Abstract
Purpose
To minimize toxicity while maintaining tumor coverage with stereotactic body radiation therapy (SBRT) for centrally or superiorly located stage I non-small-cell lung cancer (NSCLC), we investigated passive-scattering proton therapy (PSPT) and intensity-modulated proton therapy (IMPT).
Materials and Methods
Fifteen patients with centrally or superiorly located (within 2 cm of critical structures) Stage I NSCLC were treated clinically with 3-dimensional photon SBRT (50 Gy in 4 fractions). Photon SBRT plan was compared with the PSPT and IMPT plans. The maximum tolerated dose (MTD) was defined as the dose that exceeded the dose-volume constraints in the critical structures.
Results
Only 6 photon plans satisfied the >95% planning target volume (PTV) coverage and MTD constraints, compared to 12 PSPT plans (p = 0.009) and 14 IMPT plans (p = 0.001). Compared with the photon SBRT plans, the PSPT and IMPT plans significantly reduced the mean total lung dose from 5.4 Gy to 3.5 Gy (p < 0.001) and 2.8 Gy (p < 0.001) and reduced the total lung volume receiving 5 Gy, 10 Gy and 20 Gy (p < 0.001). When the PTV was within 2 cm of the critical structures, the PSPT and IMPT plans significantly reduced the mean maximal dose to the aorta, brachial plexus, heart, pulmonary vessels, and spinal cord.
Conclusions
For centrally or superiorly located stage I NSCLC, proton therapy, particularly IMPT, delivered ablative doses to the target volume and significantly reduced doses to the surrounding normal tissues compared with photon SBRT.
Keywords: stereotactic body radiation therapy, non-small cell lung cancer, centrally located lesion, proton therapy, stage I
INTRODUCTION
Stereotactic body radiation therapy (SBRT) appears to be as effective as surgery for these patients with medical inoperable early stage lung cancer, with local control rates typically exceeding 80% [1–3]. However, SBRT of patients with tumors located centrally or superiorly in the lung and near critical structures is particularly challenging due to the potential of complications from radiation-induced damage to these critical structures. Timmerman et al. found that patients treated with 54 to 60 Gy in 3 fractions for tumors in the hilar/pericentral regions (defined as within 2 cm of the bronchial tree) had a higher chance of developing grade 3 to 5 adverse effects and a lower 2-year rate of freedom from severe toxicity compared with peripheral lesions [2]. Our previous study showed that when we used an individualized approach and respected critical normal-tissue-dose volume constraints, a fractionation scheme of 50 Gy in 4 fractions was safe and practical [3]. However, the planning target volume (PTV) coverage had to be compromised in some cases to keep the dose to critical normal tissues below the dose-volume constraints. In addition, our recent publication [4] for SBRT using 40 or 50 Gy in 4 fractions in patients who received prior chest radiation for lung cancer showed 50% of patients experienced worsening of dyspnea after SBRT, 30% of patients experienced chest wall pain and 8% grade 3 esophagitis.
Proton therapy may be able to reduce toxicity, given its ability to deposit most of the proton energy when the end of the proton beam’s finite range is reached, a phenomenon that has the potential to increase normal tissue sparing and reduce the radiation dose to the lung compared to photon therapy [5, 6]. Passive-scattering proton therapy (PSPT) uses 3-dimensional proton treatment planning with beam range shifter to determine distal edge of beam, compensator to form conformal dose distribution and aperture to limit the field size [5]. In contrast to PSPT, intensity-modulated proton therapy (IMPT) using scanning beam therapy can simultaneously optimize the intensities and energies of all pencil beams using an objective function that takes into account the targets and normal tissue constraints [6].
However, using PSPT or IMPT to treat clinically challenging, centrally or superiorly located, Stage I NSCLC can pose some challenges. PSPT is essentially 3-dimensional conformal RT, and its ability to create a complicated dose distribution to avoid the intervening and nearby critical normal structures is limited. For scanning beam proton therapy, however, the in-air full-width half maximum of the scanning spots in our proton therapy facility ranges from 1.2 cm to 3.5 cm, and it is still unclear whether such large scanning spots will allow clinically significant sparing of the critical structures near the tumor.
In this virtual clinical study, we investigated the benefit of PSPT and IMPT compared to photon SBRT to maximize normal tissue sparing and preserve target volume coverage in patients with clinically challenging, centrally or superiorly located, Stage I NSCLC.
