Abstract
Background
Several disease processes of the colon and rectum, including constipation and incontinence, have been associated with abnormalities of the autonomic nervous system. However, the autonomic innervation to the colon and rectum are not fully understood. The aims of this study were to investigate the effect of stimulation of vagus nerves, pelvic nerves (PN) and hypogastric nerves (HGN) on colorectal motility in rats.
Methods
Four strain gauge transducers were implanted on the proximal colon, mid colon, distal colon and rectum to record circular muscle contractions in rats. Electrical stimulation was administered to the efferent distal ends of the cervical vagus nerve, PN and HGN. Motility index (MI) was evaluated before and during stimulation.
Key Results
Electrical stimulation (5–20 Hz) of the cervical vagus elicited significant contractions in the mid colon and distal colon, whereas less pronounced contractions were observed in the proximal colon. PN stimulation elicited significant contractions in the rectum as well as the mid colon and distal colon. Atropine treatment almost completely abolished the contractions induced by vagus nerve and PN stimulation. HGN stimulation caused relaxations in the rectum, mid colon and distal colon. The relaxations in response to HGN stimulation were abolished by propranolol.
Conclusions & Inferences
Vagal innervation extends to the distal colon, while the PN has projections in the distribution of the rectum through the mid colon. This suggests a pattern of dual parasympathetic innervation in the left colon. Parasympathetic fibers regulate colorectal contractions via muscarinic receptors. The HGN mainly regulates colorectal relaxations via beta-adrenoceptors.
Keywords: gastrointestinal motility, vagus nerve, pelvic nerve, sympathetic nerve, electrical stimulation
INTRODUCTION
During the past decades, much attention has been paid to abnormalities of autonomic nerves that are associated with colorectal motility disorders, including constipation and incontinence. Such problems may occur spontaneously or after childbirth, hysterectomy and colorectal surgery. Postoperative functional disorders in colorectal motility and defecation such as frequent and irregular evacuation, sensory disorders, and fecal incontinence are closely related to autonomic nerve injury 1–4. The autonomic nervous system can be divided into parasympathetic and sympathetic components. Parasympathetic, general visceral efferent innervation of the large bowel is derived from the dorsal motor nucleus of vagi (DMV) and the sacral parasympathetic nucleus.
It is generally believed that vagal innervation to the large bowel terminates at the level of the splenic flexure 5–9, while the remainder of the colon, including the rectum, receives parasympathetic input from the pelvic nerves (PN) 10–12. However, the extent of innervation from the vagus and PN is still a matter of debate. Electrophysiological studies have resulted in conflicting data. Hulten and Jodal 13 reported that efferent vagal stimulation caused contraction of the right colon in cats, while others showed that the entire colon responded to vagal stimulation in rats 14 and monkeys 15. In contrast, Gillis et al. 16 demonstrated that vagal stimulation did not cause any colonic contractions in cats.
The role of the PN on colonic motility, especially for the proximal and mid colon, remains unclear. As early as 1895, Langley and Anderson 17, 18 demonstrated that stimulation of the PN induces contractions in the distal colon. Since then, it has been well established that PN stimulation elicits rectal and distal colon contractions in dogs 19, 20 and cats21. This was supported by a recent retrograde labeling study which showed that the left colon receives parasympathetic input from the PN in cats 11. In contrast, others have demonstrated that contractile responses were observed in the entire colon, including the proximal colon, secondary to PN stimulation in cats 22.
The distal colon and rectum also receives sympathetic input from the hypogastric nerves (HGN) 7. The sympathetic fibers to the large intestine are mainly derived from the lumbar preganglionic outflow that runs to the inferior mesenteric ganglia, which is also called hypogastric ganglion. Caudally, the continuation of the inferior mesenteric ganglion is the HGN, a bilateral sympathetic nerve that reaches the pelvic plexus. The innervation and functional role of the HGN on the internal anal sphincter have well been studied 23. However, it still remains unclear how the HGN regulates colorectal motility.
Our recent study indicates that transection of pelvic nerves leads to a dramatic decrease in rectal motility whereas transection of HGN causes an increase in rectal motility in rats 24. The aims of the present study were to investigate the effect of stimulation of vagus nerves, pelvic nerves (PN) and hypogastric nerves (HGN) on colorectal motility in rats.
