Abstract
The purpose of this study was to determine whether myofibroblasts or other cells in the stroma in the cornea produce interleukin (IL)-1α or IL-1β that could modulate myofibroblast viability in corneas with haze after photorefractive keratectomy (PRK). Twenty-four female rabbits had haze-generating PRK for 9 diopters of myopia and were sacrificed at 1 week, 2 weeks, 3 weeks or 4 weeks after surgery. Corneal rims were removed, frozen in OCT at −80°C, and analyzed by immunocytochemistry using primary antibodies to IL-1α, IL-1β and alpha smooth muscle actin (SMA). Double immunostaining was performed for the co-localization of SMA with IL-1α or IL-1β. Central dense haze and peripheral slight haze regions of each cornea were analyzed. SMA+ cells that expressed IL-1α protein were detected in both regions of the corneas at most time points following PRK. However, in the haze region at the 1, 3 and 4 week time points, significantly more (p<0.01) SMA+ cells did not express IL-1α. Also, in the haze region at all three time points, significantly more (p<0.01) SMA− cells than SMA+ cells expressed interleukin-1α protein. IL-1β expression patterns in SMA+ and SMA− stromal cells was similar to that of IL-1α after PRK. Previous studies have demonstrated that IL-1α or IL-1β triggers myofibroblast apoptosis in vitro, depending on the available concentration of apoptosis-suppressive TGFβ. This study demonstrates that SMA− cells such as corneal fibroblasts, keratocytes, or inflammatory cells may produce IL-1α and/or IL-1β that could act in paracrine fashion to regulate myofibroblast apoptosis—especially in the region where there is haze in the cornea after PRK was performed and SMA+ myofibroblasts are present at higher density. However, some SMA+ myofibroblasts themselves produce IL-1α and/or IL-1β, suggesting that myofibroblast viability could also be regulated via autocrine mechanisms.
1. Introduction
Corneal injury, including surgery or infection, may trigger a loss of corneal transparency associated with the generation of stromal myofibroblasts that produce disordered matrix components such as collagen and glycosaminoglycans (Masur, et al., 1996; Jester, et al., 1999a; Wilson, et al., 1999; Netto, et al., 2006). TGFβ and PDGF have been shown to have important roles in modulating the development of corneal myofibroblasts from precursor cells (Masur, et al., 1996; Jester, et al., 1999b; Jester, et al., 2002; Kaur, et al., 2009b). The appearance and persistence of the myofibroblasts has been hypothesized to occur when structural and/or functional defects in the regenerated epithelial basement membrane allows penetration of TGFβ and PDGF from the epithelium into the stroma at sufficient levels required for receptor activation (Netto, et al., 2006; Kaur, et al., 2009b).
Over a period of years following surgery, many cases of corneal haze that occur after photorefractive keratectomy resolve spontaneously (Rajan, et al., 2004). Recent work demonstrated that myofibroblast apoptosis has an important role in the resolution of corneal haze through the removal of the cellular contribution to the opacity (Wilson, Chaurasia and Medeiros, 2007). Remaining anomalous extracellular matrix is subsequently removed by keratocytes that repopulate the anterior stroma. In a recent in vitro study, Kaur, et al., (2009a) demonstrated that exogenous interleukin (IL)-1 alpha or IL-1 beta triggers apoptosis of corneal myofibroblasts and that TGFβ blocks this effect. The current study examines potential in situ sources of IL-1 alpha or IL-1 beta in the corneal stroma following haze-generating photorefractive keratectomy in a rabbit model.
2. Materials and Methods
2.1. Animals and Surgery
All animals were treated in accordance with the tenets of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The Animal Control Committee at the Cleveland Clinic approved the animal studies included in this work. Anaesthesia was obtained by intramuscular injection of ketamine hydrochloride (30 mg/kg) and xylazine hydrochloride (5 mg/kg). In addition, topical proparacaine hydrochloride 1% (Alcon, Ft. Worth, TX, USA) was applied to each eye at the time of photorefractive keratectomy. Euthanasia was performed with an intravenous injection of 100 mg/kg pentobarbital while the animal was under general anaesthesia.
Twenty-four 12-week-old to 15-week-old female New Zealand white rabbits weighing 2.5–3.0 kg each were included in this study. One eye of each rabbit, selected at random, had epithelial scrape performed with a #64 Beaver (BD, Franklin Lakes, NJ USA) blade received a −9 diopter (D) photorefractive keratectomy (PRK) with a 6.0 mm ablation zone using a Summit excimer laser (Alcon, Ft. Worth, TX). Previous experiments demonstrated that 100% of rabbit corneas receiving −9 diopter PRK with this laser develop severe late stromal haze (Mohan et al., 2003). Four groups of 6 animals each were included in this study (1 week, 2 weeks, 3 weeks and 4 weeks groups, respectively, after PRK). Contralateral eyes were used as unwounded control eyes since the previous studies did not show any contralateral effects of PRK surgery (Mohan et al., 2003).