METHODS AND MATERIALS
Patients, Treatment, and Study Design
For this study, we identified 15 patients with medically inoperable, centrally (all cases) and superiorly located (6 cases), Stage I NSCLC who had actually undergone 3-dimensional photon SBRT at The University of Texas M. D. Anderson Cancer Center. Centrally located tumors were defined as tumors within 2 cm of critical structures, including the tracheal (above carina) bronchial tree (carina, right and left main bronchi, right and left upper lobe bronchi, bronchus intermedius, right middle lobe bronchus, lingular bronchus, right and left lower lobe bronchi), esophagus, heart, major vessels, and/or spinal cord. Superiorly located tumors were defined as tumors in the lung apices or within 2 cm of the brachial plexus. These patients were chosen to demonstrate the unique challenges in sparing these critical structures.
Photon Stereotactic Body Radiation Therapy
Target volume delineation
All patients underwent 4-dimensional computed tomography simulation as we have described before [3]. The gross tumor volume (GTV) was contoured as the envelope of motion of the GTV on a reconstructed maximal intensity projection (MIP) image at the lung window level and verified across all phases of the 4D-CT dataset. We defined the clinical target volume (CTV) as the gross tumor volume (GTV) plus an 8-mm margin to account for microscopic disease. We defined the PTV as the CTV plus a 3-mm margin to account for setup uncertainty and residual tumor motion. Normal tissues were contoured in the free breath CT dataset. Contours for the major vessels, bronchial tree, esophagus, heart, spinal cord, and brachial plexus were added to the photon SBRT plans as needed and used in the virtual proton plans. The dosimetric data were extracted for the physician-approved photon SBRT plans generated using the Pinnacle treatment planning system (Philips Medical Systems, Bothell, WA). The photon SBRT plans were generated by our experienced thoracic dosimetrists based on our institutional SBRT guidelines for NSCLC [3]. The most current dose-volume constraints used at our institution are shown in Table 1. All photon SBRT plans prescribed 50 Gy in 12.5-Gy fractions to the PTV with heterogeneity corrections using Pinnacle’s superposition-convolution algorithm. All photon SBRT plans were normalized such that 95% of the PTV received 100% of the prescription dose. The isodose line distributions and dose-volume histograms for the photon SBRT plans served as the standard for comparison with the PSPT and IMPT plans.
Table 1.
SBRT maximum tolerated doses for critical structures
| Organ | Maximum Tolerated Dose, Gy |
|---|---|
| Brachial plexus | 40 |
| Bronchial tree | 40 |
| Esophagus | 35 |
| Heart | 40 |
| Major vessels | 40 |
| Spinal cord | 20 |
| Trachea | 35 |
| Total lung, V5* | 40 |
| Total lung, V10* | 30 |
| Total lung, V20* | 20 |
% of total lung volume receiving 5, 10, or 20 Gy, respectively.
Passive-Scattering Proton Therapy Planning
The same 4-dimensional computed tomography images, contours, and dose-volume constraints used for the photon SBRT plans were used for generating the PSPT and IMPT plans. The proton plans were designed using the Varian Eclipse planning system (Eclipse Version 8.1.20, Varian Medical Systems, Inc., Palo Alto, CA). The CTV from the photon SBRT plans was used for plan design, and the PTV was used for target volume coverage evaluation. For the PSPT plans, at least 95% of the PTV had to receive 100% of the prescription dose and 100% of the PTV must receive 95% of the prescription dose. Each plan had 3–4 coplanar beam angles designed in an attempt to minimize the exit dose into the lung parenchyma. For each beam, we designed an aperture block 1.3 cm from the outer border of the CTV. Then, we calculated the beam-line properties to determine the user-defined proximal margin/distal margin, designed a compensator to shape the distal margin of the spread-out Bragg peak and added a smearing margin to the compensator to smear out the dose as described by our previous publications and Moyers et al [5–7].
Intensity-Modulated Proton Therapy Planning
The same beam angles used in the PSPT plans were used for generating the IMPT plans. These plans were generated using an inverse planning technique on Varian’s Eclipse planning software. The PTV was used for both plan design and evaluation. We assigned a lateral margin of 0.2 cm and set the distal margin to 0 cm. The dose grid used in the calculations was set to 0.4 cm. Dose-volume objectives were entered into the planning software for specific critical structures, and the target volume was based on the anatomical relationships and the assessment of risk. The critical structure objectives were prioritized based on the risk that the dose to the structure would exceed the MTD. The IMPT plans were normalized in a manner similar to that used for the PSPT plans.
Statistical Analyses
We used a 2-tailed paired-samples t test for statistical analysis (SPSS software v.16.0, SPSS Inc., Chicago, IL). We considered a p value of ≤ 0.05 to be statistically significant.
RESULTS
For all 15 patients, the median GTV was 6.49 cc (range, 1.63–50.92 cc). The median number of critical structures within 2 cm of the PTV was 3 (range, 1–6).