METHODS
Preparation of Animals
Eighteen adult male Sprague Dawley (SD) rats weighing 280–350 g were housed in individual cages under conditions of controlled temperature (22–24°C) and illumination (12-hr light cycle starting at 6:00 AM) for at least 7 days prior to experimentation. Rats were given ad libitum access to food and water. Protocols describing the use of rats were approved by the Animal Care and Use Committee of Clement J. Zablocki Veterans Affairs Medical Center (Milwaukee).
The rats were divided into 3 groups: vagal stimulation, PN stimulation and HGN stimulation. Animals were placed on a thermal mattress and a heat lamp was directed at the operative field to ensure adequate body temperature.
Operative Procedure
Under anesthesia by isoflurane, a tracheal cannula was inserted. A median abdominal incision was made and four strain-gauge transducers (SGs) were implanted on the colon and rectum. Each SG was calibrated before the operation with 10 g weights. The four SGs were sutured to the seromuscular layer, perpendicular to the longitudinal axis of the colon and rectum. The four SGs were affixed at the following sites: SG1 was sutured on the proximal colon, 1 cm distal to the ileocecal junction; SG2 on the mid colon, 5 cm distal to SG1; SG3 on the distal colon, 5 cm proximal to the sacral promontory; and SG4 on the rectum, 3 cm proximal to the anus.
For vagus nerve stimulation, the left cervical vagus was isolated under a dissecting microscope and transected. The distal cut end was stimulated with bipolar electrodes. For PN stimulation, the right PN was dissected free and sectioned where it emerged from the sacral roots. The peripheral end was prepared for subsequent efferent electrical stimulation. The right HGN was transected distal to the IMG at the level of the sacral promontory.
Nerve stimulation and motility recordings
SGs were connected to a recording system (Power Lab 8SP, AD Instruments, Colorado Springs, CO). Colorectal motility was recorded for 1h prior to electrical stimulation. Electrical stimulation was administered at the distal ends of the left cervical vagus nerve, right PN and right HGN. Electrical stimulation parameters were 30 sec at 10V with 2 ms pulse duration (Harvard Apparatus, 62020 stimulator, England). Frequency of electrical stimulation was serially increased from 1–20 Hz with a 5 min interval between stimulations. After finishing the first series of electrical stimulation, rats were administered atropine, phentolamine or propranolol accordingly, and similar electrical stimulations (1–20 Hz) were repeated.
Administration of drugs
To investigate whether beta-adrenoceptors are involved in the response to sympathetic nerve stimulation, propranolol hydrochloride (1 mg/kg) were injected intramuscularly (IM) 15 min before the nerve stimulation. To investigate whether muscarinic receptors are involved in the response to parasympathetic nerve stimulation, atropine sulfate (1 mg/kg) was injected (IM) 15 min prior to nerve stimulation. All chemicals were purchased from Sigma Chemical Company (St. Louis, MN, USA).
Statistical analysis
Quantification of colorectal motility was achieved by calculating a motility index (MI) 30 sec prior to stimulation and again during the 30 sec stimulation. The MI was calculated by integrating the area under the transducer tracing from baseline. (PowerLab, ADInstruments Colorado Springs, CO), as previously reported 25. The percent increase or decrease in MI (MI of during the stimulation/MI prior to the stimulation) was calculated to compare the stimulation induced motility change. Statistical analysis was performed with SPSS (SPSS Inc., Chicago, IL, USA) system for Windows, version 17.0. Differences between the prestimulation and stimulation at serial frequency (1–20 Hz) were examined by repeated measures analysis of variance (ANOVA), followed by Bonferroni test. A p value <0.05 was considered statistically significant. Results are expressed as mean±SE of the groups
RESULTS
Effects of vagus nerve stimulation on colorectal motility
Vagal nerve stimulation at 1 Hz did not cause contractions throughout the colon. Vagal nerve stimulation at 5, 10, and 20Hz elicited significant contractions in the mid colon and distal colon, while less pronounced contractions were observed in the proximal colon. Contractions in the proximal colon, mid colon and distal colon, secondary to vagal stimulation, were rapid in onset and simultaneous. This suggests a non-propagated colonic contraction (Fig. 1a). Maximum effects were observed at 10 Hz, which increased MI to 232±47% (n=6, P<0.05), 692±154% (n=6, P<0.01) and 331±103% (n=6, P<0.01) of basal in the proximal colon, mid colon and distal colon respectively (Fig. 1b).
