Abstract
The AIDS Malignancy Consortium (AMC) undertook a pilot trial of valproic acid (VA) in patients with AIDS-associated Kaposi’s sarcoma (KS). Treatment was associated with low toxicity, but the KS clinical response and KS herpesvirus lytic induction rates were not sufficiently high to meet pre-defined criteria for efficacy.
Keywords: Valproic acid, AIDS, HIV, KSHV, HHV-8, Kaposi sarcoma
INTRODUCTION
Kaposi’s sarcoma (KS) is an AIDS-defining neoplasm that may occur even when HIV RNA levels are effectively suppressed by antiretroviral therapy[1, 2]. KS is consistently associated with the KS herpesvirus (KSHV or HHV-8)[3]. In tumors, latent viral gene expression is detected consistently, whereas lytic viral genes are infrequently expressed. Histone deacetylase (HDAC) inhibitors are important laboratory tools for activating KSHV lytic cycle gene expression[4]. Viral lytic induction in vivo might directly lead to death of latently-infected tumor cells or lead to expression of antigenic viral proteins that would render tumor cells more susceptible to T cells. Valproic acid (VA), an agent used to treat seizure and mood disorders, activates lytic gene expression in KSHV-infected cell lines[5].
VA has properties of a HDAC inhibitor[6] and the literature suggests a variety of possible effects in AIDS-KS patients. VA has been variably reported to rescue replication-competent HIV-1 from resting CD4+ T cells and thus reduce the size of the HIV latency reservoir[7, 8].
Induction of KSHV lytic infection also raised concerns that VA treatment could lead to KS progression or development of KS in seropositive patients without tumor[5]. With these rationales and concerns in mind, the AIDS Malignancy Consortium (AMC) in conjunction with the AIDS and Cancer Specimen Resource (ACSR) undertook a pilot clinical study to determine the safety and efficacy of VA in AIDS-KS patients.
METHODS
Patients
Eligible patients had biopsy-confirmed KS and documented HIV infection. Patients with visceral or rapidly progressive KS were excluded as were those with a Karnofsky performance status <60 or life expectancy <3 months. Patients on antiretroviral therapy were required to be on a stable regimen for ≥4 weeks before enrollment. Because VA and zidovudine have been associated with lactic acidosis, patients with a history of lactic acidosis and those receiving zidovudine-containing regimens were excluded[9].
Sample Size Estimation and Stopping Rule
We prospectively specified four criteria be met to conclude that VA was safe, effective and likely to be working according to the hypothesized mechanism: a low toxicity-related discontinuation rate (<35%), a low rate of accelerated KS progression (<10%), a high clinical response rate (>30%), and a high rate of induction of lytic viral gene expression (>60%). Eighteen patients were sufficient to evaluate these four measures. No adjustment for multiple testing was made.
Study design and treatment
This was a prospective, open-label pilot study to determine the safety of VA in patients with AIDS-KS and evaluate the effect of VA on KSHV gene expression. Secondary endpoints included evaluation of the effects of VA on HIV and KSHV in blood and clinical response. After giving written informed consent, patients received VA for 28 days followed by a 2-week taper. VA (250mg capsules) was administered twice daily. The dose was escalated over the first six days from 500mg to 1000mg. Thereafter dosing was adjusted to achieve the therapeutic range established for epilepsy (50-100mg/liter). Although VA treatment ended after 6 weeks, patients were monitored for 24 weeks or until KS progression.
Schedule of events
Clinical assessments, including history and physical examinations, tumor assessments, complete blood count, serum electrolytes, renal and liver function tests were performed at baseline, on days 8, 15, 29; and monthly thereafter. CD4 T-lymphocyte counts were obtained at baseline. HIV-1 RNA was measured at baseline, on days 45-50, and every 3 months thereafter. Tumor punch biopsies were obtained at baseline, and days 8 and 29. Plasma and PBMC for KSHV copy number were obtained at baseline, days 8, 15, 29 and 1 month later. Tumor assessments were performed at baseline, at days 8, 15, 29 and monthly thereafter.
Assays for KSHV gene expression
Punch biopsy specimens (3mm) were snap frozen in liquid nitrogen for RNA studies or formalin fixed for immunohistochemistry. Specimens forwarded to the ACSR were coded and batched before transfer to laboratories for blinded evaluation. KSHV protein expression (LANA, vIL6, ORF8.1, ORF59) was categorized as (-), (+/-), (+), (++), (+++) or non-evaluable based on the number of positive staining cells and stain intensity. KSHV transcription was profiled using real-time quantitative PCR as previously described[10]. RNA quality was ascertained using an Agilent Bioanalyzer. Data were normalized to the mean of 3 housekeeping mRNAs to yield dCT, a log-transformed measure of relative RNA abundance.