Based on our MTD objectives outlined in Table 1, only 6 photon SBRT plans satisfied PTV coverage and all MTDs, compared to 12 PSPT plans (p = 0.009) and 14 IMPT plans (p = 0.001). For each critical structure, the MTD was exceeded in at least one of the photon SBRT plans.
Lungs
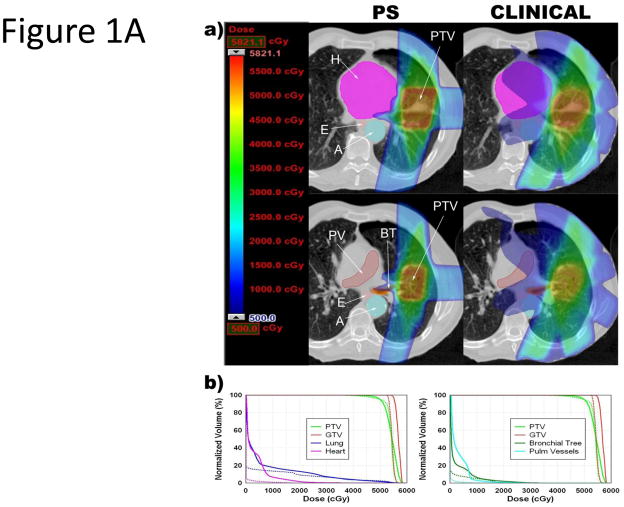
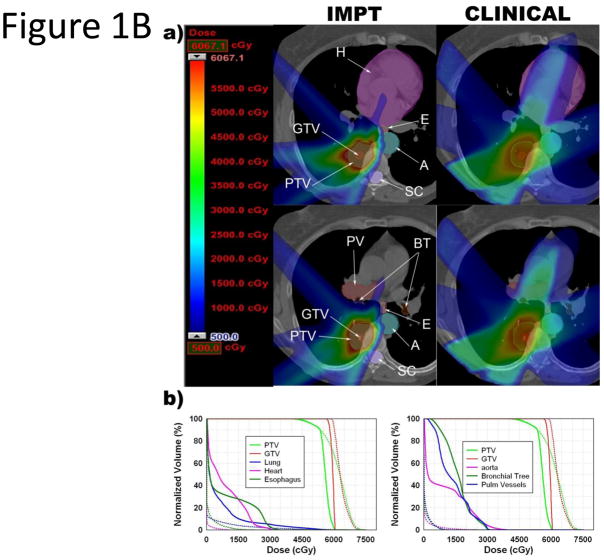
As shown in Table 2 and Figure 1A/B, the PSPT plans significantly reduced the total mean lung dose (MLD; p <0.001), mean volume of total lung receiving 5 Gy (V5; p <0.001), mean volume of total lung receiving 10 Gy (V10; p <0.001), and mean volume of total lung receiving 20 Gy (V20; p = 0.039). Similarly, the IMPT plans further significantly reduced the total MLD (p <0.001), mean total lung V5 (p <0.001), mean total lung V10 (p <0.001), and mean total lung V20 (p = 0.004).
Table 2.
Mean critical total lung volumes received on the photon SBRT, PSPT, and IMPT plans
| Critical Lung Volumes | Photon SBRT | PSPT | p* | IMPT | p† | p‡ |
|---|---|---|---|---|---|---|
| Mean total lung dose, Gy (SE) | 5.4 (0.5) | 3.5 (0.4) | < 0.001 | 2.8 (0.3) | < 0.001 | < 0.001 |
| Mean total lung V5, % (SE) | 22.9 (2.1) | 11.4 (1.1) | < 0.001 | 10.0 (1.1) | < 0.001 | 0.001 |
| Mean total lung V10, % (SE) | 15.2 (1.5) | 10.3 (1.0) | < 0.001 | 8.2 (1.0) | < 0.001 | < 0.001 |
| Mean total lung V20, % (SE) | 8.7 (1.1) | 6.7 (0.8) | 0.039 | 5.5 (0.6) | 0.004 | < 0.001 |
Abbreviations: IMPT = intensity-modulated proton therapy; PSPT = passive-scattering proton therapy; SBRT = stereotactic body radiation therapy; SE = standard error; V5 = volume of total lung receiving 5 Gy; V10 = volume of total lung receiving 10 Gy; V20 = volume of total lung receiving 20 Gy.
Photon SBRT vs PSPT
Photon SBRT vs IMPT
PSPT vs IMPT
Figure 1.
