Abstract
Molecular mechanisms that contribute to sex bias in the development of systemic lupus erythematosus (SLE), an autoimmune disease, remain unknown. We found that the expression levels of interferon regulatory factor 5 (IRF5), a lupus susceptibility factor, depend on gender of mice. We found that steady-state levels of the Irf5 mRNA were relatively higher in splenic cells from certain autoimmune-prone mice (for example, NZB and NZB/W F1) than in non-autoimmune C57BL/6 mice. Additionally, levels of Irf5 mRNA and protein were higher in females than in strain and age-matched males. Accordingly, splenic cells from estrogen receptor-alpha (ERα) knockout, when compared with the wild-type (ERα+/+), female mice expressed relatively lower levels of Irf5 mRNA and the treatment of splenic cells with 17β-estradiol increased the levels. Furthermore, splenic B cells from the female mice had relatively more IRF5 protein in the nucleus than the male mice. Collectively, our observations demonstrate a gender bias in the expression and sub-cellular localization of the murine IRF5.
Keywords: lupus susceptibility, gender bias, estrogen, interferon, IRF5
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease (Kotzin, 1996; Crispín et al., 2010). The disease is characterized by production of pathogenic autoantibodies against certain nuclear antigens. The disease involves multiple organs, including the kidneys. On the basis of genetic studies involving SLE patients and mouse models of the disease, it is evident that SLE is a polygenic disease (Kotzin, 1996; Crispín et al., 2010). Of note, the disease has a strong sex bias and develops at a female-to-male ratio of 10:1 (Whitacre, 2001; Rider and Abdou, 2001; Cohen-Solal et al., 2006; Zandman-Goddard et al., 2007). Moreover, increased serum levels of type I interferon (IFN-α) and induction of ‘IFN-signature’ genes are associated with active SLE disease in patients (Theofilopoulos et al., 2005; Banchereau and Pascual, 2006).
Like sex bias in the development of SLE in patients, (NZB × NZW)F1 female mice also display sex bias: the female mice develop disease much earlier and have shorter life spans than males (Rider and Abdou, 2001; Cohen-Solal et al., 2006; Zandman-Goddard et al., 2007). Accordingly, castrated male (NZB × NZW)F1 mice have earlier onset of lupus and shorter life span than their intact littermates (Roubinian et al., 1978). In addition, treatment of male mice with 17β-estradiol exacerbates the disease activity and causes early mortality (Roubinian et al., 1978). Although studies involving various mouse models of SLE have revealed that the female sex hormone estrogen and estrogen receptor-alpha (ERα) and increased serum levels of type I interferon (IFN-α) are associated with SLE disease (Grimaldi et al., 2001; Couse et al., 2003; Li and McMurray, 2007; Bynote et al., 2008; Gubbels et al., 2008; Svenson et al., 2008), the molecular mechanisms that contribute to the sex bias remain unclear.
One of the genes that control expression of the type I IFNs, interferon regulatory factor 5 (IRF5), has been found to be a significant genetic risk factor for lupus susceptibility (Graham et al., 2006; Niewold et al., 2008; Moser et al., 2009; Feng et al., 2010). The human IRF5 gene falls into key signal transduction pathways, such as immune complex-induced signaling, host immune signal transduction and interferon signaling pathways (Kozyrev and Alarcon-Riquelme, 2007). Accordingly, the IRF5 SLE risk haplotype is associated with higher serum IFN-α activity in SLE patients, and this effect is most prominent in patients who test positive for autoantibodies (Niewold et al., 2008). It has been reported that functional SNPs in the human IRF5 gene result in expression of multiple unique isoforms of IRF5 mRNA and increased steady-state level of mRNAs encoding the IRF5 (Graham et al., 2006; Feng et al., 2010).
The expression of the human IRF5 is detected in cells of lymphoid origin and can be further induced by type I IFN treatment of cells (Barnes et al., 2001, 2002a, b). In the splenic B cells, monocytes and particularly in precursor dendritic cells that are high producers of IFN-α, IRF5 is expressed constitutively. Expression of the human IRF5 gene is induced by type I IFNs and by p53 (Kozyrev and Alarcon-Riquelme, 2007). Human IRF5 is activated by phosphorylation by certain viruses and/or ligands that bind to toll-like receptors (TLRs), such as TLR3, TLR4 and TLR7 (Barnes et al., 2001, 2002b). Upon activation, IRF5 protein translocates to the nucleus and functions either as a transcriptional activator or repressor (Barnes et al., 2002a, 2002b). Upon activation, the IRF5 contributes to the transcriptional regulation of various IFNA genes (Barnes et al., 2003). In addition, IRF5 has a distinct role in the differentiation of lymphoid cells and apoptosis (Barnes et al., 2002a, 2002b).
Unlike the heavily spliced human IRF5 gene, the murine Irf5 gene is primarily expressed as a full-length transcript, with only a single splice variant that is detected in low levels in the bone marrow (BM) of C57BL/6J mice (Paun et al., 2008). This BM Mu Irf5 transcript contains a 288-nucleotide deletion in the coding region. Therefore, it encodes an isoform of the IRF5 with an impaired transcriptional activity. In vitro, the murine IRF5 can be activated by both TBK1 and MyD88 to form homodimers and to activate transcription of type I interferon and inflammatory cytokine genes (Paun et al., 2008). Moreover, the IRF5 also undergoes ubiquitination, which is important for nuclear translocation (Balkhi et al., 2008). However, the BM Mu IRF5 is not ubiquitinated (Balkhi et al., 2008). In the nucleus, the activated murine IRF5 stimulates transcription of the Ifna genes and the Blimp-1 gene, which encodes a master regulator of the B cell differentiation (Barnes et al., 2003; Lien et al., 2010; Crotty et al., 2010).
