Abstract
The hippocampus, a major site of neurogenesis in the adult brain, plays an important role in memory. Based on earlier observations where exposure to high-intensity noise not only caused hearing loss but also impaired memory function, it is conceivably that noise exposure may suppress hippocampal neurogenesis. To evaluate this possibility, nine rats were unilaterally exposed for 2 h to a high-intensity, narrow band of noise centered at 12 kHz at 126 dB SPL. The rats were also screened for noise-induced tinnitus, a potential stressor which may suppress neurogenesis. Five rats developed persistent tinnitus-like behavior while the other four rats showed no signs of tinnitus. Age-matched sham controls showed no signs of hearing loss or tinnitus. The inner ear and hippocampus were evaluated for sensory hair cell loss and neurogenesis 10 weeks post-exposure. All noise exposed rats showed severe loss of sensory hair cells in the noise-exposed ear, but essentially no damage in the unexposed ear. Frontal sections from the hippocampus were immunolabeled for doublecortin to identify neuronal precursor cells, or Ki67 to label proliferating cells. Noise-exposed rats showed a significant reduction of neuronal precursors and fewer dividing cells as compared to sham controls. However, we could not detect any difference between rats with behavioral evidence of tinnitus versus rats without tinnitus. These results show for the first time that high intensity noise exposure not only damages the cochlea but also causes a significant and persistent decrease in hippocampal neurogenesis that may contribute to functional deficits in memory.
Keywords: doublecortin, Ki67, hearing loss, tinnitus, memory, subgranular zone
The hippocampus is a major site of neurogenesis in the adult brain (Altman and Das, 1965, Kaplan and Hinds, 1977, Kuhn et al., 1996). Approximately 9000 new cells are born each day in the rat hippocampus; most cells differentiate into neurons, form synapses and generate electrical responses (Kaplan and Bell, 1984, Cameron et al., 1993, Hastings and Gould, 1999, Cameron and McKay, 2001, Song et al., 2002, van Praag et al., 2002). The hippocampus is known to play important roles in learning, memory, mood, and spatial navigation (Squire, 1982, Gould et al., 1999, Moscovitch et al., 2005, Becker and Wojtowicz, 2007). Recent studies have shown a link between hippocampal neurogenesis and memory (Kempermann, 2002b, Aimone et al., 2006, Leuner et al., 2006; Ehninger and Kempermann, 2008), particularly the consolidation and retention of long-term memory and trace-fear conditioning (Shors et al., 2002, Snyder et al., 2005, Winocur et al., 2006).
Neurogenesis is upregulated by environmental enrichment, learning, exercise and brain injury, and may play a role in central plasticity and recovery of function (Kempermann et al., 1997, Jin et al., 2001, Brown et al., 2003a, Prickaerts et al., 2004, Ernst et al., 2006, Olson et al., 2006, Taupin, 2006, Uda et al., 2006, Brene et al., 2007, Ploughman, 2008). In contrast, anti-cancer drugs, irradiation and alcohol suppress neurogenesis (Crews et al., 2006, Dietrich et al., 2006, Duffner, 2006, Nixon, 2006). In animal models of depression, neurogenesis is decreased and conversely, treatment with anti-depressant drugs increases neurogenesis (Kempermann 2002a, Campbell and Macqueen, 2004, Duman, 2004, Chen et al., 2006, Paizanis et al., 2007, Sahay et al., 2007, Vollmayr et al., 2007, Pittenger and Duman, 2008). Chronic exposure to stressors such as isolation, crowding, or unpredictable aversive conditions such as immobilization or forced swimming depress neurogenesis (Joels et al., 2004, Lucassen et al., 2006, Warner-Schmidt and Duman, 2006).
Rats exposed to high intensity blast waves showed cognitive deficits in behavioral testing (Cernak et al., 2001, Saljo et al., 2009). Recent data from combat personnel exposed to excessive noise levels, explosions and blast waves show not only severe hearing loss and tinnitus (Cave et al., 2007) but also cognitive and memory impairment (Myers et al., 2008, Belanger et al., 2009). The hippocampus responds to auditory stimuli (Bickford-Wimer et al., 1990, Ehlers et al., 1994, Xi et al., 1994, Sakurai, 2002) and is thus directly affected by noise. Intense noise levels have been shown to cause cell death of granule and pyramidal neurons (Saljo et al., 2002), and alter the firing patterns of hippocampal place cells (Goble et al., 2009). In this study, we wanted to determine if intense noise may impair hippocampal neurogenesis.
Subjective tinnitus, a phantom auditory sensation (e.g., ringing, buzzing) is strongly correlated with noise-induced hearing loss, stress and depression (Halford and Anderson, 1991, Dobie et al., 1992, Henry and Wilson, 1995, Folmer et al., 1999, Nicolas-Puel et al., 2002, Holgers, 2003) and may therefore be able to suppress neurogenesis. The emotions and memories evoked by severe tinnitus have been postulated to involve the hippocampus and the limbic system. Human brain-imaging studies showing changes in hippocampal activity by tinnitus or tinnitus-like acoustic stimuli provide support for this hypothesis (Jastreboff and Hazell, 1993, Lockwood et al., 1998, Andersson et al., 2000, Mirz et al., 2000, Jastreboff and Jastreboff, 2007). Moreover, a decrease in hippocampal grey matter has been observed in tinnitus patients (Landgrebe et al., 2009), possibly related to impaired neurogenesis. Thus, noise exposure could affect hippocampal neurogenesis indirectly through noise-induced tinnitus and stress.
To evaluate the hypothesis that noise exposure or noise-induced tinnitus may affect hippocampal neurogenesis, we unilaterally exposed rats to a high intensity noise designed to damage the inner ear and induce tinnitus-like behavior in some but not all animals. The microtubule-associated protein doublecortin (DCX) and the mitosis marker Ki67 were used as tools for visualization of neurogenesis. DCX is expressed in neuronal precursor cells, but not in glial cells nor in neural stem cells from which the precursors develop. Upon migration and maturation into functional neurons, DCX is down-regulated (Brown et al., 2003b, Couillard-Despres et al., 2005). Ki67, a marker for cell proliferation, is expressed during all active phases of the cell cycle but is absent in resting cells (Scholzen and Gerdes 2000).
Experimental Procedures
Animals
Twelve adult male Sprague-Dawley rats (Sasco, Charles River Laboratories International, Inc., Wilmington, MA) were used for this study. Rats used for DCX and Ki67 immunolabeling were divided into two groups, Sham Controls (N=3) and Noise Trauma rats (N=9 for DCX staining and N=5 for Ki67 staining). To determine if noise trauma caused persistent changes in neurogenesis, Noise Trauma rats were unilaterally exposed to noise, screened for tinnitus, and sacrificed 10 weeks after noise exposure. Sham Controls underwent the same kind of treatment (i.e. tinnitus screening procedures and anesthetic exposure) except for noise trauma. The age of controls and experimental rats was matched: On day of sacrifice the average age was 174.3 ± 2.7 days for Sham Controls, 189.4 ± 7.8 days for Noise Trauma rats used for DCX immunolabeling, and 187.4 ± 10.9 days for Noise Trauma rats used for Ki67 labeling.