2.2. Cornea tissue preparation
Rabbits were euthanized and the corneoscleral rims of ablated and unablated control eyes were removed with 0.12 forceps and sharp Westcott scissors. For histological analyses, the corneas were embedded in liquid OCT compound (Sakura FineTek, Torrance, CA, USA) inside 24 mm×24 mm×5 mm tissue moulds (Fisher, Pittsburgh, PA, USA). Cornea specimens were centered within the moulds so that blocks could be bisected and transverse sections cut from the center of each cornea. The mould and tissue were rapidly frozen and stored at −80 °C until sectioning was performed.
Central corneal sections (7 µm thick) were cut with a cryostat (HM 505M, Micron GmbH, Walldorf, Germany). Sections were placed on 25 mm×75 mm×1 mm microscope slides (Superfrost Plus, Fisher) and maintained frozen at −80 °C until immunocytochemistry was performed.
2.3. Immunocytochemistry in corneal tissues
Several different antibodies purported to be useful for immunocytochemistry for IL-1α or IL-1β were tested in preliminary experiments and one antibody that showed the best staining for each cytokine with the lowest background staining was used in subsequent experiments. Double immunofluorescent staining was performed to study the co-expression of IL-1α with α-SMA or IL-1β with α-SMA in the rabbit corneas after −9 diopter PRK. The polyclonal antibody for IL-1α was goat anti-rabbit IL-1α IgG (Cat. #SC 1279, Santa Cruz Bio, Inc., Santa Cruz, CA) and the polyclonal antibody for IL-1β was goat anti-rabbit IL-1β IgG (Cat. #SC 1251, Santa Cruz Bio, Inc.). Either antibody was diluted 1:50 in 1% bovine serum albumin (Santa Cruz Bio, Inc.) and incubated on the sections at room temperature for 90 minutes. Sections were washed in phosphate buffered saline (PBS) and the secondary antibody, donkey anti-goat IgG, F(ab')2FITC (fluorescein green: Cat. #SC 3853, Santa Cruz Bio, Inc., CA) was applied at a concentration of 1:100 in PBS at room temperature for 60 minutes. The sections were then incubated for 60 minutes with goat normal serum (Cat. # 005-000-121, Jackson ImmunoResearch, West Grove, PN) diluted 1:5 with PBS.
Alpha-smooth muscle actin (α-SMA) was detected on the same slides using a monoclonal mouse anti-human smooth muscle actin clone1A4 (Cat. # M0851, Dako, Carpinteria, CA) that also recognized rabbit antigen. The antibody was used on the sections at 1:50 dilution in 1% BSA and incubated at room temperature for 90 minutes. Sections were washed with PBS and then incubated at room temperature for 60 minutes in Alexa Fluor 568 (Cat. # A11031, Invitrogen, Carlsbad, CA) secondary antibody, goat anti-mouse IgG (H+L) (Red) diluted 1:100 in PBS. Coverslips were mounted with Vectashield containing DAPI (Vector Laboratories Inc., Burlingame, CA) to allow visualization of all nuclei in the tissue sections. Negative controls were included with secondary antibody alone since no antigen was available for pre-absorption. The sections were viewed and photographed with a Leica DM5000 microscope equipped with Q-Imaging Retiga 4000RV (Surrey, BC, Canada) camera and ImagePro software.
2.4. Quantification of cells
The SMA and IL-1α or IL-1β positive cells were counted by a single observer for six different corneas from each time point after PRK in randomly selected fields of the region of the corneas that had dense haze (see Fig. 1), with counts being made in full-thickness stromal columns under 400X microscopic field, as previously described (Mohan et al., 2003).
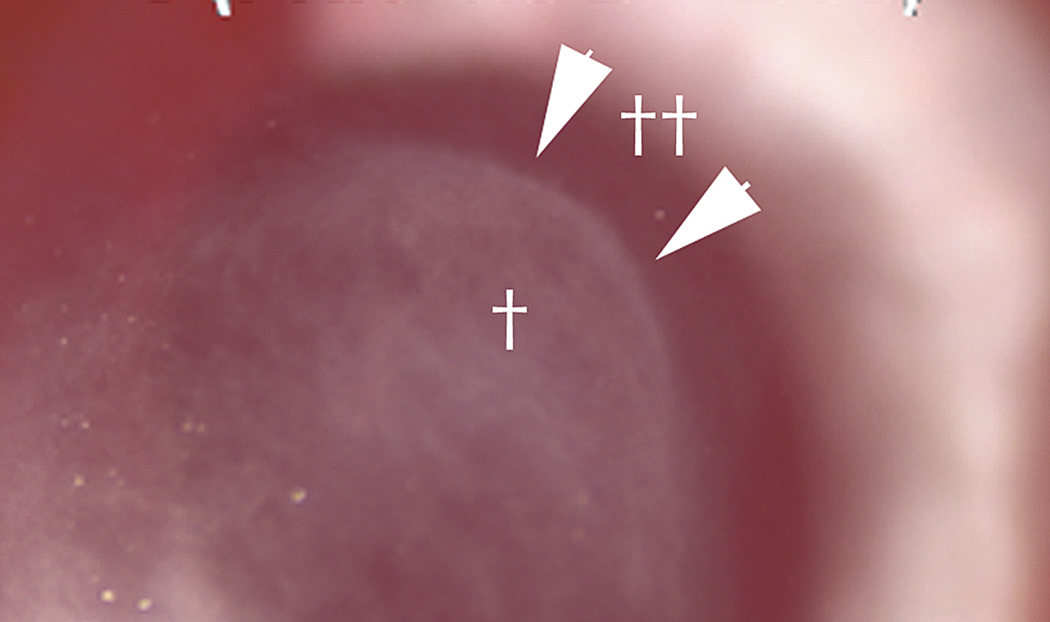
Fig. 1.
