Abstract
The development of the craniofacial system occurs, among other reasons, as a response to functional needs. In particular, the deficiency of the proper masticatory stimulus affects the growth. The purpose of this study was to relate alterations of muscle activity during postnatal development to adaptational changes in the muscle fibers. Fourteen 21-day-old Wistar strain male rats were randomly divided into two groups and fed on either a solid (hard-diet group) or a powder (soft-diet group) diet for 63 days. A radio-telemetric device was implanted to record muscle activity continuously from the superficial masseter, anterior belly of digastric and anterior temporalis muscles. The degree of daily muscle use was quantified by the total duration of muscle activity per day (duty time), the total burst number and their average length exceeding specified levels of the peak activity (5, 20 and 50%). The fiber type composition of the muscles was examined by the myosin heavy chain content of fibers by means of immunohistochemical staining and their cross-sectional area was measured. All muscle fibers were identified as slow type I and fast type IIA, IIX or IIB (respectively, with increasing twitch contraction speed and fatigability). At lower activity levels (exceeding 5% of the peak activity), the duty time of the anterior belly of the digastric muscle was significantly higher in the soft-diet group than in the hard-diet group (P < 0.05). At higher activity levels (exceeding 20 and 50% of the peak activity), the duty time of the superficial masseter muscle in the soft-diet group was significantly lower than that in the hard-diet group (P < 0.05). There was no difference in the duty time of the anterior temporalis muscle at any muscle activity level. The percentage of type IIA fibers of the superficial masseter muscle in the soft-diet group was significantly lower than that in the hard-diet group (P< 0.01) and the opposite was true with regard to type IIB fibers (P< 0.05). The cross-sectional area of type IIX and type IIB fibers of the superficial masseter muscle was significantly smaller in the soft-diet group than in the hard-diet group (P < 0.05). There was no difference in the muscle fiber composition and the cross-sectional area of the anterior belly of the digastric and anterior temporalis muscles. In conclusion, for the jaw muscles of male rats reared on a soft diet, the slow-to-fast transition of muscle fiber was shown in only the superficial masseter muscle. Therefore, the reduction in the amount of powerful muscle contractions could be important for the slow-to-fast transition of the myosin heavy chain isoform in muscle fibers.
Keywords: electromyography, fiber type transition, jaw muscle, myosin heavy chain
Introduction
Skeletal muscles contain a heterogeneous fiber type composition consisting of fibers with different physiological properties. These properties are mainly related to the expression of various myosin heavy chain (MyHC) isoforms (Cobos et al. 2001; Pette & Staron, 2001). MyHC-I is found in infatigable slow-type fibers. MyHC-IIA, MyHC-IIX and MyHC-IIB can be found in fast-type fibers. IIB fibers show the fastest contraction velocities and extreme fatigability, whereas the fast-contracting IIA fibers show some fatigue resistance but not to the same level as observed in slow-type fibers. IIX fibers demonstrate intermediate physiological properties relative to type IIA and IIB fibers. Muscles can adapt to altered functional demands by changing the fiber cross-sectional area and their MyHC-type composition. Long-term electrical stimulation of the skeletal muscle has been demonstrated to cause an increase in the percentage of slow-type fatigue-resistant fibers (Kernell et al. 1987; Caiozzo, 2002; Woo et al. 2002; Pae et al. 2007) and cross-sectional area of muscle fibers (Delp & Pette, 1994). Inactivity of the muscle is followed by an increase in fast-type fibers and decrease in the fiber cross-sections (Grossman et al. 1998).
In jaw muscles, foods with different consistencies have been used to change the loading to muscles (Kiliaridis et al. 1985, 1988; Liu et al. 1998; Saito et al. 2002; Langenbach et al. 2003; Larsson et al. 2005). Kiliaridis et al. (1988) reported that, in rats fed on a soft diet, a slow-to-fast transition of myosin types was observed in the anterior deep masseter muscle but not in the anterior digastric muscle. The transition in the superficial masseter muscle was confirmed by the measurement of the expression of MyHC mRNA (Saito et al. 2002). These studies clearly show that a decrease in functional demand causes morphological changes in some jaw muscle fibers. However, it is unknown how these functional changes affect the daily use of the muscle fibers.