Recent studies have provided evidence that the female sex hormone estrogen through the ERα up-regulates expression of IFN-γ (Li and McMurray, 2007; Bynote et al., 2008). The IFN-γ can up-regulate expression of IRF9, a component of the type I IFN-inducible ISGF3 complex (Bluyssen et al., 1996). Thus, making it likely that female sex hormone estrogen can activate the expression of certain type I IFN-inducible genes. Given that certain lupus-prone female mice exhibit IFN-inducible gene ‘signature’ and have increased serum levels of type I IFNs (Jørgensen et al., 2007; Lu et al., 2007; Nacionales et al., 2007), we investigated whether the expression of Irf5 gene is gender-dependent. Here, we report that the expression of the murine Irf5 in immune cells depends on the gender of mice.
Results
Mouse strain-dependent regulation of the Irf5 mRNA levels
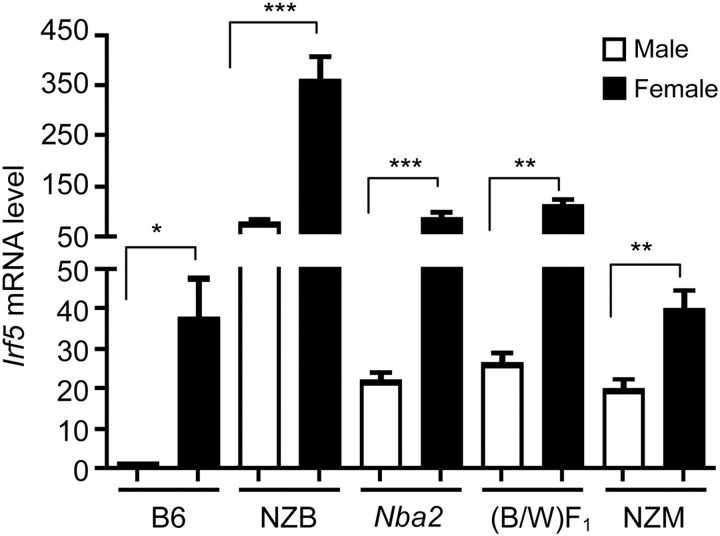
A previous study using semi-quantitative PCR approach had indicated that steady-state levels of Irf5 mRNA may vary among certain strains of mice (Paun et al., 2008). Therefore, to investigate the potential role of the murine IRF5 in lupus susceptibility, we compared steady-state levels of Irf5 mRNA among several strains of male and age-matched female mice. These strains of mice included a non-lupus-prone strain of mice (C57BL/6) and several known strains of lupus-prone mice [NZB, (NZB/W)F1, NZM2410 and B6.Nba2]. The B6.Nba2 congenic (congenic for the NZB-derived Nba2 interval) female mice develop detectable levels of autoantibodies against nuclear antigens beginning ∼6-months of age (Jørgensen et al., 2004). Interestingly, B6.Nba2 female mice that are deficient in the IFN-α/β-receptor fail to develop autoantibodies (Jørgensen et al., 2004, 2007). As shown in Figure 1, steady-state levels of Irf5 mRNA were relatively lower in C57BL/6 splenic cells than the age-matched most lupus-prone strains of mice. Levels of Irf5 mRNA in B6.Nba2 splenic cells were moderately higher when compared with age- and gender-matched C57BL/6 mice. Interestingly, irrespective of the mouse strain, all females had significantly higher steady-state levels of Irf5 mRNA than the strain- and age-matched males in several experiments. However, the difference between male and female mice varied among the strains that were tested. Therefore, these observations indicated that the genetic factors may contribute to differential expression of the Irf5 gene in certain strains of mice.
Figure 1.
Steady-state levels of Irf5 mRNA vary among mouse strains. Total RNA isolated from splenic cells (splenic cells from two or more age-matched male or female mice were pooled) prepared from male (M) or age-matched female (F) C57BL/6, NZB, B6.Nba2, NZB × NZW)F1 or NZM2410 mice were analyzed for the Irf5 mRNA levels by quantitative real-time PCR. The ratio of the Irf5 mRNA to β2-microglobulin mRNA was calculated in units (one unit being the ratio of the Irf5 mRNA to β2-microglobulin mRNA in splenocytes). The relative levels of Irf5 mRNA in male C57BL/6 mice are indicated as 1. Results are the mean values of triplicate measurements and error bars represent standard deviation (*P < 0.05; **P < 0.01; ***P < 0.001).