Comparison of PSPT, IMPT and photon SBRT for tumors near critical structures. A. PSPT (PS) or clinical photon (Clinical) SBRT for lesion near the bronchial tree and heart. B. IMPT or clinical photon (Clinical) SBRT for lesion near the bronchial tree, aorta, esophagus, and heart. (a) Color wash dose distributions shown with corresponding scale; maximum determined by plan with lower PTVmax. In the IMPT images, the gray zone seen inside the PTV represents where the IMPT plan had a dose higher than the scale maximum. Abbreviations: BT = bronchial tree; E = esophagus; SC = spinal cord; A = aorta; H = heart; PV = pulmonary vessel; PTV = plan target volume; GTV = gross target volume. (b) Dose-volume histograms. Dotted lines = PSPT or IMPT plan; solid lines = photon SBRT plan.
Major Vessels
The MTD to the aorta was exceeded in 5 photon SBRT plans, 2 PSPT plans, and 1 IMPT plan. The aorta and pulmonary vessels were within 2 cm of the PTV in 11 and 7 cases, respectively, in the photon SBRT plans. For those cases, the PSPT and IMPT plans significantly reduced the mean aorta maximal dose received (Dmax; p = 0.02 and p = 0.014, respectively) and the pulmonary vessels Dmax (p = 0.002 and p = 0.003, respectively; Table 3 and Figure 1A/B) when compared to the photon SBRT plans.
Table 3.
Maximum doses received on the photon SBRT, PSPT, and IMPT plans
| Critical normal structures | Cases | Mean maximum dose received on photon SBRT plan, Gy (SE) | Mean maximum dose received on PSPT plan, Gy (SE) | p* | Mean maximum dose received on IMPT plan, Gy (SE) | p† | p‡ |
|---|---|---|---|---|---|---|---|
| Aorta | 11 | 34.3 (3.8) | 27.9 (5.3) | 0.02 | 25.5 (5.7) | 0.014 | 0.292 |
| Brachial plexus | 6 | 24.1 (5.3) | 11.1 (2.8) | 0.01 | 7.0 (2.8) | 0.006 | 0.007 |
| Bronchial tree | 8 | 22.9 (4.3) | 18.2 (4.4) | 0.221 | 13.9 (4.1) | 0.014 | 0.093 |
| Esophagus | 7 | 27.2 (5.7) | 25.8 (3.6) | 0.698 | 15.6 (4.1) | 0.005 | 0.019 |
| Heart | 4 | 45.1 (8.8) | 25.5 (6.5) | 0.036 | 23.6 (7.3) | 0.032 | 0.202 |
| Pulmonary vessels | 7 | 36.2 (3.4) | 24.3 (2.7) | 0.002 | 18.0 (4.7) | 0.003 | 0.115 |
| Spinal cord | 9 | 20.7 (1.0) | 17.4 (1.1) | 0.02 | 12.6 (1.3) | <0.001 | 0.006 |
| Trachea | 3 | 18.4 (5.3) | 19.6 (5.0) | 0.909 | 13.5 (4.6) | 0.598 | 0.215 |
Abbreviations: IMPT = intensity-modulated proton therapy; PSPT = passive-scattering proton therapy; SBRT = stereotactic body radiation therapy; SE = standard error.
Photon SBRT vs PSPT
Photon SBRT vs IMPT
PSPT vs IMPT
Brachial Plexus
The MTD was exceeded in the brachial plexus in 1 photon SBRT plan and 0 PSPT or IMPT plans. For the 6 cases in which the tumor was located in the upper half of the upper lobes, the PSPT and IMPT plans significantly reduced the mean brachial plexus Dmax (p = 0.01 and p = 0.006, respectively, Table 3) compared to the photon SBRT plans.
Spinal Cord
The MTD was exceeded in the spinal cord in 5 photon SBRT plans, 1 PSPT plan, and 0 IMPT plans. The spinal cord was within 2 cm of the PTV in 9 cases. For these cases, the PSPT and IMPT plans significantly reduced the mean spinal cord Dmax (p = 0.02 and p < 0.001, respectively, Table 3).