The craniofacial system development occurs as a response to functional needs and here the jaw muscle function has a considerable influence on craniofacial morphology. It has been reported that the development of craniofacial morphology can be affected by a diet habit in human (Sardi et al. 2006) and in experimental animals (Kiliaridis et al. 1985; Larsson et al. 2005). Bone loadings are predominantly caused by muscle contractions. Therefore, muscular strains are considered inductive for adaptive processes in the underlying skeletal system (Burr, 1997; Turner, 2000). In order to know the influence of environmental factors on craniofacial system development, it is important to examine the responses of jaw muscles to diet consistency.
The purpose of this study was to relate alterations of muscle activity during postnatal development to adaptational changes in the jaw muscle fibers. Rats were randomly divided into two groups at weaning (3 weeks old) and fed on either a solid (hard-diet group) or a powder (soft-diet group) diet for 63 days. At 12 weeks of age, daily activities of the superficial masseter, anterior belly of digastric and anterior temporalis muscles were determined, whereafter the fiber type composition of these muscles was characterized by immunohistological analysis. We hypothesized that the amount of muscle activity would be less in the soft-diet group combined with a larger amount of fast-type fibers with smaller cross-sectional areas. The scale of this divergence between groups will probably differ between the three muscles depending on their role in the generation of occlusal forces. From this, it can be assumed that the largest modifications will be found in the masseter, the muscle with the most prominent role during mastication (Weijs & Dantuma, 1975).
Materials and methods
Experimental animals
Fourteen 21-day-old Wistar strain male rats were randomly divided into two groups (n = 7 in each group) and fed on either a solid (hard-diet group) or a powder (soft-diet group) diet for 63 days. The hard-diet group was fed an ordinary pellet (CE-2; CLEA Japan, Inc., Tokyo, Japan), whereas the soft-diet group was fed a powder diet that contained the same constituents (CE-2; CLEA Japan, Inc.). All animals were weighed once per week to monitor growth.
At 12 weeks of age, a telemetric device was implanted in the shoulder area for the recording of jaw muscle activities. Each animal was housed individually in a cage (45 × 22 × 18 cm) and fed with pellets and water ad libitum. Day–night rhythm was ensured by automatic dimmed lighting (08:00–20:00 h). Except for the daily care and regular physical examination, they were left undisturbed to minimize any external influence. After the recording period, the animals were killed with an overdose of sodium pentobarbital (300 mg/kg Nembutal; Dinabott, Osaka, Japan) and the jaw muscles (the superficial masseter, anterior belly of digastric and anterior temporalis muscles) were dissected for immunohistochemical analysis of their muscle fibers. The protocol of the experiment was approved by the Animal Care and Use Committee at Hiroshima University.
Telemetric system
The current system for electromyographic (EMG) recording has been described in previous studies (Langenbach et al. 2004; van Wessel et al. 2005; Kawai et al. 2007). Briefly, implantable three-channel transmitters for biopotential recording (F50-EEE, 45 × 17 × 10 mm, 14 g; Data Sciences International, St Paul, MN, USA) were used to record muscle activity. For the implantation of this device, each animal (n = 7, 12 weeks old) was anesthetized with intra-abdominal injections of sodium pentobarbital at a dose of 50 mg kg−1 body weight. The transmitter was placed under the skin of the shoulder area and the three pairs of bipolar electrodes were subcutaneously led to an incision in the right submandibular region. From here, the bipolar electrodes were inserted into the center of the right superficial masseter, anterior belly of digastric and anterior temporalis muscles, and sutured at the muscle surface to prevent them from dislodging. The distance between the two tips of the bipolar electrodes (each consisting of a double helix, diameter: 0.45 mm) was 1–2 mm and the effective electrode tip length was 7 mm. These procedures were performed under sterile conditions. An antibiotic (100 mg/kg phosphomycin disodium salt; Sigma-Aldrich Co., St Louis, MO, USA) was administered for 3 days preceding and 2 days following surgery. An analgesic (5 μg/kg buprenorphine; Lepetan, Otsuka Pharmaceutical Co., Ltd, Tokyo, Japan) was provided immediately after surgery. Muscle activities were continuously recorded for 1 week, starting at 7 days after surgery.
In the device, the biopotentials were filtered (first-order low-pass filter, 158 Hz) and sampled (250 Hz) on the input of each channel. The transmitted data were then collected by a receiver (RPC-1; Data Sciences International) placed under the cage. The signals were stored on a personal computer hard disk, using the Dataquest A.R.T. data acquisition system (Data Sciences International).
