Abstract
Biological, psychological, and social processes interact over a lifetime to influence health and vulnerability to disease. Those interested in studying and understanding how and why racial/ethnic and social disparities emerge need to focus on the intersection of these processes. Recent work exploring molecular epigenetic mechanisms of gene expression (in humans as well and other mammalian systems) has provided evidence demonstrating that the genome is subject to regulation by surrounding contexts (eg, cytoplasmic, cellular, organismic, social). The developing stress axis is exquisitely sensitive to regulation by social forces represented at the level of the epigenome. Old assumptions about an inert genome are simply incorrect. Epigenetic processes may provide the missing link that will allow us to understand how social and political conditions, along with individual subjective experiences, can directly alter gene expression and thereby contribute to observed social inequalities in health. Developmental neurogenomics may provide the direct link between the biological and social/psychological worlds. These biological mechanisms of plasticity (at the level of gene expression and regulation) may play a profound role in how we conceptualize health inequalities by informing our concepts regarding the somatization or embodiment of social inequalities.
Keywords: disparities, development, stress, epigenetics
Biological, psychological, and social processes interact over a lifetime to influence health and vulnerability to disease. A wealth of epidemiologic data have documented the relationship between socioeconomic status (SES) and health, with low-SES groups faring most poorly across multiple health-outcome measures.1 The probabilistic relationship between social phenomena and biological vulnerability seems to be in direct contrast to the commonly shared belief that the fixed genome (or genotype) plays a larger “deterministic” role in health outcomes. Indeed, a common approach to understanding disparities by exploring “social” and “biological” factors as independent agents divorced from one another has been extremely limiting. The ubiquitous study of gene × environment interactions provides a ready example. The assertion is made that vulnerability for a given outcome measure is caused by the interaction of genes with environments, the classic nature-versus-nurture debate. Inherent in the framing of this equation is an unacknowledged directionality; genes come first in time and environments then act on them, resulting in a given vulnerability. Equally plausible but rarely explored at a molecular or mechanistic level is the hypothesis that environmental factors are acting on the genome to create differences in vulnerability (or resilience). The same variables are factored into the equation; however, the function can now be described as an environment × gene interaction.
Recent work exploring molecular epigenetic mechanisms of gene expression (in humans and other mammalian systems) has provided evidence that the genome is subject to regulation by surrounding contexts (eg, cytoplasmic, cellular, nutritional, organismic, and sociopolitical). Environments are capable of regulating how genes are expressed. Epigenetic processes may be the key to understanding how forces distal to an individual, such as social and political conditions, along with more proximate individual (subjective) experiences can directly alter gene expression and thereby contribute to observed social inequalities in health. The very recent and powerful new results demonstrating that the epigenome is subject to environmental regulation could provide the direct link between the biological and social or psychological worlds. Understanding how genes are differentially regulated by experience will play a profound role in how we conceptualize health inequalities by informing our concepts of the somatization or embodiment of social inequalities. As our knowledge of epigenetic processes grows, so too does our capacity to develop early-life interventions to prevent and mitigate child health disparities.
PLASTICITY OF THE GENOME
Ecological models and theories of human health that emphasize the interaction of biological, behavioral, and environmental determinants are plentiful. In reality, however, few truly multilevel research programs or projects translate at a practical level. For those studying the genome, imagining the ways in which social and societal forces might alter how genes are regulated and expressed seems formidable. Conversely, those who are interested in social disparities might find it fantastical to assume that a working knowledge of biological mechanisms, such as gene regulation and gene expression (ie, genomic plasticity), might directly inform how they fundamentally conceptualize the embodiment of social experience.
For many social scientists a working knowledge of genetics is limited to a classical mendelian perspective; genes are the physical units of heredity, they are transmitted across generations, alleles are different “versions” of a gene (they exist in 2 forms; dominant or recessive), and a given genotype is related to an observable phenotype. This view of genetics has expanded dramatically since the early 1900s, yet most scientists who do not work at the level of the genome have had little or no exposure to recent findings in the field. As a consequence, we remain saddled with the antiquated central dogma that (1) the flow of information is unidirectional, from genes to environment, and (2) biological vulnerabilities are classically inherited (in contrast to being created by “vulnerable” environments or experience).2 This limited perspective of the genome has had a large influence on the theoretical framework of many academic disciplines. Table 1 shows the units of study or focus for some classic academic disciplines.
