Abstract
To test the hypothesis that the outcome of hematopoietic stem cell grafts is at least partially determined by the cellular composition of the graft, the National Marrow Donor Program analyzed the correlation of cellular phenotypes of unrelated grafts with graft outcome. Samples from 94 bone marrow (BM) and 181 peripheral blood progenitor cell (PBPC) grafts for transplantations at 40 U.S. transplant centers between 2003 and 2005 were analyzed at a single immunophenotyping reference laboratory. Samples were shipped from transplant centers upon receipt of graft. Graft cellular composition included analysis of leukocyte total cell numbers, and subsets of myeloid [CD34+, CD34+ CD38−], lymphoid [CD3+, CD3+ CD4+, CD3+ CD8+], and activated lymphoid cells [CD3+ CD25+, CD3+ CD69+, CD3+ HLA-DR+] coexpressing CD3+. There was substantial variability in the cellular composition of BM and PBPC grafts before and after graft processing by red blood cell (RBC) removal or plasma depletion in preparation for transplant. With BM grafts, cellular composition was not associated with hematopoietic recovery, graft-versus-host disease (GVHD), or survival. With PBPC grafts, survival rates were higher with CD34 + >5 × 106/kg, 59% compared to 34% with CD34+ ≤5 × 106/kg at 1-year. Platelet recovery was higher with PBPC containing CD3+ CD8+ >8 × 107/kg. Neutrophil recovery or GVHD could not be predicted by any cellular subsets of PBPC grafts. Though survival was superior with PBPC grafts containing >5 × 106 CD34+/ kg an optimal graft mix of myeloid, lymphoid and activated lymphoid subsets was not identified.
Keywords: Graft composition, unrelated donor transplant, hematopoietic recovery, overall survival
INTRODUCTION
The clinical outcome of hematopoietic cell transplantation (HCT) is determined by a variety of patient, disease and graft factors. While donor-recipient HLA matching is the major determinant of transplant-outcome after unrelated donor transplantation, among graft factors, product cellular composition, cell dose, and the effects of post harvest, pre-infusion processing are considered sufficiently important to influence transplant-outcome. However, after almost three decades of allogeneic HCT using volunteer unrelated bone marrow (BM) or peripheral blood progenitor cells (PBPC), the optimal cellular composition of these grafts has not been defined. Prior trials using diverse patient populations have reported conflicting results in correlating the composition of BM and PBPC grafts (1- 8) with transplant-outcome. Therefore, this study was designed to examine the composition of volunteer unrelated donor grafts and the effect on graft outcomes.
The various methods of graft processing may affect the cellular composition of the final product. The most common graft manipulations are red blood cell (RBC) removal, plasma depletion, and mononuclear cell concentration. The aim of these simple manipulations is to volume reduce or remove erythrocyte antigens or plasma antibodies (isohemagglutinins) from the graft without significant loss of hematopoietic progenitor cells or lymphocyte subsets. In contrast, the more complex procedures of T-cell depletion or CD34+ cell selection profoundly affect graft composition with the specific aim of modifying numbers of certain cell types to improve engraftment or decrease graft-versus-host disease (GVHD) ( 6,7). Flow cytometric analysis of the graft may provide a rapid predictive measure of graft outcome (2- 5, 7,8). However, inter-laboratory standardization in analyzing grafts is difficult, as gating and subset enumeration still remains an art rather than a precise science, particularly for complex cellular samples such as BM or mobilized PBPC. The use of a single reference laboratory offers the advantages of consistency and efficiency, but the logistics and costs of sample collection and shipment to the reference laboratory can limit the feasibility of standardized data collection. Furthermore, the quality of the data obtained may be compromised by the effects of transport time and temperature on overall sample integrity and cellular viability (9).
MATERIALS AND METHODS
Study population: patient, disease and transplant characteristics
This study was developed to prospectively determine the relationship between graft myeloid and lymphoid subset cell dose and clinical outcome for BM and PBPC grafts, using data from a single immuno-phenotyping reference laboratory. The study was developed and coordinated by the National Marrow Donor Program, (Minneapolis, MN), following approval of the protocol from the NMDP Institutional Review Board. Forty-five participating transplant centers were recruited through the NMDP, however, only 40 transplant centers provided patient consent, samples and complete phenotypic and clinical data (see list of participating transplant centers, Appendix 1). Consequently, the study population (94 BM and 181 PBPC recipients) were transplanted at 40 centers in the U.S between July 2003 and March 2005. Recipients of T-cell depleted (BM; n=29) and CD34 selected (PBPC; n=17) transplants from these centers were excluded as the most centers did not send a sample to the reference laboratory post-processing.
All grafts were characterized for cellular quantity and quality and studied to determine the effects of laboratory processing on graft composition. Specifically, we examined the impact of graft composition measured by total WBC numbers, and the number of cells belonging to the myeloid subset [CD34+, CD34+ CD38−], the lymphoid subset [CD3+, CD3+ CD4+, CD3+ CD8+], and activated lymphoid cells [CD3+ CD25+, CD3+ CD69+, CD3+ HLA-DR+] coexpressing CD3+. Constraints of cell numbers dictated that other subpopulations of lymphocytes, notably Tregs, were not analyzed. The outcome parameters of hematological recovery, acute and chronic GVHD, and survival were analyzed, with a median follow-up of 2 years. Data were adjusted for patient, disease and transplant characteristics.
Definition of outcomes
The primary outcome of this analysis was overall survival defined as time from infusion of graft to death from any cause. Surviving patients were censored at last follow-up. Secondary endpoints evaluated include: 1) neutrophil recovery defined as achieving neutrophil count ≥0.5 × 109/L for three consecutive measurements, 2) platelets recovery to ≥20 × 109/L for seven days, unsupported, 3) grade 2-4 acute GVHD as defined by the Glucksberg scale (10) and 4) chronic GVHD (11).
