Abstract
In this study we successfully constructed a full-length cDNA library from Siberian tiger, Panthera tigris altaica, the most well-known wild Animal. Total RNA was extracted from cultured Siberian tiger fibroblasts in vitro. The titers of primary and amplified libraries were 1.30×106 pfu/ml and 1.62×109 pfu/ml respectively. The proportion of recombinants from unamplified library was 90.5% and average length of exogenous inserts was 1.13 kb. A total of 282 individual ESTs with sizes ranging from 328 to 1,142bps were then analyzed the BLASTX score revealed that 53.9% of the sequences were classified as strong match, 38.6% as nominal and 7.4% as weak match. 28.0% of them were found to be related to enzyme/catalytic protein, 20.9% ESTs to metabolism, 13.1% ESTs to transport, 12.1% ESTs to signal transducer/cell communication, 9.9% ESTs to structure protein, 3.9% ESTs to immunity protein/defense metabolism, 3.2% ESTs to cell cycle, and 8.9 ESTs classified as novel genes. These results demonstrated that the reliability and representativeness of the cDNA library attained to the requirements of a standard cDNA library. This library provided a useful platform for the functional genomic research of Siberian tigers.
Keywords: Siberian tiger, Panthera tigris altaica, Fibroblast cell line, SMART cDNA library, Expressed sequence tags.
1. Introduction
Tiger (Panthera tigris Linnaeus, 1758) is a special species only found in Asia and considered as a symbol of beauty, power, and bravery 1. There are four generally accepted tiger subspecies in China, Siberian tigers (P. t. altaica), Indochinese tigers (P. t. corbetti), South China tigers (P. t. amoyensis), and Indian or Bengal tigers (P. t. tigris). Today an estimated fewer than 20 Siberian tigers now survive in northeastern China 2,3. The tiger is warranted the highest level of protection by the Convention on International Trade in Endangered Species of Wild Fauna & Flora (CITES). In 1989, the Chinese government brought Siberian tiger into category I of National Protected Animal breed.
To preserve the wealth of biodiversity in China, there is a very urgent need to commence rigorous conservation of endangered species. However, only about 13 functional genes of P. t. altaica were cloned and partially studied according to the latest data of NCBI and information with reference to cDNA library of P. t. altaica is scarce. To identify more genes of Siberian tiger, including the characterization of specific expressed, new or unknown functional genes and further study their functions, construction of full-length cDNA library is an efficient method 4,5.
In this paper, we have cryopreserved Siberian tiger this international protected genomic resource for the purposes of reviving endangered breed by cloning and supplying a convenient and effective resource for genomic research. Firstly, we established the Siberian tiger fibroblasts cell line to save its genomic resources at the cell level. Secondly, a study on the cDNA library construction and preliminary analysis of ESTs from Siberian tiger fibroblast cells conducted in our laboratory is hereby described.
2. Materials and methods
Materials and reagents
Siberian tiger ear tissue samples (16 male and 18 female) were sampled from The Northeast Tiger Wooden Land of Heilongjiang.
Cell culture and analysis
Siberian tiger ear tissue samples (about 1 cm2 in size) were chopped finely into 1 mm3 in size added DMEM medium with 10% fetal bovine serum in a 37℃ incubator with 5% CO2 to culture fibroblast line. Characteristic tests for established cell line with cell viability, microorganism detection and chromosome analysis: for details of the procedure used see Liu et al 6.
cDNA library construction
Cells were harvested and total RNA was extracted with Trizol reagent (Invitrogen, USA) when they were in the period of passage 3. First and double-strand cDNAs were synthesized according to the protocol of the SMART cDNA Library Construction kit (Clontech, USA). Subsequently approximately 2μl of first strand cDNA sample was amplified using long distance PCR (LD-PCR). The first four peak fractions containing cDNA (>500bp) were pooled together using column chromatograph with CHROMA SPIN-400 medium. The cDNA was ligated to λTriplEx2 vector (1:1.5) and the ligation was packaged with Gigapack Ⅲ Gold Packaging extract.
Titration of the primary library
The number of clones was counted to calculate the library titer according to the formula: pfu/ml = number of plaques × dilution factor × 103μl/ml (μl of diluted phage plated). The recombination efficiency was identified by blue/white screening in E.coli XL1-Blue. Colony PCR was used to confirm the size of inserted fragments in the library. After amplification, the completed cDNA libraries were stored in 7% dimethyl sulfoxide at -80°C.
Sequencing and analysis
cDNA clones were selected randomly from the cDNA library and single-pass sequenced at the 5′ end on an ABI 3730 Genetic Analyzer (Applied Biosystems). A large-scale EST sequencing project for Siberian tiger was initiated to identify and functionally annotate as many unique transcripts as possible. The processed cDNA sequences were used to perform the BLAST search at the GenBank database to compare all available ESTs and genes to date (http://www.ncbi.nlm.nih.gov/blast). BLASTX results with bit scores greater than 80 and E-values of less than 10-10 were generally regarded as significant match 7,8. Multiple sequence alignment between the amino acid sequences of candidate clones and their homologues of the other species were also analyzed by using CLUSTAL W 9.
