Abstract
Renal, adrenal, gonadal, ureteral and inferior phrenic arteries vary in their level of origin and in their calibre, number and precise anatomical relationship to other structures. Studies of the origin and early development of these arteries have evoked sharp disputes. The ladder theory of Felix, which states that ‘All the mesonephric arteries may persist; from them are formed the phrenic, suprarenal, renal and internal spermatic arteries’ has been generally quoted in the anatomical textbooks without rigorous verification for 100 years. In this study, we re-examined this theory by performing micro-injection of dye and resin into rat (Rattus norvegicus) embryos. Our results revealed that most of the mesonephric arteries had degenerated before the metanephros started its ascent. The definitive renal, adrenal, gonadal, ureteral and inferior phrenic arteries appeared as new branches from the gonadal artery and/or directly from the abdominal aorta to the para-aortic ridge. Coincidental to this, the anatomical architecture of the inter-renal vascular cage, which consists of the interlobar and arcuate arteries and their collateral veins, was completed within the developing metanephros. We demonstrated that the delicate renal vascular cage switched from the primary renal artery to the definitive renal artery and that the route of venous drainage changed from the posterior cardinal vein to the inferior (caudal) vena cava.
Keywords: adrenal gland, gonad, kidney, micro-injection, para-aortic ridge, rat, renal artery, renal vein
Introduction
Anatomical textbooks describe how the renal artery gives off a variable number of inferior adrenal arteries and branches to the perinephric tissue, which includes the renal capsule, pelvis and the proximal part of the ureter. The primary branch of the renal artery divides into an anterior and posterior division near the renal hilum. Accessory renal arteries are common and these usually arise from the aorta above or below the main renal artery and follow it to the renal hilum. On rare occasions, accessory renal arteries arise from the coeliac or superior mesenteric arteries near the aortic bifurcation or from the common iliac arteries (Dyson, 1995).
Renal arteries vary in their level of origin and in their calibre, number, and precise anatomical relations. Merklin & Michels (1958) reviewed these features in almost 11 000 kidneys and they concluded that the standard textbook description of the renal blood supply is only true for approximately one-third of the population. They reported multiple variant renal and suprarenal blood supplies with data from the inferior phrenic, ureteral and gonadal arteries. In addition, they revealed that the blood supply to the kidney and suprarenal glands is intertwined intimately and shows marked and frequent anatomical differences. They noted that ‘Frequently the renal arteries give off from two to five slender branches to the suprarenal gland, while inferior suprarenal arteries stemming from the aorta or the renal artery often supply from one to four small branches to the superior pole of the kidney and its fat body (capsular branches)’.
Investigations with regard to the origin and early development of the renal artery have led to different and often contradictory theories. Hochstetter (1893) proposed that the ascending metanephros acquires a new branch directly from the dorsal aorta, which becomes the definitive renal artery. Walter Felix proposed an alternative model, which is known as the ladder theory (Felix, 1912). He reconstructed the mesonephric arteries from an 18-mm human embryo and superimposed the diagram onto a contour drawing of the suprarenal gland, mesonephros, metanephros and gonad of a 19.4-mm human embryo (see Discussion). Using the superimposed diagram, he claimed that ‘All the mesonephric arteries may persist; from them are formed the phrenic, suprarenal, renal, accessory renal, internal spermatic and accessory spermatic arteries and also the branches to the lymph nodes and the sympathetic ganglia in the region between the superior and inferior mesenteric arteries’. Although Abe (1956) and Salama et al. (1982) obtained evidence in support of the model of Hochstetter (1893) using both hamsters and humans, Felix’s ladder theory has been accepted widely because it provides a convenient embryological explanation for the frequent and marked variations that are seen in the renal, adrenal and gonadal arteries in humans. However, as Felix himself declared, his theory was only speculation and, although it has been quoted in anatomical textbooks for over 100 years, it has not been verified in vivo. Here, we carefully re-examine the origins of the renal artery in rat (Rattus norvegicus) embryos. Our results do not support the ladder theory but rather verify other theories for the origin of the renal artery.
Materials and methods
Rats
The embryos used in this study were obtained from specific pathogen-free/virus antibody-free white Wistar rats (Rattus norvegicus; Charles River, Japan). The rats were aged between 6 and 10 weeks; the females (n = 82) weighed 180–200 g and the males (n = 10) weighed 300–350 g. Animal care and procedures followed the ‘Guide for Animal Experimentation’ guidelines of the animal care ethical committees of Iwate Medical University. The rats were housed in metal cages with access to food (standard pellets) and water ad libitum under the following conditions: temperature, 23 ± 1.5 °C; humidity, 55 ± 5%; and illumination, 06:30–20:30 h day/night rhythm. They were weighed to assess their physical condition at 15:00 h twice per week.
The vascular system develops very quickly during the early stages of development and the vascular anatomy changes rapidly until embryonic day (E)13. Therefore, we collected embryos that had been staged precisely for every 0.1-day interval from E10.0 to E15.0. Beyond E13.0, vascular development progresses more slowly in rats and therefore we collected embryos for every 1-day interval between E15.0 and E20.0 (Table 1). Empirically, it is known that the oestrus cycle of female rats becomes synchronized in groups that are housed under the same non-stressful conditions (Abe, 1956; Aizawa et al. 1999). After the rats had acclimatized for 1 week, we checked vaginal smears closely at 15:00 h every day to set the date for mating. Females that were found to be in a proestrus state were paired with a male rat from 00:00 to 06:00 h. The female rats were then checked for copulation plugs and sperm in the vaginal smear, and the embryonic day was designated as E0 from 09:00 h.
Table 1.
The number of embryos injected with dye or resin at each developmental stage.
| E day | Numb. Dye | Numb. Resin | E day | Numb. Dye | Numb. Resin | E day | Numb. Dye | Numb. Resin | E day | Numb. Dye | Numb. Resin | E day | Numb. Dye | Numb. Resin | E day | Numb. Dye | Numb. Resin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10.0 | 17 | 0 | 11.0 | 27 | 14 | 12.0 | 28 | 15 | 13.0 | 11 | 8 | 14.0 | 15 | 10 | 15.0 | 38 | 21 |
| 10.1 | 10 | 0 | 11.1 | 21 | 2 | 12.1 | 16 | 0 | 13.1 | 0 | 0 | 14.1 | 6 | 0 | 16.0 | 23 | 21 |
| 10.2 | 27 | 0 | 11.2 | 31 | 16 | 12.2 | 4 | 5 | 13.2 | 17 | 17 | 14.2 | 10 | 10 | 17.0 | 29 | 16 |
| 10.3 | 13 | 0 | 11.3 | 8 | 0 | 12.3 | 15 | 0 | 13.3 | 14 | 7 | 14.3 | 7 | 0 | 18.0 | 32 | 12 |
| 10.4 | 0 | 0 | 11.4 | 5 | 8 | 12.4 | 20 | 5 | 13.4 | 4 | 3 | 14.4 | 6 | 0 | 19.0 | 37 | 14 |
| 10.5 | 29 | 0 | 11.5 | 20 | 0 | 12.5 | 0 | 4 | 13.5 | 18 | 10 | 14.5 | 6 | 12 | 20.0 | 15 | 11 |
| 10.6 | 17 | 12 | 11.6 | 14 | 3 | 12.6 | 11 | 5 | 13.6 | 8 | 0 | 14.6 | 6 | 0 | |||
| 10.7 | 16 | 0 | 11.7 | 15 | 2 | 12.7 | 25 | 8 | 13.7 | 6 | 0 | 14.7 | 6 | 0 | |||
| 10.8 | 7 | 0 | 11.8 | 13 | 0 | 12.8 | 4 | 4 | 13.8 | 6 | 0 | 14.8 | 6 | 0 | |||
| 10.9 | 10 | 6 | 11.9 | 6 | 9 | 12.9 | 2 | 5 | 13.9 | 6 | 0 | 14.9 | 6 | 0 |
E day, embryonic day; Numb. Dye, number of dye-injected embryos; Numb. Resin, number of resin-injected embryos
Injection of dye
Our preliminary research suggested that the basic anatomical pattern of the renal vascular system is completed within 1 day (E14–E15). To reveal this process in detail, we applied the micro-dye injection technique (Ura, 1943) to embryos and prepared specimens at every 0.1-day interval during E10–E15 and at every 1-day interval during E15–E20 (Table 1).
