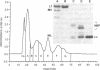
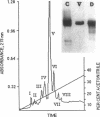
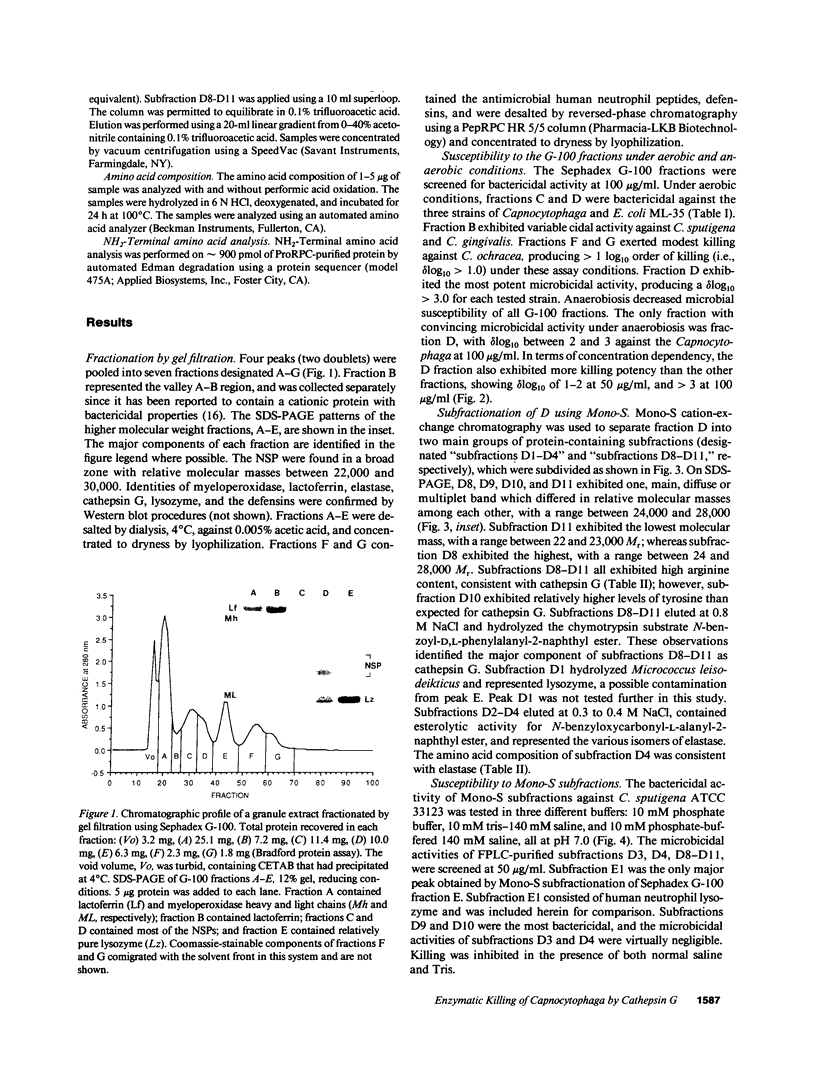
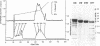Abstract
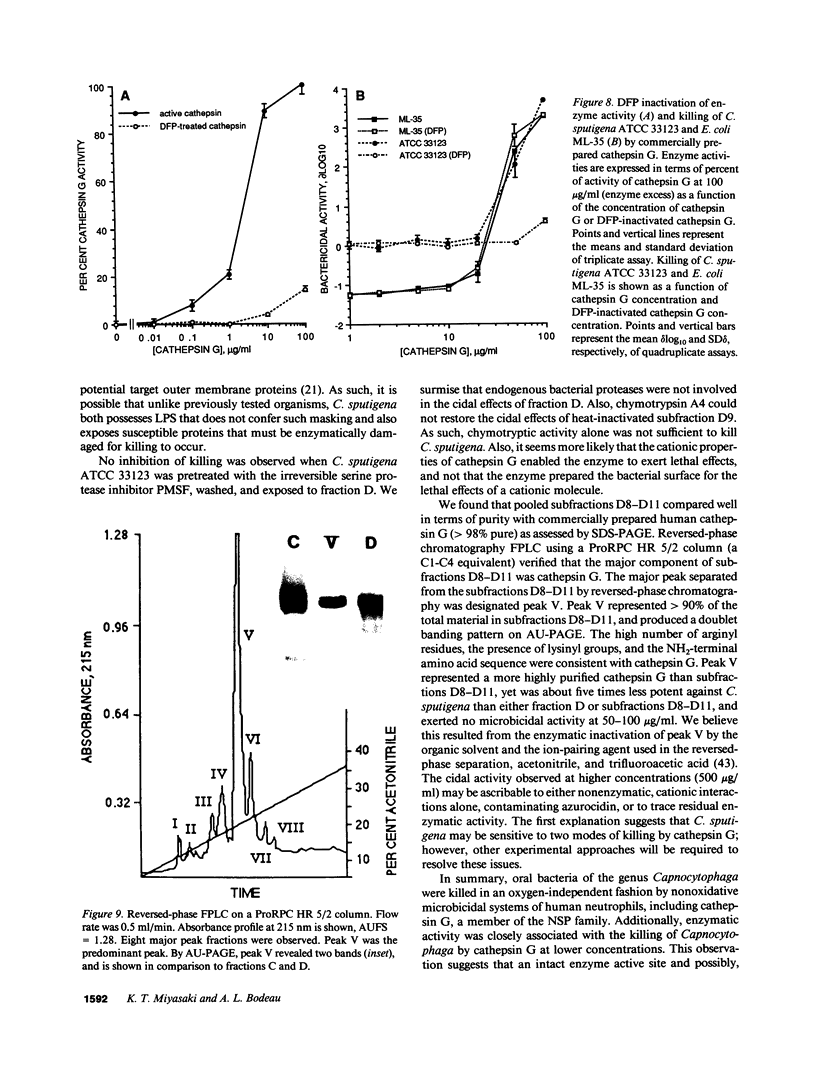
The Capnocytophaga are inhabitants of the hypoxic human gingival crevice that are normally prevented by neutrophils from causing periodontal and systemic infection. To identify potential nonoxidative bactericidal mechanisms against Capnocytophaga within human neutrophils, gel filtration chromatography was used to fractionate neutrophil granule extracts. Seven granule fractions, designated A through G, were obtained. The Capnocytophaga were most sensitive to killing by fraction D. Fraction D exhibited substantial bactericidal activity under aerobic and anaerobic conditions. The bactericidal activity associated with ion-exchange subfractions D8-D11, which contained primarily cathepsin G as assessed by enzymatic activity, amino acid composition, and NH2-terminal sequence. Heat-inactivation, diisopropylfluorophosphate, PMSF, and N-benzyloxycarbonylglycylleucylphenylalanyl-chloromethyl ketone inhibited bactericidal activity against Capnocytophaga sputigena but not Escherichia coli. We conclude that (a) human neutrophil cathepsin G is an important antimicrobial system against the Capnocytophaga, (b) the bactericidal activity of cathepsin G against Capnocytophaga is oxygen independent, and (c) an intact enzyme active site is involved in the killing of C. sputigena but not E. coli. We suggest that human neutrophil cathepsin G is an important antimicrobial system against certain oral bacteria and that cathepsin G kills bacteria by two distinct mechanisms.
