Abstract
Type XI collagen is a quantitatively minor yet essential constituent of the cartilage extracellular matrix. The amino propeptide of the α1 chain remains attached to the rest of the molecule for a longer period of time after synthesis than the other amino propeptides of type XI collagen and has been localized to the surface of thin collagen fibrils. Yeast two-hybrid system was used to demonstrate that a homodimer of α1(XI) amino propeptide (α1(XI)Npp) could form in vivo. Interaction was also confirmed using multi-angle laser light scattering, detecting an absolute weight average molar mass ranging from the size of a monomer to the size of a dimer (25,000–50,000 g/mol), respectively. Binding was shown to be saturable by ELISA. An interaction between recombinant α1(XI)Npp and the endogenous α1(XI)Npp was observed, and specificity for α1(XI)Npp but not α2(XI)Npp was demonstrated by co-precipitation. The interaction between the recombinant form of α1(XI)Npp and the endogenous α1(XI)Npp resulted in a stable association during the regeneration of cartilage extracellular matrix by fetal bovine chondrocytes maintained in pellet culture, generating a protein that migrated with an apparent molecular mass of 50–60 kDa on an SDS-polyacrylamide gel.
Type XI collagen is a constituent of the extracellular matrix of cartilage. It belongs to the family of fibrillar collagens, which includes types I, II, III, V, and XI (1). As with all of the fibrillar collagens, type XI contains a 300-nm uninterrupted triple helical domain (2). Type XI collagen forms the heterotypic collagen fibrils characteristic of cartilage along with types II and the nonfibrillar type IX collagen (3). Although minor in quantity, type XI collagen is thought to play a critical role in collagen fibril assembly and extracellular matrix organization in early development as demonstrated by the chondrodystrophic mouse (cho/cho) (4). Fibrils of developing cartilage are of uniform 20 nm in diameter and oriented more randomly than fibrils of adult articular cartilage.
Collagen fibril assembly in embryonic cartilage is known to rely upon the ratio of the collagenous constituents, types II and XI (5). Initially, type XI collagen may function to nucleate the formation of new collagen fibrils (6). The role of type XI collagen in limitation of lateral growth of collagen fibrils is well established, although the molecular mechanism is not fully understood. Type XI collagen may also play a role in the maturation of thin fibrils into the larger diameter fibrils of the territorial zone of cartilage matrix, either by regulation of the accretion of more collagen molecules onto the surface of thin fibrils or by mediating the fusion of thin fibrils.
Type XI collagen is initially synthesized as a procollagen, which is subsequently proteolytically processed at both the amino and carboxyl termini (7). The amino-terminal processing site was identified 7 amino acids from the variable region (8, 9). Recently, type XI α1 pro-collagen was identified as a substrate for the enzyme bone morphogenetic protein 1 (BMP-1)1 (10). The carboxyl propeptide of the α1 chain of type XI collagen is homologous to the other fibrillar collagens, whereas the amino propeptide of type XI α1 pro-collagen is markedly different. In addition to the characteristic minor triple helix, the α1 chain of XI contains a large non-triple helical domain at the amino terminus.
The α1 chain of type XI collagen exists as a set of isoforms that arise by alternative splicing of the primary RNA transcript (11). The portion encoding the variable region gives rise to biochemically unique domains. However, common to all isoforms is the amino propeptide, the distal globular 233 amino acids, which is ultimately proteolytically removed during the process of extracellular matrix assembly and fibril formation. Proteolytic removal of the amino propeptide may rely on the action of BMP-1, as it does in the case of α1(V) collagen chain (12).
The amino propeptide domain is structurally related to other amino propeptides including that of the α2(XI) and α1(V) collagen chains. The position of four cysteine residues are conserved, and sequence analysis predicts a conserved secondary structural motif rich in β-strand (13). This prediction has been supported by analysis of circular dichroism spectra, demonstrating a relatively high percentage β-strand and a markedly low percentage of α helix (14). The dimensions of the globular amino propeptide domain of α1(XI) collagen chain has been estimated by rotary shadowing to be at least 8 nm in diameter. This domain has been localized to the surface of collagen fibrils by immunoelectron microscopy (15).