For all dye injection procedures, embryos were removed surgically from a pregnant rat that had been anaesthetized with ketamine intravenously (12 mg kg−1 via the caudal vein) after tranquilization (Xylazine 10 mg kg−1 intramuscularly). They were then placed in cooled phosphate-buffered saline (PBS) to reduce their metabolic rate and heart rate. Just prior to the injection of dye, each embryo was soaked in warm PBS at 37 °C. The atrium (or the umbilical vein after E13) was incised under a stereomicroscope to allow the blood to be drained. The anaesthetized embryo was perfused fully with Berlin blue solution [0.5 or 0.75% in distilled water (DW)] via a glass needle that cannulated the ventricle (or umbilical artery after E13). The embryo was then fixed in 4% paraformaldehyde diluted with 0.1 m PBS (pH 7.3). The time period for the preparation of embryos was limited to within 1 h from the start of extraction of a litter in order to maintain the precise staging of the specimens. The dye-injected specimens were dissected under a stereomicroscope (Olympus SZX12) and photographed. To observe the vessels that had been injected with dye clearly, the whole specimen was placed in a graded series of glycerol (30, 50, 70 and 95%; 1 day in each concentration) before being preserved in pure glycerol.
Injection of resin
We adopted the micro-resin casting technique (Isogai & Horiguchi, 1996) to visualize the 3D morphogenesis of the fine vascular architecture within the metanephric primordium. Embryos were prepared for every 0.5- and 1-day interval during E14–E15 and E15–E20, respectively, as described above for the dye injection procedure. The umbilical vein was incised under a stereomicroscope to enable perfusion with Locke’s solution initially to remove the blood and then with 2% glutaraldehyde to fix the specimen. The perfused specimens were stored temporarily in 1% paraformaldehyde that was diluted with 0.1 m PBS (pH 7.3, 4 °C) for several days. For the injection of resin, we used embryos that were prepared within 30 min from the start of extraction of a litter because, beyond that period, tiny clots of blood form in the fine capillaries that prevent the perfusion of these vessels. A mixture of 30% (v/v) methyl, 17.5% (v/v) ethyl and 52.5% (v/v) 2-hydroxypropyl methacrylate monomer (Nisshin EM) was mixed with 1.5% (w/v) benzoyl peroxide 75% (Katayama Chemical) and 1.5% (v/v) N, N-dimethylaniline (Nacalai Tesque) immediately prior to the resin injection. The resin medium was then infused via a glass needle that cannulated the umbilical artery until it hardened in a plastic syringe. The injected embryos were macerated in 20% KOH solution at 40 °C overnight and rinsed gently with DW. To remove tissues that remained adherent to the casts, we used a handmade fine water jet stream generator or an ultrasonic generator (Iuchi VS-100III). The casts were trimmed to expose the renal vascular system and frozen in water before being freeze-dried (Eiko ID-2). The casts were prepared for observation by a scanning electron microscope (SEM) as described below.
Scanning electron microscope observations
Several non-injected embryos at each developmental stage were fixed prior to SEM observation. The embryos were rinsed with 0.1 m phosphate buffer, pre-fixed with 2.5% glutaraldehyde/0.1 m PBS for 2 h at 4 °C, rinsed with 0.1 m PBS and post-fixed with 1% OsO4 in PBS for 2 h at 4 °C. They were dehydrated with 50, 70, 80, 90 and 100% ethanol, and then frozen in 100% t-butanol (−20 °C) for freeze drying (Eiko ID-2). All dried specimens were mounted on metal stubs and coated with osmium (Filgen OPC 60A) before observation with an SEM (Hitachi S-4700) using an acceleration voltage of 8–10 kV.
Histology
Haematoxylin and eosin staining
A series of injected embryos that had been fixed with 4% paraformaldehyde were embedded in paraffin, sectioned serially (5-μm-thick sections), stained with haematoxylin and eosin, and photographed using a microscope (Olympus BX51).
Immunohistochemical staining
Prior to fixation, the abdominal cavity was opened and embryos were fixed with 4% paraformaldehyde in PBS at 4 °C. Before further processing, whole embryos or tissue blocks that contained the adreno-genito-nephric region were washed (3 × 2 h) with 0.1 m PBS.
Tissue blocks were transferred into a graded series of saccharose solution (10, 20 and 30%), oriented in the desired position on brass holders, enclosed in Tissue TEK OCT compound (Sakura Finetechnical, Japan) and frozen in liquid nitrogen. Cryostat sections (20 μm thick) were mounted on slides, air-dried for 24 h, rinsed in PBS for 3 h and placed in PBS that contained 10% fetal bovine serum and 0.1% Triton X-100 (PBS-ST 0.1%) for 60 min at room temperature (18°C ∼ 23°C). Slides were incubated with mouse anti-rat CD31 (PECAM-1; BD Biosciences 550300), which had been diluted (× 50) in PBS-ST 0.1%, as the primary antibody for 2 h at room temperature. The slides were then rinsed with PBS several times, incubated with secondary antibody (Alexa Fluor 594 goat anti-mouse IgG), which had been diluted (× 200) with PBS-ST 0.1%, for 60 min and rinsed again with PBS several times. Alternatively, slides were incubated with the primary antibody rabbit anti-tyrosine hydroxylase (Chemicon AB152), which had been diluted (× 100) with PBS-ST 0.1%, for 2 h at room temperature and rinsed with PBS several times. They were then incubated with Alexa Fluor 488 goat anti-rabbit IgG, which had been diluted (× 200) with PBS-ST 0.1%, for 60 min and rinsed with PBS several times. Sections were post-fixed with 15% formalin in PBS for 10 min, rinsed several times with DW and mounted with aqueous mounting medium.
For whole-mount analysis, embryos were washed with DW (2 × 20 min), transferred into 100% methanol (2 × 20 min) and placed in methanol that contained 10% H2O2 overnight at 4 °C. They were then washed with DW twice and with PBS/1% Triton X-100 (PBS-ST 1%) (2 × 2 h) and placed in PBS-ST 1% overnight at 4 °C. After two rinses for 20 min with PBS/0.2% Triton X-100 (PBS-ST 0.2%), specimens were incubated with the primary antibody (rabbit anti-tyrosine hydroxylase; Chemicon AB152), which had been diluted (× 100) with carrier solution, for 1–2 days at 4 °C. After washing (four to eight times for 30 min) with PBS-ST 0.2%, specimens were incubated with goat anti-rabbit antibody (KPL074-1506), which had been diluted (× 200) with PBS-ST 0.2%, for 1–2 days at 4 °C. The samples were then washed with PBS-ST 0.2% (8 × 30 min) and with Tris-HCl buffer (4 × 30 min). A diaminobenzidine substrate kit (Dako) was used to develop the colour for 20 min at room temperature. The embryos were rinsed several times in DW and then cleared with a graded series of glycerol (30–100%).