Slit lamp photo of haze in rabbit cornea at one month after -9D PRK. Arrows not edge of dense haze (†) that corresponds to the edge of ablation by the excimer laser. Peripheral to the ablated zone (††) haze is far less pronounced but the corneal stroma is not as clear as unwounded control corneas (not shown). Magnification 40X.
2.5. Statistical analysis
Statistical comparisons between the groups were performed using the non-parametric Mann-Whitney U test. p values less than 0.01 were considered statistically significant.
3. Results
In response to −9 diopter PRK in the rabbit, little haze can be noted in corneas 1 week or 2 weeks after PRK (not shown). At week 3, a significant haze response to the PRK is present (not shown). By 4 weeks after the PRK there is dense haze that obscures iris details (Fig. 1).
Immunocytochemistry (Fig. 2) and cell counts performed on double-stained slides (Table) from the areas of the stroma with dense haze in all of the corneas treated with PRK revealed the presence of SMA+ and SMA− stromal cells. Some of the SMA+ cells in the stroma at each time point were also IL-1α+. However, when large numbers of sections were examined and quantified in the areas with haze, significantly more of SMA+ cells at each time point were IL-1α− than were IL-1α+ (Table 1, p<0.01). Note that the particular section that was selected for Fig. 2 was one where most of the SMA+ cells were also IL-1α+. There were significantly more cells in the haze region of each cornea that were SMA−/IL-1α+ than were SMA+/IL-1α+ (Table 1, p<0.01) and, thus, the majority of stromal cells that produce IL-1α in the dense haze regions are probably not myofibroblasts. The largest number of cells present in the dense haze region of the cornea expressed neither SMA nor IL-1α.
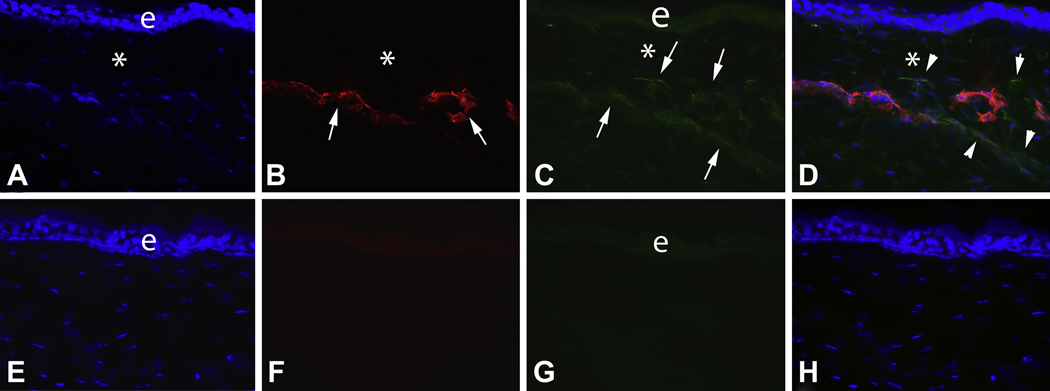
Fig. 2.
Double immunocytochemistry for α-smooth muscle actin (SMA) and IL-1α in the region of the cornea with dense haze (* in Fig. 1) at 4 weeks after −9 D PRK in the rabbit. A. Cell nuclei of all cells stained blue with DAPI. B. SMA+ myofibroblasts (arrows) are present in the anterior stroma. C. IL-1α+ cells (arrows) are also detected in the anterior stroma. Note that IL-1α is also detected in the epithelium (e). D. The overlay of A thru C demonstrates that all of the SMA+ myofibroblasts in this section also express IL-1α, but that there are adjacent SMA− cells that also express IL-1α protein (arrowheads). Note that there is an artifactual separation (*) between the epithelium and stroma that is typically formed in an area with severe haze when corneas with prominent myofibroblasts are sectioned for immunocytochemistry. Panels E to H are corresponding −9D PRK cornea sections in which primary antibody to SMA (F, H) or IL-1α (G, H) was omitted. Mag. 300X.