Figure 1.
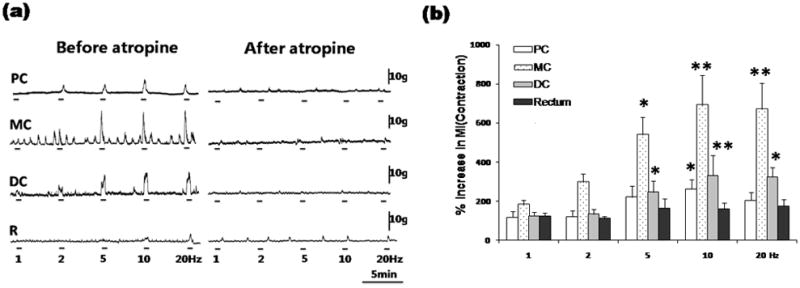
Colorectal responses to vagal stimulation (a). Vagus efferent stimulation elicited significant contractions in the entire colon, which could be antagonized by atropine administration. MI changes in response to the vagal stimulation (b). ANOVA for repeated measures (p<0.001, F=12.09, r2=0.3482) followed by posthoc Bonferroni test compared to pre-stimulation (n=6, *P<0.05, **P<0.01). PC: proximal colon, MC: mid colon, DC: distal colon, R: rectum.
Following atropine (1 mg/kg) administration, the contractile responses after vagal stimulation were almost completely abolished (Fig. 1a).
Effects of PN stimulation on colorectal motility
PN stimulation elicited significant contractions in the rectum as well as distal colon and mid colon. Maximum effects were observed at 10 Hz, which increased MI to 931±157% of baseline in the rectum (n=6, p<0.01), to 364±162% in the mid colon (n=6, p<0.05) and to 255±54% (n=6, p<0.05) in the distal colon. No responses were observed in the proximal colon (Fig. 2a and 2b).
Figure 2.
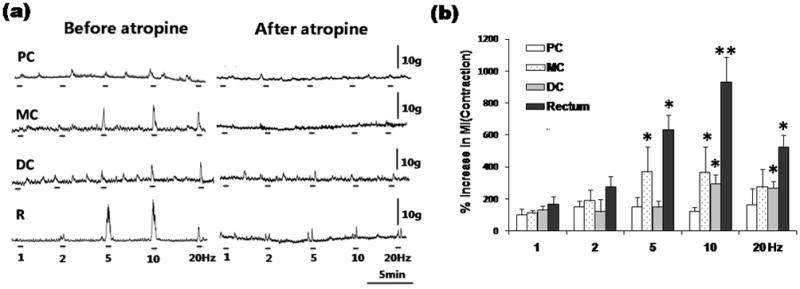
Colorectal responses to PN stimulation (a). PN efferent stimulation caused prominent contractions from the rectum extending to the mid colon, which could be antagonized by atropine administration. MI changes in response to PN stimulation (b). ANOVA for repeated measures (p<0.001, F=7.917, r2=0.2267) followed by posthoc Bonferroni test compared to pre-stimulation (n=6, *P<0.05, **P<0.01). PC: proximal colon, MC: mid colon, DC: distal colon, R: rectum.
After atropine (1 mg/kg) administration, the contractile responses evoked by PN stimulation were almost completely abolished (Fig. 2a).
Effects of HGN stimulation on colorectal motility
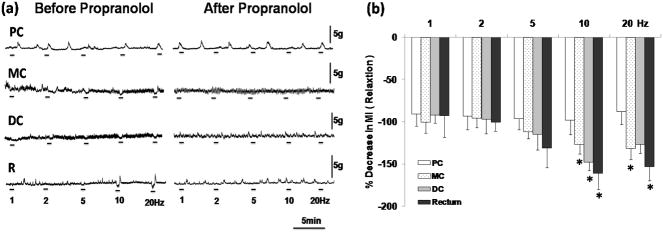
Following 2 Hz to 20 Hz stimulation, obvious relaxations were observed in the rectum, mid colon and distal colon, but not in the proximal colon (Fig. 3a). Maximum effects were observed at 10 Hz, which decreased the MI to 161±18% of baseline (n=6, p<0.05) in the rectum. HGN stimulation at 10 Hz slightly attenuated the MI to 127±11% (n=6, p<0.05) in the mid colon and 148±11% (n=6, p<0.05) in the distal colon, (Fig. 3b).