Esophagus
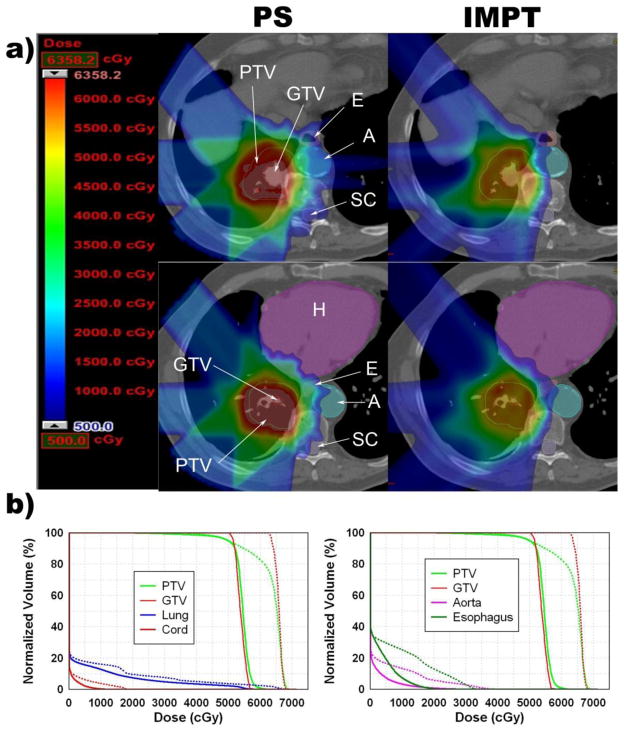
The MTD was exceeded in the esophagus in 2 photon SBRT plans, 2 PSPT plans, and 0 IMPT plans (Table 3 and Figure 1B). The esophagus was within 2 cm of the PTV in 7 cases. For these cases, the PSPT plans showed a trend toward the reduction of the mean esophagus Dmax, but this did not reach statistical significance (p = 0.698). In contrast, the IMPT significantly reduced the mean esophagus Dmax (p = 0.005). In 1 patient, the IMPT plan kept the MTD in the aorta, spinal cord, bronchial tree and esophagus below the dose-volume constraints, but the photon or PSPT SBRT plan did not (Figure 2).
Figure 2.
Improvement of IMPT compared with PSPT SBRT for a tumor close to the bronchial tree, esophagus, heart, spinal cord and aorta. (a) Color wash dose distributions shown with corresponding scale; maximum determined by plan with lower PTVmax. In the IMPT images, the gray zone seen inside the PTV represents where the IMPT plan had a dose higher than the scale maximum. (b) Dose-volume histograms. Dotted lines = PSPT plan; solid lines = IMPT SBRT plan.
Heart
The MTD was exceeded in the heart in 3 photon SBRT plans and 0 PS or IMPT plans. The heart was within 2 cm of the PTV in 4 cases. For these cases, the PSPT and IMPT plans reduced the mean heart Dmax (p = 0.036 and p = 0.032, respectively, Table 3). In 1 patient, the MTD was exceeded in the heart and bronchial tree in the photon SBRT plan, but these structures were spared in the PSPT plan (Figure 1A/B).
Bronchial Tree
The MTD was exceeded in the proximal bronchial tree in 1 photon SBRT plan and 0 PSPT or IMPT plans. The MTD was not exceeded in the trachea in any of the plans. The proximal bronchial tree and trachea were within 2 cm of the PTV in 8 and 3 cases, respectively. For the 8 proximal bronchial tree cases, the PSPT plans showed a trend toward reducing the mean bronchial tree Dmax, though this did not reach statistical significance (p = 0.221). In contrast, the IMPT plans were able to significantly reduce the mean bronchial tree Dmax (p = 0.014) (Table 3, Figure 1A/B). For the 3 trachea cases, the PSPT plans showed a trend toward increasing the mean trachea Dmax, (p = 0.909), while the IMPT plans showed a trend toward reducing the mean trachea Dmax, but neither achieved statistical significance (p = 0.598).
As shown in Tables 2, 3 and Figure 2, IMPT significantly reduced the dose to the lungs and several critical structures near the lungs when compared to PSPT.
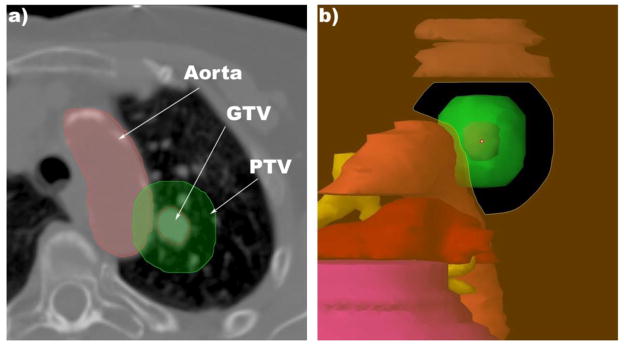
In our study, PSPT achieved desirable normal tissue sparing without sacrificing target coverage in most cases. However, we observed 2 cases in which PSPT fell short of achieving the requisite normal tissue sparing: in 1 case, a significant amount of the PTV overlapped with a critical structure (i.e., the aorta); and in another case, the tumor location was in a cul-de-sac surrounded on several sides by critical structures, thus limiting the beam angle choices. Because IMPT is capable of modifying the dose distribution to specifically avoid critical structures, IMPT was able to successfully achieve the dose-volume objectives for the 2 patients with tumors in a pseudo cul-de-sac (Figure 2). However, for the case with significant PTV/aorta overlap (Figure 3), both the PSPT and IMPT plans had to sacrifice the PTV coverage to below 95% to keep the aorta dose under the MTD.
