Abstract
Humans evolved in a world with high levels of infection resulting in high mortality across the life span and few survivors to advanced ages. Under such conditions, a strong acute-phase inflammatory response was required for survival; however, inflammatory responses can also promote chronic diseases of aging. We hypothesize that global historical increases in life span at older ages are partly explained by reduced lifetime exposure to infection and subsequent inflammation. To begin a test of this hypothesis, we compare C-reactive protein (CRP); levels in two populations with different epidemiological environments: the Tsimane of Bolivia and persons in the United States. High CRP is significantly more prevalent among the Tsimane up through middle age; by age 35, the Tsimane have spent more years with high CRP than have Americans at age 55. Further testing of the links among infection, inflammation, and chronic diseases of aging among the Tsimane requires collection of age-specific indicators of atherosclerosis and cardiac function.
Keywords: Inflammation, Infection, C-reactive protein
Explanations of increases in life expectancy at older ages often focus on the role of relatively recent medical advances and behavioral changes. The historical reduction of infections is recognized as a major factor in the decline of early age mortality in the past two centuries: We and others have noted that cohorts who experienced lower mortality when young also experienced reduced mortality among survivors in old age (1–4). It is further hypothesized that population levels of inflammation have decreased over time in parallel with the reduction in mortality from infectious diseases (1,2). A reduction in the prevalence and duration of time spent with infectious conditions should lower the levels of lifetime inflammation. Because chronically elevated blood levels of inflammatory cells and proteins are independent factors in the atherosclerotic process, reductions in lifetime levels of inflammation should slow deterioration in the cardiovascular system with aging, as well as delay the onset of chronic cardiovascular diseases of old age (5–9). Cohort reductions in inflammation should result in increased ages at mortality, heart attack, stroke, and cognitive loss (8,9). The hypothesis that lifetime inflammation has decreased in modern populations and that aging processes are consequently delayed relative to historical populations cannot be directly tested because of the lack of tissue samples from these earlier eras. As an approach to testing the inflammation–aging hypothesis in modern populations, we compare C-reactive protein (CRP) levels in two populations with different epidemiological environments: the Tsimane of the Bolivian Amazon versus Americans.
Methods
Lacking statistically valid blood or tissue samples from historical populations, we sought a contemporary population that had limited exposure to modern medicine with a high level of mortality that would provide a useful calibration for understanding past populations and modern epidemiologic transitions. The Tsimane are indigenous foragers in the Bolivian Amazon with limited access to modern medicine, low life expectancy, and high mortality from infection. We examined CRP, a serum marker of inflammation, across the age range in this population for comparison with age-specific CRP and mortality levels in the United States. These two populations represent extremes in contemporary levels of infectious mortality, exposure to inflammation, and life expectancy.
In July 2002, Kaplan and Gurven initiated long-term research on Tsimane life history. Data were collected on demographic, economic, social, and health characteristics of >2200 Tsimane living in 17 study villages (9). These forager-horticulturalists live in about 60 small villages of extended family clusters located in a forested lowland tropical area. Despite their exposure to Jesuit missionaries in the late 17th century, the Tsimane were never successfully settled in missions and are relatively unacculturated. Their isolation will not last much longer because of increasing access to market towns, especially as supplies of forest game and fish diminish with increased encroachment by loggers and colonists. The life history data provide the basis for estimating life expectancy over the past 15 years in this population (10).
Inflammation is indicated in both countries by levels of serum CRP. Elevation of CRP is an acute phase response to infection that mediates bacterial clearance and is widely used to indicate systemic inflammation. However, chronic exposure to even moderately high CRP is associated with increased risk of cardiovascular events and other disabilities of aging in long-lived populations (9,11). We examined age-specific prevalence of high CRP and estimated the average number of years spent with high CRP for survivors up to specified ages.
For the Tsimane, CRP was assayed in whole blood collected by venipuncture from 607 people over the age of 4 during medical examinations in four villages during the summers of 2004 and 2005. Whole blood was hand-centrifuged on site at an ambient temperature and then frozen and stored in liquid nitrogen pending transport to the United States for assay. Assays were done using the Immulite 2000, high-sensitivity CRP assay, a solid-phase, chemiluminescent immunometric assay with a mean replicate coefficient of variation of 5.6% (Diagnostics Products Corporation, Siemens, Deerfield, IL). An indication of the reliability of this assay is that the percentages of high serum CRP reported for Tsimane children resemble those reported for Tsimane children of the same age from assays of dried blood spots after using the conversion formula (12).