Sample collection and analysis
Unrelated donor BM or filgrastim-mobilized PBPC grafts were couriered by standard NMDP shipment protocols from the collection center to the transplant center’s processing laboratory. Upon arrival at the transplant center processing laboratory, a sample of the original product was shipped to the reference laboratory (sample 1). If the graft underwent further processing (RBC or plasma depletion) at the transplant center, the processing laboratory sent a sample of the graft after processing (sample 2). Both samples were packaged and shipped (in insulated cool packs) to the central reference laboratory, Baylor College of Medicine Center for Cell and Gene Therapy (CAGT). The samples were shipped inside secondary containers with frozen gel packs in Class 6.2 shipping containers and transported by overnight delivery service to the reference laboratory.
The transplant center processing laboratory reported data on type of processing (no processing, mononuclear cell concentration, plasma depletion, red blood cell removal), time of collection and time of processing. Upon receipt of the samples, the reference laboratory recorded the condition of the sample, the packaging condition (orientation, temperature and number of gel packs), and the time from collection and processing. Each sample was assessed for overall sample integrity and filtered using a 40μm Cell Strainer (Falcon #352340) if cellular aggregates were present. Only samples that were free of visible clumps and were at least 70% viable (by 7 AAD staining) were included in the analysis. Leukocyte count of the sample was enumerated using a Coulter AcT 8/10 Analyzer (Beckman-Coulter, Miami FL). In preparation for immunophenotyping each sample was adjusted to 1×107 cells per mL. Saturation concentrations of conjugated monoclonal antibodies (BDIS : CD45, CD34+, CD38, CD3+, CD3+ CD4+, CD3+ CD8+, CD3+ CD69+, CD3+ CD25+ & CD3+ HLA-DR+) were added to 12×75mm polystyrene tubes (Falcon 352008), followed by 100μL (106) cells and incubated 15 minutes at ambient temperature (18°C-22°C) in the dark. The antibodies and reagents are ASR reagents purchased from Becton-Dickinson Biosciences and include CD45 clone 2D1, CD34 class III clone 8G12, CD3 clone SK7, CD4 clone SK3, CD8 clone SK1, CD69 clone L78, CD25 clone 2A3, and HLA-DR clone L243. Also included, but not requested in the study design, CD19 clone 4G7 and CD16 clone B73.1 + CD56 clone MY31, to serve as internal Lympho-Sum control. All antibodies were used a saturated concentrations against the antigen concentration and determined by in-house checkerboard titration and/or used at the manufacturers suggested concentration. Commercial reference controls (CD-Chex Plus from Streck Laboratories, Inc) were run each day of sample evaluation as quality control of the staining-acquisition-and analysis for CD34, CD3, CD4, CD8 and HLA markers, in addition to the B-cell and NK-cell markers. There were no commercial controls available for the activation markers CD69 and CD25. Additionally, a combination of CD45 and CD14 was included to provide a 3-part differential which was also used as an internal control to ensure that the sum of T+B+NK cells was equal to the lymphocytes. The samples were processed using a programmable Lyse-Wash Assistant (BDIS), lysing the erythrocytes with ammonium chloride-based solution (PharM Lyse, BDIS). Viability after phenotyping was assessed using 7AAD (ViaProbe, BDIS). Data were acquired using single laser (488nm) flow cytometer with Cell-Quest Pro™ software. For progenitor cells, a total of 100,000 events were stored in list-mode; and 20,000 total events for lymphocyte and viability data. The acquisition software was also used for the analysis of the list-mode data. The CD34+ enumeration was performed using ISHAGE gating strategy (12). The lymphocytes were enumerated by defining a light scatter region around the lymphocyte population. The region was confirmed by CD3-backgating and the subsets co-expressing CD3 were enumerated (CD3+ CD4+, CD3+ CD8, CD3+ CD69+, CD3+ CD25+, and CD3+ HLA-DR+).
Statistical analysis
Median value and ranges are reported for continuous variables and percentages for categorical variables. The probability of overall survival was calculated using the Kaplan-Meier estimator (13). The probabilities of neutrophil and platelet recovery and acute and chronic GVHD were calculated with the cumulative-incidence estimator (14). Death was considered a competing risk for hematopoietic recovery and GVHD. Patients were censored if they underwent a second transplant, or at last follow-up. 95% confidence intervals (CI) were calculated with log transformation.
To analyze the association of cellular composition (white blood cell count, CD34+, CD34+ CD38−, CD3+, CD3+ CD4+, CD3+ CD8+, CD3+ CD25+, CD3+ CD69+ and CD3+ HLA-DR+) and clinical outcomes, multivariate logistic regression models were constructed for each cellular component for hematopoietic recovery, and Cox proportional hazards regression models (15) were constructed for acute and chronic GVHD, and overall survival. All the cell dose effects were analyzed as a binary variable. To dichotomize the continuous variables, Martingale residuals were plotted for each cell dose in order to determine the optimal cut point. We concluded that the medians were good cut points for all cell doses and thus a binary variable with median as cut point was used for all subsets analyzed. Other variables tested included age, performance score, disease (malignant vs. non-malignant), disease status at transplantation (early vs. intermediate vs. advanced disease vs. non-malignant disease), conditioning regimen (myeloablative vs. reduced intensity conditioning vs. conditioning for non-malignant diseases), donor-recipient HLA match (well matched vs. partially matched vs. mismatched) (16), donor-recipient CMV serostatus, ABO mismatch, donor age and graft processing (mononuclear cell concentration vs. plasma depletion vs. red blood cell removal vs. none). Due to the multiple comparisons included in the statistical analysis, a p<.01 was defined as statistically significant. All p-values were two-sided. All analyses were performed using SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
The demographic, disease and transplant characteristics of the 94 BM and 181 PBPC recipients are outlined on Table 1. The median age was 20 years (range <1-66) for BM recipients and 45 years (<1-70) for PBPC recipients. Most patients had high performance scores; performance scores were ≥90 for 81% of BM and 71% of PBPC recipients. Nearly all transplants (85% BM and 94% PBPC) were for treatment of malignancy. Transplant conditioning regimens for hematological malignancies included both myeloablative (70% of BM and 54% of PBPC transplants) and reduced intensity regimens (15% of BM and 40% of PBPC transplants). Only 14 (15%) BM and 10 (6%) PBPC transplants were for non-malignant diseases. Donor and recipient were HLA well-matched for 73% of BM and 66% of PBPC transplants.