Assays for KSHV DNA
KSHV copy number in plasma and PBMC was assessed by real-time PCR as previously described[11]. Blood was collected into heparin tubes, transported at ambient temperature and processed within 30 hours. DNA was isolated using the QIAGEN Blood Kit (QIAGEN Inc., Valencia, CA).
Response criteria
Tumor assessments and grading of responses as complete, partial, stable or progression were performed as previously described[12].
Toxicity
Adverse events were classified as possibly, probably or definitely related to VA, and their severity was graded using the NCI Common Toxicity Criteria version 3.0.
Statistical Methods
The Wilcoxon signed rank test was used to evaluate changes from baseline for laboratory correlates. The Spearman correlation coefficient was used to evaluate bivariate correlations.
RESULTS
Patients
Nineteen patients were enrolled. One patient withdrew before receiving treatment. The remaining 18 patients completed the planned 28 days of therapy. All patients were men. At entry, most were receiving antiretroviral therapy, had an undetectable HIV viral load, had previously received treatment for KS, and had tumor-associated edema. Half of the patients had ≥50 lesions. Patient and tumor characteristics are summarized in Table 1.
Table 1.
Selected Patient and Tumor Characteristics
| Characteristic | Number |
|---|---|
| Male Gender (%) | 18 (100) |
| Age (Median, Range) | 37 (29-60) |
| Absolute CD4 (Median, Range) | 286 (82-1119) |
| HIV Load Undetectable (%) | 13 (72) |
| 50 lesions or greater (%) | 9 (50) |
| No. of raised lesions (median) | 6 (33) |
| No. of flat lesions (median) | 13 (72) |
| Oral lesions present (%) | 4 (22) |
| Tumor-associated edema (%) | 10 (56) |
Toxicity
The only adverse event that occurred in more than one patient was mild diarrhea, which occurred in two patients. No patient discontinued treatment due to toxicity.
Clinical outcomes
One patient achieved a partial response that persisted without further intervention at last follow-up 5 months after therapy. Two other patients achieved partial responses 4 months and 6 months post-treatment without any intervening KS treatment. One patient showed an approximately 30% increase in the size of marker lesions in the 4 weeks of therapy, while at the same time the total number of lesions decreased from 128 to 99. No patients developed rapid KS progression.
KSHV gene expression in tumor biopsies
Viral gene expression in tumor biopsies was assessed by immunohistochemical staining. Latent (LANA) and lytic (vIL6, ORF8.1, ORF59) antigens were detected in most specimens at baseline and following VA treatment (Supplementary Table 2). Paired t-tests showed no significant differences in expression between baseline and either day 8 or day 29 for any of these antigens, Using real-time QPCR to assess KSHV mRNAs in biopsies, significant changes were not detected by t-test. We hypothesized that higher VA trough levels might be associated with more robust lytic induction. Although we saw no correlation between day 8 VA trough levels and antigen expression assessed by immunohistochemical staining, the VA trough level on day 8 correlated with quantities of several KSHV mRNAs (Fig 1). The top 5% of KSHV mRNAs were ordered by individual regression coefficients to build a multiple regression model. Orf 48 (unknown function), orf8 (gB) and orf 22 (gH, two primers) correlated with VA levels at day 8 with p≤0.02 (n = 12 patients).
Figure 1.