Cellular IL-1 alpha in stromal region with haze
| WEEKS | TOTAL CELLS DAPI | SMA+/IL1α+ | SMA+/IL1α− | SMA−/IL1α+ |
|---|---|---|---|---|
| 1 WEEK | 48.3±4.2 | 0.3±0.3 | 0.8±0.7 | 3.5±0.8† |
| 2 WEEKS | 50.3±7.2 | 2.8±1.4 | 2.8±1.3 | 5.0±1.2† |
| 3 WEEKS | 53.1±4.2 | 0.2±0.2 | 3.3±2.4* | 3.7±1.1† |
| 4 WEEKS | 45.3±5.3 | 1.8±0.8 | 3.2±2.6* | 3.2±1.5† |
Cells/400x field, (n=6 corneas that had −9D PRK) at each time point
indicates that the SMA+IL-1α− is significantly different from SMA+IL-1α+ at that time point (p <0.01) Mann-Whitney U test
indicates that the SMA−IL-1α− is significantly different from SMA+IL-1α+ at that time point (p <0.01) Mann-Whitney U test
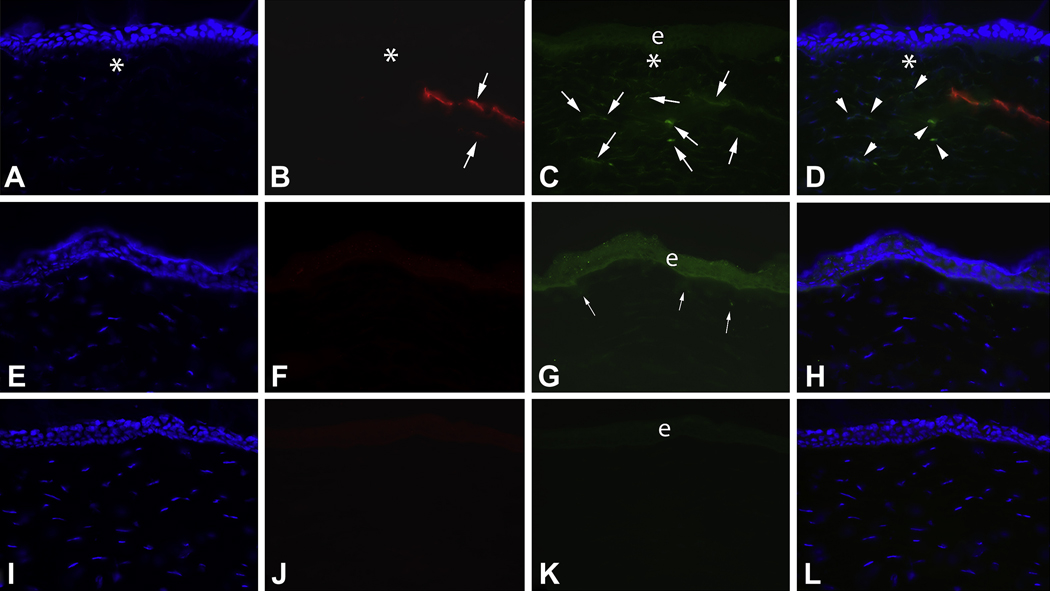
In the peripheral cornea outside the region of haze (Fig. 3), SMA+ and SMA− stromal cells are also present. Again, however, more cells that are IL-1α+ are SMA− than SMA+. Similar to the dense haze area of the corneas, the majority of cells were neither SMA+ nor IL-1α+ in the peripheral cornea with limited haze. SMA+ cells were very rare in the stroma of unwounded corneas (Fig. 3F), as were IL-1α+ cells (Fig. 3G).
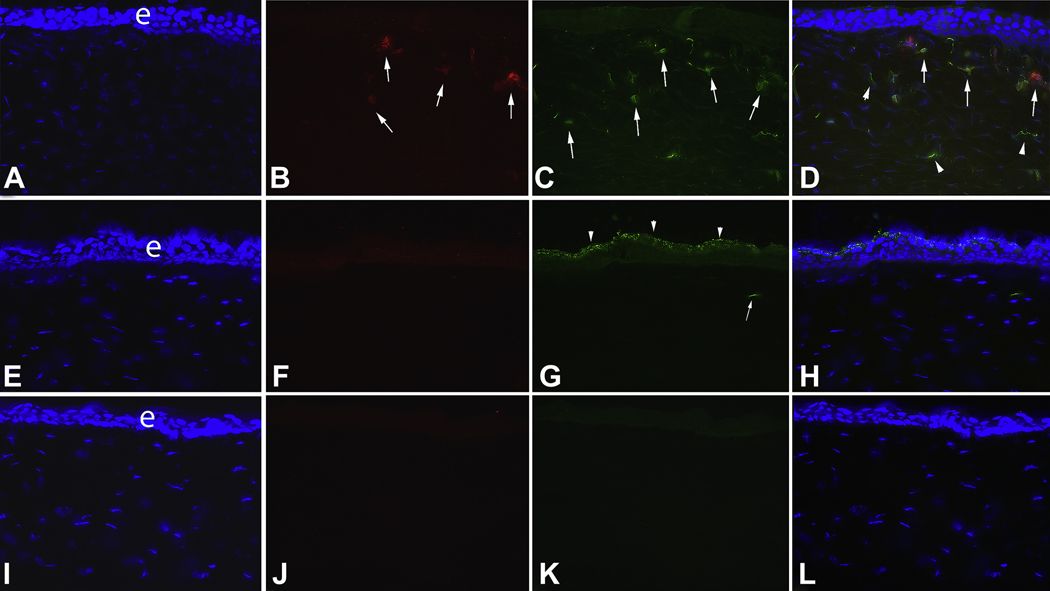
Fig. 3.