Muscle activity analysis
The method of analysis was similar to that performed previously (van Wessel et al. 2005; Kawai et al. 2007). Briefly, muscle activities of a 24-h period were analyzed using Spike2 software (Cambridge Electronic Design, Cambridge, UK). After motion artifacts had been removed (5-Hz high-pass filter), the signal was rectified, averaged and downsampled (20-ms window, i.e. five samples). To eliminate possible artifacts, 0.001% of the samples (i.e. 43 samples) with the largest amplitudes were excluded. The peak EMG activity was defined as the largest of the remaining samples. Daily muscle use was characterized by means of the total duration of muscle activity (duty time), the total burst number and their average length, determined for muscle activities exceeding 5, 20 and 50% of the day’s peak activity. A burst was defined as a series of consecutive samples exceeding the aforementioned activity levels (van Wessel et al. 2005; Kawai et al. 2007). Note that the duty time for activations exceeding a certain level includes the duty times for activations exceeding all higher levels. Duty time exceeding 5% of the peak EMG level was assumed to represent the overall muscle use including all levels and types of muscle activities. Muscle activity exceeding 50% of the peak EMG level was considered as representative of the most forceful muscle usage.
Immunohistochemical analysis
After the EMG recording period, the left jaw muscles were isolated, rapidly frozen in liquid nitrogen-cooled isopentane and stored at −80 °C until further processing. For each of the three muscle portions (superficial masseter, anterior belly of digastric and anterior temporalis muscles), the muscles were brought to −20 °C and serial transverse sections (10 μm) were made with a cryomicrotome. They were obtained from the belly of the muscles perpendicular to the main direction of the muscle fibers.
After overnight fixation at −20 °C in a mixture of methanol : acetone : acetic acid : water (35 : 35 : 5 : 25) (Wessels et al. 1991), five consecutive sections were incubated with monoclonal antibodies raised against purified myosin (antibody 219-1D1 recognized MyHC-I, antibody 333-7H1 recognized MyHC-IIA, antibody 332-3D4 recognized MyHC-IIA and MyHC-IIX, antibody 340-3B5 recognized all fast MyHC isoforms, and antibody 249-5A4 recognized MyHC-cardiac α myosin) (Sant’Ana Pereira et al. 1995; Korfage et al. 2001; Kawai et al. 2009). The indirect unconjugated immunoperoxidase technique (peroxidase antiperoxidase complex technique) was applied to detect the specific binding of the different antibodies and nickel-diaminobenzidine was used to visualize the staining (Hancock, 1986).
Evaluation of the fiber type distribution was performed at the same locations as the electrode insertions in the right jaw muscles. This selection was carefully executed because a heterogeneity in the fiber type distribution exists in the jaw muscles (Cobos et al. 2001; Sano et al. 2007; Kawai et al. 2009). At each sample area, photographs were taken using a digital camera attached to a microscope. The fibers were copied onto a sheet and fiber outlines were drawn. On average, each sample area consisted of about 200 fibers. Fibers were classified by means of the five consecutive incubated sections. To measure the cross-sectional area of the fibers, the drawn sheets were read into a personal computer via a flatbed scanner, together with a grade mark for correction of enlargement. A custom-made program computed the cross-sectional area of each muscle fiber from the reproduced image. In total, more than 8000 fibers were analyzed in the 42 muscles recorded.
Statistics
The daily duty time, total burst number and mean burst length were averaged for each muscle and activity level. The proportions and mean cross-sectional areas of the different fiber types were calculated for each animal and their mean values for each muscle were determined. The averaged body weight of rats in each group was also calculated every week. Results were expressed as means ± SDs. To identify differences between hard- and soft-diet groups in these parameters, all muscles were compared by a Student’s t-test. In all tests, a P-value of < 0.05 was considered statistically significant.
Results
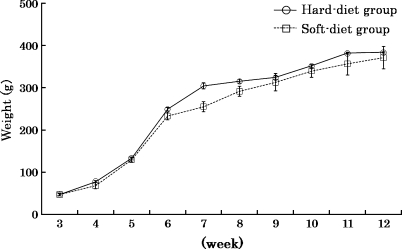
Throughout the experimental period, no significant difference in body weight between the two groups was found, although some temporary dissimilarities could be seen (Fig. 1).
Fig. 1.