Figure 3.
Effects of HGN stimulation on colorectal motility (a). HGN efferent stimulation evoked apparent relaxation in the rectum, distal colon as well as mid colon, which could be abolished by propranolol administration. MI changes in response to HGN stimulation (b). ANOVA for repeated measures (p<0.01, F=4.495, r2=0.2725) followed by posthoc Bonferroni test compared to pre-stimulation (n=6, *P<0.05). PC: proximal colon, MC: mid colon, DC: distal colon, R: rectum.
Relaxation in response to HGN stimulation was almost completely abolished by the administration of propranolol (1 mg/kg) (Fig. 3a).
DISCUSSION
It has been assumed that in humans the vagus nerve innervates the right and transverse colon 5, 7, 9, 12. Our knowledge concerning extrinsic innervations of the human colon is mainly derived from dissection studies. For this reason the distribution of autonomic nerves still presents uncertainties and therefore remains controversial.
In animals, the nerves conveying the parasympathetic or sympathetic outflow to the large bowel vary across species. These differences in anatomy between and within species of laboratory animals are highlighted in the exhaustive work of Langley and Anderson 17, 18. In cats, Gillis et al. 16 demonstrated that vagal stimulation did not cause any contractions in the colon. In contrast, Hulten and Jodal 13 observed contractions of the right colon following vagus stimulation, whereas Rostard26 showed responses in the entire colon. In monkeys, vagus nerve stimulation also causes contractions in the entire colon 15.
Our current study showed that vagus nerve stimulation evoked contractions from the proximal colon to the distal colon, which suggests that vagal innervation extends throughout the entire colon in rats. This is supported by a retrograde neuronal tracer study in rats14, 27. A recent immunohistochemical study also described vagal projections throughout the colon in zebra fish 28.
Vagus nerve stimulation caused significant contractions in the mid colon and distal colon, whereas less pronounced contractions were observed in the proximal colon. This may due to the variable distribution of neurotransmitters within the myenteric plexus as well as vagal projection density 29. There are regional differences in acetylcholine release in response to nerve stimulation between the proximal colon and distal colon in guinea pigs; more acetylcholine is released in the distal colon than the proximal colon 30.
While vagus nerve stimulation did not cause any response in the rectum, PN stimulation elicited significant contractions in the rectum. Simultaneously, prominent contractions were observed in the mid colon and distal colon, but not in the proximal colon. Our result from rats is consistent with the previously studies in canines 19, 20 and cats 21. In contrast, Fasth 22 observed contractile response in the entire colon of cats secondary to PN stimulation, and Ishikawa 31 reported reduced proximal colon motility after PN denervation in dogs. Thus the functional role of the PN in the proximal colon remains to be studied. Fukai and Fukuda 32 suggested that some rectal branches of the PN ascend between the longitudinal and circular muscular layers of the colon to about 60% of its length, which were called ascending intramural PN in dogs. This may be the morphological basis for long distance innervation of the PN. After atropine treatment, the contractile responses evoked by vagus and PN stimulation were almost completely abolished. This suggests that both parasympathetic nerves regulate colorectal contractions via muscarinic receptors.
Taken together, the present study suggests a pattern of dual parasympathetic innervation in the left colon by both the vagus and PN in rats. This area appears to extend from the distal portion of the mid colon through the end of distal colon. This pattern of overlapping innervation of parasympathetic nerves has also been described in cats and dogs 17, 18. This pattern of dual parasympathetic innervation in the left colon may contribute to the compensatory mechanism of colonic motility following colorectal surgery. The manner in which colorectal motility is coordinated given this pattern of dual innervation and its adaptive response to injury require further exploration.
The effects of HGN stimulation on rectal motility have not been fully studied in rats. Hedlund 33 reported that sympathetic nerves to the rectum exert both excitatory and inhibitory responses in cats, while others reported excitatory responses to HGN stimulation are only occasionally observed 23. The present study revealed that HGN stimulation induced colorectal relaxations, followed by rebound contractions. This suggests a predominant inhibitory role of HGN innervation in rats. It has been assumed that rebound or post-stimulus contractions result from either release of an excitatory effect (normally masked by inhibition during stimulation) or to be a myogenic rebound phenomenon 34, 35.