All animal procedures were carried out in accordance with the NIH guidelines for use and care of laboratory animals and approved by the University of Buffalo Institutional Animal Care and Use Committee (protocol HER05080Y).
Noise exposure
Under isofluorane anesthesia (4% induction, 1.5-2% maintenance), rats were unilaterally exposed to narrowband noise (bandwidth 100 Hz) centered at 12 kHz, 126 dB SPL for 2 hours. Sound stimuli were generated by Tucker-Davis Technology (TDT) hardware and presented by a super compression driver (D-59; GMI Sound Corp) positioned 10 mm from the opening of the rat's left ear. The SPL was calibrated with a sound level meter coupled to a half-inch condenser microphone (Model 824 Audiometer, Larson Davis). The contralateral ear was plugged in order to maintain normal hearing in the right ear. The normal hearing ear allowed the animals to respond to the acoustic stimuli used for the tinnitus behavioral tests.
Tinnitus Assessment
Gap-prepulse inhibition of the acoustic startle (GPIAS) was utilized to obtain behavioral evidence of tinnitus as described in our previous publication (Yang et al., 2007). Each rat was positioned in an acoustically transparent wire mesh cage mounted on an acrylic base attached to a sensitive piezoelectric transducer. The sound level within each cage was measured and calibrated (Model 824 sound level meter, one-half inch microphone, Larson Davis). The output of the piezoelectric transducer was sent to a low-pass filter (1000 Hz, LPF-300, World Precision Instruments) and then to an A/D converter on a digital signal processing module (RP2.1, TDT). Using MATLAB software (The MathWorks Inc.) and custom software (generously provided by J. Ison and P. Allen, University of Rochester), the root mean square (RMS) amplitude of the startle response was calculated over a 100 ms interval following the onset of the startle stimulus. Sound stimuli were generated by two digital signal processors (RP2.1, TDT) controlled by custom software, amplified (SA1; TDT), and presented by a high frequency dome tweeter (FT28D, Fostex) located 25 cm above the rat. The startle eliciting stimulus consisted of a broadband noise burst presented at 100 dB SPL (1 to 30 kHz bandwidth, 20 ms duration, 0.1 ms rise/fall time, 100 kHz sampling rate).
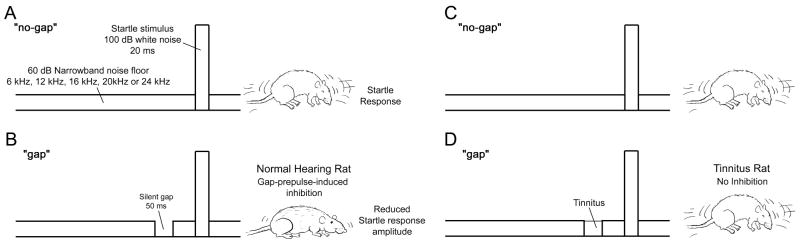
GPIAS
The amplitude of the acoustic startle response was measured in the Noise Trauma rats and Sham Controls under two different test conditions. For GPIAS testing, the startle stimulus was embedded in a continuous background noise (60 dB SPL); the center frequency of the narrow band was varied across GPIAS test conditions. On half the trials, the continuous noise contained a silent gap (50 ms). The onset of the gap preceded the onset of the startle stimulus by 100 ms. The RMS amplitude of the acoustic startle response was measured in the presence of continuous noise with “no gap” (Fig. 1A) or with a “gap” (Fig. 1B). When the gap preceded the startle stimulus, there was a reduction in the amplitude of the startle response in normal hearing rats (Fig. 1A vs. 1B), i.e. the gap serves as a pre-pulse and inhibits the startle response (Ison et al., 2002, Yang et al., 2007). The presence of significant GPIAS indicated that the rat was able to detect the silent gap. In contrast, gap detection and GPIAS were expected to be greatly reduced in rats with severe tinnitus since the phantom sound of tinnitus presumably fills in the silent gap. Therefore in rats with tinnitus, the startle amplitude would no longer differ significantly between the “gap” and “no-gap” conditions (Fig. 1C, D). To estimate the pitch of the tinnitus, GPIAS tests were run with narrow bands of noise (1000 Hz bandwidth) centered at 6, 12, 16, 20 or 24 kHz. For each frequency the test set contained 20 pairs of “no-gap” and “gap” trials, with the test order randomized. The inter-trial interval was randomly varied from 9 to 15 seconds.
Figure 1.
Gap prepulse-induced inhibition of the acoustic startle (GPIAS) paradigm used for tinnitus screening in rats after noise trauma: A noise burst at 100 dB SPL, embedded in a continuous noise floor of moderate SPL (60 dB), results in an acoustic startle response (A). When the background noise floor contains a short gap immediately before the startle-eliciting stimulus, the amplitude of the startle is reduced (B) showing that the rat is able to detect the silent gap by inhibiting its response to the subsequent startle stimulus. A rat with tinnitus however, shows no difference in startle amplitudes in the “no-gap” (C) and “gap” (D) condition, since the perceived phantom sound fills the gap and thus prevents the rat from detecting the silent gap thereby eliminating pre-pulse inhibition of the startle response.
Ten GPIAS tests were run during a 2 week period before the noise exposure (“baseline”). The results from the first three days were considered as acclimation tests and discarded from the analysis. After noise exposure, rats were again tested at 1-10 days post-exposure (“short-term”, 7 test days in total) and then at 8-10 weeks post-exposure (“long-term”, 7 test days in total). Age-matched Sham Controls were tested along with the Noise Trauma rats. For each day of testing, amplitudes were normalized to the average amplitudes at the “no-gap” condition, which were set to 1, and the average amplitudes were presented as mean ± standard error of mean (SEM). For each testing period, the average startle response amplitude in the “gap” condition was compared to the average amplitude in the “no-gap” condition using the two-tailed Student's t-test (P<0.05).
Fixation
On the day of sacrifice, rats used for immunolabeling received a lethal dose of sodium pentobarbital and were transcardially perfused with phosphate buffered saline (PBS, 0.1M, pH 7.4) for 5 minutes and then with 10% phosphate buffered formalin (Fisher Scientific, Pittsburgh, PA) for 15 minutes at room temperature. The brain and the bulla containing the cochleae were removed from the skull. The brains were post-fixed in 10% phosphate buffered formalin for one week.
Cochleograms
The organ of Corti was carefully dissected out of the cochlea as a flat surface preparation and stained with Harris' hematoxylin solution as described in detail in our previous publications (Ding et al., 2001, Ding et al., 2002). The basal, middle and apical turns of the organ of Corti were carefully removed, mounted on glass slides in glycerin, coverslipped and examined from base to apex under a microscope (Zeiss Standard, 400X). Cochleograms were prepared showing percent missing outer hair cells (OHC) and inner hair cells (IHC) as a function of percent distance from apex of the cochlea using lab norms. Hair cells were counted in 0.24 mm intervals along the entire length of the cochlea. Hair cells were counted as present when the cell body and cuticular plate were intact.