Double immunocytochemistry for α-smooth muscle actin (SMA) and IL-1α in the peripheral cornea outside of the dense haze zone (** in Fig. 1) at 4 weeks after −9D PRK and in control unwounded corneas in the rabbit. A to D are from a −9D PRK cornea. A. Cell nuclei of all cells stained blue with DAPI. e is the epithelium. B. SMA+ myofibroblasts (arrows) are present in the anterior stroma. C. IL-1α+ cells (arrows) are also detected in the anterior stroma. D. The overlay of A thru C demonstrates that many (arrows), but not all, of the SMA+ myofibroblasts in this section also express IL-1α. There are also many adjacent SMA− cells that also express IL-1α protein (arrowheads). E to H are corresponding tests performed on unwounded control corneas. Note that no SMA+ cells were detected in the unwounded corneal stroma (F). Very light staining in the epithelium in F is background staining. Very few IL-1α+ cells were detected in the stroma of unwounded corneas (G). In this particular section, only one was noted (arrow, G). Note the heavy IL-1α expression in apical cells (arrowheads) of the unwounded corneal epithelium. This pattern of epithelial expression had not recovered by 1 month after −9D PRK (C). I to L are representative −9D PRK cornea sections in which primary antibody to SMA and IL-1α were omitted. Magnification 300X.
The expression pattern of IL-1α noted in the epithelium in this study was also of interest. In unwounded corneas, epithelial IL-1α expression was highest in the apical epithelial cells (Fig. 3G). Even at 1 month after PRK, this pattern of expression had not been re-established in the corneas that had PRK (Fig. 3C).
Fig. 4 shows a higher magnification view of the central cornea (A) and the peripheral cornea (B) in which SMA+ myofibroblasts have staining for IL-1α that appears to be localized in organelles or vesicles within the cells. Note there are also numerous SMA− stromal cells that are IL-1α+.
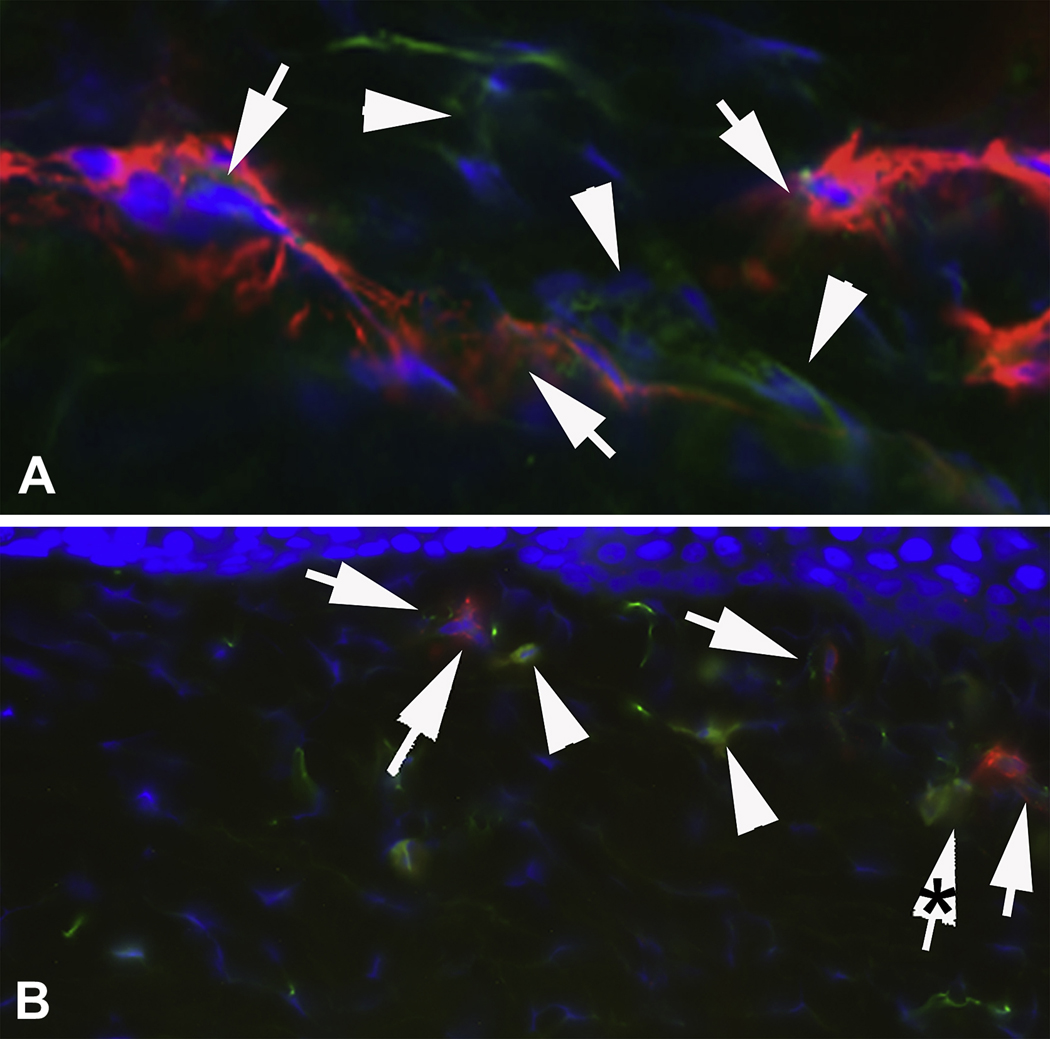
Fig. 4.