Results
Embryonic day 14.0
At E14.0, the urogenital/para-aortic ridges appeared on either side of the dorsal mesentery and protruded into the dorsolateral parts of the peritoneal cavity (Fig. 1, E14.0; Fig. 2, E14.0-1). Whereas the mesonephroi that were located on the lateral aspect of the ridges were regressing, the gonadal primordia on the medial aspect of the ridges continued to increase in size. E14.0 was the earliest stage that the sex of an embryo can be determined from histological analysis. Medial to the urogenital ridge, another ridge could be recognized on either side of the dorsal mesentery (Fig. 1, E14.0; Fig. 2, E14.0-1). Zukerkandle (1912) described the paired ridges as the abdominal sympathetic paraganglia. Wrobel and Süß (1999) revealed that the segmentally-organized intermediate mesoderm between the cranial mesoderm and coelomic cavity fuses into a longitudinally-oriented continuous joined blastema. They called this blastema of intermediate mesodermal (nephric) origin the ‘common blastema’; the cranial portion represents the ‘adrenocortical anlage’ and the following caudal portion represents the ‘gonadal rete blastema’. Here, we term the cortical common blastema and medullary abdominal sympathetic paraganglia as the ‘para-aortic ridge’ (see Discussion). The nerves and adrenomedullary cells that were derived from the neural crest were revealed by the presence of tyrosine hydroxylase in the ridges. The sympathetic trunks and their branches, the abdominal sympathetic paraganglia, and the adrenal medulla could also be seen (Figs 3 and 4). The primordial metanephroi were still located caudal to the para-aortic ridges in their original position in the pelvic region (Fig. 1, E14.0; Fig. 2, E14.0-2; Fig. 4, E14.0; Fig. 5, E14.0-1).
Fig. 1.
Scanning electron microscope images of the urogenital/para-aortic ridge and metanephros in embryonic day (E)14.0, E14.4, E14.5, E15.0 and E16.0 rat embryos. All images are viewed from the ventral side, cranial to the top and caudal to the bottom. Nude area (not covered with peritoneum) is coloured in yellow. A, para-aortic ridge; Ad, adrenal gland; Dm, dorsal mesentery; G, genital ridge; K, metanephros; M, mesonephric ridge. White scale bar represents 500 μm in each panel.
Fig. 2.
Haematoxylin and eosin sections of the urogenital/para-aortic ridge and metanephros in embryonic day (E)14.0-1, E14.0-2, E14.5-1, E14.5-2 and E15.0 embryos. The arrowhead in E14.5-1 identifies the venous rete within the gonadal rete blastema. All sections are viewed from the cranial side with the dorsum to the top of the image. The white bars in the scanning electron microscope images represent the transverse cutting levels. A, para-aortic ridge; ci, common iliac artery; da, dorsal aorta (abdominal aorta); G, genital ridge; K, metanephros; M, mesonephric ridge; mi, inferior mesenteric artery; ms, superior mesenteric artery; pc, posterior cardinal vein. Black scale bar: 100 μm.
Fig. 3.
Nerves and adrenomedullary cells derived from the neural crest detected by tyrosine hydroxylase reactivity (green fluorescence) around the abdominal aorta (red fluorescence) in an embryonic day 14.0 rat embryo with the dorsum of the embryo towards the top of the image in all sections. The sequence of numerals in the ventral view indicates the transverse cutting levels along the craniocaudal axis. All sections are viewed from the caudal side. da, dorsal aorta (abdominal aorta); K, metanephros.
Fig. 4.
Adrenal medulla, splanchnic ganglia, sympathetic trunk and nerve, and hypogastric plexus demonstrated by the presence of tyrosine hydroxylase in embryonic day (E)14.0, E14.25, E14.5, E15.0 and E16.0 embryos. The images are viewed from the ventral side with the craniocaudal axis running from the top to bottom of the images. To show the relative position of the urogenital/para-aortic ridges and the metanephros, the colour background has been adjusted. A, para-aortic ridge; G, genital ridge; K, metanephros; M, mesonephric ridge. White scale bar: 500 μm.
Fig. 5.
Vascular resin casts of embryonic day (E)14.0 (-1, ventral view; -2, dorsal view), E14.5 (-1 and -2) and E15.0 (-1 and -2) embryos. E14.0-1 is viewed from the cranioventral aspect to show the metanephros in the pelvis. The arterial branch to the adrenocortical anlage (E14.0-1 and 14.5-1) is coloured in pink, the gonadal rete blastema (14.0-1) and renal arteries (14.5-1) are coloured in red, and the metanephric vascular cage is coloured in blue. The white arrowhead (14.5-1 and 15.0-1) indicates the venous rete within the gonadal rete blastema. All images show the cranial aspect towards the top. Ad, adrenal gland; ca, caudal (median sacral) artery; ci, common iliac artery; da, dorsal aorta (abdominal aorta); G, gonadal sinusoid plexus; ia, intersegmental (lumbar) artery; iv, intersegmental (lumbar) vein; M, mesonephric sinusoid plexus; mi, inferior mesenteric artery; ms, superior mesenteric artery; pcv, posterior cardinal vein; tc, coeliac trunk; u, umbilical artery; vc, inferior (caudal) vena cava. White scale bar: 1 mm.
The dorsal (abdominal) aorta bifurcated in a ventrolateral direction into the robust common iliac arteries to leave a single caudal (median sacral) artery (Fig. 5, E14.0-2). The right posterior cardinal vein became thicker, whereas the left one regressed. The intersegmental (lumbosacral) arteries and veins branched dorsally from the abdominal aorta or the middle sacral artery and posterior cardinal veins, respectively. The coeliac, superior and inferior mesenteric arteries stemmed from the ventral wall of the aorta. Arterial blood was supplied to the mesonephric ridge, which was still highly vascularized, by only a few branches, which originated from the medial edge of the umbilical artery (shown in green in Fig. 9) at points distal to the division of the external iliac artery. The mesonephric sinusoid drained into the posterior cardinal or the subcardinal veins (Fig. 5). Felix speculated that the primitive kidney (metanephros) ascends to the mesonephric arteries, as if it were on a ladder (see Discussion). However, most of the remnant arteries seen in earlier stages (white arrowheads in Fig. 6, E12.1, E13.1) had already become disconnected from the mesonephric ridges at this stage and had degenerated, except for a few that provided connections for the coarse vascular network within the gonadal primordium and remained as gonadal arteries (Fig. 5, E14.0-1; Fig. 6, E14.0). The vascularization of the para-aortic ridge also became conspicuous at this stage. The cranial half of the ridge (adrenal primordium) was invested with new arterial branches that emerged from the gonadal artery and/or directly from the ventrolateral wall of the abdominal aorta (pink arrowhead in Fig. 6, E14.0). Caudal branches (red arrowhead in Fig. 6, E14.0) from the gonadal artery and/or the aorta also penetrated into the caudal half of the ridge (gonadal rete blastema). These branches arose separately from the aorta and reached the adrenocortical anlage and the gonadal rete blastema (Fig. 6, E14.0). However, often a single branch appeared, bifurcated cranially and caudally, and reached both portions (Fig. 9, E14.0). The exact pattern of branching depended on the side (right or left) of the embryo on which it occurred.
Fig. 9.