Immunohistochemistry
Brain tissue containing the left or right hippocampus was immersed in 30% sucrose in 0.1 M PBS pH 7.4 (Sigma-Aldrich) overnight at 4° C. The following day the tissue was cut on a cryostat into 30 μm frontal sections at -30 °C. Free floating sections were collected and washed in 0.1 M PBS, pH 7.4, pretreated with H2O2 for peroxidase deactivation, and then pre-incubated in blocking buffer containing 10% normal horse serum and 0.05% Triton X-100 in 0.1M PBS, pH 7.4 for 30 minutes at room temperature. Subsequently, sections were exposed for 2 h at room temperature to an antibody against DCX (polyclonal; made in goat; sc-8066; Santa Cruz Biotechnology, Inc; 2 μg/ml) or against Ki67 (polyclonal, made in rabbit; Novocastra Laboratories Ltd, UK; 1μl/ml) in 0.1 M PBS, pH 7.4 with 1.0% normal horse serum and 0.05% Triton. DCX or Ki67 was visualized through the indirect staining method utilizing a biotinylated secondary antibody (anti-goat IgG or anti-rabbit IgG, Vector Laboratories, Burlingame, CA), the avidin-biotin-peroxidase complex technique (Elite-ABC, Vector Laboratories), and diaminobenzidine tetrahydrochloride (DAB, 0.05%, Sigma-Aldrich) with nickel ammonium sulfate (0.3%) and H2O2 (0.0015%) in 0.1 M Tris buffer, pH 7.2 (Trizma-base, Sigma). The secondary antibody and ABC incubation steps lasted for 1 h each at room temperature. Between all incubation steps, sections were washed in 0.1 M PBS, pH 7.4. After staining, sections were washed in PBS and mounted on gelatin-coated slides (SuperFrost plus; Fisher Scientific) and dried overnight. Then sections were dehydrated in increasing concentrations of ethanol, cleared in xylene and sealed with DPX.
Photomicrographs, quantification and statistical analysis
Images visualized under bright field illumination (Axioskop, Carl Zeiss Inc) were photographed using a digital camera (SPOT Insight, Diagnostic Instruments Inc) and processed with imaging software (SPOT Software, version 4.6) into 8-bit grayscale. Assembly of images was done with Adobe Photoshop 5.5. Statistical evaluations were done with GraphPad Prism (Version 5.01; GraphPad Software Inc).
Cell bodies in the subgranular zone (SGZ) immunopositive for DCX or Ki67 were counted at high magnification (400X). A cell immunopositive for DCX was counted when the cell body was recognizable as such by morphology, either by a clearly visible nucleus or by size and shape characteristic for this type of cell. Specific-stained cells were clearly darker than its surroundings, and with cytoplasm homogenously stained. Cells immunopositive for Ki67 were recognized by a dark globular or oval structure with size comparable to the nucleus. Immunopositive cells in the SGZ in the dorsal dentate gyrus were counted in sections throughout the rostral-caudal axis of the hippocampus (-2.0 to -5.6 mm from bregma; Paxinos and Watson, 2004). For DCX quantification, cells were counted from 20 sections from the left or right side. For every section, the number of cell profiles was divided by the length of the SGZ to obtain cell density. Average cell densities were then normalized to the numbers obtained in Sham Controls, which were set to 100% (1.0 in figures). For Ki67 quantification, cells were counted in at least 25 sections from each animal and the number divided by the length of the SGZ. Average Ki67 cell densities were based on the median of 20 or 21 values in the samples. All numbers are presented as averages ± SEM and statistical comparisons were performed with the two-tailed Student's t-test (P<0.05).
Results
Inner ear damage after unilateral noise trauma
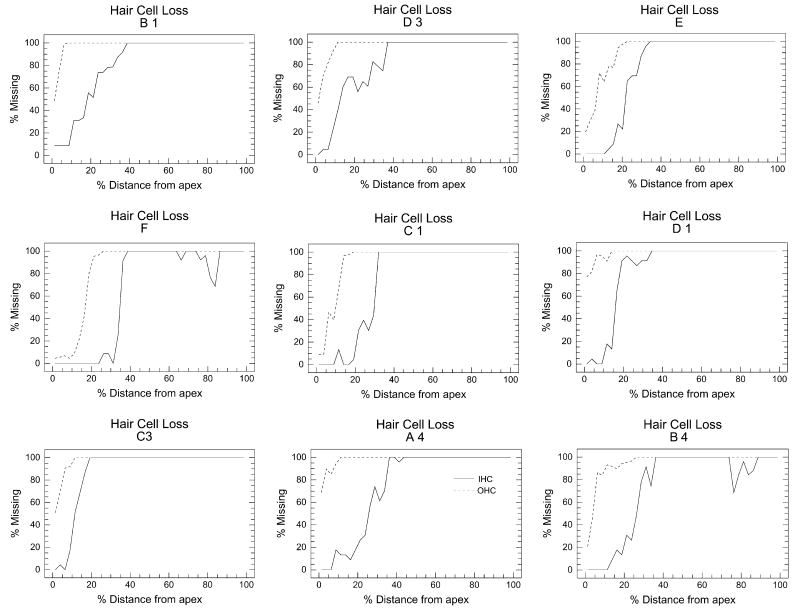
All rats unilaterally exposed to the 126 dB SPL noise showed nearly complete loss of OHC and IHC in the exposed ear between 20% and 100% distance from the apex of the cochlea (Fig. 2). This region corresponds roughly from 2 to > 50 kHz on the rat cochlear frequency-place map (Müller, 1991, Greenwood, 1996). OHC loss extended further towards the apex than IHC. The pattern and extent of OHC and IHC damage showed relatively little variability across rats. Importantly, contralateral ears of all noise exposed rats showed no signs of damage (data not shown).
Figure 2.
Cochleograms showing degree of outer hair cell (OHC, dashed line) and inner hair cell (IHC, solid line) loss as function of percent distance from the apex of the noise exposed cochlea (126 dB, 100 Hz narrowband noise centered at 12 kHz, 2 hours) in nine rats allowed to survive for 10 weeks. All nine Noise Trauma rats showed severe loss of both IHC and OHC throughout most of the cochlea except for the most apical, low-frequency region. OHC loss was greater than IHC loss.