TABLE 1.
Focus of Classic Academic Disciplines
| Academic Discipline | Focus | Environment |
|---|---|---|
| Genetics | Gene transmission and genotypes | Cell nucleus, DNA |
| Molecular and cellular biology | Gene-cell regulation | System, cell, RNA, and DNA |
| Neuroscience | Nervous system and neurons | Brain |
| Medicine | Individual diseases | Individual person |
| Psychology and social psychology | Behavioral and individual differences | Individual person or animal and social space (SES, gender, race) |
| Sociology | Group processes | Individuals in groups |
| Ecology | Organisms and their environment | Physical world |
| Public health | Health protection, promotion, and restoration | Populations |
| Public policy | Effects of government decisions and indecision | Decision-making bodies |
The number of genes predicted (by molecular biologists and geneticists) to exist in the human genome was far higher (~150 000) than the number reported (~25 000) in the first draft of the Human Genome Project report.3,4 To provide an example, humans and the worm Caenorhabditis elegans have a similar number of genes, 20 000 to 25 000, despite large differences in organism size and complexity. Both species have significantly fewer genes than the corn plant, which has 40 000.5
Clearly, humans are greater than the sum of their genes. The emerging field of epigenetics focuses on the study of changes in gene expression that are not caused by changes in DNA sequence.6 The epigenome consists of DNA marks and modifications that control gene expression.7 The epigenome is innately plastic and can be programmed or reprogrammed by environmental experiences such as nutrition and stress.8–10 These epigenetic mechanisms provide the means through which social experiences can fundamentally and profoundly alter the regulation and expression of the genome without altering genotypes. Epigenetic processes are extremely active during early developmental windows when a young organism is growing. The epigenome, therefore, is extremely sensitive to dysregulation during early development when DNA synthesis rates are highest.7
The environment column in Table 1 helps in visualizing and understanding how environmental characteristics at every level (individual, family, community, etc) can potentially affect genome regulation. Again, the salient role of early life experiences is evident. We begin to mechanistically understand how both biological vulnerability and resilience can emerge from differences in the social experience.
STRESS AXIS AS A LOCUS OF VULNERABILITY OR RESILIENCY
Stress is a risk factor for several illnesses such as cardiovascular disease, type 2 diabetes, and depression.11 The pathways through which stressful events can promote the development of such divergent illnesses and compromised health seem to be mediated by activation of the same systems that ensure survival. Activation of the hypothalamic-pituitary-adrenal (HPA) axis in response to stress is a basic adaptive mechanism in mammals. This response governs the metabolic and cardiovascular responses to the challenges of everyday stressors and those associated with more prevailing chronic stress.
During stress, the hypothalamus (in the brain) releases corticotropin-releasing factor (CRF). CRF provokes the release of corticotropin from the pituitary gland, which, in turn, causes the release of glucocorticoids from the adrenal gland.12 The highly catabolic glucocorticoids act in synergy with catecholamines to produce lipolysis, glycogenolysis, and protein catabolism, which result in increased blood glucoses. These processes contribute to the survival of an organism during stress, in part by increasing the availability of energy substrates.13
Prolonged exposure to elevated stress hormones, however, can become problematic. Glucocorticoids, along with catecholamines, promote the suppression of anabolic processes, muscle atrophy, decreased insulin sensitivity, hypertension, amenorrhea, impotence, and impaired tissue repair. Cognitive and emotional states also change during stress.11 CRF release in the brain activates pathways that enhance vigilance when the organism is challenged. Again, activation of these pathways is quite adaptive under acute challenge or stress, but continued activation of these circuits can lead to impairments.14 Increased “wear and tear” on an organism that is subjected to repeated challenge or stress might chronically tax the HPA axis and ultimately lead to disease vulnerability (both mental and physical).