Arterial branches from the abdominal aorta and iliac artery to the urogenital/para-aortic ridge and the metanephros in embryonic day (E)14.0, E14.2, E14.4, E14.6 and E14.8 rat embryos. Each arterial contour is traced from the dye-injected specimen and photographed from the ventral side. The adrenal arteries are shown in pink, the gonadal arteries are shown in blue, the mesonephric arteries are shown in green and the primary renal (ci) and renal (da) arteries are shown in red. ca, caudal (median sacral) artery; ci, common iliac artery; da, dorsal aorta (abdominal aorta); ei, external iliac artery; ia, intersegmental (lumbar) artery; mi, inferior mesenteric artery; ms, superior mesenteric artery; tc, coeliac trunk; u, umbilical artery.
Fig. 6.
Arterial branches from the abdominal aorta to the urogenital/para-aortic ridge and metanephros in embryonic day (E)12.1, E13.1, 14.0, 14.1, E14.3, E15.0 and E16.0 rat embryos visualized by injection of dye (ventral views). The white arrowheads show the remnant mesonephric arteries in the urogenital ridges, the pink arrowheads show the arterial branches to the adrenocortical anlage (E14.0, E14.1 and E14.3 embryos) or adrenal arteries (E15.0 and E16.0 embryos) and the red arrowheads demonstrate the arterial branches to the gonadal rete blastema (E14.0 and E14.1 embryos) or renal arteries (E14.3, E15.0 and E16.0 embryos). A, para-aortic ridge; Ad, adrenal gland; G, genital ridge; K, metanephros; M, mesonephric ridge; mi, inferior mesenteric artery; ms, superior mesenteric artery; tc, coeliac trunk. White scale bar: 500 μm.
At E14.0, the metanephroi are at an early stage in their ‘ascent’ from the original pelvic site. Saxén (1987) showed the condensation of the mesenchyme and its subsequent splitting via secondary and tertiary condensations into the ureteric bud and its branches within the metanephric rudiment of the mouse. We recognized seven distinct mesenchymal condensations and their branches at this stage. The metanephric rudiment was supplied by a few branches (primary renal arteries) that stemmed from the cranial edge of the common iliac artery (Fig. 7, E14.0-d; Fig. 10-1). From the dorsocaudal aspect, the proximal branch (or branches) reached the ureter at the renal hilum and formed an O-shaped vascular ring around it (Fig. 7, E14.0-v; Fig. 10-2). The distal branch (or branches) penetrated into the rudiment from its dorsal (cortical) side and formed a coarse network among the mesenchymal condensations. The network merged ventrally (medullary) with the vascular O-ring of the renal hilum. As a consequence, a vascular cage that represented the primary renal vascular architecture appeared within the metanephric rudiment at this stage (Fig. 7, E14.0; Fig. 10). We noticed that, in several embryos, a branch (the presumptive renal artery) from the aorta to the gonadal rete blastema often reached the renal vascular cage from the cranioventral aspect even at this stage (right branch labelled in red in Fig. 9, E14.0).
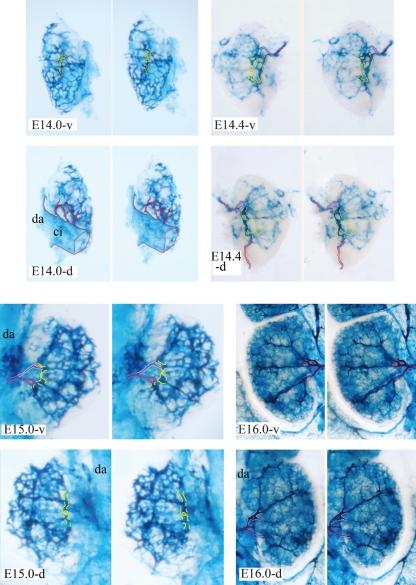
Fig. 7.
Stereo view of vascular architecture within right metanephric rudiments of embryonic day (E)14.0 (-v, ventral view; -d, dorsal view), E14.4 (-v and -d) and E16.0 (-v and -d) embryos and left metanephric rudiments of an E15.0 (-v and -d) embryo visualized by injection of dye. The primary renal artery that stems from the common iliac artery is traced in red, the definitive renal artery and its anterior division are traced in pink, and the posterior division is traced in light pink. The O-shaped vascular ring around the renal hilum is traced in yellow. ci, common iliac artery; da, dorsal aorta.
Fig. 10.
Angiogenesis of primary intrametanephric circulation. The venous drainage route to the mesonephric sinusoid is not shown. (i) A primary renal artery that stems from the common iliac artery penetrates the metanephric rudiment from the dorsal (cortical) side and forms a coarse network among the mesenchymal condensations, whereas another primary renal artery forms an O-shaped vascular ring around the renal hilum. (ii) A fine vascular cage that represents the primary circulatory architecture appears within the metanephric rudiment in the pelvic region. A presumptive renal artery from the abdominal aorta reaches the vascular cage from the cranioventral side. The metanephros is shown in light green and the ureter is shown in yellow.
Embryonic day 14.3 (14.1–14.4)
With the intensive growth of the liver, the width of the abdominal cavity more than doubled by E16.0 but the craniocaudal length of the cavity remained unchanged during E14.0–16.0 (Figs 1, 4 and 6). The urogenital ridge shifted laterally in its position on both sides. The cranial half of the para-aortic ridges, which differentiates into the adrenal glands, expanded laterally, whereas the caudal half of the ridges that were located on both sides of the aorta migrated medially to fuse into the gonadal rete blastema and the preaortic abdominal sympathetic paraganglia of Zukerkandle (1912) (Fig. 2, E14.5-1; Fig. 4, E14.25). The metanephros ascended in position from the pelvis to the lumbar region and occupied the area vacated by the migrating blastema cells (Fig. 1, E14.0; Fig. 2, E14.5-2; Fig. 4, E14.25; Fig. 6, E14.1 and E14.3). The base of the dorsal mesentery became thinner from the caudal to the cranial aspect (Fig. 1, E14.4).
As shown in Fig. 9, arterial blood for the regressing mesonephric ridge was supplied by branches of the umbilical artery. A few branches of the gonadal artery and/or the abdominal aorta that supplied the cranial half of the para-aortic ridge (the developing adrenal anlage) became thickened and formed the definitive adrenal arteries (pink arrowheads in Fig. 6, E14.1 and E14.3). The most cranial adrenal artery (superior adrenal artery), which was also the prospective inferior phrenic artery, reached the diaphragm at E14.2 (left side of Fig. 9). These arteries continued to branch in order to supply arterial blood to the diaphragm bilaterally (Fig. 9, E14.4, E14.6 and E14.8). Several caudal branches from the gonadal artery and/or the abdominal aorta reached the gonadal rete blastema, preaortic abdominal sympathetic paraganglia of Zukerkandle (1912) and the ascending metanephric rudiment. They then thickened and formed definitive renal arteries (red arrowheads in Fig. 6, E14.1 and E14.3). The genital ridge was supplied by the branches to the cranial half of the para-aortic ridge at E14.0 and E14.2 (Fig. 9) but the differentiating testicular or ovarian primordia were supplied by branches to the caudal half of the para-aortic ridge in later stages (Fig. 9, E14.4, E14.6 and E14.8). The definitive renal artery at first came into contact with the metanephros at its cranioventral edge. However, the intensive growth of the metanephros caused the definitive renal artery to shift this point of connection, putting the ureter between its anterior and posterior renal divisions to the O-shaped vascular ring around the ureter at the renal hilum (Fig. 7, E14.4-v and E14.4-d). The mesenchymal condensations increased until there were approximately 11 and the cortical and medullary zones differentiated within the metanephric anlage until E14.5. Presumptive interlobar vessels along each condensation flowed into the polygonal vascular ring (the presumptive arcuate vessels) between the cortical and medullary zones. The metanephros remained connected to the primary renal arteries that were derived from the common iliac artery at the O-shaped vascular ring of the renal hilum (Fig. 7, E14.4-d). It must be noted that the root position of the primary renal arteries on the common iliac artery shifted proximally towards the abdominal aorta until they became disconnected from the iliac artery (Fig. 9, E14.0, E14.2 and E14.4). The metanephros received a dual arterial supply from both the primary and definitive renal arteries during E14.1–E14.4 (Fig. 7, E14.4-d). Venous blood was drained laterally by the gonadal and mesonephric sinusoids at this stage (Figs 5 and 6), whereas the venous rete began to appear within the gonadal rete blastema, which was now located just ventral to the abdominal aorta and caudal to the supramesenteric artery (Fig. 2, E14.5-1; Fig. 5, E14.5-1).