Tinnitus screening
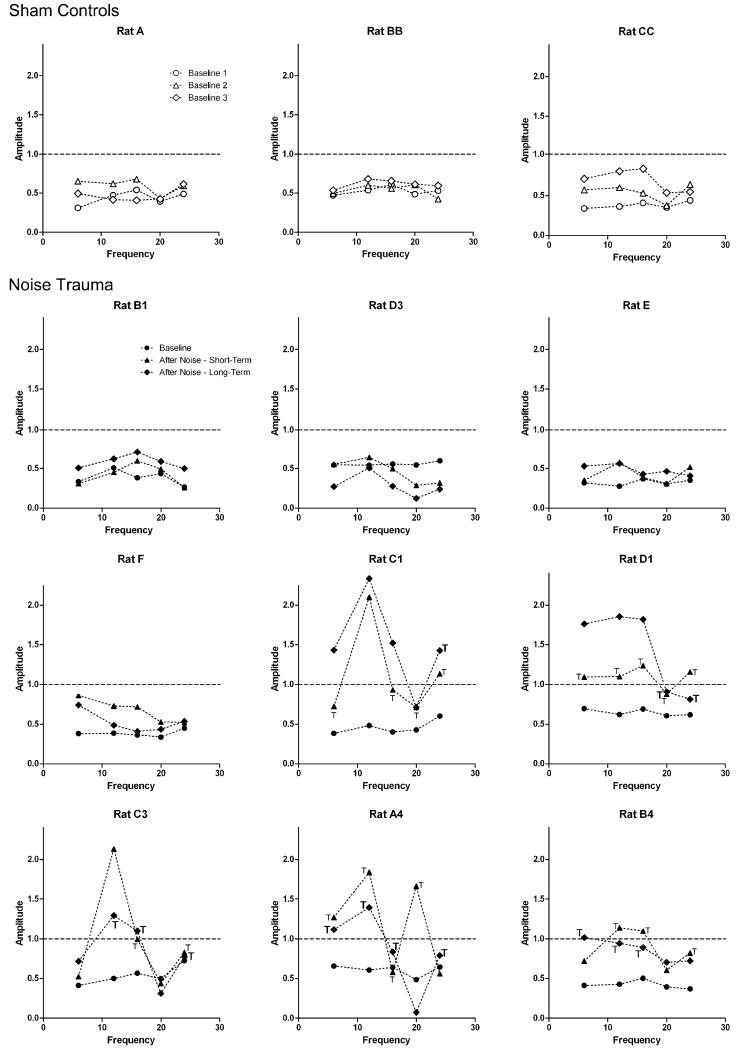
Noise Trauma rats and Sham Controls showed significant GPIAS before noise exposure (Baseline; Fig. 3; Circles). These results indicated that the rats could detect the silent gap and were not experiencing tinnitus, consistent with previous research (Turner et al., 2006, Yang et al., 2007). The differences in startle amplitudes between the “no-gap” and “gap” conditions (Fig. 1) were significant at all frequencies (P < 0.05). However, the extent of GPIAS shown by startle amplitude reduction in percent varied among rats from approximately 30% to 60%.
Figure 3.
Gap pre-pulse induced inhibition of the acoustic startle (GPIAS) in Sham Controls (open symbols) and Noise Trauma rats (filled symbols): For each rat, frequency, and testing period, the diagrams show the average amplitude at the “gap” condition, relative to the average amplitude at the “no-gap” condition, which was normalized and set to 1 (horizontal dashed line). In case of gap detection, the average amplitude at the “gap” condition was significantly smaller than the amplitude at the “no gap” condition (inhibition). Circles: Before any treatment, all rats showed significant inhibition at the “gap” condition at all frequencies tested (“Baseline”). Average inhibition ranged from approximately 30% to 60% and varied among individual rats, but was significant at all frequencies tested for all rats. Triangles: At days 1 – 10 after noise exposure, five Noise Trauma rats (Rat C1, D1, C3, A4 and B4) showed changes in gap detection. At one or more frequencies tested, these rats now showed no significant difference in amplitudes at “no-gap” conditions and “gap” conditions (“T”), demonstrating no or only very poor gap-detection and thus signs of severe tinnitus. Some of these rats showed a significant increase of amplitudes (facilitation) which may be related to increased stimulus aversiveness. The other four Noise Trauma rats (B1, D3, E and F), along with age-matched Sham Controls (A, BB and CC), continued showing significant inhibition at all frequencies. Diamonds: When rats were re-tested at week 8-10 after the noise exposure, the five Noise Trauma rats with tinnitus-like behavior during days 1-10 again showed evidence of tinnitus (bold “T”) and facilitation. The other four Noise Trauma rats and Sham Controls continued showing significant inhibition at all frequencies.
After noise exposure, the Noise Trauma rats and Sham Controls were tested for a period from day 1 to day 10 post-exposure for “short term” effects (Fig. 3; Triangles) and re-tested from week 8 to week 10 for “long-term” effects (Fig. 3; Diamonds). The age-matched, normal hearing Sham Controls continued to show significant GPIAS at both testing periods and at all frequencies. Rats in the Noise Trauma group showed varying outcomes. In both post-noise testing periods, four rats (B1, D3, E and F) continued showing significant GPIAS at all frequencies similar to the results from Sham Control rats, and thus no signs of tinnitus. Five animals (C1, D1, C3, A4 and B4) showed a lack of GPIAS at one or more frequencies. There was no significant difference in startle amplitudes in “no-gap” versus “gap” conditions at one or more frequencies in these rats (P > 0.05). Therefore rats C1, D1, C3, A4 and B4 were considered to have behavioral evidence of tinnitus (Turner et al., 2006, Yang et al., 2007). Additionally, there was one other change after the noise exposure. At the lower frequencies, particularly 12 kHz (i.e., the exposure frequency), rats C1, C3 and D1 showed a significant increase in startle amplitudes during the “gap” condition as compared to the “no-gap” condition, that is, the gap signal enhanced the response to the startle stimulus (facilitation) instead of reducing it. Rat A4 and B4 showed a similar tendency, but it was not significant. These changes may possibly be related to loudness recruitment or a collapse of sound tolerance, which sometimes accompanies tinnitus, and which may increase stimulus aversiveness (Sun et al. 2009). This facilitation-like behavior was only observed in rats with behavioral evidence of tinnitus.