Figure 3.
SBRT for a lesion attached to aorta. (a) This axial cut shows the proximity of the GTV to the aorta and the degree of overlap between the aorta and the PTV. (b) Eye view of the beam for 1 of the fields for the PSPT plan.
Compared with photon SBRT, we did not see significant increased dose delivered to chest wall including skin and ribs in proton therapy. In fact, in the most cases, the chest wall dose was reduced in proton compared with photon (see Fig. 1 and 2).
DISCUSSION
Our results show that for most patients with clinically challenging, centrally or superiorly located, Stage I NSCLC, proton therapy was capable of delivering ablative doses to the target volume and significantly reduced doses to the surrounding normal tissues compared with photon SBRT. In particular, IMPT achieved significant dose reductions for the lungs and select critical structures beyond those achieved with PSPT.
Several studies using both standard fractionated and hypofractionated schemes support our conclusion on using proton therapy for treating Stage I NSCLC [8–10]. In these studies, when a high dose of protons was delivered, the reported local control rates were > 90% at 2 years. Most of these studies reported no acute or severe late toxicities or treatment-related fatalities [8–10]. Our recent data using 87.5-Gy proton therapy in 2.5-Gy fractions in centrally located or T2-T3 NSCLC showed a local control rate of 93%, with no Grade 3 or above toxicity except Grade 3 skin reaction [11].
There were few papers addressing the issue of IMPT in lung cancer. Georg et al. conducted a dosimetric study of PSPT and IMPT compared to 3-dimensional conformal photon therapy delivered with 45-Gy SBRT in 3 fractions [12]. The authors reported 7–9% and >10% improvement of lung V20 using PSPT and IMPT, respectively. Both proton techniques achieved full sparing of the contralateral lung and superior sparing of the heart. Georg et al. concluded that only small dosimetric differences were found between photons and protons for SBRT of lung lesions. Improved dosimetric data might not be clinically significant for patients for whom the clinical objectives are easily met by both photon and proton therapy. However, for patients with clinically challenging tumors, such as patients with centrally or superiorly located lesions, for whom it is hard to remain within clinical dose-volume constraints using photon therapy, additional sparing of surrounding critical structures from high-dose proton therapy may prevent severe or even life-threatening adverse effects [2–4].
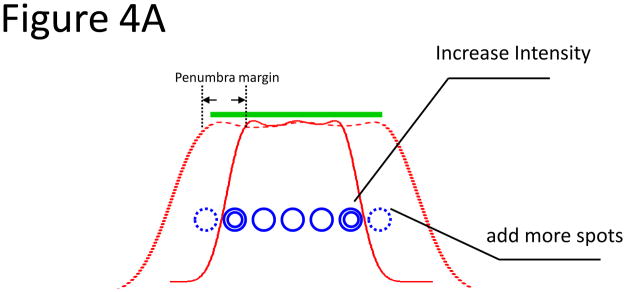
Although IMPT can reduce the dose to critical structures by optimizing the intensity of individual pencil beam spots, the finite size of the scanned pencil beam spots (1–3.5 cm) combined with the scattering in air downstream of the vacuum window (low density of lung parenchyma) degrades the lateral penumbra, which can be inferior to that of the passively scattered beam collimated by the aperture in the machine [13]. If the lateral penumbra is still the primary tool to spare the organs at risk, we would expect that the IMPT plans would not offer better normal tissue sparing in small lesions (< 4 cm). However, for IMPT, the intensity of the beam spots just inside the field edge may be increased to partially compensate for the scattered radiation flowing out of the target region, thus sharpening the penumbra (Figure 4). In our study, we took advantage of this special margin reduction capability offered by IMPT to achieve a small lateral margin of 0.2 cm. The lateral margin in the treatment planning was primarily used to compensate for the penumbra (about 1.0 cm in size) for our scanning beam if the intensity of beam spots just inside the field edge was not increased. In Figure 4b, we compared the dose-volume histograms for the plan designed using a lateral margin of 0.2 cm (solid line) and 1.0 cm (dashed line). When the larger lateral penumbra margin of 1.0 cm was used, sparing of the spinal cord, aorta, and pulmonary vessels was compromised. For this plan, the MTD to the spinal cord increased from 13.2 Gy to 21.5 Gy, and the dose-volume criteria were not met.
Figure 4.