Embryonic day 14.5 (14.4–14.7)
With the coincidental growth of the metanephros and the adrenal primordium along the dorsomedial aspects of the lumbar region, the urogenital ridges (mesonephros and gonadal) were shifted laterally in the peritoneal cavity at E14.5 (Fig. 1, E14.5; Fig. 2, E14.5-1). The preaortic splanchnic ganglia emerged from the fusion of paired sympathetic paraganglia within the cortical gonadal rete blastema, and the sympathetic trunk and nerve and hypogastric plexus became evident in the lumbar and pelvic regions (Fig. 4, E14.5). The dorsal mesentery of the hindgut became visible along its entire extent (Fig. 1, E14.5).
The regressing mesonephros still retained a thick sinusoidal vascular plexus that was supplied by the branches from the umbilical artery (Fig. 5, E14.5; Fig. 9). The adrenal, renal and gonadal (testicular or ovarian) arteries acquired their definitive branching patterns during this morphogenetic period but the manner and the number of arteries varied among embryos. As shown in Fig. 9, the renal artery shared a common trunk with the gonadal artery in E14.0-right and E14.6-left, whereas the adrenal artery shared a common trunk with the gonadal artery in E14.0-left and E14.4-right. The renal artery and the adrenal artery shared a common root in Fig. 9, E14.4-right, E14.6-right and E14.8-right. In Fig. 9, E14.2-right and E14.8-left, all of the adrenal, renal and gonadal arteries had a single common root.
By E14.6, the metanephros was no longer connected via the primary renal arteries with the common iliac artery and/or the caudal end of the abdominal aorta (Fig. 9, E14.6). The definitive renal artery reached the developing metanephros from the ventral side, forked into the anterior and posterior divisions, which positioned the ureter between them, and then connected to the O-shaped vascular ring around the renal hilum (Fig. 7, E14.4 and E15.0-v). An extra branch or branches from the abdominal aorta to the metanephros was often observed (Fig. 9, E14.2-right and E14.4-right). It appeared that an aberrant accessory renal artery (or arteries), which accessed the metanephros out of the renal hilum, originated from it. The renal lobes (mesenchymal condensations and their ureteric branches) increased in number, up to approximately 14, within the metanephros until E15.0. The collecting tubules also differentiated within the metanephros amongst the interstitial cells of the cortical zone (Fig. 2, E14.5-2 and E15.0). Presumptive interlobar arteries extended cortically along the ureteric branches and thus it appeared that the interlobar arteries radiated out from the O-shaped vascular ring (Fig. 7, E14.4 and E15.0). The polygonal ring-shaped presumptive arcuate vessel began to split into paired vessels, in which an artery was accompanied by a vein within the border between the cortical and medullary zones (Fig. 8c). Sporadic fenestrations also became evident within the interlobar and arcuate vessels (Fig. 8a,b).
Fig. 8.
(A) Fenestrations (black arrowhead) in interlobar vessels at embryonic day (E)15.0 visualized by injection of dye (stereo view). (B) Fenestrations (white arrowhead) in the arcuate vessel at E15.0, as demonstrated by a resin cast. (C) Double polygonal vascular rings that consist of an arcuate artery (A) and vein (V) at E19.0, as demonstrated by a resin cast. White arrow: interlobular artery. Black scale bar: 250 μm.
The regression of the mesonephros and the ascent and intense growth of the metanephros and adrenal gland, and relative descent of the gonads, forced drastic changes not only on their respective arterial systems but also on their venous drainage routes to the inferior vena cava, which consisted of the posterior and subcardinal veins. The mesonephric sinusoid degenerated and the superficial venous connections between the metanephros and the gonads became detached. As a consequence, the adrenal gland and metanephros abandoned their lateral drainage routes via the gonadal and mesonephric sinusoids. Instead, the highly vascularized adrenal gland and metanephros acquired a new medial venous drainage route via the venous rete within the gonadal rete blastema (Fig. 2, E14.5-1; Fig. 5, E14.5-1).
Embryonic day 15.0
The coincidental growth of the metanephros and adrenal gland meant that these structures approached each other rapidly but did not make direct contact at this point in development (Fig. 1, E15.0; Fig. 5, E15.0; Fig. 6, E15.0). The relative position of the mesonephros and gonads became more lateral within the widened peritoneal cavity. The splanchnic ganglia, the sympathetic trunk and nerves, and the hypogastric plexus assumed their definitive positions within the lumbar and pelvic regions (Fig. 4, E15.0). Leaving the nude area (not covered with a peritoneum), the dorsal mesentery also acquired a more definitive structure (Fig. 1, E15.0).
As the mesonephros underwent its final regression from the cranial side, the sinusoid was still supplied by branches from the common iliac artery but this blood supply degenerated gradually. The adrenal, renal and gonadal arteries had already established their definitive branching patterns that are observed in the adult. The single renal artery became robust and extended to the renal hilum from its ventral side. The O-shaped vascular ring around the renal hilum was divided into separate segments that became integrated into segmental arteries along the ureteric branches, of which there were approximately 14, at E15.0 (Fig. 7, E15.0-v). As a consequence, the anterior and posterior divisions connected to the respective interlobar arteries via the segmental arteries. The sporadic fenestrations of the interlobar and arcuate vessels merged into the capillary clefts to form a paired vessel at E15 (Fig. 8a,b), each individual vessel of which assumed an arterial or venous nature during the later stages of development (Fig. 8c). Caduff et al. (1986) showed that small holes that were observed in sheet-like regions of the microvasculature enlarge to form new capillary meshes and they proposed to name this type of expansion of the capillary network as an ‘intussusceptional’ growth of the microvascular bed. It appears that the arcuate and interlobar arteries and their collateral veins are generated directly by a mechanism that is similar to the angiogenic process of intussusception.
The polygonal double vascular rings, which consisted of arcuate arteries and veins, were assembled into a vascular cage, which gave a honeycomb-like appearance to the border between the cortical and medullary zones (Fig. 5, E15.0). The left caudal (inferior) vena cava had already disappeared and the venous rete within the gonadal rete blastema began to converge at the single left renal vein. On the left side, thick adrenal and metanephric veins drained into the right caudal vena cava via the renal vein, whereas they connected directly to the thick posterior vena cava on the right side (Fig. 5, E15.0).
Embryonic day 16.0
By E16.0, the abdominal cavity had increased in width to more than double its width at E14.0. The enlarged metanephroi and adrenal glands were directly in contact each other at this stage and occupied the medial aspects of the abdomen fully. In addition, the urogenital ridges appeared to be displaced by these structures to the lateral aspects of the cavity (Fig. 1, E16.0). The sympathetic trunk, splanchnic nerve and ganglia, and hypogastric plexus had already established their definitive anatomical position (Fig. 4, E15.0). The peritoneum covered all of the retroperitoneal organs, which include the kidney, adrenal gland and gonads (Fig. 1, E16.0).