The relaxation and rebound contraction induced by HGN stimulation was antagonized by propranolol. This supports the previous findings that the inhibitory response elicited by sympathetic nerve stimulation is a result of activation of postganglionic fibers acting on beta adrenoceptors of the colorectal smooth muscle cells 33, 36. It seems that the influence of the HGN on colonic motility is not limited to the rectum but extends to the distal colon and mid colon.
In addition to HGN, the colon and rectum also receive sympathetic input from lumbar colonic nerves (LCN) 7. It has been shown that stimulation of LCN inhibits rectal motility in guinea pigs 37, 38 and cats 33. LCN stimulation-induced relaxations are antagonized by yohimbine (an alpha2-adrenoceptor antagonist) and propranolol of the guinea pig rectum 38 and cat rectum 39, respectively. In contrast, the excitatory effects of LCN stimulation have been shown in the cat rectum 33, 39, 40. LCN stimulation-induced contractions were antagonized by phentolamine in the cat rectum 39. Others showed that LCN stimulation-induced contractions were antagonized by atropine 33 in the cat rectum. This suggests that LCN contains excitatory cholinergic fibers (postsynaptic parasympathetic efferents and/or presynatptic sympathetic efferents) innervating to the rectum in cats. Thus, the functional role of LCN innervation needs be determined.
In conclusion, the vagus nerve has a major influence on the entire colon, while the PN has projections from the rectum through the distal colon and mid colon. Both the vagus and PN regulate colorectal contractions via muscarinic receptors, while the HGN mainly regulates colorectal relaxations via beta-adrenoceptors in rats. A pattern of dual, coordinated, parasympathetic innervation in the left colon may regulate motor activity between the proximal colon and rectum. To our knowledge, this is the first demonstration of the pattern of dual parasympathetic innervation, maintained by the vagus and PN, in the rat colon. This information may have important physiological implications and may help to explain the adaptive mechanism frequently observed following colorectal surgery.
Supplementary Material
Acknowledgments
This study was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (RO1 DK62768, T.T.).
The results of this study were presented in part at the Neurogastroenterology and Motility 2009 Joint International Meeting.
Abbreviations
- PN
pelvic nerves
- HGN
hypogastric nerves
- LCN
lumbar colonic nerves
- MI
motility index
- IMG
inferior mesenteric ganglia
- SGs
strain-gauge transducers
Footnotes
No conflicts of interest exist
References
- 1.Mochiki E, Asao T, Kuwano H. Gastrointestinal motility after digestive surgery. Surg Today. 2007;37(12):1023–32. doi: 10.1007/s00595-007-3525-5. [DOI] [PubMed] [Google Scholar]
- 2.Iizuka I, Koda K, Seike K, et al. Defecatory malfunction caused by motility disorder of the neorectum after anterior resection for rectal cancer. Am J Surg. 2004;188(2):176–80. doi: 10.1016/j.amjsurg.2003.12.064. [DOI] [PubMed] [Google Scholar]
- 3.Dorofeeva AA, Panteleev SS, Makarov FN. Parasympathetic innervation of the initial segments of the large intestine in cats. Neurosci Behav Physiol. 2008;38(9):923–7. doi: 10.1007/s11055-008-9073-7. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu K, Koda K, Kase Y, et al. Induction and recovery of colonic motility/defecatory disorders after extrinsic denervation of the colon and rectum in rats. Surgery. 2006;139(3):395–406. doi: 10.1016/j.surg.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Devroede G, Lamarche J. Functional importance of extrinsic parasympathetic innervation to the distal colon and rectum in man. Gastroenterology. 1974;66(2):273–80. [PubMed] [Google Scholar]