Higher magnification localization of (A) α-smooth muscle actin (SMA) and IL-1α in the region of the cornea with dense haze (* in Fig. 1) at 4 weeks after −9 D PRK and (B) SMA and IL-1α in the peripheral cornea outside of the dense haze zone (** in Fig. 1) at 4 weeks after −9D PRK. Arrowheads in (A) and (B) show SMA+ myofibroblasts that have simultaneous IL-1α. Arrowheads in (A) and (B) indicate SMA− stromal cells near myofibroblasts that express IL-1α. Note that the IL-1α in many SMA+ cells appeared to be compartmentalized within these cells, perhaps within vesicles or organelles. This is perhaps best seen in B where the arrow with the asterisk is a part of the SMA+ cell also indicated by the nearest adjacent arrow. In this cell the IL-1α is clearly within organelles of some type, probably vesicles. This was best seen in real-time at the microscope. Magnification 1000X.
The findings for IL-1β were similar to those for IL-1α (Fig. 5). At one month after −9D PRK, both SMA+ and SMA− cells in the anterior stroma produced IL-1β (Fig. 5D). In unwounded control corneas, there was heavy epithelial expression of IL-1β (Fig. 5 G). Notice that this typical epithelial expression pattern had not recovered by 4 weeks after −9D PRK (Fig 5C). In the cornea shown in Fig. 5G, a few anterior stromal cells expressed IL-1β. These cells were SMA− and could be inflammatory or other cells.
Fig. 5.
Double immunocytochemistry for α-smooth muscle actin (SMA) and IL-1β in the peripheral cornea at the junction of the ablated dense haze zone (* in Fig. 1) and the low haze zone (** in Fig. 1) at 4 weeks after −9D PRK and in control unwounded corneas in the rabbit. A to D are from a −9D PRK cornea. Note that there is an artifactual separation (*) between the epithelium and stroma of A to D that is typically formed in an area with severe haze when corneas with prominent myofibroblasts are sectioned for immunocytochemistry. A. Cell nuclei of all cells stained blue with DAPI. e is the epithelium (in C, G and K). B. SMA+ myofibroblasts (arrows) are present in the anterior stroma to the right where haze was present. C. IL-1β+ cells (arrows) are also detected in the anterior stroma. D. The overlay of A thru C demonstrates that many (arrows), but not all, of the SMA+ myofibroblasts in this section also express IL-1β. There are also many adjacent SMA− cells that also express IL-1β protein (arrowheads). E to H are the corresponding tests performed on unwounded control corneas. In G, there is heavy IL-1β production in the unwounded corneal epithelium. Note that the IL-1β production in epithelium had not returned to normal at one month after PRK in panel C. A few cells in the anterior stroma of the unwounded cornea (G) express IL-1β (arrows). I to L are corresponding −9D PRK cornea sections in which primary antibody to SMA (J, L) or IL-1β (K, L) was omitted. Magnification 300X
When large numbers of sections were examined and quantified in the haze regions of the corneas, significantly more of SMA+ cells at each time point were IL-1β− than were IL-1β+ (Table 2, p<0.01). There were significantly more cells in the haze region of each cornea that were SMA−/IL-1β+ than were SMA+/IL-1αβ (Table 2, p<0.01) and, thus, the majority of stromal cells that produce IL-1β in the dense haze regions are probably not myofibroblasts. The largest number of cells present in the dense haze region of the cornea expressed neither SMA nor IL-1β.
Cellular IL-1 beta in stromal region with haze
| WEEKS | TOTAL CELLS DAPI | SMA+/IL1β+ | SMA+/IL1β− | SMA−/IL1β+ |
|---|---|---|---|---|
| 1 WEEK | 50.7±5.2 | 1.3±1.1 | 2.8±1.6* | 2.5±1.4† |
| 2 WEEKS | 50.7±5.2 | 0.2±0.2 | 1.3±1.3* | 3.0±0.6† |
| 3 WEEKS | 38.7±0.4 | 0.3±0.3 | 2.7±0.8* | 1.3±0.6† |
| 4 WEEKS | 38.8±6.0 | 0.1±0.1 | 2.0±0.9* | 0.8±0.6† |
Cells/400x field, (n=6) at each time point
indicates that the SMA+IL-1β− is significantly different from SMA+IL-1β+ at that time point (p <0.01) Mann-Whitney U test
indicates that the SMA−IL-1β− is significantly different from SMA+IL-1β+ at that time point (p <0.01) Mann-Whitney U test
4. Discussion
Severe haze (or opacity) sometimes develops in the corneal stroma after photorefractive keratectomy (PRK) and other surgeries or injuries. Myofibroblasts have an important role in the development of haze after they are generated following injury to some corneas. Large numbers of these cells result in altered transparency through the production of disordered collagen and other extra cellular matrix materials produced by these cells and the opacity of the cells themselves (Jester, Petroll and Cavanaugh, 1999a; Mohan, et al., 2003). Interestingly, even very severe haze, similar to that shown in Fig. 1, will often resolve spontaneously over time measured in years. Spontaneous resolution of haze is associated with disappearance of the myofibroblasts and resorption of the disordered matrix by repopulating keratocytes. Late apoptosis of the myofibroblasts is thought to have an important role in the disappearance of these cells (Wilson, Chaurasia and Medeiros, 2007).