Gender-dependent factors regulate expression of the Irf5 gene
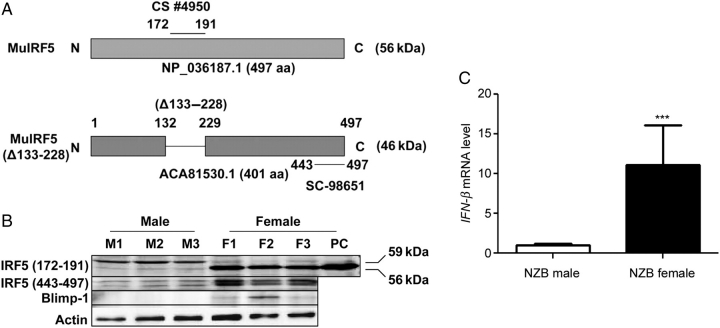
Given that the steady-state levels of Irf5 mRNA were significantly higher in females than the age-matched males in total splenic cells (Figure 1), we compared IRF5 protein levels between males and females. Given that splenic cells from the NZB mice express relatively higher levels of Irf5 mRNA than other strains of mice (Figure 1), we chose the NZB splenic cells to compare the IRF5 protein levels. As shown in Figure 2A, one of the commercially available antibodies (from Cell Signaling Biotech.) against murine IRF5, which was raised against a peptide (amino acid residue 172–191), detected a major expected murine IRF5 size (∼56 kDa) protein band in the splenic cells from female mice, but not male mice (Figure 2B). However, this antibody detected a protein band of ∼59 kDa in males. Given that this antibody is not predicted to detect the BM-derived isoform of the Mu IRF5 (Figure 2A), we decided to use another antibody (from Santa Cruz Biotechnology), which was raised against a peptide (amino acid residue 443–497) corresponding to the human IRF5 protein (Figure 2A). Using this antibody, we also detected increased levels of the two isoforms (the 56 and 59 kDa) of the IRF5 in splenic cells from the female mice than the age-matched males (Figure 2B). Notably, both commercially available antibodies to IRF5, under our experimental conditions, detected two isoforms of the IRF5 protein in total splenic cells from the NZB male and female mice. Moreover, levels of Blimp-1 protein, expression of which is induced by the IRF5 transcription factor (Lien et al., 2010), were detectable in females. However, Blimp-1 protein was not detectable in extracts from age-matched males. Accordingly, steady-state levels of Blimp-1 mRNA were 1.5–2-fold higher in all females than strain- and age-matched males and levels varied with the age of mice (∼50% less in 15 weeks old females than 9 weeks old females; data not shown). Furthermore, consistent with increased expression levels of the IRF5 protein in females compared with the age-matched males, the female mice had ∼10 times more type I IFN-β mRNA levels in splenic cells than the age-matched males (Figure 2C). Together, these observations suggested that mouse gender-dependent factors contribute to the up-regulation of IRF5 expression in female splenic cells.
Figure 2.
Steady-state levels of Irf5 mRNA and protein in splenic cells are gender-dependent. (A) A schematic representation of two isoforms of the murine IRF5 proteins and their predicted molecular weights. An internal deletion (from amino acid residue 133–228) in BM-derived IRF5 protein results in an isoform that is defective in transcriptional activation. Epitopes for two commercially available antibodies (#4950 and sc-98651) that were used to detect murine IRF5 in immunoblotting are also shown. (B) Total protein lysates from splenic cells isolated from male (M1, M2 and M3) and age-matched female (F1, F2 and F3) NZB mice (age 8 weeks) were analyzed by immunoblotting using antibodies specific to the indicated proteins. IRF5 (CS), the IRF5 antibody, which is directed against amino acid residue 171–191 of mouse IRF5, was from Cell Signaling Technology; IRF5 (SC), the IRF5 antibody, which is directed against amino acid residue 443–498 of human IRF5, was from Santa Cruz Biotech. PC, positive control: an extract from murine RAW264.7 macrophage cell line. (C) Total RNA isolated from splenic cells prepared from male or age-matched female NZB mice were analyzed for the IFN-β mRNA by quantitative real-time PCR. The ratio of the IFN-β mRNA to β2-microglobulin mRNA was calculated in units (one unit being the ratio of the IFN-β mRNA to β2-microglobulin mRNA). The relative levels of IFN-β mRNA in the NZB male mice are indicated as 1. Results are the mean values of triplicate measurements and error bars represent standard deviation (***P < 0.0005).
Estrogen through ERα increases the Irf5 mRNA levels
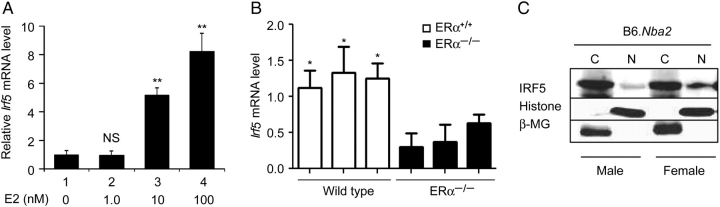
Increased steady-state levels of Irf5 mRNA and protein in female splenic cells when compared with males (Figure 2) prompted us to test whether 17β-estradiol, a female sex hormone, could regulate the expression of Irf5 gene. As shown in Figure 3A, treatment of splenic cells with increasing concentration of 17β-estradiol (from 0 to 100 nM) for 24 h resulted in increases in steady-state levels of Irf5 mRNA. This observation prompted us to compare steady-state levels of Irf5 mRNA in splenic cells between wild-type (ERα+/+) and ERα-knockout (NZB × NZW)F1 mice (Bynote et al., 2008). As shown in Figure 3B, levels of Irf5 mRNA were relatively higher in the wild-type than age-matched ERα−/− females. Given that the splenic B cells (CD45R+) express relatively higher levels of ERα mRNA and protein than T cells (Panchanathan et al., 2009) and the increased levels IRF5 protein in NZB females are associated with increased levels of Blimp-1 (Figure 2B), a transcriptional target of IRF5, we also compared steady-state levels of the IRF5 protein and the extent of its nuclear localization in splenic B cells between male and female mice. As shown in Figure 3C, the IRF5 protein was detected in the cytoplasmic and nuclear fractions of B cells isolated from both male and age-matched female B6.Nba2 mice. However, more IRF5 protein was detected in the nucleus of female B cells than males. Together, these observations suggested that sex-related factors also contribute to the activation and the nuclear localization of the IRF5 protein in female splenic B cells.