Although the amino propeptides are ultimately removed from the collagen triple helical molecule by proteolytic processing, the rate and extent of removal vary. Although the processing of α1(XI) collagen chain is relatively slow, thus achieving 50% completion by 18 h after synthesis in pulse-chase experiments, the processing of the α2(XI) collagen chain is much faster, 75% complete by 5 h after synthesis (16). This raises the possibility that the amino propeptides carry out different functions and that the α1(XI) amino propeptide may function while still retained on the surface of collagen fibrils for the extended period of time after synthesis. Globular domains poised on the surface of newly synthesized collagen fibrils may play a role in both the mechanism of growth of an individual fibril as well as lateral fusion of neighboring collagen fibrils or interaction between the collagen fibrils and other constituents of the extracellular matrix.
We report the interaction between amino propeptide domains of α1(XI) collagen chains. The interaction had a dissociation constant on the order of 130 nm. This interaction was stabilized in cell culture by an association that was not disrupted by reducing agents, EDTA, SDS, or heat. Such an interaction may explain, in part, how collagen XI functions to regulate growth of collagen fibrils in developing tissues.
EXPERIMENTAL PROCEDURES
Synthesis and Purification of Recombinant Rat α1(XI)Npp Domain
A cDNA fragment encoding the amino propeptide of α1 chain of type XI collagen was inserted into expression vector pET11a (Stratagene). BL21 DE3 transformants were screened for expression of recombinant protein on SDS-polyacrylamide gels and subsequent transfer to polyvinylidene difluoride membrane for detection of recombinant proteins by nickel affinity blot using nickel-NTA conjugated to alkaline phosphatase (Qiagen). Cells from transformant cultures were collected by centrifugation, and proteins were solubilized using bacterial protein extraction reagent (Pierce) with 1 unit of DNase I per 10 ml of cell suspension. This extraction solubilized the majority of cellular proteins, leaving recombinant α1(XI) amino propeptide (α1(XI)Npp) insoluble in the inclusion bodies. Protein from inclusion bodies was solubilized and unfolded in 20 mm Tris-HCl, pH 7.5, with freshly prepared 8 m urea, and 14 mm β-mercaptoethanol at a protein concentration of ~1 mg/ml. Refolding was initiated by diluting solubilized unfolded protein dropwise and with stirring into 100 volumes of 20 mm Tris-HCl, pH 7.5, containing 100 mm KCl, 20% glycerol, and 2 m urea at 4 °C. Incubation at 4 °C was continued for 16 h. Refolded protein was concentrated by ultrafiltration using a Centriprep filtration unit (Amicon). Insoluble material, if present, was removed by centrifugation. Refolded protein was further purified by chelation chromatography on a Hi-Trap nickel column (Amersham Biosciences) (Fig. 1). Disulfide-bonded cysteine pairs were verified by tryptic digest followed by protein sequencing of those bands present in the absence of 10 mm DTT but absent when the sample was treated with reducing agent as described previously (17). Refolded protein was characterized by circular dichroism and compared with native protein isolated from tissue (17). Npp without a His6 tag was generated by removing the carboxyl portion of a recombinant α1(XI) amino-terminal domain containing the variable region (α1(XI)NTD), which contains a functional BMP-1 proteolytic processing site (10). Recombinant protein was incubated in the presence of purified recombinant human BMP-1 (FibroGen, Inc.) as described previously (10).
Fig.1. Expression of recombinant rat α1(XI)Npp.
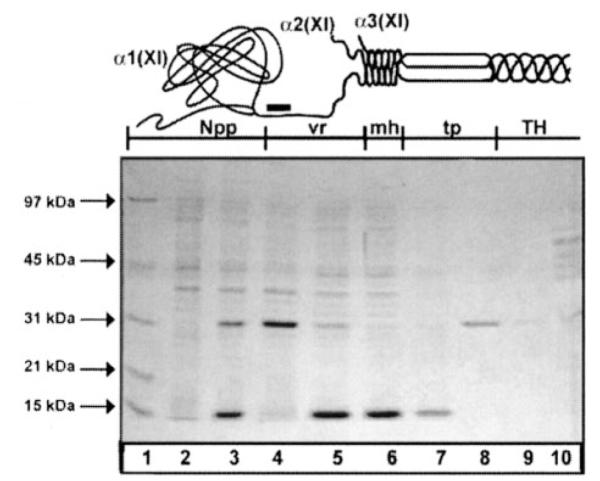
A, schematic representation of the amino-terminal domain of a type XI collagen molecule. The Npp of the α1(XI) chain is shown as a terminal globular domain attached to the minor helix (mh) via an extended variable region (vr). The α2(XI)Npp is absent from this schematic to represent the rapid proteolytic processing of this chain. Between the minor helix and the major triple helix (TH) lies the amino telopeptide (tp). Black bar represents location of antibody recognition site. B, extracts of E. coli cells grown in the presence of IPTG were resolved by SDS-PAGE on a 12% polyacrylamide gel stained with Coomassie Blue. Lane 1, molecular weight markers; lane 2, mid-log culture grown in the absence of IPTG; lane 3, mid-log culture grown in the presence of IPTG; lane 4, insoluble protein after extraction of E. coli cell pellet; lane 5, soluble protein after protein extraction; lane 6, flow-through from nickel-affinity column; lane 7, column wash; lanes 8 and 9, elution of purified, refolded recombinant protein; lane 10, recombinant protein markers.