The mesonephric arteries from the common iliac artery continued to distribute arterial blood into the mesonephric ducts and the testicular or ovarian arteries began to show their characteristic oblique patterns of distribution. The presumptive branching patterns of the adrenal arteries, which include the super, medial and inferior suprarenal arteries and the inferior phrenic artery, were established. The renal artery branched from the lateral wall of the dorsal aorta at the approximate level of the supermesenteric arterial stem and migrated to the renal hilum (Fig. 6, E16.0). It forked into the anterior and posterior divisions and then into the segmental and the interlobar arteries. The inter-renal vascular cage completed the anatomical architecture, consisting of the interlobar and arcuate arteries and their collateral veins by E16.0 (Fig. 7, E16.0). We detected interlobular arteries that arose from the arcuate artery and glomeruli in the renal cortex at E19 (Fig. 8c).
The single left renal vein received drainage from the adrenal and inferior phrenic veins and then drained into the right caudal (inferior) vena cava, whereas they directly connected to the vena cava on the right side.
Discussion
Classical dispute
After examining reconstructions of the vessels of human embryos, Felix (1912) proposed the ladder theory of abdominal vascular development (Fig. 11). At the level of the suprarenal (adrenal) body, the arteries are divided into three groups: (i) a cranial group (the first and second arteries on either side), (ii) a middle group (the third and fourth arteries on the right side and the third to fifth arteries on the left), and (iii) a caudal group (the fifth and sixth arteries on the right and the sixth and ninth arteries on the left). The fifth to ninth or seventh to ninth mesonephric arteries form the rete arteriosum urogenitale, which is an extensive arterial network. From this network, the mesonephros, reproductive glands and metanephros are supplied with arterial branches. The occurrence of this network explains why all of the arteries that arise from the roots of this network and that persist later show, within certain limits, variability in their point of origin from the aorta. Each of the remaining ninth to eleventh mesonephric arteries can become an artery spermatica interna because all supply the gonad. The suprarenal arteries arise from the second group and, on rare occasions, from the first. The renal arteries are not new formations but each is formed from a mesonephric artery. The kidney ascends to the mesonephric arteries, as if it were on a ladder. As soon as a sufficient cranial blood supply is assured, the caudal branches separate from it. By the time that the kidney has acquired its definitive position, it is supplied by several renal arteries; one of these becomes enlarged greatly to form the definitive artery, whereas the others either degenerate or persist as accessory renal arteries. The relationship between the urogenital rete and the metanephros demonstrates how the accessory renal arteries might develop and explains their multiple varied relationships with the kidney. Given that the mesonephric arteries of the third group also provide branches to the lymphatic nodes and to the symphathetic ganglia of this region, they are retained at least as far as the point of origin of the small branches even if they do not become renal or internal spermatic arteries.
Fig. 11.
A reconstruction of the mesonephric arteries of an 18-mm human embryo superimposed onto a contour drawing of a 19.4-mm embryo, after the style of Felix.
Prior to Felix’s theory, Hochstetter (1893) had proposed a model in which the ascending metanephros acquired a new branch directly from the dorsal aorta, which became the definitive renal artery. Abe (1956) and Salama et al. (1982) obtained evidence in support of this less popular model with the use of hamsters and humans, respectively, but they failed to show the morphogenetic process in these specimens. Felix’s ladder theory has been accepted widely because it provides a convenient embryological explanation for the frequent and marked variations that are seen in the renal, adrenal and gonadal arteries in humans. However, it should be noted that Felix simply superimposed the reconstructed mesonephric arteries onto the configuration of the meta- and mesonephros, the suprarenal body, and the gonad that was drawn from a different embryo. During this short morphogenetic period, the size and relative position of each primordium within the peritoneal cavity changes rapidly; concurrently, the vascular system both within and outside the primordium is reconstructed dynamically. For instance, the fine 3D architecture of the vascular cage that is composed of the segmental, interlobar and arcuate vessels is established coincidentally within the growing and differentiating metanephros. Felix did not address how the delicate renal vascular cage might switch arteries as the metanephros climbs up the mesonephric ladder because, as Felix declared, his theory was only speculative.
Verification for the contradictory theories
Here, we investigated the contradictory theories that are described above with the use of rat embryos. Both theories were based on the assumption that the metanephric rudiment ascends from the pelvis to a definitive lumbar position. Felix proposed that the attainment of this definitive position is completed by the growth of the ureter. Otani et al. (2004) suggested that this ‘ascent’ due to the convergent extension (Wolpert, 1998) of the ureter is only limited during its early stages. We prepared a series of specimens at closely-spaced time intervals from E13.0 to E16.0 for SEM observation to examine whether this assumption was correct. We observed that the metanephros ascended from the pelvic to the lumbar region during the earlier morphogenetic process as Otani et al. (2004) suggested. During later morphogenesis, the metanephros did not ascend to the adrenal gland but the two structures approached each other and came into direct contact owing to their intensive and coincidental growth in the bilateral dorsomedial aspects of the lumbar region.
The ladder theory was also based on the assumption that the higher-numbered mesonephric arteries persist as a ladder. We confirmed the presence of vestigial arteries in E12 and E13 rat embryos but most of these had degenerated before the metanephros commenced its ascent. Although the degree of segmentation of the metanephroi and the timing of regression of the mesonephros relative to metanephric development differ between species, Salama et al. (1982) reconstructed the nephric arteries and the metanephros with the use of a single 20-mm human embryo and revealed that the definitive renal artery appears as a new branch directly from the dorsal aorta. Using serial sections, Murakami (Dr. Gen Murakami, Iwamizawa Koujinkai Hospital, Iwamizawa, Japan; personal communication) confirmed that the remnant mesonephric arteries have disappeared in human embryos at stages of renal morphogenesis that are comparable to the stages by which they have disappeared in rats.
During the late 19th and early 20th centuries, gross anatomists reported large numbers of variations in the blood vascular system of humans and sought developmental explanations for these. In 1909, Herbert Evans proposed his famous ‘Network theory’, which claimed that arteries and veins are chosen from primitive capillary beds by blood flow (Thoma, 1893; Evans, 1909). The ladder and network theories are both based on the persistence of high-numbered branches that are derived from the major axial artery because it was believed that the dorsal aorta loses the ability to sprout at a very early stage (Evans, 1912). Modern vascular biologists have accepted the classical network theory that was derived from observations of the vitelline vascular network of the chick as a morphological foundation (Risau & Flamme, 1995), as they were generally less familiar with the anatomy of embryonic vascular development and may not have fully appreciated opportunities to study the morphogenesis of the circulatory system at later stages. Therefore, it is believed that the primitive capillary network is remodelled into the hierarchical vascular system, which consists of arteries, veins and capillaries (Conway et al. 2001). However, as Hochstetter (1893), Abe (1956) and Salama et al. (1982) claimed previously, we observed that the metanephros in rat embryos acquired new branches directly from the dorsal aorta without remodelling or the persistence of high-numbered remnant mesonephric arteries. The definitive renal and adrenal arteries appeared as new branches from the gonadal artery and/or directly from the abdominal aorta into the cranial part (adrenal anlage) and the caudal part (gonadal rete blastema) of the para-aortic ridge. The branching pattern and the number of the branches varied among embryos.
