6 and CRP into high-risk categories, designating those in the top tertile of either marker as high risk (9,10). Results were similar to those discussed above in terms of the significance and direction of relationships. Finally, using logistic regression, we tested the relationship between high inflammation (indicated by the top tertile of IL-6 or CRP) and incident cognitive decline, defined as a decline in the bottom tertile of change on each measure over 7 years. Results are displayed in Table 4. Consistent with previous research in the same sample (9), we found no relationship between CRP levels and risk of decline, but there was a significantly increased risk associated with high IL-6 levels. Participants in the top IL-6 tertile (IL-6 > 3.8 pg/mL) had 52% increased odds of declines in abstraction (odds ratio [OR] = 1.52, 95% confidence interval [CI], 1.01–2.27), 62% increased odds of declines in global cognitive function (OR = 1.62, 95% CI, 1.07–2.45), and 88% increased odds of cognitive impairment, indicated by a decline of ≥2 on the SPMSQ (OR = 1.88, 95% CI, 1.20–2.94), relative to those with lower levels of IL-6. After adjustment for confounders, only the relationship between high IL-6 and SPMSQ decline was significant (OR = 1.67, 95% CI, 1.04–2.67). In short, a significant relationship between inflammation and cognitive change was observed only when examining the ends of the distribution or participants with the greatest decline.
Table 4.
Results of Logistic Regression Models: Effects of High Inflammatory Markers on Risk of Being in the Bottom Tertile of Cognitive Decline (N = 533)
| Cognitive test | Inflammation Model* | Full Model† |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Abstraction (≤−2) | ||
| IL-6 > 3.8 pg/mL | 1.52 (1.01–2.27) | 1.41 (0.92–2.15) |
| CRP > 2.7 mg/dL | 0.74 (0.48–1.12) | 0.75 (0.48–1.17) |
| Language (≤−1) | ||
| IL-6 > 3.8 pg/mL | 1.36 (0.90–2.06) | 1.31 (0.85–2.01) |
| CRP > 2.7 mg/dL | 0.66 (0.43–1.02) | 0.70 (0.44–1.11) |
| Spatial ability (≤−2) | ||
| IL-6 > 3.8 pg/mL | 1.23 (0.82–1.84) | 1.12 (0.73–1.70) |
| CRP > 2.7 mg/dL | 1.23 (0.81–1.86) | 1.35 (0.86–2.10) |
| Verbal recall (≤−2) | ||
| IL-6 > 3.8 pg/mL | 1.29 (0.86–1.92) | 1.32 (0.87–2.00) |
| CRP > 2.7 mg/dL | 0.98 (0.65–1.48) | 0.87 (0.56–1.35) |
| Spatial recognition (≤−4) | ||
| IL-6 > 3.8 pg/mL | 0.96 (0.62–1.48) | 0.96 (0.61–1.52) |
| CRP > 2.7 mg/dL | 0.82 (0.52–1.28) | 0.74 (0.45–1.19) |
| Summary score (≤−7) | ||
| IL-6 > 3.8 pg/mL | 1.62 (1.07–2.45) | 1.49 (0.97–2.28) |
| CRP > 2.7 mg/dL | 0.74 (0.48–1.14) | 0.72 (0.45–1.14) |
| SPMSQ decline (≤−2) | ||
| IL-6 > 3.8 pg/mL | 1.88 (1.20–2.94) | 1.67 (1.04–2.67) |
| CRP > 2.7 mg/dL | 0.73 (0.45–1.19) | 0.70 (0.41–1.16) |
Notes:
Inflammation model includes age, IL-6, and CRP.
Full model includes age, IL-6, CRP, female, black, education (y), income (> $10,000), diabetes, myocardial infarction, stroke, hip fracture, systolic and diastolic blood pressure, glycated hemoglobin, high-density lipoprotein cholesterol, waist circumference, nonsteroidal antiinflammatory drug use, alcohol use in last month, physical activity, and smoking history.
OR = odds ratio; CI = confidence interval; IL-6 = interleukin-6; CRP = C-reactive protein; SPMSQ = Short Portable Mental Status Questionnaire.
Discussion
The purpose of this study was to assess the effects of two markers of inflammation, IL-6 and CRP, on cognitive function and the rate of cognitive change over 7 years. Cross-sectionally, we found a linear inverse association between inflammation and cognitive summary score, suggesting small effects on cognition across the range of values of IL-6 and CRP and no evident cutpoint representing a threshold effect of inflammation on cognitive status. Cross-sectional effects of IL-6 and CRP disappeared after fully accounting for confounders, and no effects of IL-6 or CRP on the slope of cognitive change were observed in growth curve models. However, we did observe a relationship between high levels of IL-6 and large incident declines on the SPMSQ, suggesting that high levels of inflammation are associated with incident cognitive impairment.
Taken together, these results suggest that although high levels of inflammation are associated with incident cognitive impairment, these results may not generalize to cognitive decline across the full range of cognitive scores. There may be an inverse relationship between levels of inflammation and late-life cognition in specific areas (e.g., abstraction, language), but this relationship is relatively modest in the noncognitively impaired population and is largely accounted for by known predictors of cognition such as age, socioeconomic status, and health status. The effects of inflammation found in this and other cohort studies on risks for large cognitive decline may reflect increased inflammation in older adults with underlying pathology or preclinical cognitive impairment associated with incident dementia.