- 6.Frantzides CT, Condon RE, Schulte WJ, Cowles V. Effects of morphine on colonic myoelectric and motor activity in subhuman primates. Am J Physiol. 1990;258(2 Pt 1):G247–52. doi: 10.1152/ajpgi.1990.258.2.G247. [DOI] [PubMed] [Google Scholar]
- 7.Corman ML. Colon and Rectal Surgery. Lippincott Williams & Wilkins; 2005. p. 1743. [Google Scholar]
- 8.Martin JH. Neuroanatomy: text and atlas. 3. McGraw-Hill; 2003. [Google Scholar]
- 9.Willard FH. autonomic nervous system. In: Robert C, Ward RJH, John A, Jerome, editors. Foundations for osteopathic medicine. Lippincott Williams & Wilkins; 2002. pp. 105–111. [Google Scholar]
- 10.Jorge JM, Wexner SD. Anatomy and physiology of the rectum and anus. Eur J Surg. 1997;163(10):723–31. [PubMed] [Google Scholar]
- 11.Dorofeeva AA, Panteleev SS, Makarov FN. Involvement of the sacral parasympathetic nucleus in the innervation of the descending colon and rectum in cats. Neurosci Behav Physiol. 2009;39(2):207–10. doi: 10.1007/s11055-009-9104-z. [DOI] [PubMed] [Google Scholar]
- 12.Gonella J, Bouvier M, Blanquet F. Extrinsic nervous control of motility of small and large intestines and related sphincters. Physiol Rev. 1987;67(3):902–61. doi: 10.1152/physrev.1987.67.3.902. [DOI] [PubMed] [Google Scholar]
- 13.Hulten L. Extrinsic nervous control of colonic motility and blood flow. An experimental study in the cat. Acta Physiol Scand Suppl. 1969;335:1–116. [PubMed] [Google Scholar]
- 14.Altschuler SM, Escardo J, Lynn RB, Miselis RR. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology. 1993;104(2):502–9. doi: 10.1016/0016-5085(93)90419-d. [DOI] [PubMed] [Google Scholar]
- 15.Dapoigny M, Cowles VE, Zhu YR, Condon RE. Vagal influence on colonic motor activity in conscious nonhuman primates. Am J Physiol. 1992;262(2 Pt 1):G231–6. doi: 10.1152/ajpgi.1992.262.2.G231. [DOI] [PubMed] [Google Scholar]
- 16.Gillis RA, Dias Souza J, Hicks KA, et al. Inhibitory control of proximal colonic motility by the sympathetic nervous system. Am J Physiol. 1987;253(4 Pt 1):G531–9. doi: 10.1152/ajpgi.1987.253.4.G531. [DOI] [PubMed] [Google Scholar]
- 17.Langley JN, Anderson HK. On the Innervation of the Pelvic and Adjoining Viscera: Part I. The Lower Portion of the Intestine. J Physiol. 1895;18(1–2):67–105. doi: 10.1113/jphysiol.1895.sp000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langley JN, Anderson HK. The Innervation of the Pelvic and adjoining Viscera: Part VII. Anatomical Observations. J Physiol. 1896;20(4–5):372–406. doi: 10.1113/jphysiol.1896.sp000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirabayashi T, Matsufuji H, Yokoyama J, et al. Colorectal motility induction by sacral nerve electrostimulation in a canine model: implications for colonic pacing. Dis Colon Rectum. 2003;46(6):809–17. doi: 10.1007/s10350-004-6661-7. [DOI] [PubMed] [Google Scholar]
- 20.Maruyama S, Okabe S, Endo M, et al. The role of the rectal branches of pelvic plexus in defecation and colonic motility in a canine model. J Med Dent Sci. 2003;50(4):275–84. [PubMed] [Google Scholar]
- 21.Hedlund H, Fandriks L, Delbro D, Fasth S. On the transmission of sacral parasympathetic nervous influence on distal colonic and rectal motility in the cat. Acta Physiol Scand. 1985;125(2):225–34. doi: 10.1111/j.1748-1716.1985.tb07711.x. [DOI] [PubMed] [Google Scholar]
- 22.Fasth S, Hulten L, Nordgren S. Evidence for a dual pelvic nerve influence on large bowel motility in the cat. J Physiol. 1980;298:159–69. doi: 10.1113/jphysiol.1980.sp013073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrett JR, Howard ER, Jones W. The internal anal sphincter in the cat: a study of nervous mechanisms affecting tone and reflex activity. J Physiol. 1974;243(1):153–66. doi: 10.1113/jphysiol.1974.sp010747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridolfi TJ, Tong WD, Takahashi T, et al. Sympathetic and Parasympathetic Regulation of Rectal Motility in Rats. J Gastrointest Surg. 2009 doi: 10.1007/s11605-009-0999-z. [DOI] [PubMed] [Google Scholar]