The U.S. data on CRP are from the National Health and Examination Study (NHANES); 1999–2002, a nationally representative sample from noninstitutionalized persons collected from 1999 through 2002. Values of serum CRP were determined from samples analyzed by high-sensitivity latex-enhanced nephelometry on a BN II Nephelometer (Dade Behring, Siemens) (13). The assay used monoclonal anti-CRP antibodies and a calibrator that was traceable to the World Health Organization (WHO) reference material. The mean replicate coefficient of variation for the assay is 6.4% (13). Values of CRP for the Tsimane based on the Immulite 2000 assay were standardized to the Dade Behring assay used in NHANES (14).
This analysis defined CRP > 3.0 mg/L as the cutoff for high CRP, which indicates high cardiovascular risk (15). Because we are interested in lifetime exposure to high inflammation, we use the age-specific prevalence of high CRP to estimate the number of years lived with high CRP by specified ages for both the Tsimane and U.S. data. The calculation assumes that the prevalence of high CRP observed in an age interval represents the proportion of life lived with high CRP for persons who survive through the interval. We also assume that the prevalence of high CRP below the age of measurement (4 years for the Tsimane and 2 years for the Americans) is equal to that in the 5–9 age range.
Results
A Tsimane life table was constructed by Gurven from recent interviews representing the period from 1950–1989 (29,464 person-years of life) (10). Recent life expectancy, 42.8 years, is very close to that in 19th century Western Europe, for example, Sweden in 1843 (42.6 years) (Figure 1). In contrast to the Tsimane, the current U.S. life expectancy is almost twice as high (77.2 years) with infections responsible for only a small percentage of deaths.
Figure 1.
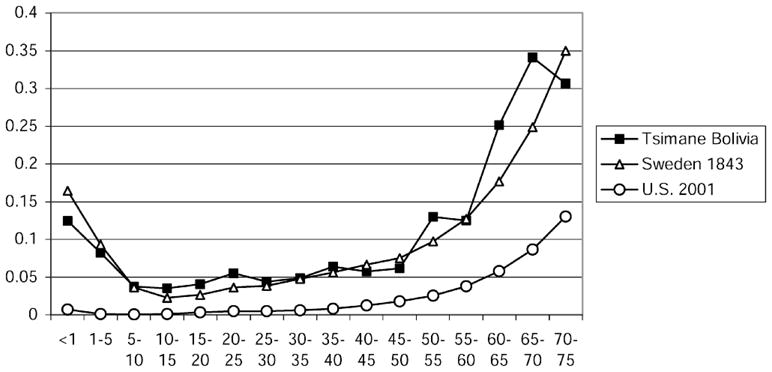
Probability of dying among Tsimane (1950–1989), Swedes (1843), and Americans (2001).
The age-specific proportions with serum CRP > 3.0 mg/L differ strikingly between the U.S. and Tsimane data (Figure 2). The Tsimane have significantly higher age-specific proportions with high serum CRP up to age 45–54 years than do the Americans. The proportion with high CRP among young Tsimane adults exceeds U.S. values for ages 65 and older. These remarkable elevations of CRP can be attributed to endemic infections in the Tsimane, as documented above. Few U.S. children have high CRP at any time relative to the Tsimane because childhood infections are less prevalent in the United States. Nonetheless, in both samples, the percentage with high CRP increases after childhood. Factors associated with chronically high CRP in low-mortality countries include obesity, smoking, and the onset of cardiovascular conditions and other chronic degenerative diseases of aging (16,17). At the older ages, the Tsimane and U.S. populations have similar serum CRP.
Figure 2.
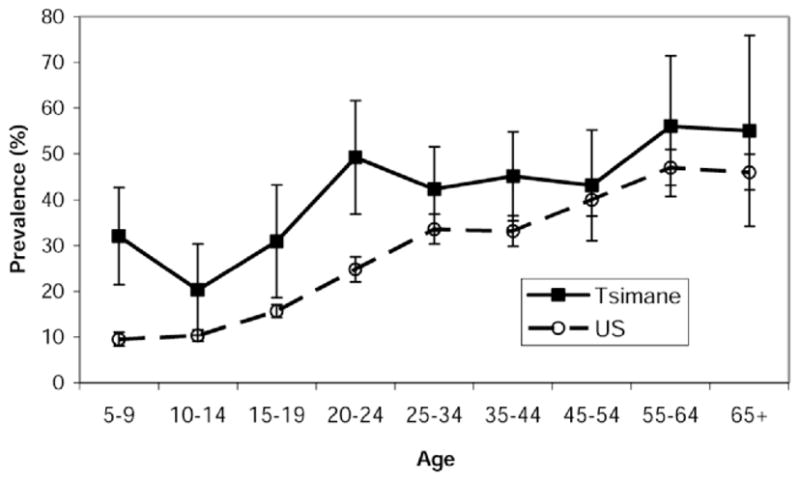
Prevalence of high-risk C-reactive protein (CRP) (>3 mg/L) in Tsimane and Americans.