Para-aortic ridge
We also studied the para-aortic ridge. The adrenal primordium is thought generally to consist principally of cortical tissue from the cellular proliferation of the coelomic epithelium, which forms two large condensations on either side of the abdominal aorta in the retroperitoneal region. The adrenal medulla consists of sympathetic nerve cells derived from the neural crest and it is located initially on the medial aspect of the adrenal gland as the preaortic abdominal sympathetic paraganglia of Zukerkandle (1912). Wrobel and Süß (1999) used bovine embryos to examine the origin of this area and then compared their results with those obtained previously in studies of fish, amphibians, reptiles, birds and mammals, which included mice and humans. They claimed that, in higher vertebrates, the segmentally-organized intermediate mesoderm between the cranial mesoderm anlage and coelomic cavity fuses into a blastema that is continuously joined longitudinally (Wrobel & Süß 1999). They called this nephric-derived blastema, which consists of a cranial part that represents the adrenocortical anlage and a caudal part that corresponds to the gonadal rete blastema, the ‘common blastema’. Wrobel and Süß (1999) described how the gonadal and adrenocortical parts of the joined blastema are separated from each other by the ascending metanephros. It has also been reported that the definitive population of haematopoietic stem cells originates intraembryologically in the region of the adreno-gonadal-mesonephros (Mullar et al. 1994; Medvinsky & Dzierzak, 1996; Bruijn et al. 2000; Minehata et al. 2002). Studies of the adreno-gonadal-mesonephros region have revealed that dynamic haemato-angiogenesis occurs and support the theory that the para-aortic ridge induces new branches from the dorsal aorta during later developmental stages.
Adrenal, renal and gonadal arteries
As we have shown, the definitive superior (inferior phrenic), medial or inferior adrenal arteries originated from the cranial branches of the gonadal artery and/or the abdominal aorta into the adrenocortical blastema. The caudal branches to the gonadal rete blastema reached the metanephros as the definitive renal artery or the preaortic abdominal sympathetic paraganglia. It appears that an accessory renal artery (or arteries) that arose from the aorta above or below the main renal artery originated from an extra branch or branches, as observed in Fig. 9 (E14.2-right and E14.4-right). As the gonads descended, the caudal branches to the gonadal blastema developed as the presumptive testicular or ovarian arteries, assuming their characteristic oblique routes at E16. We confirmed that the intimate intertwinement of the adrenal, renal and gonadal arterial systems was established during this morphogenetic period. We also showed that the marked and frequent constitutional differences in the vascular supply for the adrenal, renal and gonadal arterial systems (Merklin & Michels, 1958) originated from the pattern of branching of the arteries to the para-aortic ridge.
Aberrant accessory renal arteries
The aberrant accessory renal arteries arise rarely near the aortic bifurcation or the common iliac artery, which are often observed in a horseshoe kidney. In order to give an embryological explanation for the arterial variations and anomalies that are found in human gross anatomy, classical anatomists and embryologists have made assumptions about the developmental stage by which all of the presumptive arteries have appeared (Tandler, 1903, 1904). The existence of many remnant arteries was proposed because it was believed that the dorsal aorta is surrounded by layers of periendothelial cells and loses the ability to sprout at very early stages of development. Felix made the same assumption, although he did observe that the origins of remnant arteries shifted from the lateral surface of the aorta to the dorsal or ventral aspect during the early embryonic stages. The cranial or caudal displacement of the points of origin from the aorta were reported in the coeliac and inferior mesenteric arteries of the hamster (Tada, 1956), the primary subclavian arteries of the rat (Aizawa et al. 1999), and the pronephric glomus and coeliacomesenteric artery of the salamander (Isogai, unpublished data) during relatively late embryonic stages. However, on the whole, the developmental models of Tandler (1903, 1904) and Felix (1912) are still accepted by modern anatomists. As we have shown, the metanephros acquired the primary renal arteries from the common iliac artery in the pelvic region at an early stage in its ascent. In conjunction with this ascent and the following intensive growth of the metanephros, the primary arteries elongated. They shifted their root position from the common iliac artery towards the aortic bifurcation and ultimately degenerated. Our results suggest that aberrant accessory renal arteries, which arise from points near the aortic bifurcation or the common iliac artery, are the remnants of the primary renal arteries.
Establishment of the basic vascular architecture within the metanephros
Despite the intensive study of renal glomerulogenesis (Fourman & Moffat, 1971; Kriz & Kaissling, 2000; Woolf & Yuan, 2003), histologists have paid little attention to the establishment of the basic vascular architecture within the metanephros, which includes the mechanism of formation of the vascular cage that consists of the segmental, interlobar, and arcuate arteries and veins (Lewis, 1958; Oliver et al. 1997). How the delicate renal vascular cage switches arteries from one artery to another as the metanephros climbs up the mesonephric ladder and how the cage changes its venous drainage route from the posterior cardinal vein to the inferior (caudal) vena cava is not well understood. We observed that, in the rat, the primary renal arteries penetrated into the metanephric rudiment from the dorsocaudal aspect and a vascular cage that represented the primary renal vascular architecture appeared at approximately E14.0. The definitive renal artery (the branch to the gonadal rete blastema) made its first contact with the metanephros at the cranioventral edge. It made a direct connection with the O-shaped vascular ring around the ureter at the renal hilum, which positioned the ureter between the anterior and posterior divisions, whereas the primary renal arteries from the common iliac artery or aortic bifurcation became disconnected. The O-ring is divided into segments that are integrated into the segmental vessels along the ureteric branches. The mesenchymal condensations increased in number, and the cortical and medullary zones differentiated within the metanephric rudiment. Interlobar vessels that extended cortically along the ureteric branches flowed into the polygonal arcuate vessels between the cortical and medullary zones and drained laterally into the gonadal and mesonephric sinusoids.
The interlobar and arcuate vessels began to split into paired vessels, in which an artery was accompanied by a vein, at approximately E15.0. Sporadic fenestrations within these vessels suggest that the arcuate and interlobar arteries and their collateral veins are generated directly by an intussusceptive mechanism. Popoff (1894) showed how the collateral vein to the vitelline artery is generated in the primary vascular network of the chick. Ishida (1956) revealed that there are several sequential morphogenetic processes that are the result of ‘sprouting’ and generalized the mechanism of formation of the secondary vein for the vascular system of other organs in vertebrates. Initially, intussuception was thought to be solely an aberrant mechanism that occurred during tumour angiogenesis but later it was reported to be another mechanism of embryonic angiogenesis in the lung (Caduff et al. 1986; Burri & Tarek, 1990; Djonov et al. 2000). Our results suggest that the collateral arcuate and interlobar veins are laid down directly along the arteries by a process of splitting. The formation of the interlobar and arcuate arteries and their collateral veins by E16.0 completes the anatomical architecture that makes up the inter-renal vascular cage.
Surprisingly, we did not detect any glomerular or interlobular arteries until E19.0. Mesonephric regression, metanephric ascent, gonadal descent and the rapid growth of the adrenal glands and metanephros also force drastic changes in the routes of venous drainage (Sabin, 1915; Huntington & McClure, 1920; McClure & Butler, 1925). We observed that the adrenal gland and metanephros abandoned their lateral drainage route via the gonadal and mesonephric sinusoids into the posterior or subcardinal veins and adopted new medial drainage routes via the venous rete within the gonadal rete blastema. On the left side, the gonadal rete converged into a single renal vein that received drainage from the adrenal and inferior phrenic veins along its course before draining into the right caudal (inferior) vena cava. The right renal, adrenal and inferior phrenic veins connected directly to the vena cava.
Acknowledgments
The authors would like to thank G. Murakami (Iwamizawa Koujinnkai Hospital) for providing data on human embryos and valuable suggestions, and Y. Yoshida and K. Ishida (Bio-imaging Centre of Iwate Medical University) for their technical assistance. We are grateful to B.M. Weinstein (National Institutes of Health, USA) for his critical reading of this manuscript. This study was supported by a MEXT KAKENHI grant to each author in aid of scientific research. We dedicate this article to the late Dr Masaharu Horiguchi (Department of Anatomy, School of Medicine, Iwate Medical University) who gave us valuable suggestions and encouragement during the course of this work.