A. Illustration showing the concept of using intensity modulation to reduce the penumbra. The thick green line denotes the target to be uniformly covered, and the solid red curve shows the dose profile calculated using the spots denoted by the single blue circles. The green target was not adequately covered owing to inadequate penumbra margin. Two options were used to cover the target: (1) increase the number of spots at the edge, denoted by dashed blue circles (large penumbra margin), and (2) increase the intensity of the spots at the edge, denoted by the double circles (small penumbra margin). The red-dashed curve shows the dose profile that adequately covered the target using those 2 approaches. B. Application of the 2 approaches on patients who received proton SBRT. Dose volume histogram of a plan using a 1-cm lateral/penumbra margin (dashed line) and 0.2-cm lateral/penumbra margin (solid line).
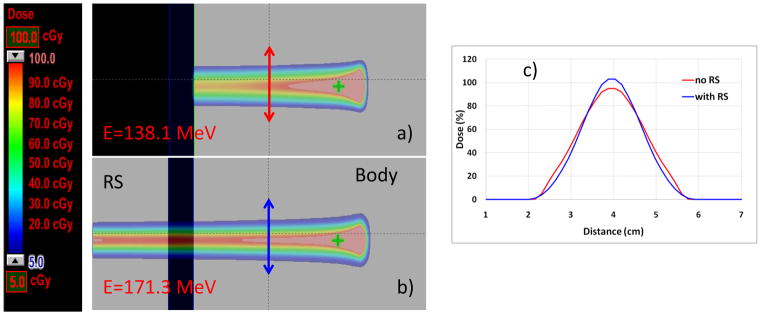
We also studied the possibility of using a range shifter to reduce the penumbra. The beam energies used were based upon the distal and proximal margins, and the spot sizes were decided by these energies. There could be different proton pencil beam energies reaching the same depth of the patient using a range shifter by, for example, using a lower-energy beam or higher-energy beam spot with a range shifter in the beam line. By the nature of manufacture of proton therapy, the lower the energy of the spot, the larger the beam spot sizes. For example, the in-air full-width half maximums of proton pencil beam spots ranging from 3.5 cm to 1.4 cm corresponds to energy levels of 72.5 MeV and 198.3 MeV, respectively. Because of that, one would expect that the spot size in the patient receiving the higher-energy pencil beam with a range shifter might be smaller than that in the patients receiving the lower energy pencil beam (Figure 5). However, when we compared the plans designed using the higher-energy beams with range shifters to produce small spot size beams and the plans designed using lower energy without a range shifter to produce large spot size beams, we found that the 2 approaches essentially led to similar dose-volume histograms. Although the GTV was only 6.49 cc (range, 1.63–50.92 cc), the PTV could be around 100 cc. Typically in IMPT, there are about 300 to 600 pencil beam spots selected for each beam. Our results indicated that the relatively larger spots could still lead to excellent normal tissue sparing for relatively smaller tumors. Our findings are significant in that the plans designed using larger spots should be relatively robust against tumor motion. Currently, we are undertaking a study to investigate the impact of beam spot size on the robustness of IMPT plans.
Figure 5.
(a) and (b) Illustrations of the concept of using a range shifter (RS) to reduce the spots size: dose distributions of a single spot with energy 138.1 MeV (a) and 171.3 MeV (b). Both spots reached the same depth in the body but with different lateral profiles at the depth of the arrows (red for the lower energy spot without RS, blue for the higher energy beam with RS). (c). The higher energy spots going through the RS demonstrated a smaller full width half maximum compared with the lower energy spot without RS in the body, indicating possible penumbra margin reduction.
The effect of motion on proton therapy dose distributions is significant and has been well documented [14–19]. This impact is particularly important for delivering proton therapy in 4 fractions of SBRT. Strategies for minimizing this effect have been discussed in the literature and include 4-dimensional computed tomography simulation and delivery techniques, such as breath hold and respiratory gating. Inter-fraction tumor motion and anatomical changes secondary to treatment response are likely less pronounced when 4 fractions are delivered on 4 consecutive days, but nonetheless should still also be considered. Current study analyzed potential advantages using proton in clinical challenging cases and compared it with photon, assuming all uncertainty about proton therapy including IMPT has been taken care of using the current approaches including 4-D CT based planning and motion/set-up uncertainty margins. Our recent study using cumulative 4-D proton planning/calculation over the course of proton radiotherapy showed that proton therapy uncertainty, including the impact of daily motion and low density in air, could be minimized by large spot size and more fractions of treatment, The uncertainty was averaged out over fractions and target coverage remained adequate [20]. However, before IMPT is used in clinic settings, particularly for hypo-fractionated stereotactic treatment, more studies are needed to validate the impact of these uncertainties since small lesions could move more significantly and there is less chance of averaged out uncertainty due to lower fraction number. In addition, most proton facilities have only on-board kilo-voltage x-ray image but lack volumetric imaging such as cone-beam CT or CT-on-rail, which has been widely used in photon SBRT. Implanted fiducial to improve clinical set up and target verification, particularly for respiratory gated treatment, may be needed. Alternatively, volumetric verification, either outside or inside proton treatment room before each fraction of treatment, should be explored.