Recent studies in non-ocular organs have shown that IL-1α and/or IL-1β are important regulators of myofibroblast function and viability, including myofibroblast apoptosis (Zhang, Gharaee-Kermani, and Phan, 1997; Bonner, et al., 1998; Zhang and Phan, 1999; Wang, et al., 2000; Shephard, et al., 2004; Werner, Krieg, and Smola, 2007). Several of these studies have also shown that IL-1 has opposing effects to TGFβ in regulating myofibroblast functions. In studies with corneal cells in vitro, we previously demonstrated that TGFβ and IL-1 have opposing effects on myofibroblast viability (Kaur, et al, 2009a). In those studies, it was found that IL-1α or IL-1β acts as an inducer of myofibroblast apoptosis, but only if the cell is not simultaneously exposed to TGFβ. TGFβ from the epithelium is thought to be the major inducer of myofibroblast development from precursor cells in the cornea (Masur, et al., 1996; Jester, et al., 1999b) and we hypothesized that stromal levels of TGFβ are maintained as long as structural and functional defects persist in the epithelial basement membrane following surgery or injury (Netto, et al., 2006). Once the epithelial basement membrane is repaired and barrier function to cytokine penetration is restored, stromal TGFβ levels likely fall and IL-1 effects on the myofibroblasts predominate.
The purpose of the present study was to determine what cells in the stroma of corneas with haze could serve as the source of IL-1α or IL-1β to trigger myofibroblast apoptosis in a rabbit model in which nearly 100% of corneas develop severe haze by one month after −9 diopter PRK (Mohan, et al., 2003). This study demonstrates that many of the myofibroblasts themselves express IL-1α or IL-1β as early as 1 week after PRK injury and during the period of haze generation to 1 month after surgery. Thus, IL-1 could modulate myofibroblast apoptosis via autocrine suicide (Mohan, et al., 1997) during this period, but this is likely suppressed in most myofibroblasts by ongoing exposure to TGFβ that penetrates into the stroma from the epithelium. However, even more stromal cells that are SMA− produce IL-1α and/or IL-1β during weeks 1 to 4 after −9 diopter PRK (Table 1 and Table 2). Presumably, most of these cells are corneal fibroblasts or keratocytes, although they could also be myofibroblast precursor cells that are not yet expressing SMA (Chaurasia, et al., 2009) or residual inflammatory cells. West-Mays and coworkers (1997) have shown that corneal fibroblasts can express IL-1. We hypothesize that these cells may modulate myofibroblast apoptosis via paracrine mechanisms, also suppressed when stromal TGFβ levels are elevated due to defective basement membrane function. Whether myofibroblast apoptosis is modulated via autocrine or paracrine mechanisms and how the overall regulation of the process is controlled will require further study.
Interestingly, in the counts shown in Table 2, there appeared to be a surge of SMA+IL-1α+ cells at week 2 after PRK, which then receded at week 3, but again rose to higher levels at week 4. This rise at week 2 could have been an artifact of counting. Alternatively, it is possible that as myofibroblasts go through differentiation from precursor cells after PRK (Chaurasia, et al., 2009), early SMA+ cells express IL-1α. Our working hypothesis is that this represents a regulatory system that modulates the number of myofibroblasts that develop early in the stroma after corneal wounding, with many early cells that develop from progenitors undergoing apoptosis in response to the endogenous IL-1α. Whether these cells develop from keratocytes or bone marrow-derived cells (Barbosa, et al., 2010), or both, remains to be determined.
We have previously demonstrated that unwounded corneal epithelium produces both IL-1α and IL-1β (Wilson, et al., 1994; Weng, et al., 1997). This study confirms that expression. It also demonstrates that the typical pattern of IL-1α and IL-1β expression in the epithelium of the unwounded rabbit cornea (Fig. 3 G and Fig. 4 G). Interestingly, the level and pattern of expression of the cytokines is not restored by 4 weeks after PRK (Fig. 3C and Fig. 4 C). It is not known how much time is required for the epithelium to be restored to its unwounded state or the physiological significance, if any, of this diminished IL-1 expression in the epithelium after corneal injury.
It is likely that IL-1α and IL-1β from the cornea epithelium is released into the corneal stroma by epithelial injury. A previous study demonstrated that application of IL-1 receptor antagonist to the stromal surface after epithelial injury in rabbits profoundly down-regulates IL-1 mediates stromal inflammatory cell infiltration (Stapleton, et al., 2008). Once the corneal epithelium is regenerated over the injury, it is unlikely that further IL-1 can penetrate from the epithelium into the stroma because neither IL-1α or IL-1β contain a signal sequence for transport from the cell and the intact basement membrane would likely serve as a barrier to penetration into the stroma of these large peptides (Stapleton, et al., 2008). Thus, although very high concentrations of IL-1α and IL-1β are noted in the epithelium of the unwounded cornea in Figures 3G and 5G, respectively, very little IL-1 signal is detected in the sub-adjacent stroma, except within a few stromal cells that are present in some sections.