Table 1.
Patient, disease and transplant characteristics
| Bone marrow | Peripheral blood progenitor cells | |
|---|---|---|
| Variables | Number (%) | Number (%) |
| Number of cases | 94 | 181 |
| Sex, male | 52 (55) | 111 (61) |
| Age, in years, median (range) | 20 (<1-66) | 45 (<1-70) |
| 10 or under 10 | 22 (23) | 13 ( 7) |
| 11-20 | 22 (23) | 16 ( 9) |
| 21-30 | 6 ( 6) | 23 (13) |
| 31-40 | 14 (15) | 22 (12) |
| 41-50 | 13 (14) | 41 (23) |
| 51-60 | 13 (14) | 44 (24) |
| over 60 | 4 ( 4) | 22 (12) |
| Karnofsky performance score 90-100 | 65 (81) | 118 (71) |
| Diseases | ||
| Acute myeloid leukemia | 29 (31) | 62 (34) |
| Acute lymphoblastic leukemia | 15 (16) | 39 (22) |
| Other leukemia | 4 ( 4) | 15 ( 8) |
| Chronic myeloid leukemia | 13 (14) | 13 ( 7) |
| Myelodysplastic syndrome | 10 (11) | 24 (13) |
| Non-Hodgkin lymphoma | 8 ( 9) | 16 ( 9) |
| Plasma cell disorder, multiple myeloma | 2 ( 2) | 3 ( 2) |
| Other malignancy | 0 | 2 ( 1) |
| Severe aplastic anemia | 7 ( 7) | 5 ( 3) |
| SCID and other immune system disorders | 1 ( 1) | 0 |
| Inherited disorder of metabolism | 2 ( 2) | 1 ( 1) |
| Histiocytic disorders | 3 ( 3) | 1 ( 1) |
| Disease status prior to transplant* | ||
| Early | 22 (23) | 45 (25) |
| Intermediate | 24 (26) | 42 (23) |
| Advanced | 34 (36) | 84 (46) |
| Non-malignant | 14 (15) | 10 ( 6) |
| Conditioning regimen | ||
| Malignant diseases, myeloablative | 66 (70) | 98 (54) |
| Malignant diseases, reduced intensity | 14 (15) | 73 (40) |
| Non-malignant diseases, other | 14 (15) | 10 ( 6) |
| HLA disparity† | ||
| Well-matched | 69 (73) | 119 (66) |
| Partially matched | 20 (21) | 41 (23) |
| Mismatched | 5 ( 5) | 21 (12) |
| Cytomegalovirus serostatus | ||
| Donor negative /recipient negative | 27 (29) | 52 (29) |
| Donor negative / recipient positive | 35 (37) | 63 (35) |
| Donor positive / recipient negative | 11 (12) | 25 (14) |
| Donor positive / recipient positive | 20 (21) | 38 (21) |
| Unknown | 1 ( 1) | 3 ( 2) |
| ABO match | ||
| Matched | 45 (48) | 67 (37) |
| Minor mismatch | 15 (16) | 55 (30) |
| Major mismatch | 28 (30) | 41 (23) |
| Bi-directional | 5 ( 5) | 18 (10) |
| Unknown | 1 ( 1) | 0 |
| Donor-recipient sex match | ||
| Male donor - male recipient | 31 (33) | 85 (47) |
| Male donor - female recipient | 34 (36) | 42 (23) |
| Female donor – male recipient | 21 (22) | 26 (14) |
| Female donor – female recipient | 8 ( 9) | 28 (15) |
| Donor age, years | ||
| 18-30 | 27 (29) | 56 (31) |
| 31-40 | 37 (39) | 68 (38) |
| 41-50 | 25 (27) | 46 (25) |
| 51-60 | 5 ( 5) | 11 ( 6) |
| Year of Transplant | ||
| 2003 | 22 (23) | 46 (25) |
| 2004 | 56 (60) | 100 (55) |
| 2005 | 16 (17) | 35 (19) |
| Graft processing type | ||
| Mononuclear cell concentration | 6 ( 6) | 0 |
| Plasma depletion | 15 (16) | 37 (20) |
| Red blood cell removal | 32 (34) | 1 ( 1) |
| None | 41 (44) | 143 (79) |
| Median follow-up survival, months (range) |
24 (6-30) | 24 (3-37) |
| Cell dose per kg, median (range) | ||
| WBC | 2.34×108 (4.33×106-9.56×108) | 7.23×108 (4.06×107-4.69×109) |
| CD34+ | 3.62×106 (1.02×105-1.84×107) | 5.41×106 (3.08×105-3.23×107) |
| CD34+ CD38− | 9.44×104 (8.76×102-7.39×105) | 1.54×105 (8.12×103-1.07×106) |
| CD3+ | 2.54×107 (6.43×105-1.21×108) | 2.37×108 (2.19×107-2.05×109) |
| CD3+ CD4+ | 1.33×107 (2.87×105-6.06×107) | 1.45×108 (1.43×107-1.29×109) |
| CD3+ CD8+ | 1.09×107 (2.94×105-7.52×107) | 8.37×107 (7.07×106-6.69×108) |
| CD3+ CD25+ | 3.05×106 (1.08×105-1.72×107) | 2.46×107 (2.34×106-2.40×108) |
| CD3+ CD69+ | 3.38×106 (1.43×105-2.64×107) | 3.55×106 (1.51×105-3.89×107) |
| CD3+ HLA-DR+ | 3.92×106 (8.59×104-3.50×107) | 2.18×107 (1.62×106-2.06×108) |
Early is defined as CR1, CP1, RA, and RARS; intermediate is defined as ≥ CR2+, AP, and ≥CP2; and advanced is defined as PIF, BP, relapse, RAEB, RAEBT, CMMoL, Durie-Salmon Stage III, and Waldenstrom macroglobulinemia.