Figure 3.
Estrogen and ERα regulate the steady-state levels of Irf5 mRNA and nuclear localization of IRF5 protein. (A) Total splenic cells isolated from female B6.Nba2 mice (age 10 weeks) were treated with the indicated increasing concentrations of the female hormone estrogen for 24 h. Total RNA was isolated after the treatment and analyzed for the Irf5 mRNA levels by quantitative real-time PCR. The ratio of the Irf5 mRNA to β2-microglobulin mRNA was calculated in units (one unit being the ratio of the Irf5 mRNA to β2-microglobulin mRNA). The relative levels of Irf5 mRNA in untreated cells are indicated as 1. Results are mean values of triplicate measurements and error bars represent standard deviation (NS, not significant; **P < 0.005). (B) Total RNA was isolated from splenic cells from wild-type (ERα+/+) or age-matched ERα-knockout (ERα−/−) female mice and RNA was analyzed for the Irf5 mRNA levels by quantitative real-time PCR. Results are mean values of triplicate experiments and error bars represent standard deviation (*P < 0.05). (C) Splenic B cells (splenic cells from two or more age-matched male or female mice were pooled) purified (using the CD45R MicroBeads) from male or female B6.Nba2 mice were fractionated into cytoplasmic and nuclear fractions. Equal amounts of proteins were analyzed by immunoblotting using the antibodies specific to the indicated proteins. Detection of histone H3 and IκBα proteins in primarily in the nuclear and cytoplasmic fraction, respectively, served as a control for the quality of cell fractionations.
Discussion
Genetic risk factors in human SLE disease include the IRF5 allele (Graham et al., 2006; Moser et al., 2009). In contrast to the human IRF5 gene that is expressed in multiple spliced variants and corresponding protein isoforms, much remains unknown about the murine Irf5 gene and the corresponding protein. Moreover, unlike the promoter region of the human IRF5 gene (Mancl et al., 2005), the promoter region of the murine Irf5 gene has not been characterized. However, it appears that the murine Irf5 gene is transcribed as a single full-length transcript and only a single splice variant, which is expressed in very low levels in the BM of C57BL/6J mice, as has been reported (Paun et al., 2008). This variant contains a 288-nucleotide deletion from exons 4–6 and exhibits an impaired transcriptional activity (Paun et al., 2008).
Given that SLE disease develops at a female-to-male ratio of 10:1 and most patients have increased serum levels of IFN-α (Whitacre, 2001; Cohen-Solal et al., 2006; Banchereau and Pascual, 2006), our observations demonstrate that the expression levels of Irf5 mRNA and protein in mice: (i) depend on the mouse strain and are relatively higher in certain lupus-prone strains than a non-lupus-prone C57BL/6 strain (Figure 1); (ii) exhibit a sex bias in the expression, being higher in females of all strains tested than the strain- and age-matched males (Figure 2); (iii) are increased upon treatment of splenic cells with 17β-estradiol (Figure 3); and (iv) depend on the expression of ERα (Figure 3). These observations are likely to provide a molecular basis for observed sex bias in SLE in mice. Moreover, detection of IRF5 protein in splenic B cells from the B6.Nba2 mice in the nuclear fraction also raises the possibility that innate immune responses, such as those initiated by RNA-containing immune complexes (Yasuda et al., 2007), may contribute to increased expression as well as the activation of IRF5 in B cells, resulting in increased expression of the IRF5 target genes.
Because the female hormone estrogen influences the development, survival and functions of the immune cells, including the B cells (Grimaldi et al., 2001; Cohen-Solal et al., 2006), T (Pernis 2007) and DCs (Hughes and Clark, 2007), it is likely that gender-dependent increased levels of estrogen in females also affect the sub-populations of immune cells in mice. Therefore, further work will be needed to determine whether the observed differences in the levels of IRF5 protein between male and age-matched female mice are associated with increases (or decreases) in certain sub-populations of the immune cells.
Our observations that steady-state levels of Irf5 mRNA in splenic cells from non-lupus-prone C57BL/6 or pre-disease (age ∼9 weeks) B6.Nba2 female mice were significantly higher than the strain and age-matched males (Figure 1) are consistent with the idea that increases in the levels of the mRNA are independent of lupus-like disease. Moreover, our observations that NZB female-derived splenic cells had significantly increased levels of IFN-β mRNA than males (Figure 2C) support the possibility that increased levels of Irf5 mRNA in splenic cells from the lupus-prone NZB mice are, in part, due to increased production of type I IFNs. Therefore, further work will be needed to identify factors that contribute to increased levels of IRF5 protein in the NZB mice.