DEVELOPMENTAL PLASTICITY OF THE STRESS AXIS
The quality of early family-life events can influence the health of human, nonhuman primate, and other mammalian offspring throughout their lifetimes. Rodent models provide the best demonstration of parental calibration of the developing stress axis in young animals. In the rat, variations in maternal care are associated with the development of differences in behavioral and endocrine responses to stress in the offspring. Naturally occurring variations in maternal licking (the largest source of tactile stimulation) are associated with the development of individual differences in the HPA axis and behavioral responses to stress in the offspring.15,16 As adults, offspring of high-licking mothers are behaviorally less fearful and exhibit a more modest HPA-axis response to stress than off-spring of low-licking mothers.16 High-licking adult offspring have lower corticotropin and corticosterone responses after an acute challenge or stressor than low-licking adult off-spring. These responses are believed to be mediated, in part, by differential regulation and expression of the glucocorticoid receptor gene in the brain.
Extremely relevant is the plasticity inherent in the quality of parent-offspring interactions. The simple procedure of rat pup cross-fostering at birth is sufficient to reverse the phenotypes described above. Rat pups born to a high-licking mother but reared by a low-licking mother exhibit stress reactivity as adults that are indistinguishable from that of offspring born to and reared by a low-licking mother.
Conversely, offspring born to a low-licking mother but reared in a high-licking and grooming maternal environment exhibit stress-reactivity profiles of offspring born to and reared by a high-licking mother.17,18 The simple environmental intervention (adoption) affected not only the existing generation of animals growing up but also subsequent generations.
The cross-fostering of offspring early in life alters adult behavioral, hormonal, and neurobiological profiles. This finding suggests that the quality of maternal care early in life is directly involved in the development and programming of the HPA axis. Events or experiences that alter maternal care are thus capable of directly altering the development of the offspring. High-licking mothers (characterized while rearing a first litter) subjected to stress during gestation for a second litter exhibit decreased licking profiles once offspring are born. Thus, these offspring are reared under low-licking conditions and, as adults, exhibit exaggerated stress-reactivity profiles.19
Early postnatal experiences can calibrate the developing brain and neuroendocrine axis in expectation of future similar environments. The HPA axis profiles described above persist into adulthood and old age if environmental conditions remain stable.20,21 Very recent findings using the laboratory rat as a model report that differences in early life events are specifically altering the epigenetic processes that regulate expression of the glucocorticoid receptor in the brain. The genotypes of the developing animals are intact; however, their epigenomes are dramatically altered.22–24
RACIAL/ETHNIC DISPARITIES AND THE STRESS RESPONSE
Conceptualizing race as a product of environment × gene interactions (mediated by epigenetic processes) allows us to retreat from the discourse that focuses on biological or psychosocial vulnerabilities. An understanding of developmental neurogenomics allows us to transcend this debate and readily observe how the social experience of chronic and pervasive racism and discrimination is experienced somatically as a chronic stressor with inevitable deleterious outcomes. Blacks have higher disease rates relative to whites even when controlling for SES.25,26 The “weathering hypothesis” suggests that racial disparities exist with respect to the burden of stressors that accumulate over the lifetime.25 Those individuals who are subjected to the greatest levels of racial discrimination (ie, stress) have the worst mental health outcomes.27,28 If we approach the question of racial/ethnic disparities in health from the perspective of developmental neurogenomics, we can begin to understand how different lived social experiences leave their epigenomic imprint on an organism. The genotype is fixed. What remains plastic, and therefore subject to environmental regulation, is the epigenome. If we hypothesize that racial discrimination is capable of directly altering the epigenomic profiles of genes that are important to the stress response, we can then predict that targeting and ameliorating discrimination and racism should have an equally direct, potent, and protective effect on the stress-axis epigenome. This hypothesis would extend to other socially disadvantaged groups subject to the stress associated with discrimination.29–31 Rather than engaging in the nature-versus-nurture debate concerning race as a genetic or social construct, we now define race as an epigenomic construct in which genotype and the socially experienced world are perpetually entwined.