Mean body weight of rats and SDs in hard-diet group (solid line) and soft-diet group (dotted line). Values are means ± SD.
Electromyographic findings
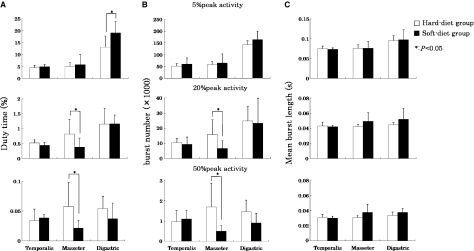
The peak activity values of all muscles in the soft-diet group were lower compared with the hard-diet group (Table 1) (superficial masseter, P < 0.05; anterior temporalis, n.s.; anterior belly of digastric, P < 0.01). At lower activity levels (exceeding 5% of the peak EMG level), the duty time of the anterior belly of the digastric muscle was significantly higher in the soft-diet group than in the hard-diet group (Fig. 2A; P < 0.05). At higher activity levels (exceeding 20 and 50% of the peak EMG level), the duty time of the superficial masseter muscle was significantly lower in the soft-diet group than in the hard-diet group (Fig. 2A; P < 0.05). For the burst numbers, a similar pattern of difference was seen between both groups (Fig. 2B). The burst length did not significantly differ between both groups for any muscle and activity level (Fig. 2C).
Table 1.
Peak activity values of the superficial masseter, anterior belly of digastric and anterior temporalis muscles in 1 day
| Hard-diet group |
Soft-diet group |
||||
|---|---|---|---|---|---|
| Mean (mV) | SD | Mean (mV) | SD | P | |
| Masseter | 1.22 | 0.54 | 0.58 | 0.27 | 0.05 |
| Digastric | 0.89 | 0.30 | 0.49 | 0.27 | 0.01 |
| Temporalis | 1.02 | 0.54 | 0.80 | 0.23 | n.s. |
Fig. 2.
(A) Duty time (%), (B) burst number and (C) mean burst length (s) in hard-diet group (open) and soft-diet group (solid). Muscle activities exceeding 5, 20 and 50% of the peak electromyographic level for all tested muscles (superficial masseter, anterior belly of digastric and anterior temporalis muscles) are shown. Values are means + SD. *Significant differences between both groups (P < 0.05).
Histometric findings
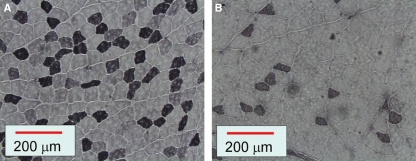
Representative sections incubated with antibody against MyHC-IIA showed that the superficial masseter muscle dissected from rats in the hard-diet group (Fig. 3A) contained more type IIA fibers than the soft-diet groups (Fig. 3B).
Fig. 3.
Immunohistochemical sections of rat superficial masseter muscles in hard-diet group (A) and soft-diet group (B) incubated with the antibody against myosin heavy chain IIA. Note that there were fewer type IIA fibers in the soft-diet group than in the hard-diet group.
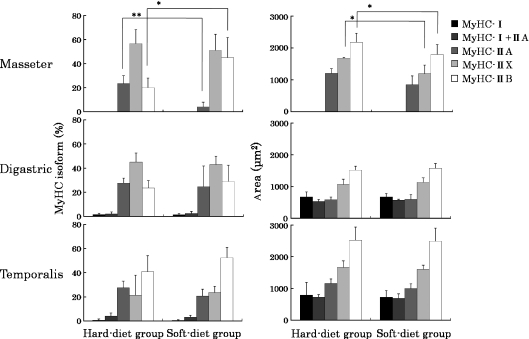
All muscles contained large numbers of fast-type fibers (type IIA, IIX and IIB) and, in particular, there were no type I fibers present in the superficial masseter muscle (Fig. 4). MyHC-cardiac α was not detected in any of the examined jaw muscles. Only the superficial masseter muscle showed significant differences in fiber type composition between the two experimental groups (Fig. 4). The percentage of type IIA fibers of the superficial masseter muscle was significantly lower in the soft-diet group (4.2%) than in the hard-diet group (23.3%; P< 0.01), whereas the percentage of type IIB fibers was significantly higher in the soft-diet group (44.9%) than in the hard-diet group (20.0%; P < 0.05).
Fig. 4.