We cannot rule out that the absence of significant effects of inflammation on the slope of cognitive change observed here is related to study limitations. First, in the domains of abstraction and spatial recognition, there was little variation in the slope over the 7 years studied. This lack of variation limited our ability to observe potential effects of explanatory variables. In other domains with significant variation, however, we found significant associations between the rate of decline and other predictors, such as systolic blood pressure and income level, suggesting that models distinguished predictors of cognitive change similar to those found in other studies (24,25). Specifically, individuals with higher incomes experienced slower cognitive decline, and having high blood pressure was marginally associated with faster cognitive decline. Second, error in the measurement of cognitive function may make it difficult to observe effects on continuous cognitive change; dichotomized indicators of incident impairment are less sensitive measures of cognitive change, but are also less likely to be affected by measurement error. Third, participants in this sample were selected to be high-functioning in old age. Those participants who had accumulated a lifetime of chronic high inflammation may have experienced higher mortality and morbidity, thereby selecting out of the sample some individuals for whom the effects of inflammation on cognitive decline might have been stronger. Finally, our ability to address chronic inflammation was limited because measures of IL-6 and CRP reflect values at only one time point. Levels of inflammatory markers vary over time, limiting the usefulness of a single measure of IL-6 and CRP.
Despite these limitations, results indicate that inflammation is only moderately related to baseline cognitive function and is not related to the average rate of cognitive decline in older persons. Although high levels of inflammation appear to be associated with incident cognitive impairment, these results do not appear to generalize to the full range of cognitive changes observed in high-functioning older adults, where the role of inflammation appears to be marginal.
Acknowledgments
This work was supported by National Institutes of Health grants P30 AG17265 and T32 AG00037.
Footnotes
Preliminary results were presented at the annual meeting of The Gerontological Society of America, November 2004.
References
- 1.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition–the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 2.Mangiafico RA, Sarnataro F, Mangiafico M, Fiore CE. Impaired cognitive performance in asymptomatic peripheral arterial disease: relation to C-reactive protein and D-dimer levels. Age Ageing. 2006;35:60–65. doi: 10.1093/ageing/afi219. [DOI] [PubMed] [Google Scholar]
- 3.Ravaglia G, Forti P, Maioli F, et al. Serum C-reactive protein and cognitive function in healthy elderly Italian community dwellers. J Gerontol A Biol Sci Med Sci. 2005;60A:1017–1021. doi: 10.1093/gerona/60.8.1017. [DOI] [PubMed] [Google Scholar]
- 4.Wright CB, Sacco RL, Rundek TR, Delman JB, Rabbani LE, Elkind MS. Interleukin-6 is associated with cognitive function: the Northern Manhattan study. J Stroke Cerebrovasc Dis. 2006;15:34–38. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: The Rotterdam Study. Arch Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up study of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 7.Teunissen CE, Lutjohann D, von Bergmann K, et al. Combination of serum markers related to several mechanisms in Alzheimer’s disease. Neurobiol Aging. 2003;24:893–902. doi: 10.1016/s0197-4580(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 8.Tilvis RS, Kahonen-Vare MH, Jolkkonen J, Valvanne J, Pitkala KH, Strandberg TE. Predictors of cognitive decline and mortality of aged people over a 10-year period. J Gerontol A Biol Sci Med Sci. 2004;59A:268–274. doi: 10.1093/gerona/59.3.m268. [DOI] [PubMed] [Google Scholar]
- 9.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 11.Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikenbloom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64:1371–1377. doi: 10.1212/01.WNL.0000158281.08946.68. [DOI] [PubMed] [Google Scholar]
- 12.Teunissen CE, van Boxtel MPJ, Bosma H, et al. Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol. 2003;134:142–150. doi: 10.1016/s0165-5728(02)00398-3. [DOI] [PubMed] [Google Scholar]
- 13.Cockrell JR, Folstein MF. Mini-Mental State Examination (MMSE) Psychopharmacol Bull. 1988;24:689–691. [PubMed] [Google Scholar]
- 14.Berkman LF, Seeman TE, Albert M, et al. High, usual and impaired functioning in community-dwelling older men and women: findings from the MacArthur Foundation Research Network on Successful Aging. J Clin Epidemiol. 1993;46:1129–1140. doi: 10.1016/0895-4356(93)90112-e. [DOI] [PubMed] [Google Scholar]
- 15.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 16.Wechsler D. Adult Intelligence Scale—Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 17.Rosen W, Moths R, Davis R. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 18.Moss M, Albert M, Butters N, Payne M. Differential patterns of memory loss among patients with Alzheimer’s disease, Huntington’s disease, alcoholic Korsakoff’s syndrome. Arch Neurol. 1986;43:239–246. doi: 10.1001/archneur.1986.00520030031008. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan E, Goodlass H, Weintraub S. Boston Naming Test. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 20.Inouye SK, Albert MS, Mohs R, Sun K, Berkman LF. Cognitive performance in a high functioning community dwelling elderly population. J Gerontol. 1993;48:M146–M151. doi: 10.1093/geronj/48.4.m146. [DOI] [PubMed] [Google Scholar]
- 21.Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Med Sci Sports Exerc. 1993;47:639–646. [PubMed] [Google Scholar]
- 22.Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 23.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Sci. 1998;24:323–355. [Google Scholar]
- 24.Anstey K, Christensen H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: a review. Gerontology. 2000;46:163–177. doi: 10.1159/000022153. [DOI] [PubMed] [Google Scholar]
- 25.Chodosh J, Reuben DB, Albert MS, et al. Predicting cognitive impairment in high-functioning community dwelling older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 2002;50:1051–1060. doi: 10.1046/j.1532-5415.2002.50260.x. [DOI] [PubMed] [Google Scholar]