Neuronal precursor cells and cell proliferation
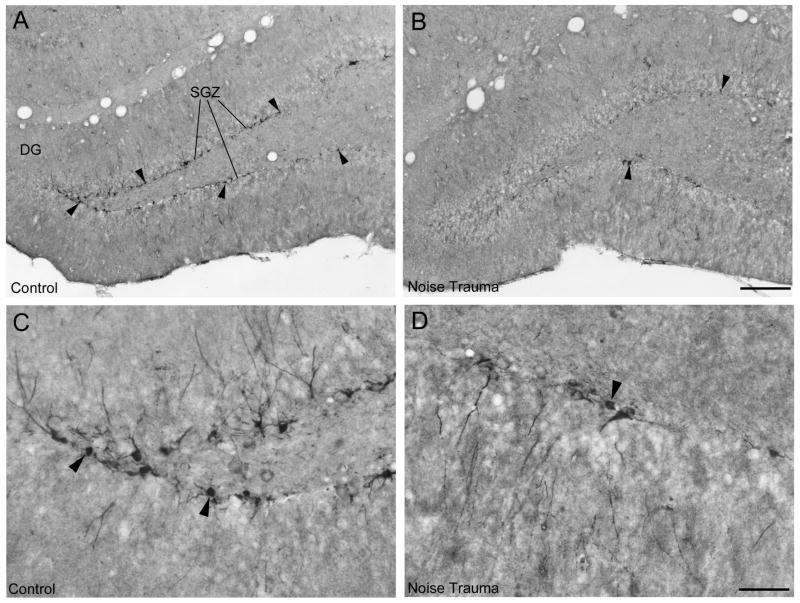
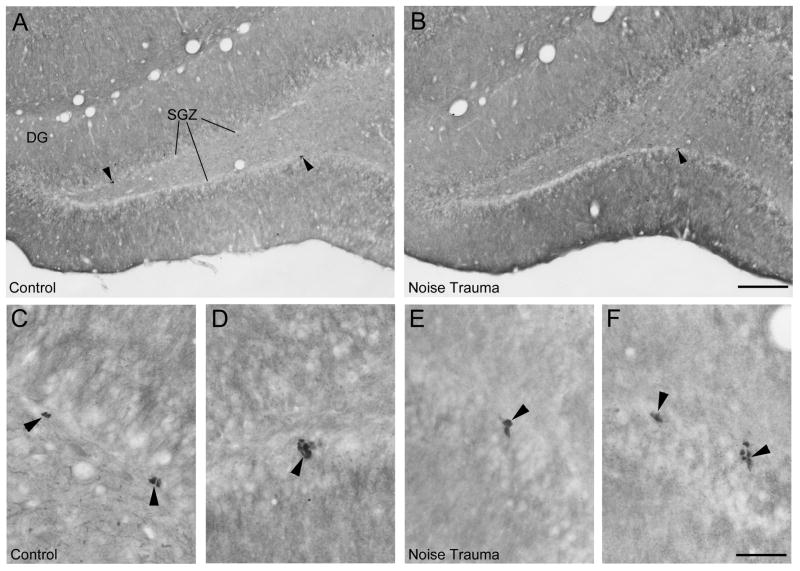
Figure 4 shows DCX immunopositive neuronal precursor cells and Figure 5 shows Ki67 immunopositive nuclei of proliferating cells in the SGZ in the dorsal dentate gyrus. In the normal hearing Sham Controls, DCX positive cell bodies form a continuous or almost continuous band along the SGZ with vertically extending dendrites (Fig. 4A, C). After noise trauma, the number of DCX-positive cells was greatly reduced (Fig. 4B). There were only a few Ki67 positive cell nuclei in each section in Sham Controls (Fig. 5A) and Noise Trauma rats (Fig. 5B), requiring cell counts throughout a high number of sections to detect any difference between normal hearing Sham Controls and Noise Trauma rats. Ki67 immunolabeled nuclei in both Sham Controls (Fig. 5C, D) and Noise Trauma rats (Fig. 5E, F) tended to occur in pairs or small cluster consistent with previous hippocampal observations (Wang and Baraban, 2007).
Figure 4.
Neuronal precursor cells immunostained for DCX in the adult rat hippocampus in a normal hearing rat (A) and a rat with severe noise trauma 10 weeks after the noise exposure (B). A: In the normal rat, cell bodies of neuronal precursor cells (arrowheads) form a thin line along the subgranular cell zone (SGZ) in the dentate gyrus (DG). B: The noise exposed rat showed a strongly reduced number of neuronal precursor cells. Arrowheads point to some of the few remaining cells. C, D: Single DCX neuronal precursor cells shown at high magnification in the normal hearing rat (C) and in the rat with noise trauma (D). Scale bars: 200 μm in Fig. 4B for Fig. 4A, B; 50 μm in Fig. 4D for Fig. 4C, D.
Figure 5.
Dividing cells immunostained for Ki67 in the adult rat hippocampus in a normal hearing rat (A, C, D) and a rat with severe noise trauma 10 weeks after the noise exposure (B, E, F). A, B: In the normal rat as well as the noise-exposed rat, Ki67-immunopositive nuclei (arrowheads) are present in the subgranular cell zone (SGZ) in the dentate gyrus (DG). Ki67 immunopositive cells were typically clustered in pairs or small groups in normal hearing rats (5C, D) as well as in noise exposed rats (5E, F). Scale bars: 200 μm in Fig. 5B for Fig. 5A, B; 50 μm in Fig. 5C for Fig. 5C-F.
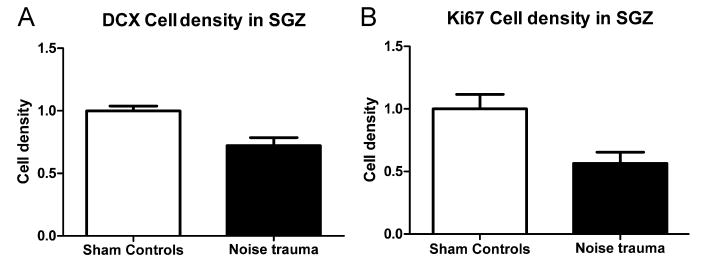
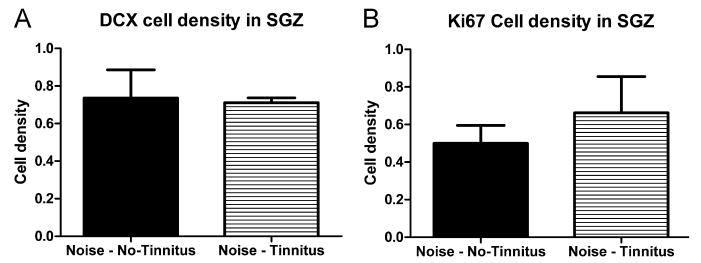
Rats in the Noise Trauma group showed a reduction in DCX immunolabeling (Fig. 4A versus B). To determine whether unilateral noise trauma exerted an asymmetrical effect on hippocampal neurogenesis, DCX cell density in the left and right hippocampus were compared. As there was no difference between counts from the left and from the right hippocampus in any of the groups (P > 0.05) counts from the left and right hippocampus were pooled. To determine whether noise trauma significantly reduced hippocampal neurogenesis, DCX cell density was compared between the Noise Trauma rats and Sham Controls. In Noise Trauma rats, the DCX cell population was significantly reduced to 72.2% ± 6.3% (P = 0.036) of Sham Control values (Fig. 6A). To determine if tinnitus was a factor regulating neurogenesis, DCX density was compared in noise trauma rats with and without behavioral evidence of tinnitus (Fig. 7A). The average DCX cell density in Noise Trauma rats with tinnitus (71.1% ± 2.6%) was similar to the density in Noise Trauma rats without tinnitus (73.6% ± 15.0%), and there was no significant difference (P = 0.86).
Figure 6.
Reduction of density of DCX labeled cells (A) and Ki67 labeled cells (B) in the hippocampus after noise trauma. Numbers were normalized to average number of cells in Sham Controls (white bars), which was set to 1. Noise Exposed rats (black bars) showed a strong and significant reduction in numbers of DCX positive cells as well as Ki67 positive cells.
Figure 7.