Yeast Two-hybrid System
A protein-protein interaction was tested in vivo using Matchmaker GAL4 (Clontech, Inc.) yeast two-hybrid system. cDNA encoding α1(XI)Npp was obtained by reverse transcription-PCR using the following primers: 5′-cca tgg ttg ata tac tga aag ctt tag at-3′ and 5′-att agg atc ctc atc tat gtg cgg ctc ctg-3′. This fragment was used to make two different gene fusions: one with the DNA binding domain of GAL4 and the other with the transcriptional activation domain of GAL4 in the plasmids pGBKT7 and pGADT7, respectively.
Yeast host strain AH109 was transformed using the lithium acetate method (17). Selection of positive transformants was carried out by plating on SD minimal medium agar plates lacking the nutrient leucine and tryptophan to select for the plasmids derived from pGBKT7 and pGADT7, respectively. Plates lacking both nutrients were used to select for double transformants.
To select for colonies in which the two-hybrid proteins interact, double transformants were plated on SD minimal medium lacking tryptophan, leucine, and histidine. Protein-protein interaction was verified by determining the expression of β-galactosidase activity by colony-lift filter assay and by determining the level of expression in liquid culture using chloro-phenol red-β-d-galactopyranoside as substrate (Clontech).
Weight Average Molecular Weight Determination by Light Scattering
Recombinant α1(XI)Npp was applied to a TSK 3000SW size exclusion column at 10 °C in the following buffer: 30 mm Na2HPO4, 45mm KH2PO4, pH 6.6, containing 100 mm KCl. The eluting peak was analyzed using a refractive index detector, a UV monitor, and by multi-angle laser light scatter (DAWN, Wyatt Technology, Santa Barbara, CA) to determine the absolute molecular weight of α1(XI)Npp in solution. A vertically polarized laser beam was passed through the flow cell containing the sample. The scattered light was simultaneously detected by 18 separate detectors at different angles. The intensity of the scattered light was digitized and transmitted to a computer for analysis by software provided by the manufacturer. The weight-average molar mass was determined from the Rayleigh ratio of scattered light, molecular concentration in grams/milliliters, and the specific refractive index increment of the dissolved molecules (dn/dc). The concentration was determined from the refractive index at each slice of the chromatogram and also from the absorbance at 280 nm and the 0.1% extinction coefficient, calculated for recombinant rat α1(XI)Npp to be 0.548 assuming all cysteine residues exist as halfcystines. The dn/dc value used was 0.185. Recombinant α1(XI)Npp was analyzed under three salt conditions: 75, 175, and 500 mm with respect to additive concentration of KCl and buffer components. Molar mass was determined for individual slices of data across the eluting size exclusion chromatographic peak and plotted as a function of elution volume. Molecular weight at the leading and trailing edges of the chromatographic peak was compared with to determine whether recombinant α1(XI)Npp multimerized under the buffer conditions of the column.
Antibodies
A peptide coding for the human α1(XI)Npp was used to make the α1(XI)Npp antibody that showed specificity for the endogenous α1(XI)Npp but not the recombinant α1(XI)Npp. Preparation and characterization of this antibody was reported previously (18). The ability of the α1(XI)Npp antibody to discriminate between endogenous and recombinant α1(XI)Npp was demonstrated by immunoblot and nickel affinity blot (Fig. 6). Samples of increasing amounts of recombinant α1(XI)Npp were loaded onto an SDS gel and subsequently transferred to polyvinylidene difluoride membrane. Nickel-NTA-alkaline phosphatase conjugate was used to detect recombinant protein from a sample that was three orders of magnitude more dilute than the concentration needed for detection by the polyclonal antibody to α1(XI)Npp. Generation of the α2(XI)Npp antibody was carried out using the sequence RERPQRQPSHRTQ from bovine sequence (18) as the immunizing peptide. Antibody to the variable region of the recombinant α1(XI) amino-terminal domain was described previously (19).