References
- Abe H. Prila disvolvigo de la angiosistemo de la reno ĉe hamsteroj (in Japanese, Esperanto summary) Kaibogaku Zasshi. 1956;36-6:677–699. [Google Scholar]
- Aizawa Y, Isogai S, Izumiyama M, et al. Morphogenesis of the primary arterial trunks of the forelimb in the rat embryos. Anat Embryol. 1999;200:573–582. doi: 10.1007/s004290050305. [DOI] [PubMed] [Google Scholar]
- Bruijn MFTR, Speck NA, Peeters MCE, et al. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 2000;19-11:2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri PH, Tarek MR. A novel mechanism of capillary growth in the rat pulmonary microcirculation. Anat Rec. 1990;228:35–45. doi: 10.1002/ar.1092280107. [DOI] [PubMed] [Google Scholar]
- Caduff JH, Fischer LC, Burri PH. Scanning electron microscope study of the developing microvasculature in the postnatal rat lung. Anat Rec. 1986;216:154–164. doi: 10.1002/ar.1092160207. [DOI] [PubMed] [Google Scholar]
- Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507–521. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- Djonov V, Schmid M, Tschnz SA, et al. Intussusceptive angiogenesis its role in embryonic vascular network formation. Circ Res. 2000;86:286–292. doi: 10.1161/01.res.86.3.286. [DOI] [PubMed] [Google Scholar]
- Dyson M. Gray’s Anatomy. 38th edn. London: Churchill Livingstone; 1995. Urinary system; pp. 1813–1845. [Google Scholar]
- Evans HM. On the development of the aorta, cardinal and umbilical veins, and the other blood vessels of vertebrate embryos from capillaries. Anat Rec. 1909;3:498–518. [Google Scholar]
- Evans HM. The development of the vascular system. In: Keibel F, Mall FP, editors. Manual of Human Embryology. Philadelphia: J. B. Lippincott Company; 1912. pp. 570–709. [Google Scholar]
- Felix W. The development of the urogenital organs. In: Keibel F, Mall FP, editors. Manual of Human Embryology. Philadelphia: J. B. Lippincott Company; 1912. pp. 752–880. [Google Scholar]
- Fourman J, Moffat DB. The Blood Vessels of the Kidney. Oxford and Edinburgh: Blackwell Scientific Publications; 1971. [Google Scholar]
- Hochstetter F. Beiträge zur Entwicklungsgeschichte des Venen-systems der Amnioten. III. Säuger. Morp Jb. 1893;20:543–648. [Google Scholar]
- Huntington GS, McClure CFW. The development of the veins in the domestic cat (Felis domestica) with especial reference, 1) to the share taken by the supra-cardinal veins in the development of the postcava and azygos veins and 2) to the interrelation of the variant conditions of the postcava and its tributaries, as found in the adult. Anat Rec. 1920;20-1:1–30. [Google Scholar]
- Ishida Z. Disvolvigo de kalateralaj vejnoj sur la ovoflavsako de kokojo (in Japanese, Esperanto summary) Kaibogaku Zasshi. 1956;31:334–348. [Google Scholar]
- Isogai S, Horiguchi M. Utility of corrosive resin casts for three-dimensional microanatomy of renal vascular system in urodela larvae. Three-Dimens Microanat. 1996;2:19–22. [Google Scholar]
- Kriz W, Kaissling B. Structural organization of the mammalian kidney. In: Seldin DW, Giebisch G, editors. The Kidney. 3rd edn. Philadelphia: Lippincott Williams and Wilkins; 2000. pp. 587–654. [Google Scholar]
- Lewis OJ. The development of the blood vessels of the metanephros. J Anat. 1958;92:84–97. [PMC free article] [PubMed] [Google Scholar]
- McClure CFW, Butler EG. The development of the vena cava inferior in Man. Am J Anat. 1925;35-3:331–383. [Google Scholar]
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Merklin RJ, Michels NA. The variant renal and suprarenal blood supply with data on the inferior phrenic, ureteral and gonadal arteries. J Int Coll Surg. 1958;29:41–76. [PubMed] [Google Scholar]
- Minehata K, Mukouyama Y, Sekiguchi T, et al. Macrophage colony stimulating factor modulates the development of hematopoiesis by stimulating the differentiation of endothelial cells in the AGM region. Blood. 2002;99-7:2360–2368. doi: 10.1182/blood.v99.7.2360. [DOI] [PubMed] [Google Scholar]
- Mullar AM, Medvinsky A, Strouboulis J, et al. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Oliver JA, Goldberg MR, Al-Awqati Q. Endothelial cell targeting during renal development: use of monoclonal antibodies. Am J Physiol. 1997;172:F153–F159. doi: 10.1152/ajprenal.1997.272.2.F153. [DOI] [PubMed] [Google Scholar]
- Otani H, Nimura M, Inoue K, et al. Organogenesis and histogenesis of the urinary system (in Japanese) Nishinihon J Urol. 2004;66-3:148–159. [Google Scholar]
- Popoff D. Die Dottersack Gefässe des Huhnes. Wiesbaden: C.W. Kreidel’s Verlag; 1894. [Google Scholar]
- Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- Sabin FR. On the fate of the posterior cardinal veins and their relation to the development of the vena cava and azygos in the embryo pig. Contrib Embryol. 1915;7:7–32. [Google Scholar]
- Salama J, Folio P, Chevrel JP. Reconstruction du métanéphros chez un embryon humain de 20 millimtres (VC) étude de l’origine de l’artère rénale. Bull Assoc Anat. 1982;66:397–406. [PubMed] [Google Scholar]
- Saxén L. Organogenesis of the Kidney. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- Tada Y. Disvolvigo de sangvazoj sur la ovoflavsako kaj ankau de la mesintesto ce hamsteroj (Cricetus anuratus) (in Japanese, Esperanto summary) Kaibogaku Zasshi. 1956;31:388–417. [Google Scholar]
- Tandler J. Zur Entwickelungsgeschichte der Menschlichen Darmarterien. 1903;23:187–210. Anatomische Hefte. [Google Scholar]
- Tandler J. Über die Varietäten der Arteria Coeliaca und deren Entwickelung. 1904;25:473–500. Anatomische Hefte. [Google Scholar]
- Thoma R. Unters über die Histogenese und Histomechanic des Gefässystems. Stuttgart: Enke Verlag; 1893. [Google Scholar]
- Ura R. Studies on the development of the blood vessels by injection (reference to the vitelline circulation) (in Japanese) Kaibogaku Zasshi. 1943;21:792–793. [PubMed] [Google Scholar]
- Wolpert L. Convergent extension and epiboly are due to cell intercalation. In: Wolpert L, et al., editors. Principles of Development. Tokyo: Oxford University Press; 1998. pp. 250–252. [Google Scholar]
- Woolf AS, Yuan HT. Development of kidney blood vessels. In: Vize PD, Woolf AS, Bard JBL, editors. The Kidney. London: Academic Press; 2003. pp. 251–266. [Google Scholar]
- Wrobel KH, Süß F. On the origin and prenatal development of the bovine adrenal gland. Anat Embryol. 1999;199:301–318. doi: 10.1007/s004290050230. [DOI] [PubMed] [Google Scholar]
- Zuckerkandl E. The development of the chromaffin organs and of the suprarenal glands. In: Keibel F, Mall FP, editors. Manual of Human Embryology. Philadelphia: J. B. Lippincott Company; 1912. pp. 157–179. [Google Scholar]