Abstract
There is an accumulating body of evidence that highlights the fact that one can identify convergence in programmes of cellular differentiation. That is, that a particular differentiated cell/tissue type can be generated via non-identical paths. Convergence is also seen in evolution and here it is termed homoplasy, thus one could term convergence in cellular differentiation, developmental homoplasy. It is important to appreciate its existence as it can confound our understanding of cellular differentiation. In particular, it highlights the point that the analysis of cellular differentiation in one region of the body may not generate an understanding that is generally applicable. The existence of the phenomenon of developmental homoplasy may lie in the evolutionary history of developmental processes, which are assembled over phylogenetic time. Such convergence in cellular differentiation may also have significance for understanding disease state and disease repair.
Keywords: cellular differentiation, convergence, dermis, evolution, muscle, sensory neurons, skeleton, smooth muscle
Introduction
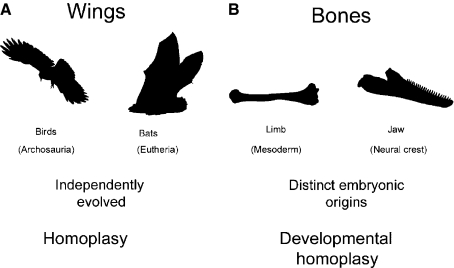
Homoplasy describes the emergence of similarities that arise as a result of convergence during evolution (Lankester, 1870). A classic example of homoplasy would be the wings of birds and the wings of bats (Fig. 1A). Although both these groups of animals possess wings that allow them to fly, their wings have evolved independently. The most recent common ancestor of birds and bats did not possess wings. Thus the modification to the development of the forelimbs that gave rise to the wings of birds occurred independently from that which generated bat wings. A number of lines of evidence would seem to suggest that homoplasy can also be detected in programmes of cellular differentiation. Thus, there is an increasing body of work on a number of diverse tissues that suggests there is convergence in processes of cellular differentiation. It has been shown that many of the differentiated cell types that are found in vertebrates do not emerge from a single precursor population but that they can have dual or multiple origins (Fig. 1B). Furthermore, it is also becoming apparent that molecular details of the paths of differentiation can also vary between different regions of the body.
Fig. 1.
Homoplasy and developmental homoplasy. (A) The wings of birds and those of bats evolved independently. Birds evolved within the archosaurs, from therapod dinosaurs, while bats are eutherian mammals. The last common ancestor of birds and bats did not have wings. Thus their evolution in these two lineages represents an example of homoplasy. (B) The bones of the limb and those of the jaw have distinct embryonic origin. Limb bones arise from lateral plate mesoderm, whereas jaw bones are neural crest-derived. The generation of bone cells from these distinct progenitor populations represents an example of developmental homoplasy.
Skeletogenesis
Skeletal tissues provide the body with its framework and underpin its morphology. It has been apparent, however, that there are differences in the routes through which bone and cartilage are generated. In the trunk, the skeleton has a mesodermal origin. The axial skeleton emerges from the somites and the appendicular skeleton from the lateral plate mesoderm, and most bones are formed by endochondral ossification via a cartilaginous template. Contrastingly, in the head, much of the skeletal tissue derives from neural crest cells (Le Douarin & Kalcheim, 1999). These are a transient multipotent embryonic progenitor population that migrates from the neural tube early in development. It is also important to note that in the head, whereas some bones are formed by endochondral ossification, others, the dermal bones, arise by direct differentiation of the neural crest cells into osteoblasts. Thus the chondrocytes and osteocytes of the trunk and head have very different developmental histories and bone can be formed via distinct paths. Yet, even when the neural crest and mesodermal precursor populations differentiate to form the same skeletal tissue, endochondral bone, differences are also apparent. Mice with a targeted mutation in Indian Hedgehog (IHH) have severely reduced endochondrally derived trunk bones, yet endochondrally derived cranial bones, such as the basioccipital and basisphenoid, are largely unaffected in these animals (Abzhanov et al., 2007). This would seem to suggest that the endochondral bones of the head have a differential requirement for IHH from those in the trunk.