Fig. 6. Characterization of specificity of antibody and recombinant α1(XI)Npp.
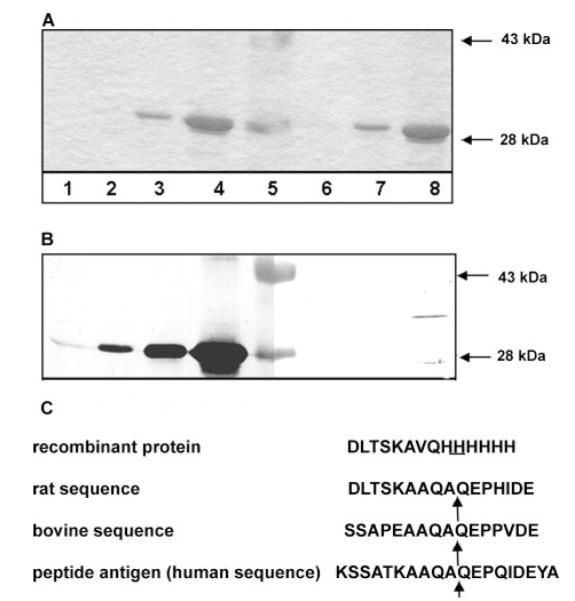
Specific changes made to the recombinant α1(XI)Npp allowed the antibody to distinguish endogenous bovine α1(XI)Npp from recombinant rat α1(XI)Npp. A, recombinant α1(XI)Npp in 10× increasing incremental concentration curve. Lane 1, 0.01-μg protein; lane 2, 0.1-μg protein; lane 3, 1-μg protein; lane 4, 10-μg protein; lane 5, molecular weight markers, 43 and 28 kDa; lane 6, 0.1-μg protein; lane 7, 1.0-μg protein; lane 8, 10-μg protein. B, nickel affinity and immunoblot of recombinant protein. Lanes 1–8, same samples as in A. Lanes 1–4, nickel affinity blot; lanes 6–8, immunoblot with α1(XI)Npp antibody. C, sequence of antigenic peptide compared with the analogous site in bovine and rat α1(XI)Npp and with the sequence of the recombinant protein. Although the antibody recognized endogenous rat α1(XI) collagen chains, it did not recognize the recombinant protein. The proteolytic processing site is indicated by the arrow.
ELISA Assay
Recombinant α1(XI)Npp was adsorbed to a microtiter plate in 96-well format at room temperature. To block unoccupied sites on plastic surface, wells were incubated with buffered skim milk powder solution for 1 h at room temperature. Recombinant α1(XI)NTD was allowed to interact with the α1(XI)Npp bound to the plate for 2 h at room temperature. Antibody to the unique variable region was used to detect interaction. Absorbance at 405 nm was plotted as a function of the concentration of bound α1(XI)NTD with variable region detected. This antibody did not recognize α1(XI)Npp. Nonspecific binding of the primary antibody was tested in the absence of antigen. Nonspecific binding of the secondary antibody was tested in the absence of primary antibody. Bovine serum albumin was bound to the 96-well plate as a control to detect nonspecific binding of α1(XI)NTD. Data was subjected to Scatchard analysis to determine KD and Bmax.
Co-precipitation of Recombinant and Endogenous α1(XI)Npp Domains
Recombinant α1(XI)Npp, synthesized with six histidine residues in tandem at the carboxyl terminus of the protein, was bound to nickel-NTA-agarose (Qiagen) to investigate the specificity of the interaction between α1(XI)Npp domains. Protein was extracted from bovine chondrocyte pellet cultures using 50 mm Tris-HCl, pH 7.5, 1 m NaCl, and 5 mm EDTA. No denaturants such as guanidine hydrochloride or urea were present during the extraction. Salt concentration was reduced to 150 mm by dialysis prior to incubation with α1(XI)Npp-bound nickel-agarose. Material that bound to the recombinant α1(XI)Npp was separated from that which did not bind by centrifugation. Samples were analyzed by SDS-polyacrylamide gel electrophoresis followed by immunoblot using an antibody that recognized α1(XI)Npp and one that recognized a very similar domain, α2(XI)Npp.