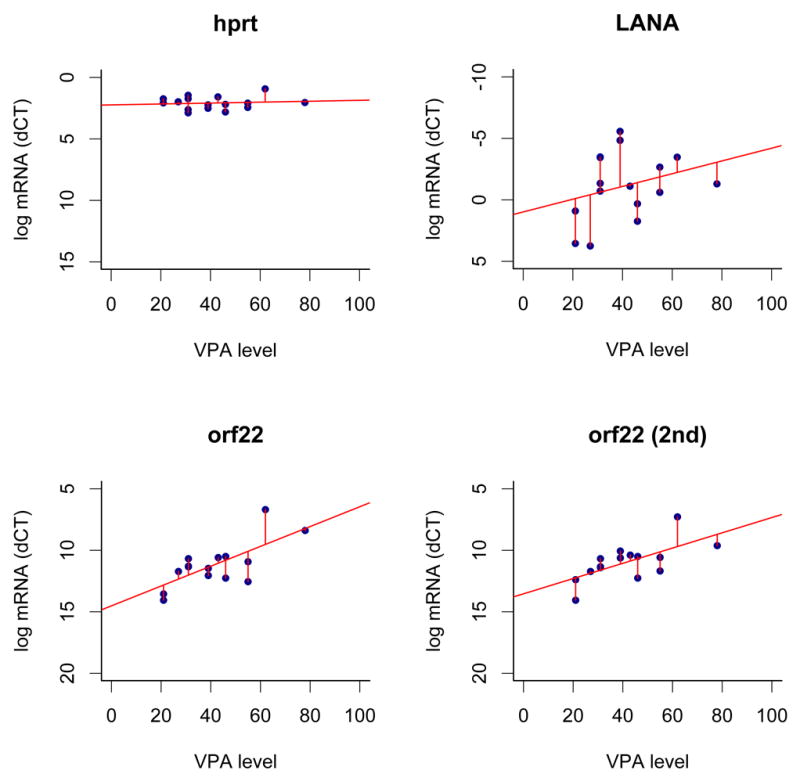
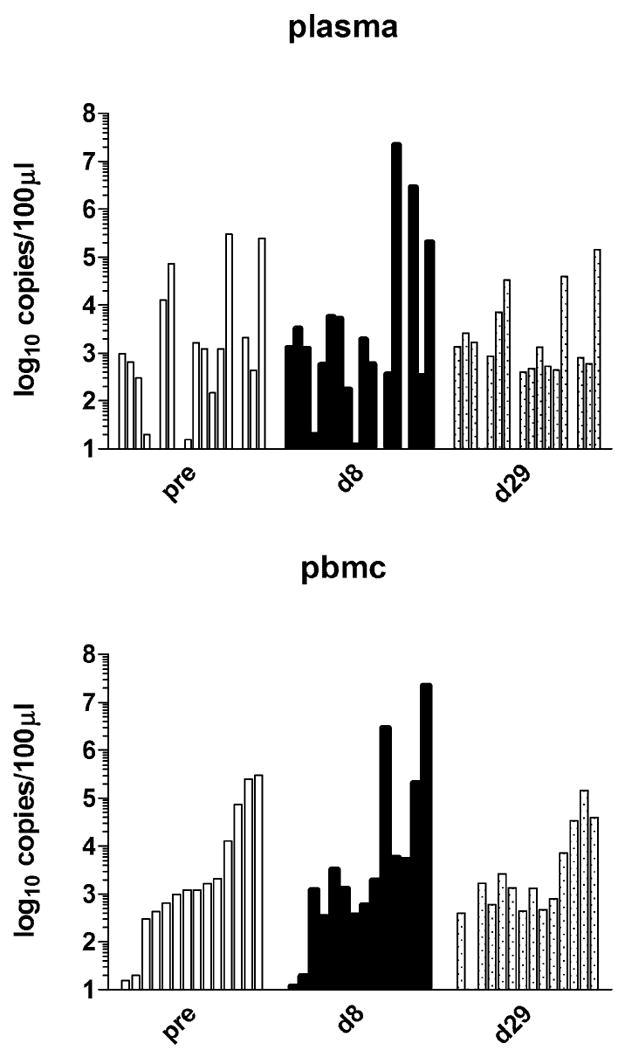
KSHV mRNA and DNA. A. Correlation of KSHV mRNA levels with VA trough levels after treatment. The VA trough level is shown on the horizontal and relative mRNA levels (dCT) on the vertical axes. The human housekeeping mRNA hprt, KSHV latent mRNA LANA, KSHV orf22, which exhibited a linear trend (p≤0.05) and orf22 as measured by a second independent primer are shown. Also shown are the linear regression lines and residuals. B. KSHV DNA copy number in blood. Vertical bars corresponding to each patient are grouped as pre-treatment, day 8, and day 29. The Y axis shows the log10 of KSHV copy number.
Viral correlates in blood
Baseline and day 29 HIV RNA levels were available for 16 patients. Two patients (13%) had detectable HIV viremia at baseline but not at day 29. One patient (6%) had HIV detected at baseline and day 29. Thirteen patients (81%) had no detectable HIV at baseline or day 29. No patients developed detectable HIV viremia during therapy. KSHV plasma and PBMC copy numbers were available for 18 patients. No significant differences were observed between baseline and either day 8 or day 29 copy numbers in either plasma or PBMC. A graphical presentation of this data is in Fig. 1B. There was also no correlation between day 8 VA levels and KSHV copy numbers in plasma or PBMC at day 8.
DISCUSSION
VA did not meet the pre-specified criteria for clinical response or KSHV lytic induction. However, insofar as none of the patients discontinued VA because of toxicity, no patients developed accelerated KS progression, and there were no detected adverse effects on HIV viremia, we believe that the study offers reassurance about using VA in HIV-infected patients with seizure or mood disorders who are seropositive for KSHV or have KS.