Full text
PDF

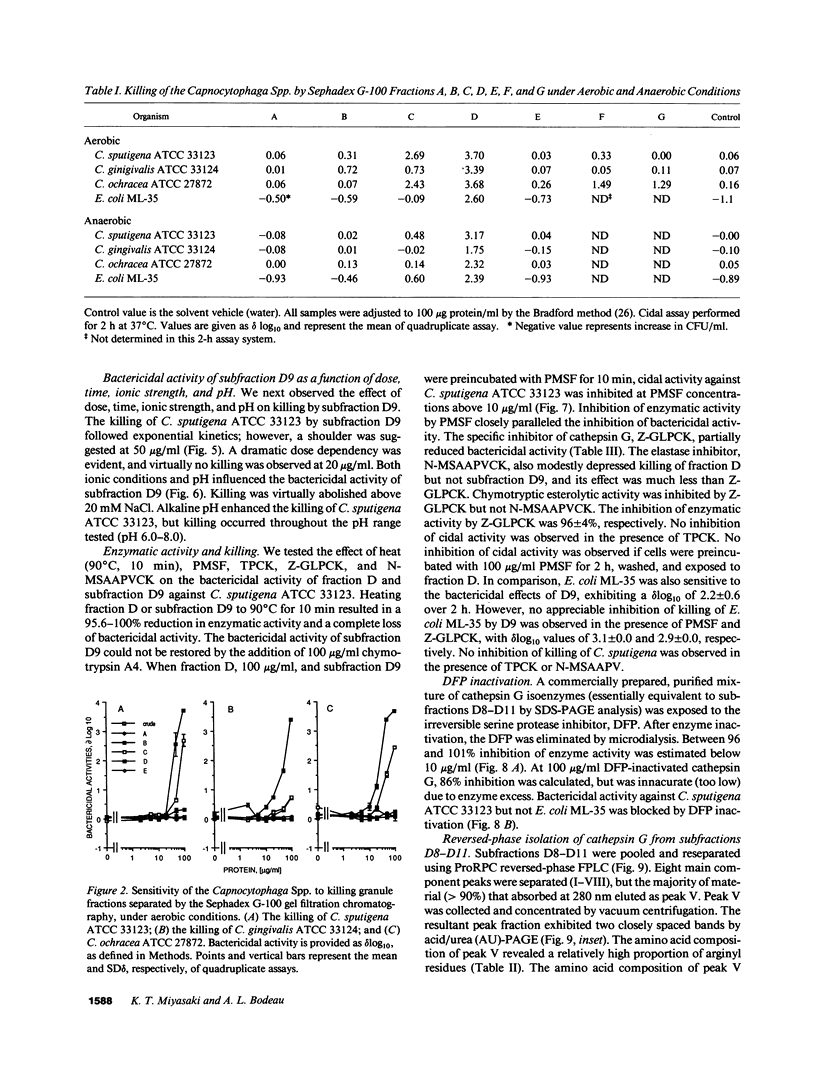
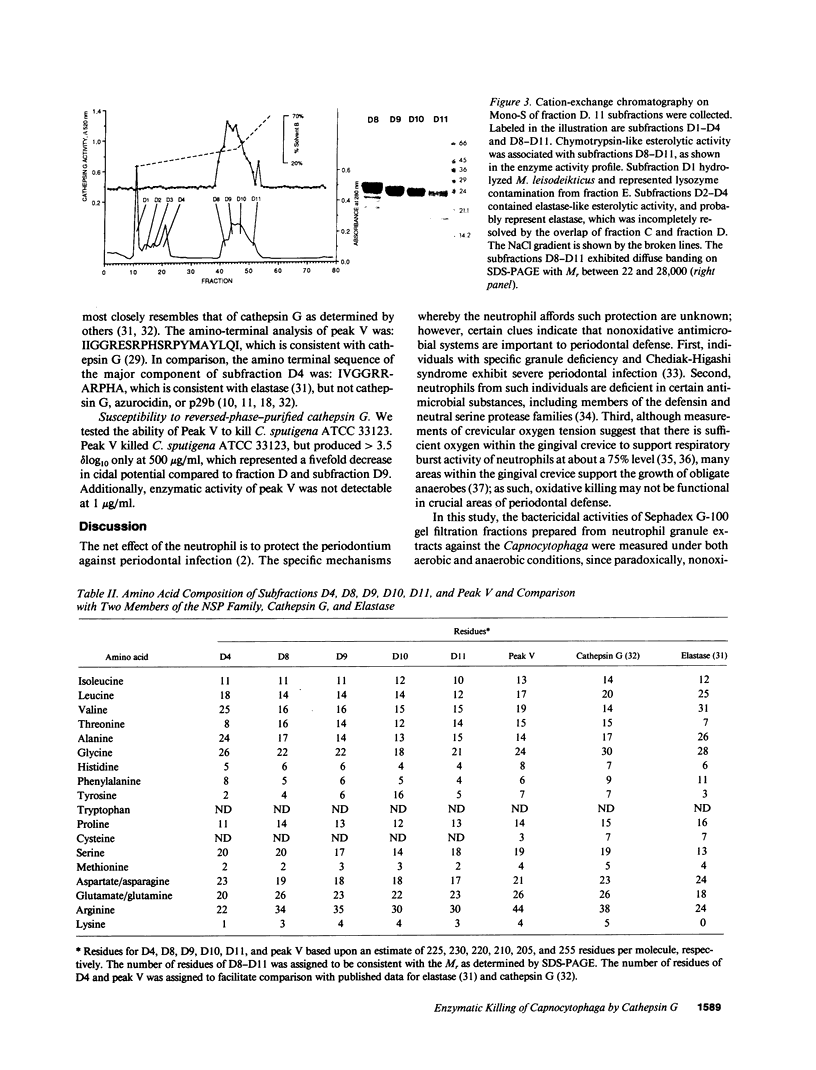
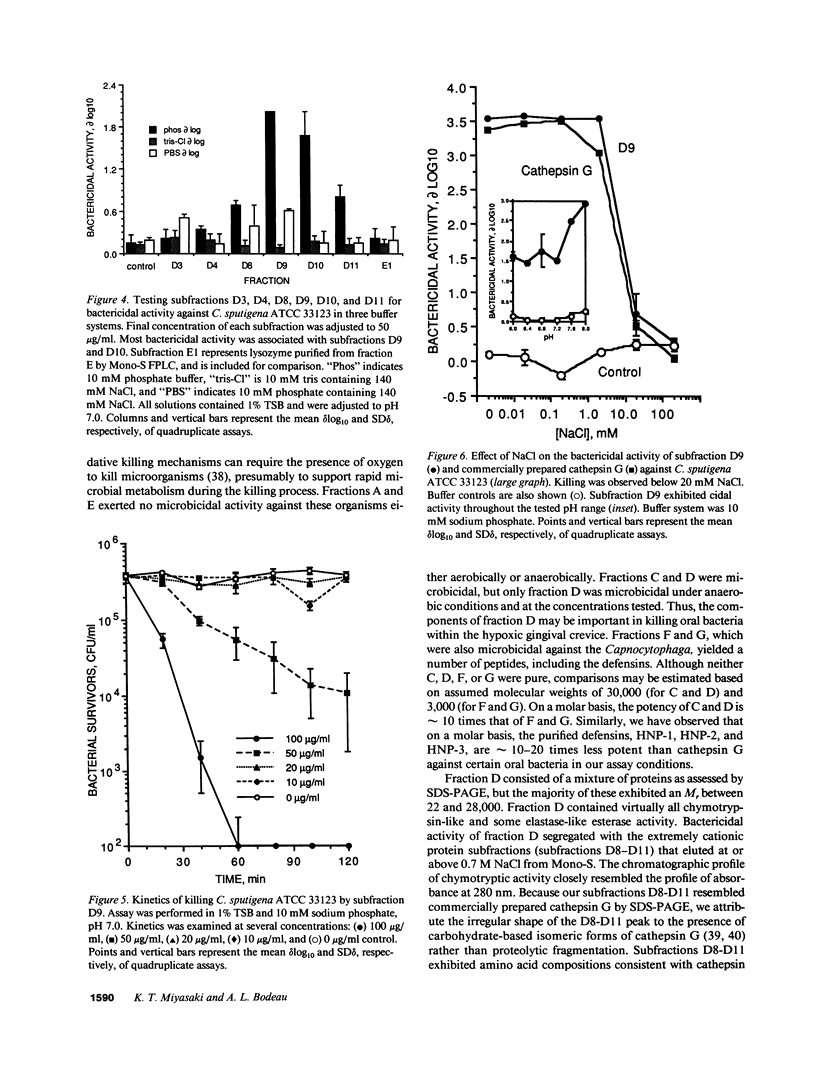
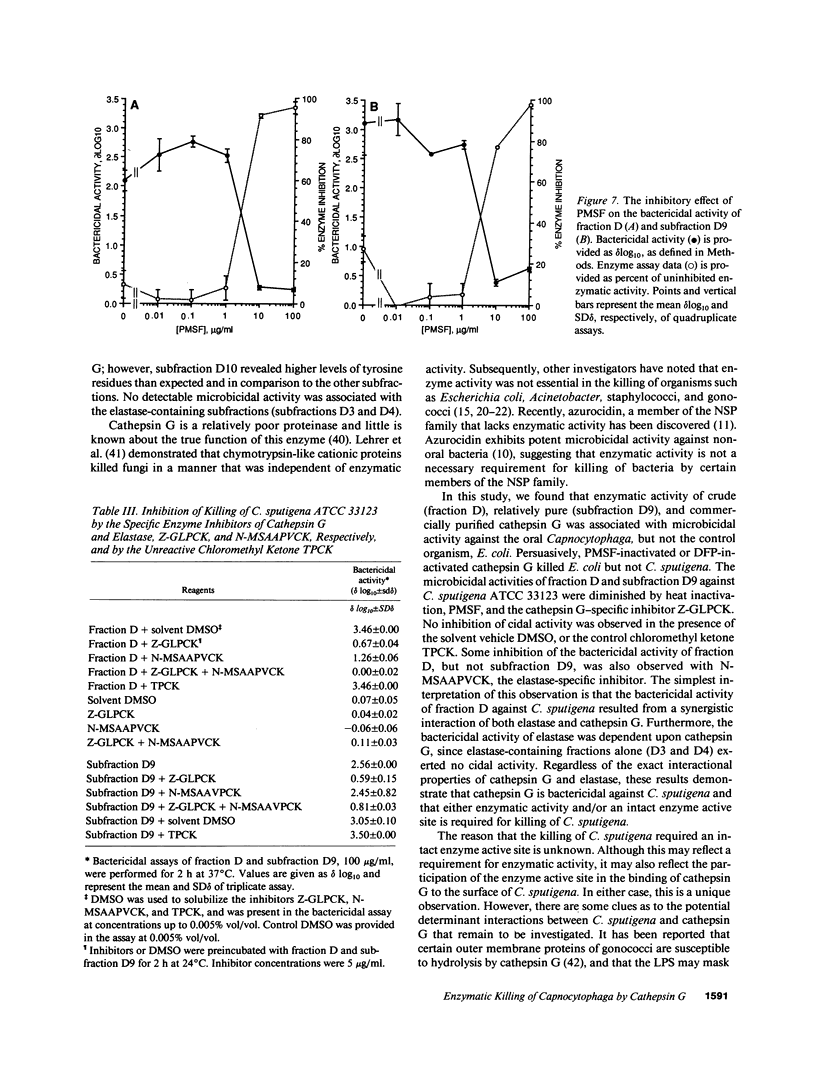

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Wilson M. E. Effect of clindamycin on neutrophil killing of gram-negative periodontal bacteria. Antimicrob Agents Chemother. 1988 Oct;32(10):1521–1527. doi: 10.1128/aac.32.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campanelli D., Detmers P. A., Nathan C. F., Gabay J. E. Azurocidin and a homologous serine protease from neutrophils. Differential antimicrobial and proteolytic properties. J Clin Invest. 1990 Mar;85(3):904–915. doi: 10.1172/JCI114518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon J. A., Mergenhagen S. E., Gallin J. I. Gingivitis and oral ulceration in patients with neutrophil dysfunction. J Oral Pathol. 1985 Feb;14(2):150–155. doi: 10.1111/j.1600-0714.1985.tb00478.x. [DOI] [PubMed] [Google Scholar]
- Dewald B., Rindler-Ludwig R., Bretz U., Baggiolini M. Subcellular localization and heterogeneity of neutral proteases in neutrophilic polymorphonuclear leukocytes. J Exp Med. 1975 Apr 1;141(4):709–723. doi: 10.1084/jem.141.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlenza S. W., Newman M. G., Lipsey A. I., Siegel S. E., Blachman U. Capnocytophaga sepsis: a newly recognised clinical entity in granulocytopenic patients. Lancet. 1980 Mar 15;1(8168 Pt 1):567–568. doi: 10.1016/s0140-6736(80)91057-0. [DOI] [PubMed] [Google Scholar]
- Gabay J. E., Scott R. W., Campanelli D., Griffith J., Wilde C., Marra M. N., Seeger M., Nathan C. F. Antibiotic proteins of human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5610–5614. doi: 10.1073/pnas.86.14.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabig T. G., Bearman S. I., Babior B. M. Effects of oxygen tension and pH on the respiratory burst of human neutrophils. Blood. 1979 Jun;53(6):1133–1139. [PubMed] [Google Scholar]