Well-matched includes: 8/8 allele-level matched (BM n=46, PBPC n=91); allele-level matched A, B, DRB1 and low res matched at HLA-C (BM n=7, PBPC n=8); low res matched at A, B and C and allele-level DRB1 (BM n=16, PBPC n=19), allele-level matched at A, B, DRB1, and HLA-C unknown (PBPC n=1)
Partially matched includes: single locus antigen or allele-level MM at A, B, C or DRB1(BM n=19, PBPC n=39); matched at low-res A, B and high-res DRB1 and HLA-C unknown (BM n=1, PBPC n=1); matched at low-res A, B, C and DRB1 (PBPC n=1).
Mismatched includes: >1 allele or antigen MM at A, B, C, DRB1 (BM=4, PBPC n=20); 1 antigen mismatch at high-res A, B, DRB1, and HLA-C unknown (BM n=1); 1 mismatch at lowres A, B, high-res at DRB1 and HLA-C unknown (PBPC n=1)
Cellular composition of grafts and effect of processing
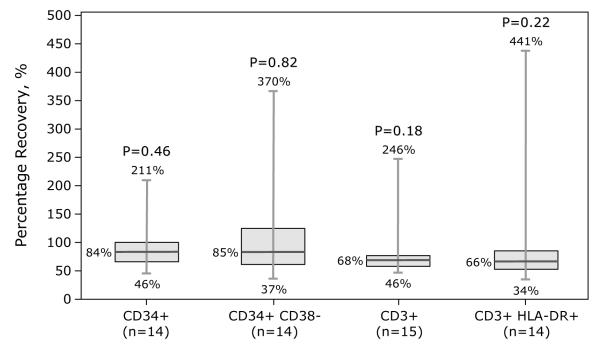
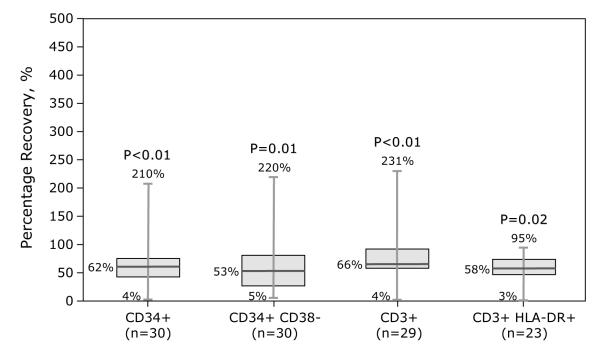
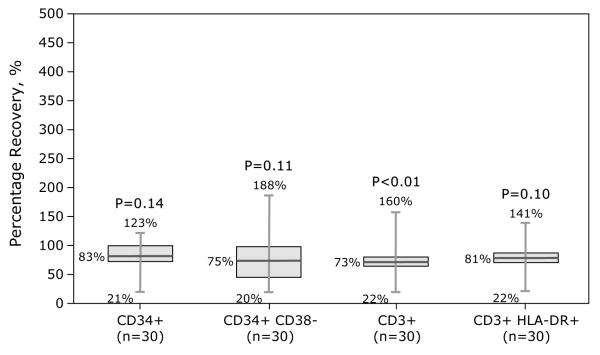
Samples were assessed for temperature of gel packs, sample integrity (absence of visible clumps after gentle agitation or clots requiring filtration), viability (>70%) and immunophenotype (CD34+, CD3+, and CD3+ T-cell subsets, CD3+ CD25+, CD3+ CD69+, CD3+ HLA-DR+). Samples from BM and PBPC products included herein met sample integrity and viability requirements. Compared to BM grafts, PBPC grafts contained more nucleated cells (7.23 × 108/kg vs. 2.34 × 108/kg), CD3+ cells (2.37 × 108/kg vs. 2.54 × 107/kg) and CD34+ cells (5.41 × 106/kg vs. 3.62 × 106/kg). Data on differences in cellular composition before and after processing (BM or PBPC) and type of processing are shown in Figures 1-2. Fifteen BM grafts were subject to plasma depletion and 32 to RBC removal. Plasma depletion of BM was not associated with changes in CD34+ and CD34+ CD38−, CD3+ and CD3+ HLA-DR+ recovery (Figure 1A). In contrast, RBC removal of BM grafts resulted in lower CD34+, CD34+ CD38− and CD3+ doses (Figure 1B). Thirty-seven PBPC grafts were subject to plasma depletion. CD3+ dose was lower but there were no differences in CD34+, CD34+ CD38− or CD3+ HLA-DR+ doses before and after plasma depletion (Figure 2).
Figure 1.
Effect of processing on BM product graft composition.
1A: Plasma depletion. 1B: RBC removal.
Figure 2.
Effect of processing on PBPC product graft composition – plasma depletion.