Whether induction of KSHV lytic infection would lead to the hoped-for therapeutic effect or, as some have feared, lead to tumor progression remains unsettled. The finding that expression of lytic viral mRNAs correlated with VA levels hints at the possibility that dose escalation might lead to increased lytic expression. However, the modest changes seen and lack of corresponding changes in either viral antigen expression assessed by immunohistochemistry or viral DNA copy number in blood suggest that robust induction of lytic viral gene expression may not be readily achievable and suggest consideration of alternative lytic activation strategies. Vorinastat, 5-azacytidine and bortezomib have all been recognized as potent lytic inducers in vitro[13] and may be more promising for future investigation.
Acknowledgments
We thank Chelsey Hilscher, Sean Gregory, Stephanie Mach, and Marie Chusa for expert technical support.
Supported in part by grants from the National Cancer Institute (UO1CA66535, U01CA121947, P01CA113239, CA109232, DE018304, U01CA083118, U01CA070062, U01CA070019, U01CA071375, U01CA083035, U01CA070047, U01CA70054)
Footnotes
All authors: no conflicts
References
- 1.Maurer T, Ponte M, Leslie K. HIV-associated Kaposi’s sarcoma with a high CD4 count and a low viral load. N Engl J Med. 2007 Sep 27;357(13):1352–3. doi: 10.1056/NEJMc070508. [DOI] [PubMed] [Google Scholar]
- 2.Krown SE, Lee JY, Dittmer DP. More on HIV-associated Kaposi’s sarcoma. N Engl J Med. 2008 Jan 31;358(5):535–6. doi: 10.1056/NEJMc072994. author reply 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambinder RF, Cesarman E. Clinical and pathological aspects of EBV and KSHV infection. In: Arvin A, Campadelli-Fiume G, Moore PS, editors. Human Herpesviruses Biology, Therapy, and Immunoprophylaxis. New York: Cambridge University Press; 2007. pp. 885–914. [PubMed] [Google Scholar]
- 4.Miller G, Heston L, Grogan E, et al. Selective switch between latency and lytic replication of Kaposi’s sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J Virol. 1997 Jan;71(1):314–24. doi: 10.1128/jvi.71.1.314-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw RN, Arbiser JL, Offermann MK. Valproic acid induces human herpesvirus 8 lytic gene expression in BCBL-1 cells. AIDS. 2000 May 5;14(7):899–902. doi: 10.1097/00002030-200005050-00021. [DOI] [PubMed] [Google Scholar]
- 6.Johannessen CU, Johannessen SI. Valproate: past, present, and future. CNS Drug Rev. 2003 Summer;9(2):199–216. doi: 10.1111/j.1527-3458.2003.tb00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehrman G, Hogue IB, Palmer S, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005 Aug 13-19;366(9485):549–55. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siliciano JD, Lai J, Callender M, et al. Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. J Infect Dis. 2007 Mar 15;195(6):833–6. doi: 10.1086/511823. [DOI] [PubMed] [Google Scholar]
- 9.Walker UA, Venhoff N. Multiple mitochondrial DNA deletions and lactic acidosis in an HIV-infected patient under antiretroviral therapy. AIDS. 2001 Jul 27;15(11):1449–50. doi: 10.1097/00002030-200107270-00020. [DOI] [PubMed] [Google Scholar]
- 10.Dittmer DP. Transcription profile of Kaposi’s sarcoma-associated herpesvirus in primary Kaposi’s sarcoma lesions as determined by real-time PCR arrays. Cancer Res. 2003 May 1;63(9):2010–5. [PubMed] [Google Scholar]
- 11.Lin L, Lee JY, Kaplan LD, et al. Effects of Chemotherapy in AIDS-Associated Non-Hodgkin’s Lymphoma on Kaposi’s Sarcoma Herpesvirus DNA in Blood. J Clin Oncol. 2009 Apr 6; doi: 10.1200/JCO.2008.20.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cianfrocca M, Cooley TP, Lee JY, et al. Matrix metalloproteinase inhibitor COL-3 in the treatment of AIDS-related Kaposi’s sarcoma: a phase I AIDS malignancy consortium study. J Clin Oncol. 2002 Jan 1;20(1):153–9. doi: 10.1200/JCO.2002.20.1.153. [DOI] [PubMed] [Google Scholar]
- 13.Fu DX, Tanhehco Y, Chen J, et al. Bortezomib-induced enzyme-targeted radiation therapy in herpesvirus-associated tumors. Nat Med. 2008 Oct;14(10):1118–22. doi: 10.1038/nm.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]