- 25.Ariga H, Imai K, Chen C, et al. Fixed feeding potentiates interdigestive gastric motor activity in rats: importance of eating habits for maintaining interdigestive MMC. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G655–9. doi: 10.1152/ajpgi.00484.2007. [DOI] [PubMed] [Google Scholar]
- 26.Rostad H. Colonic motility in the cat. II. Extrinsic nervous control. Acta Physiol Scand. 1973;89(1):91–103. doi: 10.1111/j.1748-1716.1973.tb05500.x. [DOI] [PubMed] [Google Scholar]
- 27.Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol. 1991;260(1 Pt 2):R200–7. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- 28.Olsson C, Holmberg A, Holmgren S. Development of enteric and vagal innervation of the zebrafish (Danio rerio) gut. J Comp Neurol. 2008;508(5):756–70. doi: 10.1002/cne.21705. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi T, Owyang C. Regional differences in the nitrergic innervation between the proximal and the distal colon in rats. Gastroenterology. 1998;115(6):1504–12. doi: 10.1016/s0016-5085(98)70029-0. [DOI] [PubMed] [Google Scholar]
- 30.Hasler WL, Kurosawa S, Chung OY. Regional cholinergic differences between distal and proximal colonic myenteric plexus. Am J Physiol. 1990;258(3 Pt 1):G404–10. doi: 10.1152/ajpgi.1990.258.3.G404. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa M, Mibu R, Iwamoto T, et al. Change in colonic motility after extrinsic autonomic denervation in dogs. Dig Dis Sci. 1997;42(9):1950–6. doi: 10.1023/a:1018827613809. [DOI] [PubMed] [Google Scholar]
- 32.Fukai K, Fukuda H. The intramural pelvic nerves in the colon of dogs. J Physiol. 1984;354:89–98. doi: 10.1113/jphysiol.1984.sp015364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hedlund H, Fasth S, Hulten L. Efferent sympathetic nervous control of rectal motility in the cat. Acta Physiol Scand. 1984;121(4):317–24. doi: 10.1111/j.1748-1716.1984.tb07462.x. [DOI] [PubMed] [Google Scholar]
- 34.Bennett MR. Rebound excitation of the smooth muscle cells of the guinea-pig taenia coli after stimulation of intramural inhibitory nerves. J Physiol. 1966;185(1):124–31. doi: 10.1113/jphysiol.1966.sp007975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bywater RA, Holman ME, Taylor GS. Atropine-resistant depolarization in the guinea-pig small intestine. J Physiol. 1981;316:369–78. doi: 10.1113/jphysiol.1981.sp013794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manber L, Gershon MD. A reciprocal adrenergic-cholinergic axoaxonic synapse in the mammalian gut. Am J Physiol. 1979;236(6):E738–45. doi: 10.1152/ajpendo.1979.236.6.E738. [DOI] [PubMed] [Google Scholar]
- 37.Takaki M, Neya T, Nakayama S. Sympathetic activity in the recto-rectal reflex of the guinea pig. Pflugers Arch. 1980;388(1):45–52. doi: 10.1007/BF00582627. [DOI] [PubMed] [Google Scholar]
- 38.Neya T, Takaki M, Nakayama S. Mechanism of rectal contraction mediated by sympathetic efferents from rectoanal pelvic afferents in guinea pigs. Acta Med Okayama. 1984;38(1):21–7. doi: 10.18926/AMO/30367. [DOI] [PubMed] [Google Scholar]
- 39.Carlstedt A, Fasth S, Hulten L, Nordgren S. The sympathetic innervation of the internal anal sphincter and rectum in the cat. Acta Physiol Scand. 1988;133(3):423–31. doi: 10.1111/j.1748-1716.1988.tb08425.x. [DOI] [PubMed] [Google Scholar]
- 40.Venkova K, Krier J. Stimulation of lumbar sympathetic nerves evokes contractions of cat colon circular muscle mediated by ATP and noradrenaline. Br J Pharmacol. 1993;110(3):1260–70. doi: 10.1111/j.1476-5381.1993.tb13951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.