Numbers given on the figures were minimum, median and maximum of percentage recovery, and p-value from Kruskal-Wallis test to compare cell doses between before and after processing.
Effect of graft composition on post-HCT outcomes
Hematopoietic recovery
Ninety two of 94 (98%) recipients of BM grafts and 171 of 181 (94%) recipients of PBPC grafts achieved neutrophil recovery. The median time to neutrophil recovery was 18 days and 13 days after transplantation of BM and PBPC grafts, respectively. Corresponding day-28 probabilities of neutrophil recovery were 94% (95% CI 87-98%) and 93% (95% CI 88-96%). In multivariate analysis, neutrophil recovery after BM or PBPC transplantation was not associated with white blood cell count, myeloid or lymphoid subsets or activated lymphoid cells (Table 2). 69 of 94 (73%) recipients of BM grafts and 149 of 181 (82%) recipients of PBPC grafts achieved platelet recovery. The median time to platelet recovery was 27 days and 20 days after transplantation of BM and PBPC grafts, respectively. Corresponding day-60 probabilities of platelet recovery were 67% (95% CI 57-76%) and 79% (95% CI 73-85%). In multivariate analysis, platelet recovery after BM transplantation was not associated with white blood cell count, myeloid or lymphoid subsets or activated lymphoid cells. Platelet recovery was more likely with PBPC grafts containing >8 × 107/kg CD3+ CD8+ cells (OR 2.80, 95% CI 1.25 – 6.28, p=0.01) (Table 2).
Table 2.
Multivariate analysis of hematopoietic recovery
| Bone marrow | Neutrophil recovery OR (95%CI), p-value |
Platelet recovery OR (95%CI), p-value |
|---|---|---|
| WBC >2.34 vs. ≤2.34, 108 /kg |
5.48 (0.61-48.80), 0.13 | 1.98 (0.82-4.76), 0.13 |
| CD34+ >3.62 vs. ≤3.62, 106 /kg |
1.00 (0.19-5.23), 1.00 | 0.75 (0.32-1.78), 0.51 |
| CD34+ CD38− >9.44 vs. ≤9.44, 104 /kg |
2.09 (0.36-0.41), 0.41 | 1.34 (0.57-3.17), 0.51 |
| CD3+ >2.54 vs. ≤2.54, 107 /kg |
1.00 (0.19-5.23), 1.00 | 0.75 (0.32-1.78), 0.51 |
| CD3+ CD4+ >1.33 vs. ≤1.33, 107 /kg |
1.03 (0.14-7.66), 0.98 | 0.47 (0.18-1.21), 0.12 |
| CD3+ CD8+ >1.09 vs. ≤1.09, 107 /kg |
0.32 (0.03-3.18), 0.33 | 0.80 (0.31-2.03), 0.63 |
| CD3+ CD25+ >3.05 vs. ≤3.05, 106 /kg |
1.50 (0.24-9.50), 0.67 | 1.70 (0.66-4.35), 0.27 |
| CD3+ CD69+ >3.38 vs. ≤3.38, 106 /kg |
0.65 (0.10-4.11), 0.65 | 1.55 (0.62-3.88), 0.35 |
| CD3+ HLA-DR+ >3.92 vs. ≤3.92, 106 /kg |
0.48 (0.08-2.75), 0.41 | 0.82 (0.34-1.97), 0.66 |
| Peripheral blood progenitor cells | Neutrophil recovery OR (95%CI), p-value |
Platelet recovery OR(95%CI), p-value |
|---|---|---|
| WBC >7.23 vs. ≤7.23, 108 /kg |
1.17 (0.38-3.62), 0.79 | 2.04 (0.90-4.60), 0.09 |
| CD34+ >5.41 vs. ≤5.41, 106 /kg |
1.17 (0.38-3.62), 0.79 | 2.32 (1.05-5.16), 0.04 |
| CD34+ CD38− >1.54 vs. ≤1.54, 105 /kg |
0.61 (0.19-1.94), 0.40 | 1.00 (0.46-2.17), 0.99 |
| CD3+ >2.37 vs. ≤2.37, 108 /kg |
1.64 (0.52-5.21), 0.40 | 1.92 (0.88-4.17), 0.10 |
| CD3+ CD4+ >1.45 vs. ≤1.45, 108 /kg |
0.60 (0.19-1.90), 0.38 | 1.22 (0.56-2.66), 0.62 |
| CD3+ CD8+ >8.37 vs. ≤8.37, 107 /kg |
2.36 (0.70-7.96), 0.17 | 2.80 (1.25-6.28), 0.01 |
| CD3+ CD25+ >2.46 vs. ≤2.46, 107 /kg |
6.13 (1.32-28.49), 0.02 | 2.29 (1.02-5.13), 0.04 |
| CD3+ CD69+ >3.55 vs. ≤3.55, 106 /kg |
3.62 (0.96-13.64), 0.06 | 1.18 (0.55-2.55), 0.67 |
| CD3+ HLA-DR+ >2.18 vs. ≤2.18, 107 /kg |
2.36 (0.70-7.96), 0.17 | 1.69 (0.78-3.67), 0.19 |
Abbreviations: OR=Odds Ratio; CI=Confidence Interval.
Acute and chronic GVHD
The day-100 probabilities of grade 2-4 acute GVHD were 43% and 54% after transplantation of BM and PBPC, respectively. The 1-year probabilities of chronic GVHD were 31% and 44% after transplantation of BM and PBPC, respectively. In multivariate analysis, acute and chronic GVHD were not associated with white blood cell count, myeloid or lymphoid subsets or activated lymphoid cells (Table 3).
Table 3.