Under our experimental conditions, two commercially available antibodies raised against two non-overlapping regions of the IRF5 protein (Figure 2A) detected two isoforms (∼56 and ∼59 kDa) of the murine IRF5 protein in total splenic cells from the NZB male and female mice. On the basis of the predicted size difference between the predominant form (∼56 kDa) of the murine IRF5 and the BM Mu IRF5 variant (∼46 kDa) and the predicted ability of antibodies that were raised against the peptide containing amino acid residue 172–191 of murine IRF5, it is unlikely that the slow-migrating isoform (∼59 kDa) of the IRF5 that we detected in NZB splenic cells (Figure 2B) is a previously described isoform of the IRF5 protein (Paun et al., 2008). Therefore, further work will be needed to characterize this isoform of the murine IRF5.
The Irf5 knockout mice develop splenomegaly in an age-dependent manner (Paun et al., 2008). Moreover, the development of splenomegaly is associated with an accumulation of CD19+B220− B cells with defects in functions. Furthermore, expression of Blimp-1 gene decreases in the Irf5 knockout mice (Paun et al., 2008), indicating that IRF5 transcriptionally regulates the expression of Blimp-1 gene, which encodes a plasma cell commitment factor and regulator of B cell terminal differentiation (Crotty et al., 2010). Therefore, our observations that increased levels of IRF5 in splenic cells from female NZB mice were associated with Blimp-1 expression is consistent with the possibility that increased levels of Blimp-1 in splenic B cells from the relatively young female mice contribute to an increased number of terminally differentiated B cells that secrete pathogenic autoantibodies, thus accounting for increased production of the pathogenic autoantibodies in the NZB females when compared with age-matched males. Further work will be needed to investigate the role of age-dependent factors in the expression of IRF5.
Others have shown that the expression of murine IRF5 is required for the development of lupus-like disease in the FcγRIIB−/− Yaa and FcγRIIB−/− lupus models (Richez et al., 2010). Moreover, the study also noted that type I IFN receptor subunit 1-deficient FcγRIIB−/− Yaa mice maintain a substantial level of residual disease, thus raising the possibility that IRF5 may contribute to the disease phenotype in these mouse models of SLE independent of the IFN-signaling through the receptor. Therefore, further work will be needed to identify the IFN-signaling independent role of IRF5 in SLE.
The IRF5 plays a critical role in IFN-α and IFN-β production induced not only by RNA-containing immune complexes but also by conventional TLR7 and TLR9 ligands (Schoenemeyer et al., 2005; Takapka et al., 2005). Moreover, production of IL-6 by DCs induced by these stimuli is dependent on a functional type I IFNR, indicating the need for a type I IFN-dependent feedback loop in the production of inflammatory cytokines, such as IL-6, IL-12 and TNF-α, by various TLR ligands (Takapka et al., 2005). Therefore, our observations that the female sex hormone increases Irf5 mRNA levels are consistent with the idea that female mice of certain strains, when compared with males, are prone to increased levels of proinflammatory cytokines in response to the activation of TLRs.
Disruption of ERα (or Esr1) gene in (NZB × NZW)F1 mice attenuates glomerulonephritis in females and increases survival (Bynote et al., 2008). Significantly, the ERα deficiency in these female mice also retards the development of anti-histone/DNA antibodies. Given that the Esr1-deficient splenic cells express less IRF5 (Figure 3B), our observations are consistent with the idea that ERα promotes loss of immunologic tolerance by up-regulating the expression of IRF5. Further work is in progress to test this possibility.
In summary, our observations provide evidence for sex bias in the expression and activation of the murine IRF5, which is predicted to contribute to increased levels of type I IFNs and IFN ‘signature’ observed in the lupus disease in mice (Figure 4). However, it remains to be seen whether the sex bias exists in human SLE patients with respect to the IRF5 expression. To the best of our knowledge, demonstration of gender bias in the expression of IRF5 has not been reported previously. Therefore, our observations will serve as a basis to elucidate the molecular mechanisms by which the female sex hormone estrogen increases levels of the murine Irf5 mRNA and protein in immune cells.
Figure 4.
Proposed model for gender-dependent expression of the murine IRF5 protein and its predicted role in gender bias in lupus susceptibility.
Materials and methods
Mice, splenic cells and treatments
Experimental protocols that are used in this manuscript were approved by the University of Cincinnati's Animal Care and Use Committee. Age-matched (∼6–8 weeks old) male and female C57BL/6J, NZB, (NZB × NZW)F1, NZM2410 and B6.Nba2 mice were purchased from The Jackson Laboratory and mice were housed in a pathogen-free Laboratory of Animal and Medical Services facility at he University of Cincinnati. Spleens were isolated from wild-type (Esr1+/+) or null (Esr1−/−) (NZB × NZW)F1 female mice (age ∼10 weeks) that were housed at the animal facilities of the University of Nebraska Medical Center, Omaha, NE (Bynote et al., 2008).