Acknowledgements
This study was supported by EY10056, EY015638, and Research to Prevent Blindness, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbosa FL, Chaurasia SS, Cutler A, Asosingh K, Kaur H, Medeiros FW, Agrawal V, Wilson SE. Corneal myofibroblast generation from bone marrow-derived cells. Experimental Eye Research. 2010;91:92–96. doi: 10.1016/j.exer.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JC, Lindroos PM, Rice AB, Moomaw CR, Morgan DL. Induction of PDGF receptor-alpha in rat myofibroblasts during pulmonary fibro genesis in vivo. Am J Physiol Lung Cell Mol Physiol. 1998;274:72–80. doi: 10.1152/ajplung.1998.274.1.L72. [DOI] [PubMed] [Google Scholar]
- Chaurasia SS, Kaur H, Medeiros FW, Smith SD, Wilson SE. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp. Eye Res. 2009;89:133–139. doi: 10.1016/j.exer.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, Cavanaugh HD. Transforming growth factor (beta)- mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest. Ophthalmol. Vis. Sci. 1999b;40:1959–1967. [PubMed] [Google Scholar]
- Jester JV, Huang J, Petroll WM, Cavanaugh HD. TGF beta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGF beta, PDGF and integrin signaling. Exp. Eye Res. 2002;75:645–657. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Cavanaugh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog. Retinal Eye Res. 1999a;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, Agrawal V, Wilson SE. Corneal myofibroblast viability: Opposing effects of IL-1 and TGF beta-1. Exp. Eye Res. 2009a;89:152–158. doi: 10.1016/j.exer.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, Medeiros FW, Agrawal V, Salomao MQ, Singh N, Ambati BK, Wilson SE. Corneal stroma PDGF blockade and myofibroblast development. Exp. Eye Res. 2009b;88:960–965. doi: 10.1016/j.exer.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur S, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc. Natl. Acad. Sci. USA. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AE, Choi R, Hong J, Lee J, Mohan RR, Ambrosio R, Jr, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp. Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Liang Q, Kim W-J, Helena MC, Baerveldt F, Wilson SE. Apoptosis in the cornea: further characterization of Fas-Fas ligand system. Exp. Eye Res. 1997;65:575–589. doi: 10.1006/exer.1997.0371. [DOI] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp. Eye Res. 2006;82:788–797. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan MS, Jaycock P, O'Brart D, Nystrom HH, Marshall J. A long-term study of photorefractive keratectomy; 12-year follow-up. Ophthalmology. 2004;111:1813–1824. doi: 10.1016/j.ophtha.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Shephard P, Martin G, Smola-Hess S, Brunner G, Krieg T, Smola Myofibroblast differentiation is induced in keratinocyte-fibroblast co-cultures and is antagonistically regulated by endogenous transforming growth factor-beta and interleukin-1. Am. J. Pathol. 2004;164:2055–2066. doi: 10.1016/s0002-9440(10)63764-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton WM, Chaurasia S, Medeiros FW, Mohan RR, Sinha S, Wilson SE. Topical interleukin-1 receptor antagonist inhibits inflammatory cell infiltration into the cornea. Exp. Eye Res. 2008;86:753–757. doi: 10.1016/j.exer.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-Z, Zhang P, Rice AB, Bonner JC. Regulation of interleukin-1β-induced platelet-derived growth factor receptor-α expression in rat pulmonary myofibroblasts by p38 mitogen-activated protein kinase. J. Biol. Chem. 2000;275:22550–22557. doi: 10.1074/jbc.M909785199. [DOI] [PubMed] [Google Scholar]
- Weng J, Mohan RR, Li Q, Wilson SE. IL-1 up regulates keratinocyte growth factor and hepatocyte growth factor mRNA and protein production by cultured stromal fibroblast cells: Interleukin-1 beta expression in the cornea. Cornea. 1997;16:465–471. [PubMed] [Google Scholar]
- Werner S, Krieg T, Smola H. Keratinocyte–fibroblast interactions in wound healing. J. Invest. Dermatology. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- West-Mays JA, Shadow PM, Tobin TW, Stresses KJ, Citron C, Fini ME. Repair phenotype in corneal fibroblasts is controlled by an interleukin-1 alpha autocrine feedback loop. Invest. Ophth. Vis. Sci. 1997;38:1367–1379. [PubMed] [Google Scholar]
- Wilson SE, Chaurasia SS, Medeiros FW. Apoptosis in the initiation, modulation and termination of the corneal wound healing response. Exp. Eye Res. 2007;85:305–311. doi: 10.1016/j.exer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Liu JJ, Mohan RR. Stromal-epithelial interactions in cornea. Prog. Retinal Eye Res. 1999;18:293–309. doi: 10.1016/s1350-9462(98)00017-2. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Schultz GS, Chegini N, Weng J, He Y-G. Epidermal growth factor, transforming growth factor alpha, transforming growth factor beta, acidic fibroblast growth factor, basic fibroblast growth factor, and interleukin-1 proteins in the cornea. Exp. Eye Res. 1994;59:63–72. doi: 10.1006/exer.1994.1081. [DOI] [PubMed] [Google Scholar]
- Zhang H-Y, Gharaee-Kermani M, Phan SH. Regulation of lung fibroblast alpha-smooth muscle actin expression, contractile phenotype, and apoptosis by IL-1 beta. J Immunol. 1997;158:1392–1399. [PubMed] [Google Scholar]
- Zhang H-Y, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor β-1. Am. J. Respir. Cell Mol. Biol. 1999;21:658–665. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]