Sensory neuronal differentiation
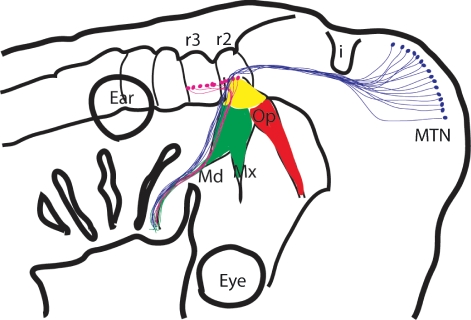
Another very clear example of convergence in cellular differentiation can be found in the generation of sensory neurons. The neurons of the dorsal root ganglia (DRGs) of the trunk and of the trigeminal ganglion of the head perform similar functions; both are concerned with relaying general somatosensory information from the periphery to the central nervous system, but they have distinct developmental histories. All of the neurons of the DRGs are neural crest-derived. The neural crest cells emerge from the dorsal neural tube and then migrate into the anterior half of each somite, wherein they subsequently differentiate as post-mitotic neurons. This developmental programme involves an increasingly well-defined cascade of transcription factors (Marmigere & Ernfors, 2007). The development of the trigeminal ganglion is somewhat more complex (Fig. 2). The first neurons to contribute to the trigeminal ganglion are not neural crest-derived but arise from focal thickening of the ectoderm, termed neurogenic placodes. There are two trigeminal placodes, the ophthalmic and the maxillomandibular, which lie alongside the midbrain hindbrain junction and are dependent on inductive signals from this region of the CNS for their formation (Canning et al., 2008). The formation of the placodes does not require the transcription factors that drive neural crest formation and the delamination of cells from the placodes is quite distinct from neural crest delamination (Graham et al., 2007). The trigeminal placodes also differ from each other both in terms of the transcription factors that they express and in the cellular behaviour of the neuronal cells they release. In the ophthalmic placode, post-mitotic neurons differentiate and express sensory neuronal transcription factors within the ectoderm prior to their migration to the site of ganglion formation (Begbie et al., 2002). Contrastingly, the maxillomandibular placodes release mitotically active neuroblasts that migrate from the ectoderm to the site of ganglion formation before differentiating. Thus there are significant differences in the ways in which the progenitors for the sensory neurons of the DRGs and the trigeminal ganglion are generated and most probably in the cues that direct their terminal differentiation.
Fig. 2.
Developmental homoplasy in the trigeminal system. The somatosensory neurons contributing to the trigeminal ganglion are derived from three distinct embryonic populations. The first-born are derived from the opththalmic placode (red), followed by cells from the maxillomanidbulare placode (green) and finally by neural crest-derived cells. The other group of sensory neurons that contribute to the trigeminal nerve are the cells of the (MTN; purple). The motor neurons, shown as red dots, are found in rhombomeres 2 and 3 (r2, r3).
There are, however, similarities in the signalling pathways used in the generation of neurons in the DRGs and the trigeminal ganglion. Proprioceptive neurons relay information about the movement and position of body parts. The proprioceptive neurons that innervate the limbs reside in the DRGs and these cells have been shown to require the transcription factor Runx3 and NT3/TrkC signalling (Marmigere & Ernfors, 2007). Runx3 expression has been detected in the neurons of the trigeminal ganglion, and in Runx3 mutants it has been reported that there is a loss of TrkC expressing neurons here, suggesting similarities in the developmental path leading to the formation proprioceptive sensory neurons in DRGs and trigeminal ganglion sensory neurons (Levanon et al., 2002). Yet, there are no proprioceptive sensory neurons in the trigeminal ganglion; they are somatosensory. The proprioceptive neurons that contribute to the trigeminal nerve reside in the CNS; these are the cells of the mesencephalic trigeminal nucleus, and these are not exclusively dependent upon TrkC signalling for their formation (Fan et al., 2000) (Fig. 2). Thus the pathway that promotes the differentiation of proprioceptive sensory neurons in the trunk affects the development of a different class of sensory neurons in the trigeminal ganglion.