Total single cell splenocytes were prepared from age-matched male or female mice as described previously (Panchanathan et al., 2009). After lysis of red blood cells, splenocytes were re-suspended in RPMI-1640 medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin/ glutamate and 1× minimal essential medium, non-essential amino acids/sodium pyruvate. Unless otherwise indicated, splenic cells from two or more age-matched male or female mice were pooled to purify splenic B cells and to prepare total RNA or protein extracts. B cells were purified from splenic cells using the CD45R (B220+) MicroBeads (purchased from Miltenayi Biotech, Auburn, CA, USA) allowing the positive selection of the splenic B cells. The purified (>90% pure) B cells were used immediately for further experiments.
For treatment of B cells with 17β-estradiol (E2), cells were cultured in phenol red-free DMEM medium (Invitrogen, Carlsbad, CA, USA) and the medium was supplemented with 10% charcoal-stripped fetal bovine serum (Invitrogen). Cells were treated in vitro with E2 at concentrations as described previously (Panchanathan et al., 2009).
Isolation of RNA from splenocytes and quantitative real-time PCR
Freshly prepared splenocytes (5–8 × 106 cells) were used to isolate total RNA using the TRIzol (Invitrogen) isolation method. Total RNA was digested with DNase I (to remove any contaminating genomic DNA in the preparation), and 0.5–2 µg of RNA was used for RT reaction. Quantitative real-time TaqMan PCR technology (7300 Real-Time PCR System, Applied Biosystems, Foster City, CA, USA) and commercially available real-time TaqMan gene expression assays were used to compare expression of genes between male and female mice. The PCR cycling program consisted of denaturation at 95°C for 10 min, 40 cycles at 95°C for 15 s, followed by annealing and elongation at 60°C for 1 min. The TaqMan assays for Irf5 (Assay Id# Mm0049677_m1; the assay allowing the detection of both full-length and truncated murine Irf5 transcript), murine interferon-beta (Assay Id# Mm00439546_s1) and the endogenous control β2-microglobulin (Assay Id# Mm00437762_m1) were purchased from Applied Biosystems and used as suggested by the supplier.
Cell fractionation and immunoblotting
Purified B220+ B cells were fractionated into the cytoplasmic and nuclear fractions as described previously (Choubey et al., 2010). Total splenocytes were collected in PBS and re-suspended in a modified radio-immune precipitation assay (RIPA) lysis buffer (50 mM Tris–HCl, pH 8.0, 250 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS), supplemented with 1× protease inhibitor (Roche Diagnostics, Mannheim, Germany) and incubated at 4°C for 30 min. Cell lysates were sonicated briefly before centrifugation at 14 000 rpm in a microcentrifuge for 10 min at 4°C. The supernatants were collected, and the protein concentration was measured by Bio-Rad protein assay kit. Equal amounts of protein were processed for immunoblotting. Two commercially available polyclonal antibodies were used to detect the murine IRF5 protein in immunoblotting (#4950, Cell Signaling Technology, Danvers, MA, USA; and SC-98651, Santa Cruz Biotech., Santa Cruz, CA, USA). Antibodies to actin (#4967) and Blimp-1 (#9115) were purchased from Cell Signaling Technology (Danvers, MA).
Statistical analyses
Values are presented as mean ± SEM. The statistical significance of differences in the measured mean frequencies between two groups was calculated using Student's two-tailed t-test. A P-value of <0.05 was considered significant.
Funding
This work was supported by a grant from the NIH (AI066261) to D.C.
Acknowledgements
We thank Dr Paula Pitha-Rowe for reading the manuscript and providing very helpful suggestions.
References
- Balkhi M.Y., Fitzgerald K.A., Pitha P.M. Functional regulation of MyD88-activated interferon regulatory factor 5 by K63-linked polyubiquitination. Mol. Cell. Biol. 2008;28:7296–7308. doi: 10.1128/MCB.00662-08. doi:10.1128/MCB.00662-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. doi:10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Barnes B.J., Moore P.A., Pitha P.M. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. J. Biol. Chem. 2001;276:23382–23390. doi: 10.1074/jbc.M101216200. doi:10.1074/jbc.M101216200. [DOI] [PubMed] [Google Scholar]
- Barnes B.J., Kellum M.J., Field A.E., Pitha P.M. Multiple regulatory domains of IRF-5 control activation, cellular localization, and induction of chemokines that mediate recruitment of T lymphocytes. Mol. Cell. Biol. 2002a;22:5721–5740. doi: 10.1128/MCB.22.16.5721-5740.2002. doi:10.1128/MCB.22.16.5721-5740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes B., Lubyova B., Pitha P.M. On the role of IRF in host defense. J. Interferon Cytokine Res. 2002b;22:59–71. doi: 10.1089/107999002753452665. doi:10.1089/107999002753452665. [DOI] [PubMed] [Google Scholar]