- Gandola C., Butler T., Badger S., Cheng E., Beard S. Septicemia caused by Capnocytophaga in a granulocytopenic patient with glossitis. Arch Intern Med. 1980 Jun;140(6):851–852. [PubMed] [Google Scholar]
- Ganz T., Metcalf J. A., Gallin J. I., Boxer L. A., Lehrer R. I. Microbicidal/cytotoxic proteins of neutrophils are deficient in two disorders: Chediak-Higashi syndrome and "specific" granule deficiency. J Clin Invest. 1988 Aug;82(2):552–556. doi: 10.1172/JCI113631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Lehrer R. I. Antimicrobial activity of phagocyte granule proteins. Semin Respir Infect. 1986 Jun;1(2):107–117. [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985 Oct;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan P. H., McCarthy L. R., Bissett B. K. Capnocytophaga ochracea septicemia. J Clin Microbiol. 1981 Apr;13(4):643–645. doi: 10.1128/jcm.13.4.643-645.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck L. W., Darby W. L., Hunter F. A., Bhown A., Miller E. J., Bennett J. C. Isolation, characterization, and amino-terminal amino acid sequence analysis of human neutrophil elastase from normal donors. Anal Biochem. 1985 Aug 15;149(1):153–162. doi: 10.1016/0003-2697(85)90488-9. [DOI] [PubMed] [Google Scholar]
- Heck L. W., Rostand K. S., Hunter F. A., Bhown A. Isolation, characterization, and amino-terminal amino acid sequence analysis of human neutrophil cathepsin G from normal donors. Anal Biochem. 1986 Oct;158(1):217–227. doi: 10.1016/0003-2697(86)90612-3. [DOI] [PubMed] [Google Scholar]