3. Results
Cell culture and Characteristic Tests
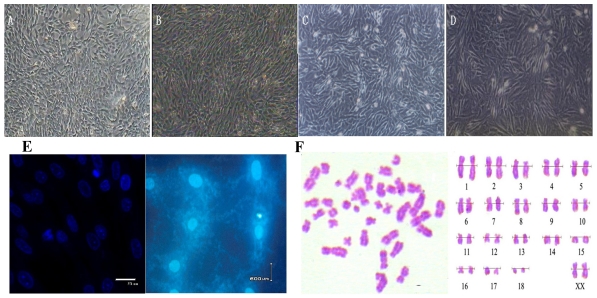
We used a primary explanting technique and cell cryogenic preservation technology to establish the Siberian tiger fibroblast cell line and proceeded to biological and genetic detection. The culture conditions seemed optimal, and the cells looked healthy (Figure 1A-D). Because we wanted to construct a cDNA library, the fibroblasts must maintain diploid character similar to the cells in vivo. Chromosome analysis showed that the frequency of cell chromosome number of 2n=38 was 90.2-91.6% in passage 1 to 3, which indicated culture in vitro effect the heritage of cells slightly, supporting that the cell line was a steady diploid one (Figure 1 F). Test results for bacteria, virus and mycoplasma were all negative (Figure 1 E).
Figure 1.
Morphology, Mycoplasma contamination and karyotype of Siberian tiger cell line. (A) Primary cells (×100), the cells were typical long spindle-shape. (B) Subcultured cells (×100). (C) Cells before cryopreservation (×100). (D) Cells after recovery (×100). (E) Mycoplasma contamination Stained with Hoechst33258 and Positive control of Mycoplasma contamination; (F) Chromosome at metaphase (left) and karyotype (right) (×1,000).
Total RNA extraction and LD-PCR
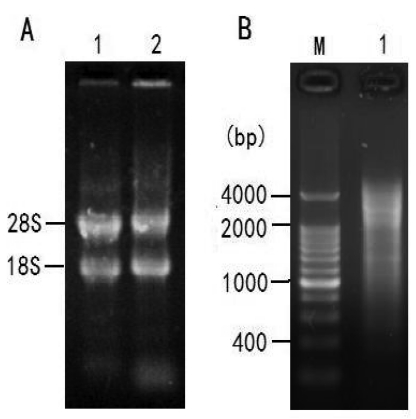
The ratio of OD260/OD280 to the total RNA was approximately 2.02 and the concentration was 1.231μg/μl. As shown in Figure 2A, two bright bands of 18S rRNA and 28S rRNA can be seen clearly, indicating that the total RNA is integrated and stable enough for cDNA library construction. Two microgram of total RNA was subjected to reverse transcription for synthesis of the first and double-stranded cDNAs for LD-PCR. As shown in Figure 2B, the ds-DNA appeared as a smear of bands of 0.5 - 4 kb on the gel.
Figure 2.
Total RNA from fibroblast cells of Siberian tiger and LD-PCR. A: Total RNA from fibroblast cells of Siberian tiger; Lane 1: a sample of 5μl total RNA; Lane 2: a sample incubated at 37℃ for 2h. B: The products of LD-PCR. M: marker; Lane 1: the products of LD-PCR with 22 cycles.
Characterization of cDNA library
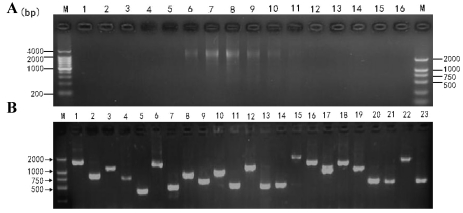
The cDNA size fractionation was carried out using CHROMA SPIN-400 column (Figure 3A). The titers of primary and amplified libraries were 1.30×106 pfu/ml and 1.62×109 pfu/ml respectively. The recombination efficiency of the amplified libraries was 90.5%. The insert ratio and the average length of inserted fragments were measured by PCR, as shown in Figure 3B. The average size was approximately 1.13 kb in average, 1-2 kb in 62.3% and 0.5-1.0 kb in 37.7%, suggesting that the insertion fragments harbored most of the mRNAs and reached the requirement for further studies on gene structure, translation, and expression.
Figure 3.
cDNA size fractionation by CHROMA SPIN-400 and Recombinant clones screening. A: cDNA size fractionation by CHROMA SPIN-400. M: DNA marker; 1-16: tube serial number. B: Recombinant clones screening within the library. M: DNA marker; 1-23: PCR products for clones selected randomly.