SES AND THE STRESS RESPONSE
Epidemiologic studies have identified a graded, continuous association between socioeconomic conditions and morbidity in adults and children of widely varied SES levels.32 This gradient of SES-health relationships leads to differences in morbidity at all SES levels. People in each class have poorer health outcomes than those in the class just above theirs and better outcomes than those in the class just below. This gradient suggests a nuanced link between SES and health that extends beyond the common effects of extreme poverty, which often includes poor nutrition, inadequate housing, environmental exposures, and lack of access to health services.
One plausible explanation for the SES-health association is that chronic activation of the stress response (stemming from the experiences of adversity that accompany lower and disadvantaged social positions) could compromise health.22,33,34 This hypothesis is consistent with evidence that relates lower SES with elevated basal activation of the stress axis in children35–37 and heightened neural reactivity to stressful challenges.38–41 An association of stress with subordinate social positions is also consistent with the previous finding that subjective estimates of social class might be a stronger predictor of health outcomes than objective indicators, such as job status, income, or wealth.42–44Previous research has also shown that dominance status in primate social hierarchies is similarly associated with health, even among captive animals with equal access to food, open environments, and veterinary care.35,45–47
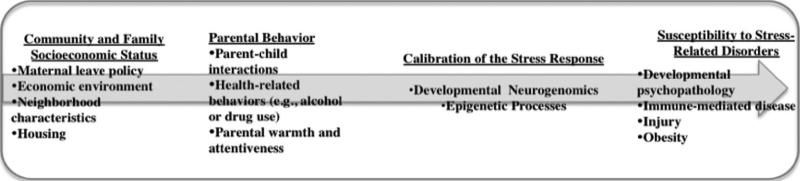
Stress and adversity among young children of low SES come in many forms and varieties. Poor children are routinely exposed to neighborhood violence; disorganized, dysfunctional schools; family turmoil; household chaos; and parental divorce.48 Among the most proximate stressors in the lives of poor children are the common experiences with harsh, unresponsive, and authoritarian parenting. Children of lower SES experience a disproportionate share of conflictive and punitive parent behavior, even in infancy.48–50 Disadvantaged homes produce not only more experiences with negative parenting but also fewer positive parent-child experiences. Class-based differences in children's disciplinary, socioemotional, cognitive, and linguistic experiences with primary caregivers are demonstrably related to brain development, particularly in the neural structures that are tied most closely to the ontogeny of reasoning capacity and language. Specifically, these experiences are linked to the executive functions involved in strategic planning and the regulation of emotion and social behavior.51 Figure 1 illustrates how broader social forces such as SES can modulate parenting profiles, which are critically involved in calibrating the stress axis in the developing child. We see how larger social forces in concert with more proximate social factors are able to impinge on the developing child to create biological vulnerabilities (another example of an environment × gene interaction).52
FIGURE 1.
Social calibration of the stress response. (Figure courtesy of W. T. Boyce, MD.)
A ROLE FOR DEVELOPMENTAL NEUROGENOMICS IN DISPARITIES RESEARCH?
Researchers, clinicians, and practitioners with an interest in understanding and ameliorating child health disparities necessarily represent a diverse group of professionals with broad notions of what matters most. By default, most of us have inherited the theoretical framework of our academic disciplines (captured in Table 1). We tend to focus on the levels of analyses most familiar to us. We acknowledge that disparities arise from the intersectionality and interactions of the genome with experiences as they occur in time; however, we rarely get to study them. The calibration and regulation of the stress axis in response to social forces provides us the opportunity to directly investigate this intersection. Developmental neurogenomic processes contribute to disparities in child health and well-being. Chronic stressors embedded in proximate family experiences (such as maternal depression and decreased parental care) are themselves subject to regulation by ultimate forces, including economic hardship, neighborhood safety, and high-quality housing. Proximate- and ultimate-level forces are biologically calibrating critical developmental processes in children, thereby influencing their vulnerability to and risk of pathology and illness. A developmental neurogenomics approach makes it possible to begin conceptualizing and understanding childhood health disparities from multiple perspectives and dimensions.