- Hovde C. J., Gray B. H. Characterization of a protein from normal human polymorphonuclear leukocytes with bactericidal activity against Pseudomonas aeruginosa. Infect Immun. 1986 Oct;54(1):142–148. doi: 10.1128/iai.54.1.142-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono V. J., Boldt P. R., MacKay B. J., Cho M. I., Pollock J. J. Lytic sensitivity of Actinobacillus actinomycetemcomitans Y4 to lysozyme. Infect Immun. 1983 May;40(2):773–784. doi: 10.1128/iai.40.2.773-784.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmar J. R., Arnold R. R. Killing of Actinobacillus actinomycetemcomitans by human lactoferrin. Infect Immun. 1988 Oct;56(10):2552–2557. doi: 10.1128/iai.56.10.2552-2557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Szklarek D., Selsted M. E. Modulation of the in vitro candidacidal activity of human neutrophil defensins by target cell metabolism and divalent cations. J Clin Invest. 1988 Jun;81(6):1829–1835. doi: 10.1172/JCI113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ladra K. M., Hake R. B. Nonoxidative fungicidal mechanisms of mammalian granulocytes: demonstration of components with candidacidal activity in human, rabbit, and guinea pig leukocytes. Infect Immun. 1975 Jun;11(6):1226–1234. doi: 10.1128/iai.11.6.1226-1234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Gusberti F., Mettraux G., Higgins T., Syed S. Relationship between oxygen tension and subgingival bacterial flora in untreated human periodontal pockets. Infect Immun. 1983 Nov;42(2):659–667. doi: 10.1128/iai.42.2.659-667.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Oxygen sensitivity of various anaerobic bacteria. Appl Microbiol. 1969 Nov;18(5):723–727. doi: 10.1128/am.18.5.723-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaki K. T., Wilson M. E., Brunetti A. J., Genco R. J. Oxidative and nonoxidative killing of Actinobacillus actinomycetemcomitans by human neutrophils. Infect Immun. 