DCX (A) and Ki67 (B) cell density in the SGZ after Noise Trauma in rats without signs of tinnitus and in rats with signs of tinnitus: We were not able to detect any difference in cell numbers between the two groups.
Rats in the Noise Trauma group showed a reduction in Ki67 immunolabeling compared to Sham Controls (Fig. 5A, B). To determine if cell proliferation was significantly reduced by the unilateral noise exposure, Ki67 cell density was compared in Noise Trauma rats versus Sham controls. In Noise Trauma rats the average Ki67 cell density was significantly reduced to 56.5% ± 9.0% (P = 0.025) of Sham Control rats (Fig. 6B). To determine if tinnitus was a factor regulating cell proliferation, Ki67 cell density was compared in noise trauma rats with and without behavioral evidence of tinnitus (Fig. 7B). Again, there was no significant difference in Ki67 cell density between Noise Trauma rats with tinnitus (66.2% ± 19.3%) and Noise Trauma rats without tinnitus (50.0% ± 9.6%; P = 0.45).
Discussion
High intensity noise exposure has long been known to cause hearing loss by damaging the sensory hair cells and supporting cells in the inner ear (Bohne, 1977, Hamernik et al., 1984). However, much is still unknown about its effects on the central nervous system, particularly in regions outside the classical auditory pathway. Our results show for the first time that acoustic overstimulation (126 dB SPL, 2 h, 12 kHz) causes a long lasting suppression of hippocampal neurogenesis in adult rats. On average, the number of DCX cells was reduced by almost 30% and the number of Ki67 cells by more than 40% 10 weeks after the noise trauma. This effect was seen in all noise exposed rats: we did not detect any difference in DCX or Ki67 cell density between rats with or without evidence of tinnitus-like behavior.
Noise exposure impairs neurogenesis
Soldiers and other personnel exposed to extremely high level noise (∼170 dB SPL; such as blast waves or explosions) often suffer from cognitive and memory impairments that are often associated with traumatic brain injury (TBI) (Rimel et al., 1981, Cernak et al., 2001, Belanger et al., 2009). Similarly, rats exposed to shock waves at 10 kPa (174 dB SPL) also demonstrate poor cognitive function on the Morris water maze test, which is most likely the result of direct injury to the brain caused by the acoustic overpressure (Saljo et al., 2009). Our results demonstrate that continuous noise (126 dB SPL, 40 Pa), much less intense than the blast waves demonstrated to cause TBI, but of longer duration, causes significant, long-term suppression of neurogenesis. Given that hippocampal neurogenesis may be related to memory (Snyder et al., 2005, Aimone et al., 2006, Winocur et al., 2006; Becker and Wojtowicz, 2007), we speculate that our noise-exposed rats would show cognitive, mood or memory impairments, and that noise at intensities much lower than those causing TBI may impair memory funtion.
Mechanism for reduced neurogenesis after noise exposure
The decrease in hippocampal neurogenesis following acoustic overstimulation may arise through different mechanisms. Noise trauma could persistently suppress cell proliferation thereby reducing neurogenesis. Our results showed a reduced number of Ki67-labeled cells as well as DCX-labeled precursor cells in Noise Trauma rats. A similar connection between total number of neuronal precursors and rate of cell-proliferation has been observed in earlier studies (Couillard-Despres et al., 2005; Kim et al., 2009), suggesting that a reduced number of precursors is often directly linked to a reduced rate of cell proliferation. Alternatively, DCX cell numbers could be reduced directly by stimuli-induced cell death, as observed for granule and pyramidal neurons after exposure to extremely high levels of impulse noise (Saljo et al., 2002), or after treatment with anti-cancer drugs like cisplatin (Dietrich et al., 2006). However, the neuronal precursor cell population fully recovers a few weeks after termination of drug treatment; therefore it seems unlikely that reduced neurogenesis seen as late as 10 weeks post-exposure is related to noise-induced cell death alone. A reduced number of neuronal precursors may also be caused by a shift of differentiation from a neuronal lineage toward a glial lineage, thereby reducing the number of DCX cells, as seen after treatment with sedatives and anticonvulsant drugs (Stefovska et al. 2008). However, since also Ki67 immunolabeling showed a large reduction in cell proliferation, altered differentiation seems unlikely to play a major role after noise exposure.
Neurogenesis can be down-regulated through neuronal hyperactivity in the hippocampus. In the case of temporal lobe epilepsy, a condition of neuronal hyperactivity, neurogenesis increases during the acute phase, but decreases in the chronic phase (Hattiangady et al., 2004, Kuruba et al., 2009). Chronic down-regulation of neurogenesis after noise exposure may be triggered by a similar mechanism. Noise exposure can cause acute (Puel et al., 1998) as well as long-term (Syka et al., 1994; Salvi et al., 2000) hyperactivity in the auditory system. Since the hippocampus responds to auditory stimuli (Bickford-Wimer et al., 1990, Ehlers et al., 1994, Xi et al., 1994, Sakurai, 2002), exposure to high intensity noise may be able to cause hyperactivity not only in the auditory system but also in the hippocampus, and cause a chronic decrease of neurogenesis. Alternatively, neurogenesis may be impaired by the unilateral noise-induced hearing loss which changes the afferent neuronal input to the brain. Intense noise-exposure was recently shown to alter the firing patterns of hippocampal place cells (Goble et al., 2009). Early onset age-related cochlear degeneration has been associated with a reduced number of hippocampal synapses and impaired spatial memory (Dai et al., 2009). In all cases, the reduced neurogenesis observed in this study would be related to abnormal auditory input to the brain, either hyperactivity during noise exposure or altered activity due to hearing loss.
Finally, neurogenesis may be reduced by stress related to hearing loss. Impaired hearing may cause aversive conditions for the animal and thus act as a chronic stressor, which in turn may down-regulate neurogenesis. Such aversive conditions may include impaired perception of the acoustic environment or increased stimulus aversiveness. We also considered tinnitus as a potential stressor, but our data obtained with the gap-startle method do not support any effect of tinnitus. Nevertheless, the results cannot rule out the possibility that tinnitus may be capable of suppressing neurogenesis under other conditions. One reason why tinnitus may not have caused a significant decrease in cell proliferation or neurogenesis in our study is that the tinnitus effect may have been masked by the stronger effects of the noise exposure itself. Alternatively, our behavioral test for tinnitus may not be sensitive enough to detect tinnitus in all of the rats with noise induced hearing loss.
Conclusions and outlook
The results presented here are the first to demonstrate that noise exposure not only damages the peripheral auditory system, but also causes a major, long-term reduction of hippocampal neurogenesis. Thus, hearing protection or noise avoidance may not only protect the ear, but also the hippocampus, which is linked to memory and emotion. Because noise-induced hearing loss is extremely prevalent in industrialized societies, an important question that needs to be answered is what level of noise or hearing loss is sufficient to suppress hippocampal neurogenesis. Moreover, understanding the mechanisms on how noise may impair hippocampal neurogenesis may be important for developing treatments for recovery of memory function after noise trauma.