The proportions (%) and mean cross-sectional areas (μm2) of the different fiber types in the superficial masseter, anterior belly of digastric and anterior temporalis muscles. Values are means + SD. Significant differences between both groups: *P < 0.05; **P < 0.01. MyHC, myosin heavy chain.
For all muscles, the cross-sectional area of the type IIB fibers was the largest (1517–2523 μm2). Again, only the superficial masseter muscle showed significant differences in the cross-sectional area between the soft- and hard-diet groups. Type IIX and IIB fibers were significantly smaller in the soft-diet group than in the hard-diet group (Fig. 4; P< 0.05).
Discussion
Alteration of food consistency has been used to study the adaptation capacity in the craniofacial region (Kiliaridis et al. 1985, 1988; Liu et al. 1998; Kakizaki et al. 2002; Saito et al. 2002; Langenbach et al. 2003; Inoue et al. 2004; Larsson et al. 2005; Tanaka et al. 2007). These studies indicate that a decrease of the amount of mastication and thus occlusal forces affects all structures, including bone and muscles. It is assumed that muscle forces play an important role in this but so far it is still controversial to what extent muscle activity is affected and how this might relate, for instance, to the modified muscle fiber type composition.
In this study, the adaptation in jaw muscles to unloading was examined using two experimental male groups fed on a food with different consistencies by means of muscle activity and immunohistochemical analysis. The changes in daily activity and fiber type composition are different across the examined muscles. Relative to the rats fed the normal hard food, a slow-to-fast transition of the MyHC isoform in the soft-food animals was detected only in the superficial masseter muscle. The superficial masseter muscle is a jaw-closing muscle that works, among other times, during the power stroke in which food is crushed (Kawai et al. 2009). This activity will be decreased in animals feeding on powdered pellets, in accordance with the lower duty time and burst numbers for activities exceeding the 50% level. This decrease in high level activities seems to result in a modification in the fiber type composition towards faster fibers. The amount of force that a muscle fiber can produce depends not only on the MyHC isoform but also on its cross-sectional area (Maughan et al. 1983). Hence, in the soft-diet-fed animals, the average cross-sectional fiber area was also decreased by the decline in powerful muscle contractions. The temporalis muscle is also a jaw-closing muscle but it works mainly in the beginning of the jaw-closing phase (Thomas & Peyton, 1983). Its role during the power stroke is less prominent. Its activity will therefore be less affected by the change in food consistency. Accordingly, its daily activity and fiber type composition did not show any difference between the two experimental groups. The digastric muscle is a jaw-opening muscle, important to stabilize both the jaw and hyoid, and involved in swallowing and other behaviors in which the hyoid or tongue are required (Cobos et al. 2001). However, its most powerful contractile forces are apparently not affected by a change in food consistency. The overall daily activity of this muscle was increased in the soft-diet group, whereas no changes were detected for the higher activity levels. Thus, in soft-diet animals this muscle showed an increased amount of low-amplitude activities. Powdered food might be processed differently. Instead of clear chewing strokes, the food might be licked or at least transported through the oral cavity by mainly tongue motions, resulting in an increased amount of low muscle activities. Apparently, this increase of mainly low-amplitude activities does not influence the fiber type composition.
Muscle fibers are dynamic structures capable of altering their phenotype under various conditions, e.g. altered neuromuscular activity, mechanical loading or unloading, altered hormonal profiles and aging (Pette & Staron, 2001). From the EMG and histometric findings, it seems reasonable to speculate that the reduction in the amount of powerful muscle contractions is important for the transition of muscle fiber types. Miyamoto et al. (1996) revealed that, in human, more than 80% of muscle activity exceeding the 25% level (and almost all muscle activity exceeding the 50% level) was observed during meal time. On the basis of that, muscle activities exceeding the 20 and 50% level reflect muscle use during specific powerful functions of the masticatory system, whereas muscle activity exceeding the 5% level was assumed to represent the overall muscle activity including low-intensity activities. The present data indicate that activities exceeding 50% of the peak EMG level have a total duration of only 30–60 s. However, disappearance of this small amount of powerful muscle contractions leads to a slow-to-fast transition of the MyHC isoform in the involved jaw muscle of male rats, accompanied by a significant decrease in the average fiber cross-section.