A fundamental understanding of the mechanisms by which social pro cesses are embodied and represented biologically free us from the constraints of having to defend a biological or psychosocial argument to explain disparities in health. The evidence at hand overwhelmingly demonstrates that the biological and psychosocial arguments are, indeed, one and the same. Moreover, because the genotype of an organism is fixed and immutable, all interventions should be targeted to optimize those variables and features that promote plasticity. For policy makers it should now be clear that efforts to eliminate or minimize social inequalities (caused by racism, SES, and class) would have direct biological effects on an individual. Moreover, interventions or programs that target the earliest developmental windows in the young child may prevent or significantly attenuate the cumulative effects of social disadvantage, which we know emerge over the life span. Funding mechanisms rarely target these most biologically plastic and labile developmental windows. Informed by biological mechanisms such as those described in this article, policies that create economic, social, or political change can all fundamentally affect the biological development of a single individual as well as whole populations. To grasp a full understanding of why and how social disparities emerge, we need to collectively move beyond the false dichotomy of the biological-psychosocial divide. An understanding of developmental neurogenomics provides us with the tools to do just that.
THE NEXT FRONTIER OF CHILD HEALTH DISPARITIES RESEARCH
Recent advances in the area of developmental biology and neurogenomics have provided evidence to demonstrate, mechanistically, how challenging or compromised early experiences can create biological vulnerabilities that affect both physical and mental health outcomes in the developing child. Shonkoff et al52 have made an excellent case for building a new framework of health promotion and disease prevention with neuroscience, molecular biology, and childhood roots of health disparities at the core. Evidence of this novel agenda is beginning to emerge at the levels of research,53 policy,54 and practice.55 However, educational or training opportunities that marry the disciplines of neuroscience, molecular biology, and childhood health disparities are virtually nonexistent (at least at the level of graduate education). A pedagogical approach inclusive of basic developmental biology, molecular biology, and neuroscience offered to the next generation of health-disparities researchers would create a powerful element for change. A breadth of biological training early in an academic trajectory should serve to “lessen” the silo-based approaches often used to address disparities-related research. No longer would the framework be one of biological, psychological, or social vulnerabilities but, rather, a framework focused on how these processes intersect to create vulnerability (or resilience). Students taught the fundamental concepts of neuroscience, developmental genomics, and HPA/stress-axis function as they relate to social inequalities and health can take the basic biological knowledge they have acquired and immediately translate it to disciplines as diverse as public policy, community health, medicine, clinical psychology, social work, and education. By providing the current generation of health-disparities researchers with a solid background in relevant biological mechanisms we will, in essence, be cultivating the next generation of researchers, practitioners, and policy makers who can readily endorse the assertion that early adversity can create health vulnerabilities.
ABBREVIATIONS
- SES
socioeconomic status
- HPA
hypothalamic-pituitary-adrenal
- CRF
corticotropin-releasing factor
Footnotes
The views presented in this article are those of the author, not the organizations with which she is affiliated.