Pellet Culture of Bovine Chondrocytes
Fetal bovine chondrocytes were maintained in pellet culture (20). Where indicated in the text, β-aminopropionitrile fumarate was included in pellet culture medium at a final concentration of 64 μg/ml, putrescine was included at a final concentration of 2 mm, AEBSF (4-(2-aminoethyl)-benzene sulfonyl-fluoride·HCl) was included at a final concentration of 0.1 mm, and recombinant α1(XI)Npp was included in tissue culture medium at a concentration of 10 μg/ml for 24 h. Proteins from medium and cell pellets were analyzed by SDS-PAGE in the presence of β-mercaptoethanol and subsequent immunoblot and nickel affinity blot.
RESULTS
Recombinant Rat α1(XI)Npp Domain
A 682-bp fragment encoding α1(XI)Npp was obtained by reverse transcription-PCR and inserted into the pET11a expression vector. Recombinant α1(XI)Npp was expressed as a fusion protein with gene-10 gene product of bacteriophage T7, resulting in the addition of amino acids MAS at the amino terminus of the protein. Protein was expressed by BL21(DE3) Escherichia coli cells upon isopropyl-1-thio-β-d-galactopyranoside induction. The DNA sequence of the insert was determined by automated fluorescence DNA sequencing. The terminal methionine was removed in the bacterial expression system as verified by LCQ™MS/MS. The sequence of interest proceeded from the first proline after the signal peptide processing site of α1(XI) and continued to the glutamine one amino acid before the amino propeptide processing site. Codons for six histidine residues were included in the downstream PCR primer immediately preceding a stop codon. The penultimate amino acid, alanine, was changed conservatively to a valine to allow the antibody to discriminate between endogenous bovine α1(XI)Npp and recombinant rat α1(XI)Npp. In addition, this change modified the sequence at the proteolytic processing site of α1(XI) collagen, reducing the chance that the histidine tag would be removed from the recombinant protein. Recombinant α1(XI)Npp with a His6 tag had a molecular weight of 24,826 g/mol and a theoretical pI of 6.91. Recombinant α1(XI)Npp was recovered from inclusion bodies. After unfolding and refolding, recombinant α1(XI)Npp was purified by nickel-chelation chromatography (Fig. 1). Purified bacterial recombinant α1(XI)Npp co-migrated with human embryonic kidney 293 recombinant α1(XI)Npp by SDS-polyacrylamide gel electrophoresis (data not shown). Unfolded and refolded α1(XI)Npp was previously characterized by spectropolarimetry, electron microscopy, and LCQ-MS/MS (17).
Detection of Interaction Using Yeast Two-hybrid System
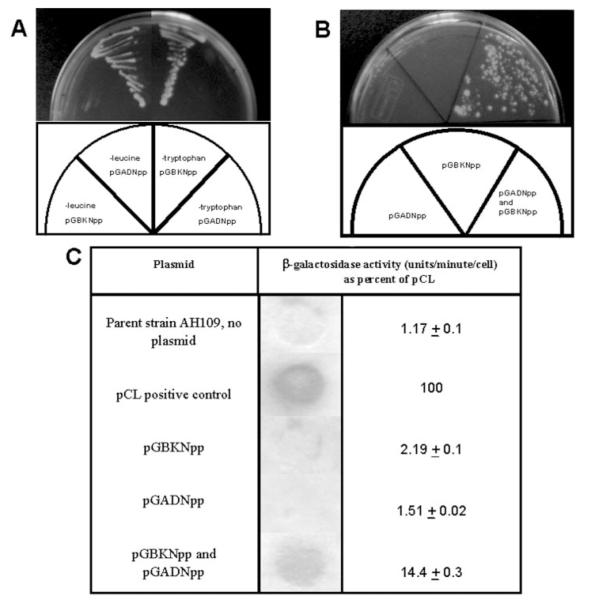
Expression vectors encoding fusion proteins between the DNA binding domain of GAL4 and α1(XI)Npp and between the transcriptional activation domain of GAL4 and α1(XI)Npp were constructed. Cells cotransfected with both constructs were able to grow in the absence of histidine (Fig. 2), indicating an interaction between the α1(XI)Npp domain portion of each of the fusion proteins. Interaction was also demonstrated by detection of β-galactosidase activity (Fig. 2C). The level of β-galactosidase activity in double transformants was ~10-fold higher than background.