Lifetime exposure to high levels of CRP is implicated in the development of atherosclerosis. Longer exposure to high inflammation early in life results in the Tsimane having lived more years with high inflammation by the time they reach adulthood. Years lived with high inflammation estimated from the prevalence is shown in Figure 3 and Table 1. By age 15, the average Tsimane has lived 6.1 years with high CRP, whereas the average time with high CRP for an American 15-year-old is only 1.5 years. By age 35, the Tsimane have spent more years with high CRP than have Americans at age 55. Over their entire life history up to age 70, the Tsimane spend more years and a greater proportion of life with high CRP than do people in the United States.
Figure 3.
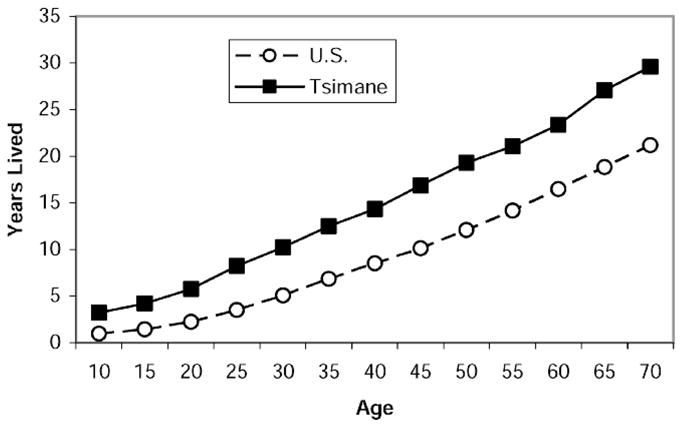
Years lived with high C-reactive protein (CRP) for those who survive to a specified age.
Table 1.
Years Lived with High C-Reactive Protein (CRP) for Those Who Survive to Specified Age; Proportion of Life Lived With High CRP Up to Specified Age
| Age | Tsimane |
United States |
||
|---|---|---|---|---|
| Years With High CRP | Proportion of Life Lived With High CRP | Years With High CRP | Proportion of Life Lived With High CRP | |
| 10 | 3.6 | 0.36 | 1.0 | 0.10 |
| 15 | 6.1 | 0.41 | 1.5 | 0.10 |
| 25 | 10.3 | 0.41 | 3.5 | 0.14 |
| 35 | 15.0 | 0.43 | 6.8 | 0.19 |
| 55 | 24.5 | 0.45 | 14.1 | 0.26 |
| 65 | 30.1 | 0.46 | 18.8 | 0.29 |
Discussion and Conclusion
The Tsimane population provides a unique opportunity to evaluate the role of CRP in an epidemiologic setting with current mortality as high as that in 19th century Europe. The similarity of the levels and the age-specific pattern of mortality for the Tsimane and Swedish suggests that both the Tsimane and those living in Northern Europe 150 years ago suffered from high levels of infectious mortality and morbidity (18,19). Medical examinations of 2800 Tsimane in 2004 and 2005 document the high levels of endemic parasitism and infection. More than two thirds (69%); were infected with at least one species of intestinal parasite at the time of the examination, and >60% had symptoms of either a gastrointestinal or respiratory illness.
Our evidence shows that persons in a high-mortality, highly infectious environment have higher CRP in the first four decades of life than persons in a low-mortality, low-infection environment have. Based on the association of CRP elevations with future cardiovascular events in populations with lower mortality and infections, we hypothesize that premature cardiovascular disease is a factor in the higher adult mortality of Tsimane. Studies of young persons in the United States have linked high CRP to vascular deterioration beginning in childhood (20,21). In the United States and other generally healthy populations, individual exposure to common infections has been associated with both CRP levels and risk of cardiovascular events (6). Thus, the greater exposure of Tsimane to inflammation could promote earlier vascular disease and other dysfunctions of aging. Conversely, the reductions in inflammatory exposure in countries like the United States may have been a significant factor in delaying aging and mortality in the past.