Acknowledgments
We are grateful to Jan Harm Koolstra and Tim van Wessel for their constructive criticism and Leo van Ruijven for technical advice. This research was supported in part by a grant (no. 19890138) for Science Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- Burr DB. Muscle strength, bone mass, and age-related bone loss. J Bone Miner Res. 1997;12:1547–1551. doi: 10.1359/jbmr.1997.12.10.1547. [DOI] [PubMed] [Google Scholar]
- Caiozzo VJ. Plasticity of skeletal muscle phenotype: mechanical consequences. Muscle Nerve. 2002;26:740–768. doi: 10.1002/mus.10271. [DOI] [PubMed] [Google Scholar]
- Cobos AR, Segade LA, Fuentes I. Muscle fibre types in the suprahyoid muscles of the rat. J Anat. 2001;198:283–294. doi: 10.1046/j.1469-7580.2001.19830283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD, Pette D. Morphological changes during fiber type transitions in low-frequency-stimulated rat fast-twitch muscle. Cell Tissue Res. 1994;277:363–371. doi: 10.1007/BF00327784. [DOI] [PubMed] [Google Scholar]
- Grossman EJ, Roy RR, Talmadge RJ, et al. Effects of inactivity on myosin heavy chain composition and size of rat soleus fibers. Muscle Nerve. 1998;21:375–389. doi: 10.1002/(sici)1097-4598(199803)21:3<375::aid-mus12>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hancock MB. Two-color immunoperoxidase staining: visualization of anatomic relationships between immunoreactive neural elements. Am J Anat. 1986;175:343–352. doi: 10.1002/aja.1001750216. [DOI] [PubMed] [Google Scholar]
- Inoue M, Harasawa Y, Yamamura K, et al. Effects of food consistency on the pattern of extrinsic tongue muscle activities during mastication in freely moving rabbits. Neurosci Lett. 2004;368:192–196. doi: 10.1016/j.neulet.2004.07.043. [DOI] [PubMed] [Google Scholar]
- Kakizaki Y, Uchida K, Yamamura K, et al. Coordination between the masticatory and tongue muscles as seen with different foods in consistency and in reflex activities during natural chewing. Brain Res. 2002;929:210–217. doi: 10.1016/s0006-8993(01)03392-3. [DOI] [PubMed] [Google Scholar]
- Kawai N, Tanaka E, Langenbach GEJ, et al. Daily jaw muscle activity in freely moving rats measured with radio-telemetry. Eur J Oral Sci. 2007;115:15–20. doi: 10.1111/j.1600-0722.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- Kawai N, Sano R, Korfage JAM, et al. Functional characteristics of the rat jaw muscles: daily muscle activity and fiber type composition. J Anat. 2009;215:656–662. doi: 10.1111/j.1469-7580.2009.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D, Eerbeek O, Verhey BA, et al. Effects of physiological amounts of high- and low-rate chronic stimulation on fast-twitch muscle of the cat hindlimb. I. Speed- and force-related properties. J Neurophysiol. 1987;58:598–613. doi: 10.1152/jn.1987.58.3.598. [DOI] [PubMed] [Google Scholar]
- Kiliaridis S, Engström C, Thilander B. The relationship between masticatory function and craniofacial morphology. I. A cephalometric longitudinal analysis in the growing rat fed a soft diet. Eur J Orthod. 1985;7:273–283. doi: 10.1093/ejo/7.4.273. [DOI] [PubMed] [Google Scholar]
- Kiliaridis S, Engström C, Thilander B. Histochemical analysis of masticatory muscle in the growing rat after prolonged alteration in the consistency of the diet. Arch Oral Biol. 1988;33:187–193. doi: 10.1016/0003-9969(88)90044-1. [DOI] [PubMed] [Google Scholar]
- Korfage JAM, Schueler YT, Brugman P, et al. Differences in myosin heavy-chain composition between human jaw-closing muscles and supra- and infrahyoid muscles. Arch Oral Biol. 2001;46:821–827. doi: 10.1016/s0003-9969(01)00042-5. [DOI] [PubMed] [Google Scholar]
- Langenbach GEJ, van de Pavert S, Savalle WP, et al. Influence of food consistency on the rabbit masseter muscle fibres. Eur J Oral Sci. 2003;111:81–84. doi: 10.1034/j.1600-0722.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- Langenbach GEJ, van Wessel T, Brugman P, et al. Variation in daily masticatory muscle activity in the rabbit. J Dent Res. 2004;83:55–59. doi: 10.1177/154405910408300111. [DOI] [PubMed] [Google Scholar]