Multivariate analysis of Grade 2-4 acute and chronic graft-versus-host disease
| Bone marrow | Acute GVHD 2-4 RR (95% CI), p-value |
Chronic GVHD RR (95% CI), p-value |
|---|---|---|
| WBC >2.34 vs. ≤2.34, 108 /kg |
0.67 (0.33-1.12), 0.11 | 1.05 (0.51-2.14), 0.90 |
| CD34+ >3.62 vs. ≤3.62, 106 /kg |
0.79 (0.43-1.44), 0.43 | 0.94 (0.47-1.90), 0.87 |
| CD34+ CD38− >9.44 vs. ≤9.44, 104 /kg |
0.85 (0.47-1.55), 0.60 | 1.24 (0.61-2.53), 0.55 |
| CD3+ >2.54 vs. ≤2.54, 107 /kg |
0.57 (0.31-1.06), 0.07 | 0.95 (0.47-1.91), 0.88 |
| CD3+ CD4+ >1.33 vs. ≤1.33, 107 /kg |
0.93 (0.49-1.76), 0.83 | 0.90 (0.43-1.91), 0.79 |
| CD3+ CD8+ >1.09 vs. ≤1.09, 107 /kg |
0.80 (0.42-1.53), 0.49 | 1.05 (0.50-2.21), 0.90 |
| CD3+ CD25+ >3.05 vs. ≤3.05, 106 /kg |
0.94 (0.50-1.91), 0.85 | 0.76 (0.36-1.61), 0.47 |
| CD3+ CD69+ >3.38 vs. ≤3.38, 106 /kg |
0.71 (0.37-1.36), 0.30 | 0.81 (0.37-1.79), 0.61 |
| CD3+ HLA-DR+ >3.92 vs. ≤3.92, 106 /kg |
0.88 (0.47-1.62), 0.67 | 0.58 (0.28-1.19), 0.14 |
| Peripheral blood progenitor cells | Acute GVHD 2-4 RR (95% CI), p-value |
Chronic GVHD RR (95% CI), p-value |
|---|---|---|
| WBC >7.23 vs. ≤7.23, 108 /kg |
1.19 (0.79-1.78), 0.41 | 1.07 (0.69-1.66), 0.77 |
| CD34+ >5.41 vs. ≤5.41, 106 /kg |
1.17 (0.78-1.76), 0.44 | 0.79 (0.51-1.22), 0.29 |
| CD34+ CD38− >1.54 vs. ≤1.54, 105 /kg |
1.63 (1.08-2.46), 0.02 | 0.98 (0.63-1.52), 0.93 |
| CD3+ >2.37 vs. ≤2.37, 108 /kg |
1.13 (0.75-1.69), 0.57 | 1.21 (0.77-1.89), 0.41 |
| CD3+ CD4+ >1.45 vs. ≤1.45, 108 /kg |
1.19 (0.79-1.78), 0.41 | 1.41 (0.90-2.21), 0.13 |
| CD3+ CD8+ >8.37 vs. ≤8.37, 107 /kg |
1.08 (0.72-1.62), 0.72 | 1.48 (0.95-2.32), 0.09 |
| CD3+ CD25+ >2.46 vs. ≤2.46, 107 /kg |
1.20 (0.80-1.82), 0.38 | 1.42 (0.91-2.23), 0.13 |
| CD3+ CD69+ >3.55 vs. ≤3.55, 106 /kg |
1.05 (0.70-1.57), 0.83 | 1.10 (0.71-1.71), 0.68 |
| CD3+ HLA-DR+ >2.18 vs. ≤2.18, 107 /kg |
0.79 (0.53-1.18), 0.25 | 0.89 (0.57-1.37), 0.58 |
Abbreviations: RR = Relative Risk; CI=Confidence Interval
Overall survival
Forty six of 94 (49%) recipients of BM and 67 of 181 (37%) recipients of PBPC transplants were alive at the last contact. In multivariate analysis of BM transplants, overall mortality was not associated with white blood cell count, myeloid or lymphoid subsets or activated lymphoid cells (Table 4). In contrast, higher CD34+ cell dose (>5 × 106/kg) was associated with lower risks of mortality (RR 0.58, 95% CI 0.39 – 0.84, p<0.01) after PBPC transplants. No other myeloid or lymphoid subset or activated lymphoid cells were associated with overall survival (Table 4). The 1-year probabilities of overall survival were 59% and 34% when PBPC grafts contained >5 × 106/kg and ≤5 × 106/kg CD34+ cells, respectively.
Table 4.
Multivariate analysis of overall mortality
| Bone marrow | Peripheral blood progenitor cells | ||
|---|---|---|---|
| Graft composition | RR (95% CI), p-value | Graft composition | RR (95% CI), p-value |
| WBC >2.34 vs. ≤2.34, 108 /kg |
0.64 (0.36-1.16), 0.14 | WBC >7.23 vs. ≤7.23, 108 /kg |
0.68 (0.46-0.98), 0.04 |
| CD34+ >3.62 vs. ≤3.62, 106 /kg |
0.71 (0.40-1.27), 0.25 | CD34+ >5.41 vs. ≤5.41, 106 /kg |
0.58 (0.39-0.84), <0.01 |
| CD34+ CD38− >9.44 vs. ≤9.44, 104 /kg |
0.67 (0.37-1.21), 0.18 | CD34+ CD38− >1.54 vs. ≤1.54, 105 /kg |
1.04 (0.71-1.51), 0.85 |
| CD3+ >2.54 vs. ≤2.54, 107 /kg |
0.72 (0.41-1.29), 0.27 | CD3+ >2.37 vs. ≤2.37, 108 /kg |
0.75 (0.52-1.09), 0.13 |
| CD3+ CD4+ >1.33 vs. ≤1.33, 107 /kg |
0.98 (0.52-1.86), 0.96 | CD3+ CD4+ >1.45 vs. ≤1.45, 108 /kg |
0.80 (0.55-1.16), 0.25 |
| CD3+ CD8+ >1.09 vs. ≤1.09, 107 /kg |
0.86 (0.45-1.65), 0.66 | CD3+ CD8+ >8.37 vs. ≤8.37, 107 /kg |
0.66 (0.45-0.96), 0.03 |
| CD3+ CD25+ >3.05 vs. ≤3.05, 106 /kg |
0.83 (0.41-1.68), 0.60 | CD3+ CD25+ >2.46 vs. ≤2.46, 107 /kg |
0.89 (0.62-1.30), 0.55 |
| CD3+ CD69+ >3.38 vs. ≤3.38, 106 /kg |
0.55 (0.29-1.04), 0.07 | CD3+ CD69+ >3.55 vs. ≤3.55, 106 /kg |
0.77 (0.53-1.12), 0.18 |
| CD3+ HLA-DR+ >3.92 vs. ≤3.92, 106 /kg |
0.83 (0.46-1.52), 0.55 | CD3+ HLA-DR+ >2.18 vs. ≤2.18, 107 /kg |
0.67 (0.46-0.98), 0.04 |
Abbreviation: RR=Relative Risk; CI=Confidence Interval.