Skeletal muscle development
A more recent example of developmental homoplasy has emerged from studies of skeletal muscle development. It has been shown that trunk and cranial muscles derive from separate genetic lineages (Harel et al., 2009). In the trunk, skeletal muscle cells arise from the somites and they can be genetically labelled through their expression of Pax3. The cranial musculature does not derive from somites but from the unsegmented cranial paraxial mesoderm and the precursors of the cranial skeletal muscles do not express Pax3. They can, however, be genetically labelled via their expression of a different transcription factor, Mesp1. It has also been shown that aspects of the myogenic programme differ between head and trunk muscle. In the trunk, it is known that Pax3 acts in a pathway parallel to Myf5 and Mrf4 and will rescue MyoD-mediated myogenesis in the absence of these two transcription factors (Tajbakhsh et al., 1997). Yet, in the head, myogenesis will ensue in Pax3:Myf5(Mrf4) mutants. Instead, Tbx1 function is required for myogenesis in pharyngeal arch muscles in the absence of Myf5, which again highlights to existence of convergence in developmental processes (Sambasivan et al., 2009). The neurons that innervate the pharyngeal muscle reside in the hindbrain, and it is important to recognize that these motor neurons can be uniquely defined through their expression of the transcription factors Phox2b and Tbx20, and are thus distinct from spinal motor neurons (Dufour et al., 2006).
Dermis formation
Although these previous examples have focussed on differences between the head and trunk, examples of developmental homoplasy can be found exclusively within the trunk. In particular, it has been shown that the dorsal and ventral dermis of the trunk arises from distinct progenitor populations (Mauger, 1972). Thus, while the dorsal dermis originates from the somites, the ventral dermis is generated by the lateral plate mesoderm. The dorsal and ventral dermis also differ in their requirement for wnt signalling during development. In both dorsal and ventral dermis, β-catenin is required for cell fate selection, but in the ventral dermis, β-catenin is additionally required for progenitor cell survival (Ohtola et al., 2008).
Vascular smooth muscle differentiation
Perhaps one of the most striking examples of convergence in cellular differentiation is that associated with the generation of vascular smooth muscle cells. These cells originate from an incredibly broad range of embryonic tissues, including, the neural crest, the secondary heart field, the somites, the splanchnic mesoderm and the mesothelium (Majesky, 2007). Significantly, it has been found that different blood vessels, and indeed different segments of the same vessel, such as the aorta, are composed of smooth muscle cells generated by distinct progenitors. The complexities in the origins of the vascular smooth muscle cells are reflected in aspects of their differentiation. Thus, although it has been shown that all smooth muscle cells express the smooth muscle myosin heavy chain (SM-MHC) gene, dissection of the regulatory apparatus of this gene has revealed the existence of distinct elements that are required for the expression of this gene in different smooth muscle cell populations (Manabe & Owens, 2001).