Acknowledgments
We thank Dr. Ison and Dr. Allen at the University at Rochester for generously sharing the custom software for startle reflex testing. This project was supported in part by grants from NIH (R01DC00909101; 1R01DC009219-01) and Tinnitus Research Initiative.
List of abbreviations
- DCX
Doublecortin
- GPIAS
Gap prepulse-induced inhibition of the acoustic startle
- IHC
Inner hair cells
- OHC
Outer hair cells
- PBS
Phosphate buffered saline
- RMS
Root mean square
- SGZ
Subgranular zone
- SPL
Sound pressure level
- TBI
Traumatic brain injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Andersson G, Lyttkens L, Hirvela C, Furmark T, Tillfors M, Fredrikson M. Regional cerebral blood flow during tinnitus: a PET case study with lidocaine and auditory stimulation. Acta Otolaryngol. 2000;120:967–972. doi: 10.1080/00016480050218717. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Becker S, Wojtowicz JM. A model of hippocampal neurogenesis in memory and mood disorders. Trends Cogn Sci. 2007;11:70–76. doi: 10.1016/j.tics.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Belanger HG, Kretzmer T, Yoash-Gantz R, Pickett T, Tupler LA. Cognitive sequelae of blast-related versus other mechanisms of brain trauma. J Int Neuropsychol Soc. 2009;15:1–8. doi: 10.1017/S1355617708090036. [DOI] [PubMed] [Google Scholar]
- Bickford-Wimer PC, Nagamoto H, Johnson R, Adler LE, Egan M, Rose GM, Freedman R. Auditory sensory gating in hippocampal neurons: a model system in the rat. Biol Psychiatry. 1990;27:183–192. doi: 10.1016/0006-3223(90)90648-l. [DOI] [PubMed] [Google Scholar]
- Bohne BA. Growth of cochlear damage with increasing severity of exposure. Trans Am Acad Ophthalmol Otolaryngol. 1977;84:420–421. [PubMed] [Google Scholar]
- Brene S, Bjornebekk A, Aberg E, Mathe AA, Olson L, Werme M. Running is rewarding and antidepressive. Physiol Behav. 2007;92:136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003a;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003b;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- Cave KM, Cornish EM, Chandler DW. Blast injury of the ear: clinical update from the global war on terror. Mil Med. 2007;172:726–730. doi: 10.7205/milmed.172.7.726. [DOI] [PubMed] [Google Scholar]
- Cernak I, Wang Z, Jiang J, Bian X, Savic J. Cognitive deficits following blast injury-induced neurotrauma: possible involvement of nitric oxide. Brain Inj. 2001;15:593–612. doi: 10.1080/02699050010009559. [DOI] [PubMed] [Google Scholar]
- Chen H, Pandey GN, Dwivedi Y. Hippocampal cell proliferation regulation by repeated stress and antidepressants. Neuroreport. 2006;17:863–867. doi: 10.1097/01.wnr.0000221827.03222.70. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Dai CF, Feng YF, Zhang R, Zhai F, Steyger P. Preliminary study of the relationship between hearing loss and synapses changes in C57BL/6 mouse hippocampus. Abstr Assoc Res Otolaryngol. 2009;32:199. [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, McFadden S, Salvi RJ. Cochlear hair cell densities and inner ear staining techniques. In: Willott J, editor. The auditory psychobiology of the mouse. Boca Raton: CRC Press; 2001. pp. 189–204. [Google Scholar]
- Ding D, McFadden SL, Woo JM, Salvi RJ. Ethacrynic acid rapidly and selectively abolishes blood flow in vessels supplying the lateral wall of the cochlea. Hear Res. 2002;173:1–9. doi: 10.1016/s0378-5955(02)00585-3. [DOI] [PubMed] [Google Scholar]
- Dobie RA, Sullivan MD, Katon WJ, Sakai CS, Russo J. Antidepressant treatment of tinnitus patients. Interim report of a randomized clinical trial. Acta Otolaryngol. 1992;112:242–247. doi: 10.1080/00016489.1992.11665412. [DOI] [PubMed] [Google Scholar]
- Duffner PK. The long term effects of chemotherapy on the central nervous system. J Biol. 2006;5:21. doi: 10.1186/jbiol51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Kaneko WM, Robledo P, Lopez AL. Long-latency event-related potentials in rats: effects of task and stimulus parameters. Neuroscience. 1994;62:759–769. doi: 10.1016/0306-4522(94)90474-x. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Kempermann G. Neurogenesis in the adult hippocampus. Cell Tissue Res. 2008;331:243–250. doi: 10.1007/s00441-007-0478-3. [DOI] [PubMed] [Google Scholar]
- Ernst C, Olson AK, Pinel JP, Lam RW, Christie BR. Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? J Psychiatry Neurosci. 2006;31:84–92. [PMC free article] [PubMed] [Google Scholar]
- Folmer RL, Griest SE, Meikle MB, Martin WH. Tinnitus severity, loudness, and depression. Otolaryngol Head Neck Surg. 1999;121:48–51. doi: 10.1016/S0194-5998(99)70123-3. [DOI] [PubMed] [Google Scholar]
- Goble TJ, Moller AR, Thompson LT. Acute high-intensity sound exposure alters responses of place cells in hippocampus. Hear Res. 2009;253:52–59. doi: 10.1016/j.heares.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- Greenwood D. Comparing octaves, frequency ranges, and cochlear-map curvature across species. Hear Res. 1996;94:157–162. doi: 10.1016/0378-5955(95)00229-4. [DOI] [PubMed] [Google Scholar]
- Halford JB, Anderson SD. Anxiety and depression in tinnitus sufferers. J Psychosom Res. 1991;35:383–390. doi: 10.1016/0022-3999(91)90033-k. [DOI] [PubMed] [Google Scholar]
- Hamernik RP, Turrentine G, Roberto M, Salvi RJ, Henderson D. Anatomical correlates of impulse noise induced mechanical damage to the cochlear. Hear Res. 1984;13:229–247. doi: 10.1016/0378-5955(84)90077-7. [DOI] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. 2004;17:473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Henry JL, Wilson PH. Coping with tinnitus: Two studies of psychological and audiological characteristics of patients with high and low tinnitus-related distress. Int Tinnitus J. 1995;1:85–92. [PubMed] [Google Scholar]
- Holgers KM. Tinnitus in 7-year-old children. Eur J Pediatr. 2003;162:276–278. doi: 10.1007/s00431-003-1183-1. [DOI] [PubMed] [Google Scholar]