Generation of expressed sequence tags and sequence analysis
EST sequencing for selected independent clones from the cDNA library has been proved to be a quick and efficient approach to assess library quality. The primary cDNA library, instead of the amplified library, was used for generation of ESTs to reduce the redundancy of cDNA clones as only a small number of ESTs were targeted through random selection. Four hundred and fifty-six white clones were picked randomly for EST sequencing. After removal of the vector sequences and low-quality sequences, 416 effective sequences from the total cDNA sequences were obtained, a total of 282 individual ESTs were analyzed and they ranged from 328 to 1,142 nucleotides in length.
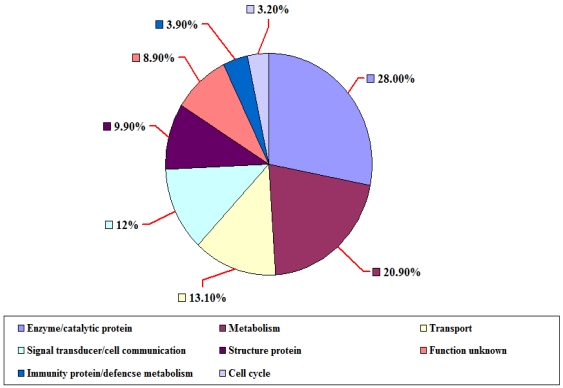
The distribution of ESTs from P. t. altaica cDNA library based on BLASTX score revealed that 152 (53.9%) of them were classified as strong, the highest match with a BLASTX score greater than 200, meanwhile 109 (38.6%) ESTs were nominal with BLASTX score for the highest match between 80 and 200, and 21 (7.4%) ESTs were weak with BLASTX score for the highest match less than 80 or no significant similarity to sequences in the database. Among the ESTs with known putative function, 79 (28.0%) ESTs were found to be related to enzyme/catalytic protein, 59 (20.9%) ESTs to metabolism, 37 (13.1%) ESTs to transport, 34 (12.1%) ESTs to signal transducer/cell communication, 28 (9.9%) ESTs to structure protein, and 11 (3.9%) ESTs to immunity protein/defencse metabolism, 9 (3.2%) ESTs to cell cycle, 25(8.9) ESTs were ''unknown protein'' with no significant matches or unknown proteins (Figure 4). 24 of 282 ESTs were found to have more than three copies and the rest ESTs have one to two copies in Siberian tiger cDNA library.
Figure 4.
The classification of ESTs from P. t. altaica cDNA library based on their putative functions
4. Discussions
Over the past years, cDNA library construction and analysis is considered to be an indispensable tool for functional genomic analysis as it provides much more detailed information on the genomic mechanisms underlying diverse processes of the organism 11. The major characteristic of cDNA construction by SMART technique could improve the ratio of full-length cDNA sequences. Although the optimal number of cycles for minimizing PCR-induced mutations has not been rigorously tested for SMART library construction, performing three cycles using a suitable polymerase mixture should be sufficient to limit the number of errors to a tolerable level 12. Conventionally generated cDNA libraries contain a high percentage of 5′- truncated clones. An important characteristic of SMART technique is that it provides a method for producing high-quality and full-length cDNA libraries that preserve the complete 5′ terminal sequence of mRNA 13.
Characterization of cDNA library of Siberian tiger
There are three chief aspects that identify the quality of a cDNA library. According to Clareke-Carbon's formula, a cDNA library should contain at least 1.7×105 independent clones to ensure that the 99% low abundance mRNA would be present in the library 14. The high recombination efficiency is another index of good quality library 15. The third aspect is that the average length of inserted cDNA should be no less than 1.0 kb to ensure the integrity of cDNA. In this paper, the full-length cDNA library constructed from Siberian tiger conformed to the requirements of a standard library 4.
The cells may be injured and changed in biological characteristics, especially hereditary characteristics, after too many passages and trypsin digestion. Improving culture procedure and decreased the passages to maintain fibroblast sample character similar with the cells in vivo, we construct the cDNA library to conserve genomic characteristics of Siberian tiger. The cDNA library constructed in this study will be affluent in the EST library, full-length analysis, the next work of expression identification, location and function in the chromosome will make great promotion for interesting genes associated with its excellent characters of Siberian tiger.
Generation and analysis of ESTs
cDNA libraries are widely used to identify genes and splice variants, and as a physical resource for full-length clones 16. The SMART library provided a useful resource for the functional genomic research of Siberian tiger and will present some new molecular material for this species as well. The regions encoding proteins of full-length cDNA are usually conserved, and may have some similarities with closely related regions, which can help us to identify and analyze the protein encoding genes. Consequently, analysis of the expression of a large number of genes combined with the knowledge of their functions can facilitate the understanding and allows us to take a glimpse of the overall picture of biological processes in P. t. altaica fibroblast cells. In this study, approximately 91.1% of the ESTs generated were sequences with known or putative functions, while the remainder was unknown proteins or sequences with no similarities to the databases. These unknown and unclassified ESTs can become candidates for discovering new interesting genes through functional analysis currently being initiated in our laboratory.