- Barnes B.J., Field A.E., Pitha-Rowe P.M. Virus-induced heterodimer formation between IRF-5 and IRF-7 modulates assembly of the IFNA enhanceosome in vivo and transcriptional activity of IFNA genes. J. Biol. Chem. 2003;278:16630–16641. doi: 10.1074/jbc.M212609200. doi:10.1074/jbc.M212609200. [DOI] [PubMed] [Google Scholar]
- Bluyssen A.R., Durbin J.E., Levy D.E. ISGF3 gamma p48, a specificity switch for interferon activated transcription factors. Cytokine Growth Factor Rev. 1996;7:11–17. doi: 10.1016/1359-6101(96)00005-6. doi:10.1016/1359-6101(96)00005-6. [DOI] [PubMed] [Google Scholar]
- Bynote K.K., Hackenberg J.M., Korach K.S., Lubahn D.B., Lane P.H., Gould K.A. Estrogen receptor-α deficiency attenuates autoimmune disease in (NZB × NZW) F1 mice. Genes Immun. 2008;9:137–152. doi: 10.1038/sj.gene.6364458. doi:10.1038/sj.gene.6364458. [DOI] [PubMed] [Google Scholar]
- Choubey D., Panchanathan R., Shen H., Duan X. Comment on ‘Development of murine lupus involves the combined genetic contribution of the SLAM and FcgammaR intervals within the Nba2 autoimmune susceptibility locus. J. Immunol. 2010;184:4051–4052. doi: 10.4049/jimmunol.1090015. doi:10.4049/jimmunol.1090015. [DOI] [PubMed] [Google Scholar]
- Cohen-Solal J.F., Jeganathan V., Grimaldi C.M., Peeva E., Diamond B. Sex hormones and SLE: influencing the fate of auto reactive B cells. Curr. Top. Microbiol. Immunol. 2006;305:67–88. doi: 10.1007/3-540-29714-6_4. doi:10.1007/3-540-29714-6_4. [DOI] [PubMed] [Google Scholar]
- Couse J.F., Yates M.M., Walker V.R., Korach K.S. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol. Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. doi:10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- Crispín J.C., Liossis S.N., Kis-Toth K., Lieberman L.A., Kyttaris V.C., Juang Y.T., Tsokos G.C. Pathogenesis of human systemic lupus erythematosus: recent advances. Trends Mol. Med. 2010;16:47–57. doi: 10.1016/j.molmed.2009.12.005. doi:10.1016/j.molmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S., Johnston R.J., Schoenberger S.P. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat. Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. doi:10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D., Stone R.C., Eloranta M.L., Sangster-Guity N., Nordmark G., Siurdsson S., Wang C., Alm G., Syvänen A.C., Rönnblom L., et al. Genetic variants and disease-associated factors contribute to enhanced interferon regulatory factor 5 expression in blood cells of patients with systemic lupus erythematosus. Arthritis Rheum. 2010;62:562–573. doi: 10.1002/art.27223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R.R., Kozyrev S.V., Baechler E.C., Reddy M.V., Plenge R.M., Bauer J.W., Ortmann W.A., Koeuth T., González Escribano M.F., et al. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat. Genet. 2006;38:550–555. doi: 10.1038/ng1782. doi:10.1038/ng1782. [DOI] [PubMed] [Google Scholar]
- Grimaldi C.M., Michael D.J., Diamond B. Expansion and activation of a population of auto-reactive marginal zone B cells in a model of estrogen-induced lupus. J. Immunol. 2001;167:1886–1890. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- Gubbels M.R., Jorgensen T.N., Kotzin B.L. Identification of candidate genes that influence sex hormone-dependent disease phenotypes in mouse lupus. Genes Immun. 2008;9:47–56. doi: 10.1038/sj.gene.6364447. [DOI] [PubMed] [Google Scholar]
- Hughes G.C., Clark E.A. Regulation of dendritic cells by female sex steroids: relevance to immunity and autoimmunity. Autoimmunity. 2007;40:470–481. doi: 10.1080/08916930701464764. doi:10.1080/08916930701464764. [DOI] [PubMed] [Google Scholar]
- Jørgensen T.N., Gubbels M.R., Kotzin B.L. New insights into disease pathogenesis from mouse lupus genetics. Curr. Opin. Immunol. 2004;16:787–793. doi: 10.1016/j.coi.2004.09.013. doi:10.1016/j.coi.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Jørgensen T.N., Roper E., Thurman J.M., Marrack P., Kotzin B.L. Type I interferon signaling is involved in the spontaneous development of lupus-like disease in B6.Nba2 and (B6.Nba2 × NZW)F(1) mice. Genes Immun. 2007;8:653–662. doi: 10.1038/sj.gene.6364430. doi:10.1038/sj.gene.6364430. [DOI] [PubMed] [Google Scholar]
- Kotzin B.L. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. doi:10.1016/S0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- Kozyrev S.V., Alarcon-Riquelme M.E. The genetics and biology of Irf5-mediated signaling in lupus. Autoimmunity. 2007;40:591–601. doi: 10.1080/08916930701510905. doi:10.1080/08916930701510905. [DOI] [PubMed] [Google Scholar]
- Li J., McMurray R.W. Effects of estrogen receptor subtype-selective agonists on autoimmune disease in lupus-prone NZB/NZW F1 mouse model. Clin. Immunol. 2007;123:219–226. doi: 10.1016/j.clim.2007.01.008. doi:10.1016/j.clim.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Lien C., Fang C.M., Huso D., Liyak F., Lu R., Pitha P.M. Critical role of IRF-5 in regulation of B-cell differentiation. Proc. Natl. Acad. Sci. USA. 2010;107:4664–4668. doi: 10.1073/pnas.0911193107. doi:10.1073/pnas.0911193107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Shen N., Li X.M., Chen S.L. Genomic view of IFN-alpha response in pre-autoimmune NZB/W and MRL/lpr mice. Genes Immun. 2007;8:590–603. doi: 10.1038/sj.gene.6364421. doi:10.1038/sj.gene.6364421. [DOI] [PubMed] [Google Scholar]