DISCUSSION
The composition of a hematopoietic progenitor cell graft is a major determinant of clinical outcome. It has been seen in grafts from both related (17,18) and unrelated (19,20) donors that when grafts are analyzed for cell dose by various phenotypic markers (e.g., CD34, DR, CD3) associations are seen with clinical outcomes such as survival, rate and type of hematologic recovery, or GVHD. The bone marrow or PBPC source for the graft has a profound effect on cellular composition and the cell dose, since mobilized peripheral blood contains a larger number but lesser percentage of primitive myeloid cells and a much greater numbers and higher percentage of mature T cells than bone marrow. Not surprisingly, this reciprocal relationship of cell types and doses between bone marrow and peripheral blood progenitor cell grafts can lead to vastly different clinical outcomes (21,22,23,24). However, though several comparative analyses have been published, including the Blood and Marrow Transplant Clinical Trials Network protocol 0201 which recently completed enrollment of 550 patients randomized to unrelated donor marrow or mobilized blood grafts. In earlier trials, prospective testing has not been done using a single reference laboratory to determine the relationship between graft composition and transplantation outcome.
Laboratory processing of bone marrow or PBPC also has a major effect on the cellular composition of the final graft. The common laboratory procedures of plasma depletion and RBC reduction are done to reduce risks of transfusion reactions in the recipient with minimal stem cell loss. The effect of processing on graft composition is necessary to plan the optimal volume of marrow cells to be collected or number of apheresis procedures.
As expected, in this study substantial variability was seen in the cellular graft composition of BM and PBPC grafts both before and after common manipulations of RBC removal or plasma depletion. In BM grafts the cellular composition was not associated with hematopoietic recovery, GVHD, or survival while in PBPC grafts, superior survival was seen when grafts contained > 5 × 106 CD34+/kg, and superior platelet recovery was seen when grafts contained > 8 × 107 CD3+ CD8+/kg. However, neither survival nor GVHD were altered by any other cellular subsets in PBPC grafts.
In this report, a central reference laboratory was used to ensure that data on the composition of the grafts examined were consistent and reliable. Importantly the study demonstrated that the data integrity is dependent on the products (samples analyzed) being an accurate representation of the transplanted graft and that the sample analyzed is relatively unaffected by storage or transportation prior to analysis. Improper storage and transport of products could differentially affect the viability of myeloid and lymphoid elements, leading to misleading interpretations. Multicenter studies using central reference laboratories must utilize uniform shipping protocols that ensure graft viability and integrity including temperature control and prompt delivery to the analyzing laboratory.
The clinical decision determining the optimal cell source and total cell number in the graft is dependent on the choice of cell source, the expected yield, how the graft is processed, and the reliability of phenotyping data obtained from samples. Determining the optimal graft composition of BM and PBPC could better serve the recipients and minimize demands on volunteer unrelated donors.
ACKNOWLEDGEMENT
Supported by funding from the National Marrow Donor Program and Department of the Navy, Office of Naval Research Cooperative Agreement #N00014-99-2-0006 to the National Marrow Donor Program. Any opinions, finding, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Office of Naval Research or the National Marrow Donor Program.
Appendix 1. Participating transplant centers
Barbara Ann Karmanos Cancer Inst.- Wayne State University
Baylor University Medical Center
Children’s Hospital Medical Center
Children’s Hospital of Orange County
Children’s Hospital of Philadelphia
Children’s Hospital of Wisconsin
Children’s Memorial Hospital
City of Hope National Medical Center
Cleveland Clinic Foundation
Duke University Medical Center
Emory University Egleston Children’s Hospital
Georgetown University Hospital
H. Lee Moffitt Cancer Center
Henry Ford Health Systems
Loyola University Medical Center
Medical College of Wisconsin/Froedtert Memorial Lutheran Hospital Cancer Center
Memorial Sloan-Kettering Cancer Center
North Shore University Hospital
Rainbow Babies & Children’s Hospital
Rocky Mountain Cancer Center/Presbyterian St. Luke’s
Roswell Park Cancer Institute
Schneider Children’s Hospital
St Louis Children’s Hospital
St. Luke’s Hospital of Kansas City
Stanford University Medical Center
The Jewish Hospital
The Ohio State University/Arthur G. James Cancer Hospital and Richard J. Solove Research Institute
University of California San Francisco Children’s Hospital
University of Chicago Children’s Hospital
University of Iowa Hospital
University of Michigan Medical Center
University of Minnesota Hospitals & Clinics
University of Mississippi Medical Center
University of Nebraska Medical Center
University of North Carolina Hospitals
University of Oklahoma Health Sciences Center/HCA Health Services of Oklahoma, Inc.