In conclusion, by cryopreserving nationally protected Siberian tiger cells, we have generated an important genomic resource that captures the genomic information of this endangered breed. First, we established the Siberian tiger fibroblasts cell line to save its genetic resources at the cell level. Second, we constructed a full-length cDNA library of Siberian tiger using the cultured cell in vitro to save the genetic resources of Siberian tiger at molecular level. This resource offers an efficient way to identify more genes of this majestic species.
Acknowledgments
This research was supported by the Ministry of Agriculture of China for transgenic research (2008ZX08009-003), the “863” National Major Research Program (2007AA10Z170), National Key Technology R&D Program (2006BAD13B08).
References
- 1.Song J, Hua S, Song K. et al. Culture, characteristics and chromosome complement of Siberian tiger fibroblasts for nuclear transfer. In Vitro Cell Dev Biol Anim. 2007;43(7):203–209. doi: 10.1007/s11626-007-9043-3. [DOI] [PubMed] [Google Scholar]
- 2.Taro S, Junco N, Vladimir V. et al. Species and sex identification from faecal samples of sympatric carnivores, Amur leopard and Siberian tiger, in the Russian Far East. Conserv Genet. 2006;7(5):799–802. [Google Scholar]
- 3.Kun W, Zhihe Z, Wenping Z. et al. PCR-CTPP: a rapid and reliable genotyping technique based on ZFX/ZFY alleles for sex identification of tiger (Panthera tigris) and four other endangered felids. Conserv Genet. 2008;9(1):225–228. [Google Scholar]
- 4.Ying SY. Complementary DNA libraries: an overview. Mol Biotechnol. 2004;27:245–252. doi: 10.1385/MB:27:3:245. [DOI] [PubMed] [Google Scholar]
- 5.Li JY, Wang HY, Liu J. et al. Transcriptome Analysis of a cDNA Library from Adult Human Epididymis. DNA Res. 2008;15:115–122. doi: 10.1093/dnares/dsn005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu CQ, Guo Y, Guan WJ. et al. Establishment and Biological Characteristic Research of Luxi Cattle Fibroblast Cell Bank. Tissue Cell. 2008;40(6):417–424. doi: 10.1016/j.tice.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Wsheeler DL, Church DM, Federhen S. et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2003;31(1):28–33. doi: 10.1093/nar/gkg033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thanh T, Chi VT, Abdullah MP. et al. Construction of cDNA library and preliminary analysis of expressed sequence tags from green microalga Ankistrodesmus convolutus Corda. Mol Biol Rep. 2010 doi: 10.1007/s11033-010-0092-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Thompson JD, Higgin DG, Gibson TJ. CLUSTAL W: improving the sensitive of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weigh matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motoi N, Norihide Y, Takashi M. et al. Construction of a Multi-Functional cDNA Library Specific for Mouse Pancreatic Islets and Its Application to Microarray. DNA Res. 2004;11(5):315–323. doi: 10.1093/dnares/11.5.315. [DOI] [PubMed] [Google Scholar]
- 11.Shao ZT, Cong X, Yuan JD. et al. Construction and characterization of a cDNA library from head kidney of Japanese sea bass (Lateolabrax japonicus) Mol Biol Rep. 2009 Sep;36(7):2031–7. doi: 10.1007/s11033-009-9536-0. [DOI] [PubMed] [Google Scholar]
- 12.Du L X, Liu S F, Zhu J. et al. Construction of SMART cDNA Library of Sheep Ovary and Identification of Candidate Gene by Homologous Cloning. Scientia Agriculture Sinica. 2007;6(11):1390–1395. [Google Scholar]
- 13.Chen XH, Chen Z, Yao HP. et al. Construction and characterization of a cDNA library from human liver tissue with chronic hepatitis B. J Zhejiang Univ SCI. 2005;6(4):288–294. doi: 10.1631/jzus.2005.B0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke L, Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- 15.Li YP, Xia RX, Wang H. et al. Construction of a full-length cDNA Library from Chinese oak silkworm pupa and identification of a KK-42-binding protein gene in relation to pupa-diapause termination. Int J Biol Sci. 2009;5:451–457. doi: 10.7150/ijbs.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiemann S, Mehrle A, Bechtel S. et al. cDNAs for functional genomics and proteomics: The German sonsortium. C R Biol. 2003;326(10-11):1003–1009. doi: 10.1016/j.crvi.2003.09.036. [DOI] [PubMed] [Google Scholar]