FINANCIAL DISCLOSURE: The author has indicated she has no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL. Socioeconomic inequalities in health: no easy solution. JAMA. 1993;269(24):3140–3145. [PubMed] [Google Scholar]
- 2.Crick FH. On protein synthesis. Symp Soc Exp Biol. 1958;12:138–163. [PubMed] [Google Scholar]
- 3.Venter JC, Adams MD, Myers EW, et al. The sequence of the human genome. Science. 2001;291(5507):1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 4.Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 5.Kim E, Goren A, Ast G. Alternative splicing: current perspectives. Bioessays. 2008;30(1):38–47. doi: 10.1002/bies.20692. [DOI] [PubMed] [Google Scholar]
- 6.Riggs A, Martienssen R. Introduction. In: Russo EA, Martienssen RA, Riggs AD, editors. Epigenetic Mechanisms of Gene Regulation. Cold Spring Harbor Laboratory Press; Plainview, NY: 1996. pp. 29–46. [Google Scholar]
- 7.Dolinoy DC, Jirtle RL. Environmental epigenomics in human health and disease. Environ Mol Mutagen. 2008;49(1):4–8. doi: 10.1002/em.20366. [DOI] [PubMed] [Google Scholar]
- 8.Weaver IC, Diorio J, Seckl JR, Szyf M, Meaney MJ. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann N Y Acad Sci. 2004;1024:182–212. doi: 10.1196/annals.1321.099. [DOI] [PubMed] [Google Scholar]
- 9.Waterland RA, Dolinoy DC, Lin JR, Smith CA, Shi X, Tahiliani KG. Maternal methyl supplements increase offspring DNA methylation at Axin Fused. Genesis. 2006;44(9):401–406. doi: 10.1002/dvg.20230. [DOI] [PubMed] [Google Scholar]
- 10.Waterland RA, Jirtle RL. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition. 2004;20(1):63–68. doi: 10.1016/j.nut.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 11.McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 12.Koob GF, Heinrichs SC, Menzaghi F, Pich EM, Britton KT. Corticotropin releasing factor, stress and behavior. Semin Neurosci. 1994;6:221–229. [Google Scholar]
- 13.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 14.Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23(3):219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- 15.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95(9):5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu D, Diorio J, Tannenbaum B, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 17.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286(5442):1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 18.Francis DD, Champagne FA, Liu D, Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann N Y Acad Sci. 1999;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- 19.Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry. 2006;59(12):1227–1235. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Meaney MJ, Aitken DH, Sharma S, Viau V. Basal ACTH, corticosterone and corticosterone-binding globulin levels over the diurnal cycle, and age-related changes in hippocampal type I and type II corticosteroid receptor binding capacity in young and aged, handled and non-handled rats. Neuroendocrinology. 1992;55(2):204–213. doi: 10.1159/000126116. [DOI] [PubMed] [Google Scholar]
- 21.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18(3):195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 22.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood) 2002;21(2):60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- 23.Szyf M, Weaver I, Meaney M. Maternal care, the epigenome and phenotypic differences in behavior. Reprod Toxicol. 2007;24(1):9–19. doi: 10.1016/j.reprotox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Weaver IC, Champagne FA, Brown SE, et al. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25(47):11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Astone NM, Ensminger M, Juon HS. Early adult characteristics and mortality among inner-city African American women. Am J Public Health. 2002;92(4):640–645. doi: 10.2105/ajph.92.4.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams DR. Race, socioeconomic status, and health: the added effects of racism and discrimination. Ann N Y Acad Sci. 1999;896:173–188. doi: 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
- 27.Kessler RC, Mickelson KD, Williams DR. The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. J Health Soc Behav. 1999;40(3):208–230. [PubMed] [Google Scholar]
- 28.Williams DR, Yan Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: socioeconomic status, stress, and discrimination. J Health Psychol. 1997;2(3):335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- 29.Krieger N. Stormy weather: race, gene expression, and the science of health disparities. Am J Public Health. 2005;95(12):2155–2160. doi: 10.2105/AJPH.2005.067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krieger N. Does racism harm health? Did child abuse exist before 1962? On explicit questions, critical science, and current controversies: an ecosocial perspective. Am J Public Health. 2003;93(2):194–199. doi: 10.2105/ajph.93.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krieger N, Davey Smith G. “Bodies count,” and body counts: social epidemiology and embodying inequality. Epidemiol Rev. 2004;26:92–103. doi: 10.1093/epirev/mxh009. [DOI] [PubMed] [Google Scholar]