The evolutionary origin of developmental homoplasy
Having established that there are a number of examples of developmental homoplasy associated with multiple tissue types, it is crucial to consider why they are present. The answer to this most probably lies in the complexity of the evolutionary history of development. Alterations to the developmental programme are vital for evolutionary change and these can include the emergence of novel features or a loss of components. Thus, it is important to appreciate that development is modular and that the developmental programme of particular modules can be altered without affecting the whole developmental process. With respect to formation of skeletal components, analysis of the fossil record has suggested that the splanchnocranial skeleton, those elements associated with the pharyngeal arches and which are likely to be neural crest derived, are ancient, whereas the appendicular skeleton, by comparison, is recent (Donoghue & Sansom, 2002). Thus, the developmental programme underpinning neural crest-derived skeletogenesis is likely to be relatively ancient. The fact that somatosensory neurons are generated from two distinct embryonic populations, neural crest cells and neurogenic placodes, also probably lies in evolutionary history. Neurogenic placodes are a region of the embryonic ectoderm in which neuronal cells are generated, and this is a widespread feature of the metazoa. In numerous animals, from flies to echinoderms, that is how neurons are generated; they are derived from the embryonic skin. However, the neural crest is a vertebrate-specific embryonic cell type. Thus, the ability to generate sensory neurons from the embryonic ectoderm is likely to have preceded the generation of sensory neurons by neural crest cells. Finally, it has been shown that homologues of the Phox2/Tbx20 motor neurons that innervate pharyngeal musculature have been identified in ascidians (Dufour et al., 2006). In Ciona, CiPhox2 expressing neurons lies in a region of the developing nervous system that abuts the domain of expression of CiOtx rostrally and overlaps with the expression of CiHox1 at its posterior end. These expression profiles define this region as being equivalent to the vertebrate hindbrain. In the post-metamorphic animals, these cells are found in the cerebral ganglion and they express CiTbx20. They also project to the muscle of the pharyngeal basket. One can therefore conclude that it is likely that the pharyngeal musculature and its associated motor neurons evolved prior to the emergence of the vertebrates, well before, for example, the spinal motor that innervates the limb musculature. Thus developmental homoplasy will have arisen as the consequence of the sequential modification of developmental processes over phylogenetic time.
Developmental homoplasy – implications for disease and repair
The fact that developmental homoplasy exists, may have ramifications for our understanding of the differential presentation of some diseases and for the correction of defects through stem cell treatment. For example, it has been noted that muscular dystrophies can affect distinct muscle groups (Emery, 2002) and this could be underlain by the fact that different muscle groups develop via distinct pathways. The lineage diversity of smooth muscle cells may also relate to vascular disease. There are differences in atherosclerotic lesion formation between thoracic and abdominal aorta, with thoracic segments being more resistant than abdominal, and this was thought to be due to differences in haemodynamic flow patterns. However, transplantation studies have shown that thoracic segments transplanted into atherosclerosis-susceptible abdominal aorta remained resistant after 1 year on an atherogenic diet, suggesting that their developmental origins may be an important factor in this behaviour (Majesky, 2007). It has also been shown recently that there is a marked difference in the ability of stem cells derived from neural crest to contribute to bone regeneration vs. those derived from mesoderm (Leucht et al., 2008). Skeletal stem cells derived from the mandible, which has a neural crest origin, can contribute to the regeneration of mandibular fractures and also to the regeneration of fractures of the mesodermally derived tibia. However, stem cells from the tibia can only help repair tibial fractures and will not contribute osteocytes to mandibular defects. Thus in some instances it may be critical to consider the fact that differentiated cell types can be generated through convergent paths and that the developmental history of the stems cells being used to repair a deficit may affect their efficacy. Finally, this of course would also apply to the generation of differentiated cell types from stem cells in vitro. It may not be enough to generate a motor neuron or a smooth muscle cell; the particular features of these cells must be considered as well.
Conclusions
As work progresses, it is probable that instances of developmental homoplasy will become more apparent in many systems, and, in particular, in areas of the body with complex developmental and evolutionary histories, such as the central nervous system and the immune system. With regards to the immune system, studies would seem to suggest that lymph nodes in different sites in the mouse, such as those associated with the nasal tissue vs. Peyer’s patch in the gut, employ different mechanisms to realize similar objectives (Kiyono & Fukuyama, 2004). However, determining the extent to which this represents an example of developmental homoplasy awaits the uncovering of the precise details of the developmental origins of these structures.
Acknowledgments
The author would like to thank Adrian Hayday, Andrew Lumsden, Paul Martin, Ivor Mason, Jo Richardson and Moya Smith for helpful discussions. Work in the author’s laboratory is supported by the Wellcome Trust and the Leverhulme Trust.