- Larsson E, Øgaard B, Lindsten R, et al. Craniofacial and dentofacial development in pigs fed soft and hard diets. Am J Orthod Dentofacial Orthop. 2005;128:731–739. doi: 10.1016/j.ajodo.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Ikeda K, Harada S, et al. Functional properties of jaw and tongue muscles in rats fed a liquid diet after being weaned. J Dent Res. 1998;77:366–376. doi: 10.1177/00220345980770020501. [DOI] [PubMed] [Google Scholar]
- Maughan RJ, Watson JS, Weir J. Strength and cross-sectional area of human skeletal muscle. J Physiol. 1983;338:37–49. doi: 10.1113/jphysiol.1983.sp014658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Yamada K, Ishizuka Y, et al. Masseter muscle activity during the whole day in young adults. Am J Orthod Dentofac Orthop. 1996;110:394–398. doi: 10.1016/s0889-5406(96)70041-0. [DOI] [PubMed] [Google Scholar]
- Pae EK, Hyatt JP, Wu J, et al. Short-term electrical stimulation alters tongue muscle fibre type composition. Arch Oral Biol. 2007;52:544–551. doi: 10.1016/j.archoralbio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochem Cell Biol. 2001;115:359–372. doi: 10.1007/s004180100268. [DOI] [PubMed] [Google Scholar]
- Saito T, Ohnuki Y, Yamane A, et al. Effects of diet consistency on the myosin heavy chain mRNAs of rat masseter muscle during postnatal development. Arch Oral Biol. 2002;47:109–115. doi: 10.1016/s0003-9969(01)00094-2. [DOI] [PubMed] [Google Scholar]
- Sano R, Tanaka E, Korfage JAM, et al. Heterogeneity of fiber characteristics in the rat masseter and digastric muscles. J Anat. 2007;211:464–470. doi: 10.1111/j.1469-7580.2007.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant’Ana Pereira JAA, Wessels A, Nijtmans L, et al. New method for the accurate characterization of single human skeletal muscle fibres demonstrates a relation between mATPase and MyHC expression in pure and hybrid fibre types. J Muscle Res Cell Motil. 1995;16:21–34. doi: 10.1007/BF00125307. [DOI] [PubMed] [Google Scholar]
- Sardi ML, Novellino PS, Pucciarelli HM. Craniofacial morphology in the Argentine Center-West: consequences of the transition to food production. Am J Phys Anthropol. 2006;130:333–343. doi: 10.1002/ajpa.20379. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Sano R, Kawai N, et al. Effect of food consistency on the degree of mineralization in the rat mandible. Ann Biomed Eng. 2007;35:1617–1621. doi: 10.1007/s10439-007-9330-x. [DOI] [PubMed] [Google Scholar]
- Thomas NR, Peyton SC. An electromyographic study of mastication in the freely-moving rat. Arch Oral Biol. 1983;28:939–945. doi: 10.1016/0003-9969(83)90090-0. [DOI] [PubMed] [Google Scholar]
- Turner CH. Muscle–bone interactions, revisited. Bone. 2000;27:339–340. doi: 10.1016/s8756-3282(00)00349-5. [DOI] [PubMed] [Google Scholar]
- Weijs WA, Dantuma R. Electromyography and mechanics of mastication in the albino rat. J Morphol. 1975;146:1–33. doi: 10.1002/jmor.1051460102. [DOI] [PubMed] [Google Scholar]
- van Wessel T, Langenbach GEJ, van Ruijven LJ, et al. Daily number and lengths of activity bursts in rabbit jaw muscles. Eur J Neurosci. 2005;21:2209–2216. doi: 10.1111/j.1460-9568.2005.04054.x. [DOI] [PubMed] [Google Scholar]
- Wessels A, Vermeulen JL, Virágh S, et al. Spatial distribution of “tissue-specific” antigens in the developing human heart and skeletal muscle. II. An immunohistochemical analysis of myosin heavy chain isoform expression patterns in the embryonic heart. Anat Rec. 1991;229:355–368. doi: 10.1002/ar.1092290309. [DOI] [PubMed] [Google Scholar]
- Woo EB, Tang AT, Jarvis JC, et al. Improved viability of latissimus dorsi muscle grafts after electrical prestimulation. Muscle Nerve. 2002;25:679–684. doi: 10.1002/mus.10099. [DOI] [PubMed] [Google Scholar]