- 32.Adler NE, Boyce WT, Chesney MA, Cohen S. Socioeconomic status and health: the challenge of the gradient. Am Psychol. 1994;49(1):15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 33.Siegrist J, Marmot M. Health inequalities and the psychosocial environment: two scientific challenges. Soc Sci Med. 2004;58(8):1463–1473. doi: 10.1016/S0277-9536(03)00349-6. [DOI] [PubMed] [Google Scholar]
- 34.Kristenson M, Eriksen HR, Sluiter JK, Starke D, Ursin H. Psychobiological mechanisms of socioeconomic differences in health. Soc Sci Med. 2004;58(8):1511–1522. doi: 10.1016/S0277-9536(03)00353-8. [DOI] [PubMed] [Google Scholar]
- 35.Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosom Med. 2006;68(3):414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- 36.Lupien SJ, Fiocco A, Wan N, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30(3):225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Lupien SJ, King S, Meaney MJ, McEwen BS. Child's stress hormone levels correlate with mother's socioeconomic status and depressive state. Biol Psychiatry. 2000;48(10):976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- 38.Suchday S, Krantz DS, Gottdiener JS. Relationship of socioeconomic markers to daily life ischemia and blood pressure reactivity in coronary artery disease patients. Ann Behav Med. 2005;30(1):74–84. doi: 10.1207/s15324796abm3001_9. [DOI] [PubMed] [Google Scholar]
- 39.Sloan RP, Huang MH, Sidney S, Liu K, Williams OD, Seeman T. Socioeconomic status and health: is parasympathetic nervous system activity an intervening mechanism? Int J Epidemiol. 2005;34(2):309–315. doi: 10.1093/ije/dyh381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steptoe A, Willemsen G, Kunz-Ebrecht SR, Owen N. Socioeconomic status and hemodynamic recovery from mental stress. Psychophysiology. 2003;40(2):184–191. doi: 10.1111/1469-8986.00020. [DOI] [PubMed] [Google Scholar]
- 41.Owen N, Poulton T, Hay FC, Mohamed-Ali V, Steptoe A. Socioeconomic status, C-reactive protein, immune factors, and responses to acute mental stress. Brain Behav Immun. 2003;17(4):286–295. doi: 10.1016/s0889-1591(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 42.Goodman E, Adler NE, Daniels SR, Morrison JA, Slap GB, Dolan LM. Impact of objective and subjective social status on obesity in a biracial cohort of adolescents. Obes Res. 2003;11(8):1018–1026. doi: 10.1038/oby.2003.140. [DOI] [PubMed] [Google Scholar]
- 43.Ostrove JM, Adler NE, Kuppermann M, Washington AE. Objective and subjective assessments of socioeconomic status and their relationship to self-rated health in an ethnically diverse sample of pregnant women. Health Psychol. 2000;19(6):613–618. doi: 10.1037//0278-6133.19.6.613. [DOI] [PubMed] [Google Scholar]
- 44.Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physio logical functioning: preliminary data in healthy white women. Health Psychol. 2000;19(6):586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- 45.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 46.Abbott DH, Keverne EB, Bercovitch FB, et al. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43(1):67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- 47.Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM. Social status, environment, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1982;2(5):359–368. doi: 10.1161/01.atv.2.5.359. [DOI] [PubMed] [Google Scholar]
- 48.Dodge KA, Pettit GS, Bates JE. Socialization mediators of the relation between socioeconomic status and child conduct problems. Child Dev. 1994;65:649–665. (2 spec No.) [PubMed] [Google Scholar]
- 49.Evans GW. The environment of childhood poverty. Am Psychol. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- 50.McLoyd VC. Socioeconomic disadvantage and child development. Am Psychol. 1998;53(2):185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- 51.Farah MJ, Shera DM, Savage JH, et al. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110(1):166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 52.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 53.McGowan PO, Sasaki A, D'Alession AC, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Middlebrooks JS, Audage NC. The Effects of Childhood Stress on Health Across the Lifespan. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2008. [Google Scholar]
- 55.Heckman JJ. Skill formation and the economics of investing in disadvantaged children. Science. 2006;312(5782):1900–1902. doi: 10.1126/science.1128898. [DOI] [PubMed] [Google Scholar]