References
- Abzhanov A, Rodda SJ, McMahon AP, et al. Regulation of skeletogenic differentiation in cranial dermal bone. Development. 2007;134:3133–3144. doi: 10.1242/dev.002709. [DOI] [PubMed] [Google Scholar]
- Begbie J, Ballivet M, Graham A. Early steps in the production of sensory neurons by the neurogenic placodes. Mol Cell Neurosci. 2002;21:502–511. doi: 10.1006/mcne.2002.1197. [DOI] [PubMed] [Google Scholar]
- Canning CA, Lee L, Luo SX, et al. Neural tube derived Wnt signals cooperate with FGF signaling in the formation and differentiation of the trigeminal placodes. Neural Dev. 2008;3:35. doi: 10.1186/1749-8104-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue PC, Sansom IJ. Origin and early evolution of vertebrate skeletonization. Microsc Res Tech. 2002;59:352–372. doi: 10.1002/jemt.10217. [DOI] [PubMed] [Google Scholar]
- Dufour HD, Chettouh Z, Deyts C, et al. Precraniate origin of cranial motoneurons. Proc Natl Acad Sci U S A. 2006;103:8727–8732. doi: 10.1073/pnas.0600805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery AE. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- Fan G, Copray S, Huang EJ, et al. Formation of a full complement of cranial proprioceptors requires multiple neurotrophins. Dev Dyn. 2000;218:359–370. doi: 10.1002/(SICI)1097-0177(200006)218:2<359::AID-DVDY9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Graham A, Blentic A, Duque S, et al. Delamination of cells from neurogenic placodes does not involve an epithelial-to-mesenchymal transition. Development. 2007;134:4141–4145. doi: 10.1242/dev.02886. [DOI] [PubMed] [Google Scholar]
- Harel I, Nathan E, Tirosh-Finkel L, et al. Distinct origins and genetic programs of head muscle satellite cells. Dev Cell. 2009;16:822–832. doi: 10.1016/j.devcel.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono H, Fukuyama S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankester ER. On the use of the term homology in modern zoology and the distinction between homogenetic and homoplastic agreements. Ann Mag Nat Hist Zool Bot Geol. 1870;6:34–43. [Google Scholar]
- Le Douarin NM, Kalcheim C. The Neural Crest. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Leucht P, Kim JB, Amasha R, et al. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development. 2008;135:2845–2854. doi: 10.1242/dev.023788. [DOI] [PubMed] [Google Scholar]
- Levanon D, Bettoun D, Harris-Cerruti C, et al. The Runx3 transcription factor regulates development and survival of TrkC dorsal root ganglia neurons. EMBO J. 2002;21:3454–3463. doi: 10.1093/emboj/cdf370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- Manabe I, Owens GK. The smooth muscle myosin heavy chain gene exhibits smooth muscle subtype-selective modular regulation in vivo. J Biol Chem. 2001;276:39076–39087. doi: 10.1074/jbc.M105402200. [DOI] [PubMed] [Google Scholar]
- Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci. 2007;8:114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- Mauger A. The role of somitic mesoderm in the development of dorsal plumage in chick embryos. 1 Origin, regulative capacity and determination of the plumage-forming mesoderm. J Embryol Exp Morphol. 1972;28:313–341. [PubMed] [Google Scholar]
- Ohtola J, Myers J, Akhtar-Zaidi B, et al. beta-Catenin has sequential roles in the survival and specification of ventral dermis. Development. 2008;135:2321–2329. doi: 10.1242/dev.021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambasivan R, Gayraud-Morel B, Dumas G, et al. Distinct regulatory cascades govern extraocular and pharyngeal arch muscle progenitor cell fates. Dev Cell. 2009;16:810–821. doi: 10.1016/j.devcel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Rocancourt D, Cossu G, et al. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]