Acknowledgments
Dr. Chang is a recipient of a Developmental Project Award from The University of Texas Specialized Program of Research Excellence (SPORE) in Lung Cancer (P50-CA70907). This research was supported in part by grant R01-CA74043 and P01-CA021239 from the National Cancer Institute.
Footnotes
Conflict of Interest Notification: Actual or potential conflicts of interest do not exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94–100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 2.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 3.Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;72:967–971. doi: 10.1016/j.ijrobp.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly P, Balter PA, Rebueno N, et al. Stereotactic body radiation therapy for patients with lung cancer previously treated with thoracic radiation. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2009.09.070. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:1087–1096. doi: 10.1016/j.ijrobp.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Li Y, Pan X, et al. Intensity-modulated proton therapy reduces normal tissue doses compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIB-non-small cell lung cancer: a vritual clinical study. Int J Rad Onc Bio Phy. 2009 Aug 4; doi: 10.1016/j.ijrobp.2009.04.028. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moyers MF, Miller DW, Bush DA, Slater JD. Methodologies and tools for proton beam design for lung tumors. Int J Radiat Oncol Biol Phys. 2001;49:1429–1438. doi: 10.1016/s0360-3016(00)01555-8. [DOI] [PubMed] [Google Scholar]
- 8.Bush DA, Slater JD, Shin BB, Cheek G, Miller DW, Slater JM. Hypofractionated proton beam radiotherapy for stage I lung cancer. Chest. 2004;126:1198–1203. doi: 10.1378/chest.126.4.1198. [DOI] [PubMed] [Google Scholar]
- 9.Hata M, Tokuuye K, Kagei K, et al. Hypofractionated high-dose proton beam therapy for stage I non-small-cell lung cancer: preliminary results of a phase I/II clinical study. Int J Radiat Oncol Biol Phys. 2007;68:786–793. doi: 10.1016/j.ijrobp.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 10.Nihei K, Ogino T, Ishikura S, Nishimura H. High-dose proton beam therapy for Stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:107–111. doi: 10.1016/j.ijrobp.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 11.Chang JY, Komaki R, Wen HY, et al. Toxicity and patterns of failure of dose-escalated proton therapy in early-stage medically inoperable non-small cell lung cancer. Journal of Thoracic Oncology. 2009;4(9):S525. doi: 10.1016/j.ijrobp.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georg D, Hillbrand M, Stock M, Dieckmann K, Potter R. Can protons improve SBRT for lung lesions? Dosimetric considerations Radiother Oncol. 2008;88(3):368–375. doi: 10.1016/j.radonc.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Safai S, Bortfeld T, Engelsman M. Comparison between the lateral penumbra of a collimated double-scattered beam and uncollimated scanning beam in proton radiotherapy. Physics in Medicine and Biology. 2008;53(6):1729–1750. doi: 10.1088/0031-9155/53/6/016. [DOI] [PubMed] [Google Scholar]
- 14.Albertini F, Bolsi A, Lomax AJ, Rutz HP, Timmerman B, Goitein G. Sensitivity of intensity modulated proton therapy plans to changes in patient weight. Radiother Oncol. 2008;86:187–194. doi: 10.1016/j.radonc.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 15.Engelsman M, Rietzel E, Kooy HM. Four-dimensional proton treatment planning for lung tumors. Int J Radiat Oncol Biol Phys. 2006;64:1589–1595. doi: 10.1016/j.ijrobp.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 16.Hui Z, Zhang X, Starkschall G, et al. Effects of interfractional motion and anatomic changes on proton therapy dose distribution in lung cancer. Int J Radiat Oncol Biol Phys. 2008;72(5):1385–1395. doi: 10.1016/j.ijrobp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang Y, Zhang X, Chang JY, et al. 4D Proton treatment planning strategy for mobile lung tumors. Int J Radiat Oncol Biol Phys. 2007;67:906–914. doi: 10.1016/j.ijrobp.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 18.Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 2: the potential effects of inter-fraction and inter-field motions. Phys Med Biol. 2008;53:1043–1056. doi: 10.1088/0031-9155/53/4/015. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, Sandison GA, Farr JB, et al. Dosimetric impact of intrafraction motion for compensator-based proton therapy of lung cancer. Phys Med Biol. 2008;53:3343–3364. doi: 10.1088/0031-9155/53/12/019. [DOI] [PubMed] [Google Scholar]
- 20.LI Y, Zhang X, Li X, Pan X, Mohan R. Evaluating 4D interplay effects for proton scanning beams in lung cancers. Medical Physics. 2009 June;36(6):2759. (abstract) [Google Scholar]