- Ison JR, Castro C, Allen P, Virag TM, Walton JP. The relative detectability for mice of gaps having different ramp durations at their onset and offset boundaries. J Accoust Soc Am. 2002;112:740–747. doi: 10.1121/1.1490352. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Hazell JW. A neurophysiological approach to tinnitus: clinical implications. Br J Audiol. 1993;27:7–17. doi: 10.3109/03005369309077884. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Jastreboff MM. Decreased Sound Tolerance. In: Snow J, editor. Tinnitus: Theory and Management. Hamilton, Canada: B.C. Decker Inc; 2007. pp. 8–15. [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, Verkuyl M, Lucassen PJ, Krugers HJ. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–231. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Bell DH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci. 1984;4:1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Regulation of adult hippocampal neurogenesis - implications for novel theories of major depression. Bipolar Disord. 2002a;4:17–33. doi: 10.1034/j.1399-5618.2002.40101.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci. 2002b;22:635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kim JS, Jung J, Lee HJ, Kim JC, Wang H, Kim SH, Shin T, Moon C. Differences in immunoreactivities of Ki67 and doublecortin in the adult hippocampus in three strains of mice. Acta Histochem. 2009;111:150–156. doi: 10.1016/j.acthis.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruba R, Hattiangady B, Shetty AK. Hippocampal neurogenesis and neural stem cells in temporal lobe epilepsy. Epilepsy Behav. 2009;14(Suppl 1):65–73. doi: 10.1016/j.yebeh.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgrebe M, Langguth B, Rosengarth K, Braun S, Koch A, Kleinjung T, May A, de Ridder D, Hajak G. Structural brain changes in tinnitus: grey matter decrease in auditory and non-auditory brain areas. Neuroimage. 2009;46:213–218. doi: 10.1016/j.neuroimage.2009.01.069. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, Murphy BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurol. 1998;50:114–120. doi: 10.1212/wnl.50.1.114. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Heine VM, Muller MB, van der Beek EM, Wiegant VM, De Kloet ER, Joels M, Fuchs E, Swaab DF, Czeh B. Stress, depression and hippocampal apoptosis. CNS Neurol Disord Drug Targets. 2006;5:531–546. doi: 10.2174/187152706778559273. [DOI] [PubMed] [Google Scholar]
- Mirz F, Gjedde A, Sodkilde-Jrgensen H, Pedersen CB. Functional brain imaging of tinnitus-like perception induced by aversive auditory stimuli. Neuroreport. 2000;11:633–637. doi: 10.1097/00001756-200002280-00039. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, Nadel L. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. J Anat. 2005;207:35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M. Frequency representation in the rat cochlea. Hear Res. 1991;51:247–254. doi: 10.1016/0378-5955(91)90041-7. [DOI] [PubMed] [Google Scholar]
- Myers P, Henry J, Zaugg T. Considerations for persons with mild traumatic brain injury. Perspectives on Audiology. 2008;4:21–35. [Google Scholar]
- Nicolas-Puel C, Faulconbridge RL, Guitton M, Puel JL, Mondain M, Uziel A. Characteristics of tinnitus and etiology of associated hearing loss: a study of 123 patients. Int Tinnitus J. 2002;8:37–44. [PubMed] [Google Scholar]
- Nixon K. Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16:287–295. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Paizanis E, Hamon M, Lanfumey L. Hippocampal neurogenesis, depressive disorders, and antidepressant therapy. Neural Plast. 2007;2007:73754. doi: 10.1155/2007/73754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson G. The rat brain in stereotaxic coordinates. Elsevier Academic Press; 2004. [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Ploughman M. Exercise is brain food: the effects of physical activity on cognitive function. Dev Neurorehabil. 2008;11:236–240. doi: 10.1080/17518420801997007. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Koopmans G, Blokland A, Scheepens A. Learning and adult neurogenesis: survival with or without proliferation? Neurobiol Learn Mem. 2004;81:1–11. doi: 10.1016/j.nlm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Puel JL, Ruel J, Gervais d'Aldin C, Pujol R. Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport. 1998;9:2109–2114. doi: 10.1097/00001756-199806220-00037. [DOI] [PubMed] [Google Scholar]
- Rimel RW, Giordani B, Barth JT, Boll TJ, Jane JA. Disability caused by minor head injury. Neurosurgery. 1981;9:221–228. [PubMed] [Google Scholar]
- Sahay A, Drew MR, Hen R. Dentate gyrus neurogenesis and depression. Prog Brain Res. 2007;163:697–722. doi: 10.1016/S0079-6123(07)63038-6. [DOI] [PubMed] [Google Scholar]
- Sakurai Y. Coding of auditory temporal and pitch information by hippocampal individual cells and cell assemblies in the rat. Neuroscience. 2002;115:1153–1163. doi: 10.1016/s0306-4522(02)00509-2. [DOI] [PubMed] [Google Scholar]
- Saljo A, Bao F, Jingshan S, Hamberger A, Hansson HA, Haglid KG. Exposure to short-lasting impulse noise causes neuronal c-Jun expression and induction of apoptosis in the adult rat brain. J Neurotrauma. 2002;19:985–991. doi: 10.1089/089771502320317131. [DOI] [PubMed] [Google Scholar]
- Saljo A, Svensson B, Mayorga M, Hamberger A, Bolouri H. Low levels of blast raises intracranial pressure and impairs cognitive function in rats. J Neurotrauma. 2009 doi: 10.1089/neu.2009.1053. in press. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–74. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J Cell Phys. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- Squire LR. The neuropsychology of human memory. Annu Rev Neurosci. 1982;5:241–273. doi: 10.1146/annurev.ne.05.030182.001325. [DOI] [PubMed] [Google Scholar]
- Stefovska VG, Uckermann O, Czuczwar M, Smitka M, Czuczwar P, Kis J, Kaindl AM, Turski L, Turski WA, Ikonomidou C. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann Neurol. 2008;64:434–445. doi: 10.1002/ana.21463. [DOI] [PubMed] [Google Scholar]
- Sun W, Lu J, Stolzberg D, Gray L, Deng A, Lobarinas E, Salvi RJ. Salicylate increases the gain of the central auditory system. Neuroscience. 2009;159:325–334. doi: 10.1016/j.neuroscience.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka J, Rybalko N, Popelar J. Enhancement of the auditory cortex evoked responses in awake guinea pigs after noise exposure. Hearing Research. 1994;78:158–168. doi: 10.1016/0378-5955(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Taupin P. Stroke-induced neurogenesis: physiopathology and mechanisms. Curr Neurovasc Res. 2006;3:67–72. doi: 10.2174/156720206775541769. [DOI] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006;120:188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Uda M, Ishido M, Kami K, Masuhara M. Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Res. 2006;1104:64–72. doi: 10.1016/j.brainres.2006.05.066. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmayr B, Mahlstedt MM, Henn FA. Neurogenesis and depression: what animal models tell us about the link. Eur Arch Psychiatry Clin Neurosci. 2007;257:300–303. doi: 10.1007/s00406-007-0734-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Baraban SC. Granule cell dispersion and aberrant neurogenesis in the adult hippocampus of an LIS1 mutant mouse. Dev Neurosci. 2007;29:91–98. doi: 10.1159/000096214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Xi MC, Woody CD, Gruen E. Identification of short latency auditory responsive neurons in the cat dentate nucleus. Neuroreport. 1994;5:1567–1570. doi: 10.1097/00001756-199408150-00006. [DOI] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W. Salicylate induced tinnitus: Behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–253. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]