1986 Jul;53(1):154–160. doi: 10.1128/iai.53.1.154-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaki K. T., Wilson M. E., Cohen E., Jones P. C., Genco R. J. Evidence for and partial characterization of three major and three minor chromatographic forms of human neutrophil myeloperoxidase. Arch Biochem Biophys. 1986 May 1;246(2):751–764. doi: 10.1016/0003-9861(86)90332-2. [DOI] [PubMed] [Google Scholar]
- Newman M. G., Socransky S. S. Predominant cultivable microbiota in periodontosis. J Periodontal Res. 1977 Mar;12(2):120–128. doi: 10.1111/j.1600-0765.1977.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Shafer W. M. Lipopolysaccharide masking of gonococcal outer-membrane proteins modulates binding of bacterial cathepsin G to gonococci. J Gen Microbiol. 1988 Mar;134(3):539–545. doi: 10.1099/00221287-134-3-539. [DOI] [PubMed] [Google Scholar]
- Shafer W. M., Martin L. E., Spitznagel J. K. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect Immun. 1984 Jul;45(1):29–35. doi: 10.1128/iai.45.1.29-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Morse S. A. Cleavage of the protein III and major iron-regulated protein of Neisseria gonorrhoeae by lysosomal cathepsin G. J Gen Microbiol. 1987 Jan;133(1):155–162. doi: 10.1099/00221287-133-1-155. [DOI] [PubMed] [Google Scholar]
- Shafer W. M., Onunka V. C. Mechanism of staphylococcal resistance to non-oxidative antimicrobial action of neutrophils: importance of pH and ionic strength in determining the bactericidal action of cathepsin G. J Gen Microbiol. 1989 Apr;135(4):825–830. doi: 10.1099/00221287-135-4-825. [DOI] [PubMed] [Google Scholar]
- Shurin S. B., Socransky S. S., Sweeney E., Stossel T. P. A neutrophil disorder induced by capnocytophaga, a dental micro-organism. N Engl J Med. 1979 Oct 18;301(16):849–854. doi: 10.1056/NEJM197910183011601. [DOI] [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Neutral proteinases of human