It is not straightforward to compare levels of CRP among the Tsimane to those in other populations. Most reports on CRP in populations come from those with low mortality and of European extraction; most do not show an age pattern of change in CRP, but report on one age group or do not differentiate by age. However, growing evidence suggests that CRP levels vary widely around the world. Tsimane children have higher levels of CRP than children in Kenya and Samoa; this finding would be consistent with relative levels of infection (12). Tsimane adults also have higher CRP than adults in the indigenous North Asian Yakut population, a group with relatively high, but recently decreasing, life expectancy due to rapid social change in the area that was once the Soviet Union (22). In fact, CRP levels among the Yakut are almost as low as levels in Japan which has much lower levels of CRP than the United States, the U.K., and Germany (22,23). In contrast, Tsimane adults 45 years old and older have a median CRP level (3.0 mg/L) close to that of the adult American Indian population (3.2 mg/L), with low levels of infection but high levels of diabetes and other cardiovascular risk factors (24).
In populations with low levels of infection and mortality, high inflammation has been strongly implicated in the pathophysiology of arterial degeneration and immunosenescence, as well as in a wide variety of chronic diseases including diabetes (25), metabolic syndrome (26), congestive heart failure (27), Alzheimer’s disease (28,29), and disability (30). These links between inflammation and most major health problems support the hypothesis that inflammation is a common mechanism of many degenerative conditions linked to aging (28,31,32). There are many links between life circumstances and inflammation in addition to the infection emphasized among the Tsimane: The importance of various paths depends on the population circumstances. Currently, in the United States, levels of inflammation among children and young adults are predicted by weight and obesity, exposure to cigarette smoke, and air pollution (33).
Further testing of our hypothesis requires new information on the role of arterial disease in adult mortality among the Tsimane. We must also test competing hypotheses about mortality from immune dysfunctions caused by high levels of lifetime infection and inflammation. CRP, for example, can impair the differentiation of antigen-presenting monocytes (dendritic cells) (34). While the Tsimane are rapidly gaining access to medicine and immunizations, we may be able to glean critical insights about the nature of mortality in a highly infectious environment that will more fully explain the doubling of life expectancy during the last 200 years and the potential for further increases in many parts of the world. In addition, a link between inflammation and late life health would provide evidence of antagonistic pleiotropy, that is, a process that enhances survival and reproductive success in the young, but with delayed adverse consequences at older ages (6,28).
Acknowledgments
This work was supported by National Institutes of Health grants R01AG024119, R01 AG13499, and P30AG17265; National Science Foundation Grant BCS-0136274; the Ellison Medical Foundation; and the Ruth Ziegler Fund.
References
- 1.Crimmins EM, Finch CE. Infection, inflammation, height, and longevity. Proc Natl Acad Sci U S A. 2006;103:499–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305:1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- 3.Janssen F, Kunst AE The Netherlands Epidemiology and Demography Compression of Morbidity Research Group. Cohort patterns in mortality trends among the elderly in seven European countries. Int J Epidemiol. 2005;34:1149–1159. doi: 10.1093/ije/dyi123. [DOI] [PubMed] [Google Scholar]
- 4.Kermack WO, Mckendrick AG, McKinley PL. Death rates in Great Britain and Sweden. Some general regularities and their significance. Lancet. 1934;31:698–703. doi: 10.1093/ije/30.4.678. [DOI] [PubMed] [Google Scholar]
- 5.Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr Opin Hematol. 2007;14:55–61. doi: 10.1097/00062752-200701000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Licastro F, Candore G, Lio D, et al. Innate immunity and inflammation in ageing: a key for understanding age-related differences. Immun Ageing. 2005;2:8. doi: 10.1186/1742-4933-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition–the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 8.Cook NR, Buring JE, Ridker PM. C-reactive protein and prediction of risk for cardiovascular disease in women. Ann Int Med. 2006;145:21–29. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- 9.Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. Br Med J. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurven M, Kaplan H, Supa AZ. Mortality experience of Tsimane amerindians of Bolivia: regional variation and temporal trends. J Hum Biol. 2007;19:376–398. doi: 10.1002/ajhb.20600. [DOI] [PubMed] [Google Scholar]
- 11.Plutzky J. Inflammatory pathways in atherosclerosis and acute coronary syndromes. Am J Cardiol. 2001;88(8A):10K–15K. doi: 10.1016/s0002-9149(01)01924-5. [DOI] [PubMed] [Google Scholar]
- 12.McDade TW. Life history theory and the immune system: steps toward a human ecological immunology. Am J Phys Anthropol. 2003;(Suppl 37):100–125. doi: 10.1002/ajpa.10398. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control. [Accessed February 21, 2007.];NHANES 1999–2000 documentation. Available at: http://www.cdc.gov/nchs/nhanes.htm.