University of Pittsburgh Medical Center Cancer Pavilion
University of Rochester Medical Center/Strong Memorial Hospital
University of Utah Medical Center
West Virginia University Hospitals, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kernan NA, Collins, Juliano L, Cartagena TS, Dupont B, O’Reilly RJ. Clonable T lymphocytes in T-cell depleted bone marrow transplants correlate with development of graft versus host disease. Blood. 1986;68:770–773. [PubMed] [Google Scholar]
- 2.Körbling M, Huh YO, Durett A, et al. Allogeneic blood stem cell transplantation: peripheralization and yield of donor-derived primitive hematopoietic progenitor cells (CD34+ Thy-1dim) and lymphoid subsets, and possible predictors of engraftment and graft-versus-host disease. Blood. 1995;86:2842–2848. [PubMed] [Google Scholar]
- 3.Kawanishi Y, Passweg J, Drobyski WR, et al. Effect of T cell subset dose on outcome of T cell-depleted bone marrow transplantation. Bone Marrow Transplant. 1997;19:1069–1077. doi: 10.1038/sj.bmt.1700807. [DOI] [PubMed] [Google Scholar]
- 4.Theilgaard-Mönch K, Raaschou-Jensen K, Palm H, et al. Flow cytometric assessment of lymphocyte subsets, lymphoid progenitors, and hematopoietic stem cells in allogeneic stem cell grafts. Bone Marrow Transplant. 2001;28:1073–1082. doi: 10.1038/sj.bmt.1703270. [DOI] [PubMed] [Google Scholar]
- 5.Panse JP, Heimfeld S, Guthrie KA, et al. Allogeneic peripheral blood stem cell graft composition affects early T-cell chimaerism and later clinical outcomes after non-myeloablative conditioning. Br J Haematol. 2005 Mar;128:659–67. doi: 10.1111/j.1365-2141.2005.05363.x. [DOI] [PubMed] [Google Scholar]
- 6.Baron F, Maris MB, Storer BE, et al. High doses of transplanted CD34+ cells are associated with rapid T-cell engraftment and lessened risk of graft rejection, but not more graft-versus-host disease after nonmyeloablative conditioning and unrelated hematopoietic cell transplantation. Leukemia. 2005;19:822–828. doi: 10.1038/sj.leu.2403718. [DOI] [PubMed] [Google Scholar]
- 7.Cao TM, Wong RM, Sheehan K, et al. CD34, CD4, and CD8 cell doses do not influence engraftment, graft-versus-host disease, or survival following myeloablative human leukocyte antigen-identical peripheral blood allografting for hematologic malignancies. Exp Hematol. 2005;33:279–285. doi: 10.1016/j.exphem.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Keever-Taylor CA, Bredeson C, Loberiza FR, et al. Analysis of risk factors for the development of GVHD after T cell-depleted allogeneic BMT: effect of HLA disparity, ABO incompatibility, and method of T-cell depletion. Blood Marrow Transplant. 2001;7:620–630. doi: 10.1053/bbmt.2001.v7.pm11760150. [DOI] [PubMed] [Google Scholar]
- 9.Durett AG, Gee AP, Collins NH, Eapen M, Weisdorf D. Flow cytometric analysis of specimens by a central reference laboratory in a multi-center study: factors affecting data quality. Blood. 2006;108:3385. [Google Scholar]
- 10.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 11.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- 12.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. J of Hematotherapy. 1996;5:213–226. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Cox DR. Regression models and life tables (with discussion) J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 16.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mavroudis D, Read E, Cottler-Fox M, et al. CD34+ cell dose predicts survival, post transplant morbidity, and rate of hematologic recovery after allogeneic marrow transplant for hematologic malignancies. Blood. 1996;88:3223–3229. [PubMed] [Google Scholar]
- 18.Bittencourt H, Rocha V, Chevret S, et al. Association of CD34 cell done with hematopoietic recovery, infections, and other outcomes after HLA-identical sibling bone marrow transplantation. Blood. 2002;99:2726–2733. doi: 10.1182/blood.v99.8.2726. [DOI] [PubMed] [Google Scholar]
- 19.Sierra J, Storer B, Hansen JA, et al. Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: the effect of leukemic burden, donor HLA-matching, and marrow cell dose. Blood. 1997;89:4226–4235. [PubMed] [Google Scholar]
- 20.Sierra J, Storer B, Hansen JA, et al. Unrelated donor marrow transplantation for acute myeloid leukemia: an update of the Seattle experience. Bone Marrow Transplant. 2000;26:397–404. doi: 10.1038/sj.bmt.1702519. [DOI] [PubMed] [Google Scholar]
- 21.Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. New England J Med. 2001;344:75–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 22.Heldal D, Tjonnfjord GE, Brinch L, et al. A randomized study of allogeneic transplantation with stem cells from blood or bone marrow. Bone Marrow Transplant. 2000;25:1129–1136. doi: 10.1038/sj.bmt.1702422. [DOI] [PubMed] [Google Scholar]
- 23.H K, Fahmy OA, Kamel A, et al. Peripheral blood vs.Bone marrow as a source for allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;24:355–358. doi: 10.1038/sj.bmt.1701906. [DOI] [PubMed] [Google Scholar]
- 24.Powles R, Mehta J, Kulkarni S, et al. Allogeneic blood and bone-marrow stem-cell transplantation in haematological malignant diseases: a randomized trial. Lancet. 2000;355:1231–1237. doi: 10.1016/S0140-6736(00)02090-0. [DOI] [PubMed] [Google Scholar]