- Mancl M.E., Hu G., Sangster-Guity N., Olshalsky S.L., Hoops K., Fitzgerald-Bocarsly P., Pitha P.M., Pinder K., Barnes B.J. Two discrete promoters regulate the alternatively spliced human interferon regulatory factor-5 isoforms. Multiple isoforms with distinct cell type-specific expression, localization, regulation, and function. J. Biol. Chem. 2005;280:21078–21090. doi: 10.1074/jbc.M500543200. doi:10.1074/jbc.M500543200. [DOI] [PubMed] [Google Scholar]
- Moser K.L., Kelly J.A., Lessard C.J., Harley J.B. Recent insight into the genetic basis of systemic lupus erythematosus. Genes Immun. 2009;10:373–379. doi: 10.1038/gene.2009.39. doi:10.1038/gene.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacionales D.C., Kelly-Scumpia K.M., Lee P.Y., Weinstein J.S., Lyons R., Sobel E., Satoh M., Reeves W.H. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 2007;56:3770–3783. doi: 10.1002/art.23023. doi:10.1002/art.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold T.B., Kelly J.A., Flesch M.H., Espinoza L.R., Harley J.B., Crow M.K. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58:2481–2487. doi: 10.1002/art.23613. doi:10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchanathan R., Shen H., Bupp M.G., Gould K.A., Choubey D. Female and male sex hormones differentially regulate expression of Ifi202, an interferon-inducible lupus susceptibility gene within the Nba2 interval. J. Immunol. 2009;183:7031–7038. doi: 10.4049/jimmunol.0802665. doi:10.4049/jimmunol.0802665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paun A., Reiert J.T., Jiang Z., Medin C., Balkhi M.Y., Fitzgerald K.A., Pitha P.M. Functional characterization of murine interferon regulatory factor 5 (IRF-5) and its role in the innate antiviral response. J. Biol. Chem. 2008;283:14295–14308. doi: 10.1074/jbc.M800501200. doi:10.1074/jbc.M800501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis A.B. Estrogen and CD4+ T cells. Curr. Opin. Rheumatol. 2007;19:414–420. doi: 10.1097/BOR.0b013e328277ef2a. doi:10.1097/BOR.0b013e328277ef2a. [DOI] [PubMed] [Google Scholar]
- Richez C., Yasuda K., Bonegio R.G., Watkins A.A., Aprahamian T., Busto P., Richards R.J., Liu C.L., Cheung R., Utz P., et al. IFN regulatory factor 5 is required for disease development in the FcgammaRIIB−/−Yaa and FcgammaRIIB−/− mouse models of systemic lupus erythematosus. J. Immunol. 2010;184:796–806. doi: 10.4049/jimmunol.0901748. doi:10.4049/jimmunol.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider V., Abdou N.I. Gender differences in autoimmunity: molecular basis for estrogen effects in systemic lupus erythematosus. Int. Immunopharmacol. 2001;1:1009–1024. doi: 10.1016/s1567-5769(01)00046-7. doi:10.1016/S1567-5769(01)00046-7. [DOI] [PubMed] [Google Scholar]
- Roubinian J.R., Talal N., Greenspan J.S., Siiteri P.K. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J. Exp. Med. 1978;147:1568–1583. doi: 10.1084/jem.147.6.1568. doi:10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenemeyer A., Barnes B.J., Mancl M.E., Latz E., Goutagny N., Pitha P.M., Fitzgerald K.A., Golenbock D.T. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J. Biol. Chem. 2005;280:17005–17012. doi: 10.1074/jbc.M412584200. doi:10.1074/jbc.M412584200. [DOI] [PubMed] [Google Scholar]
- Svenson J.L., Eudaly J., Ruiz P., Korach K.S., Gilkeson G.S. Impact of estrogen receptor deficiency on disease expression in the NZM2410 lupus prone mouse. Clin. Immunol. 2008;128:259–268. doi: 10.1016/j.clim.2008.03.508. doi:10.1016/j.clim.2008.03.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takapka A., Yanai H., Kondo S., Duncan G., Negishi H., Mizutani T., Kano S., Honda K., Ohba Y., Mak T.W., et al. Integral role of IRF-5 in the gene induction programme activated by toll-like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A.N., Baccala R., Beutler B., Kono D.H. Type I interferon (α/β) in immunity and autoimmunity. Annu. Rev. Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. doi:10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- Whitacre C.C. Sex differences in autoimmune disease. Nat. Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. doi:10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- Yasuda K., Richez C., Maciaszek J.W., Agrawal N., Akira S., Marshak-Rothstein A., Rifkin I.R. Murine dendritic cell type I IFN production induced by human IgG-RNA immune complexes is IFN regulatory factor (IRF) 5 and IRF7 dependent and is required for IL-6 production. J. Immunol. 2007;178:6876–6885. doi: 10.4049/jimmunol.178.11.6876. [DOI] [PubMed] [Google Scholar]
- Zandman-Goddard G., Peeva E., Shoenfeld Y. Gender and autoimmunity. Autoimmun. Rev. 2007;6:366–372. doi: 10.1016/j.autrev.2006.10.001. [DOI] [PubMed] [Google Scholar]