- 14.Diagnostics Products Corporation. Immulite/Immulite 1000/Immulite 2000: summary of safety and effectiveness. memo dated Dec. 22, 2006. [Google Scholar]
- 15.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: applications to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 16.de Maat MP, Kluft C. Determinants of C-reactive protein concentration in blood. Ital Heart J. 2001;2:189–195. [PubMed] [Google Scholar]
- 17.Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related pro-inflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.United Nations. Model Life Tables for Developing Countries (United Nations publication, Sales No. E.81.XIII.7) 1982. [Google Scholar]
- 19.Preston S, Keyfitz N, Schoen R. Causes of Death: Life Tables for National Populations. New York: Seminar Press; 1972. [Google Scholar]
- 20.Järvisalo MJ, Harmoinen A, Hakanen K, et al. Elevated serum C-reactive protein levels and early arterial changes in healthy children. Arterioscler Thromb Vasc Biol. 2002;22:1323–1328. doi: 10.1161/01.atv.0000024222.06463.21. [DOI] [PubMed] [Google Scholar]
- 21.Zieske AW, Tracy RP, McMahan A, et al. Elevated serum C-reactive protein levels and advanced atherosclerosis in youth. Arterioscler Thromb Vasc Biol. 2005;25:1237–1243. doi: 10.1161/01.ATV.0000164625.93129.64. [DOI] [PubMed] [Google Scholar]
- 22.Snodgrass JJ, Leonard WR, Tarskaia LA, et al. Anthropometric correlates of C-reactive protein among indigenous Siberians. J Physiol Anthropol. 2007;26:241–246. doi: 10.2114/jpa2.26.241. [DOI] [PubMed] [Google Scholar]
- 23.Yamada S, Gotoh T, Nakashima Y, et al. Distribution of serum C-reactive protein and its association with atherosclerotic risk factors in a Japanese population: Jichi Medical School Cohort Study. Am J Epidemiol. 2001;153:1183–1190. doi: 10.1093/aje/153.12.1183. [DOI] [PubMed] [Google Scholar]
- 24.Best LG, Zhang Y, Lee ET, et al. C-reactive protein as a predictor of cardiovascular risk in a population with a high prevalence of diabetes: the Strong Heart Study. Circulation. 2005;112:1289–1295. doi: 10.1161/CIRCULATIONAHA.104.489260. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt MI, Duncan BD, Sharett AR, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerois Risk in Communities): a cohort study. Lancet. 1999;353:1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 26.Tracy RP. Inflammation, the metabolic syndrome and cardiovascular risk. Int J Clin Pract Suppl. 2003;134:10–17. [PubMed] [Google Scholar]
- 27.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 28.Finch CE. Inflammation, Nutrition, and Aging in the Evolution of Lifespans. San Diego: Academic Press; 2007. The Biology of Human Longevity. [Google Scholar]
- 29.Finch CE, Morgan T. Systemic inflammation, infection, ApoE alleles, and Alzheimer disease: a position paper. Curr Alzheimer Res. 2007;4:185–189. doi: 10.2174/156720507780362254. [DOI] [PubMed] [Google Scholar]
- 30.Semba RD, Lauretani F, Ferrucci L. Carotenoids as protection against sarcopenia in older adults. Arch Biochem Biophys. 2007;458:141–145. doi: 10.1016/j.abb.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NIH Roadmap. [Accessed May 1, 2007.];Inflammation as a Common Mechanism of Disease. Available at: http://nihroadmap.nih.gov.
- 32.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition–the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 33.Ford ES. C-reactive protein concentration and cardiovascular disease risk factors in children: findings from the National Health and Nutrition Examination Survey 1999–2000. Circulation. 2003;108:1053–1058. doi: 10.1161/01.CIR.0000080913.81393.B8. [DOI] [PubMed] [Google Scholar]
- 34.Zhang R, Becnel L, Li M, Chen C, Yao Q. C-reactive protein impairs human CD 14+ monocyte-derived dendritic cell differentiation, maturation and function. Eur J Immunol. 2006;36:2993–3006. doi: 10.1002/eji.200635207. [DOI] [PubMed] [Google Scholar]