spleen. Purification and criteria for homogeneity of elastase and cathepsin G. Biochem J. 1976 May 1;155(2):255–263. doi: 10.1042/bj1550255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Oliver R. C., Barrett A. J. Lysis and killing of bacteria by lysosomal proteinases. Infect Immun. 1976 Aug;14(2):555–563. doi: 10.1128/iai.14.2.555-563.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Sasagawa T., Resing K., Walsh K. A. A simple and rapid purification of commercial trypsin and chymotrypsin by reverse-phase high-performance liquid chromatography. Anal Biochem. 1982 Jul 1;123(2):408–412. doi: 10.1016/0003-2697(82)90465-1. [DOI] [PubMed] [Google Scholar]
- Van Dyke T. E., Wilson-Burrows C., Offenbacher S., Henson P. Association of an abnormality of neutrophil chemotaxis in human periodontal disease with a cell surface protein. Infect Immun. 1987 Sep;55(9):2262–2267. doi: 10.1128/iai.55.9.2262-2267.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watorek W., Farley D., Salvesen G., Travis J. Neutrophil elastase and cathepsin G: structure, function, and biological control. Adv Exp Med Biol. 1988;240:23–31. doi: 10.1007/978-1-4613-1057-0_3. [DOI] [PubMed] [Google Scholar]
- Weiss J., Elsbach P., Olsson I., Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978 Apr 25;253(8):2664–2672. [PubMed] [Google Scholar]
- Wilde C. G., Snable J. L., Griffith J. E., Scott R. W. Characterization of two azurphil granule proteases with active-site homology to neutrophil elastase. J Biol Chem. 1990 Feb 5;265(4):2038–2041. [PubMed] [Google Scholar]
- Wilson M. E., Jonak-Urbanczyk J. T., Bronson P. M., Dudas K. C., Apicella M. A., Genco R. J. Capnocytophaga species: increased resistance of clinical isolates to serum bactericidal action. J Infect Dis. 1987 Jul;156(1):99–106. doi: 10.1093/infdis/156.1.99. [DOI] [PubMed] [Google Scholar]