Fig.2. Detection of α1(XI)Npp: α1(XI)Npp interaction by yeast two-hybrid system.
A, yeast transformants plated on selective media (minus tryptophan and minus leucine) as indicated in schematic. B, single and double transformants plated in the absence of tryptophan-, leucine-, and histidine-selective medium. C, β-galactosidase activity detected by colony lift and liquid culture assay.
Size Exclusion Chromatography and Multi-angle Laser Light Scatter
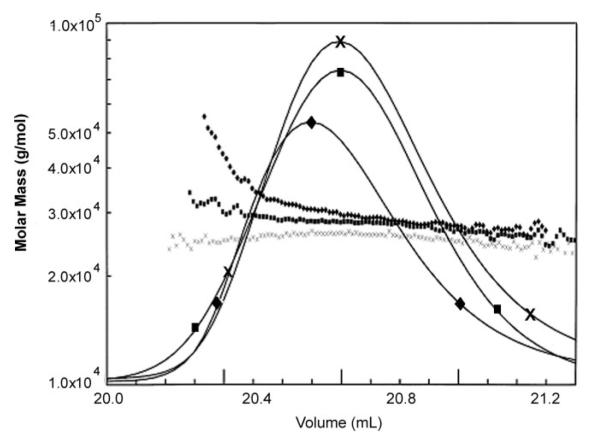
At physiological ionic strength (175 mm), the weight average molar mass varied from the molecular mass of a monomer (25,000 g/mol) at the trailing edge of the size exclusion chromatography peak to 40,000 g/mol, an average molar mass lying between that of a dimer (50,000 g/mol) and a monomer at the leading edge of the peak (Fig. 3). Interaction was favored by low ionic strength (75 mm) and disrupted by high ionic strength (500 mm). Maximum molecular weight observed corresponded to a dimer with no indication of higher multimerization. Multimerization was inferred by an increase in molar mass of purified recombinant α1(XI)Npp.
Fig.3. Npp:Npp interaction detected by size exclusion chromatography and light scattering.
Weight average molar mass plotted as a function of elution volume from TSK 3000SW column. Buffer conditions varied from low ionic strength, 75 mm (diamonds), physiological ionic strength, 175 mm (squares), and high ionic strength, 500 mm (×). Average molecular weight values are shown for the trailing and leading edges of chromatographic peaks.
ELISA
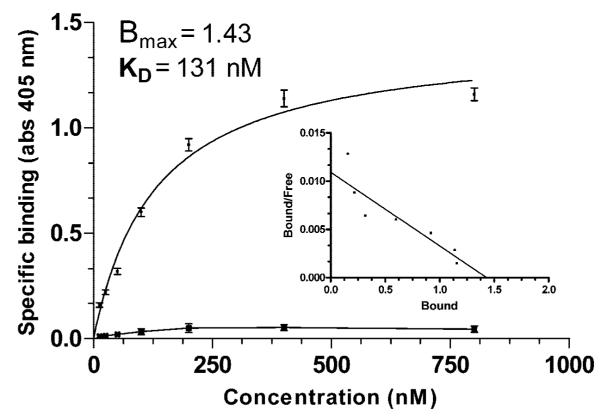
Binding was shown to be saturable by ELISA (Fig. 4) in which recombinant α1(XI)Npp was bound to the plate and a tagged recombinant isoform of α1(XI) amino-terminal domain was allowed to interact with the α1(XI)Npp bound to the plate. Antibody to the unique region of the alternate isoform was used to detect interaction. This antibody did not recognize α1(XI)Npp. The dissociation constant was determined to be on the order of 0.13 μm.
Fig.4. ELISA assay.
Binding was shown to be saturable by ELISA assay in which recombinant α1(XI)Npp was bound to the plate and tagged recombinant α1(XI) amino-terminal domain was allowed to interact with the bound α1(XI)Npp. Antibody to the unique tag region was used to detect interaction. Absorbance at 405 nm was plotted as a function of tagged α1(XI) concentration (■). This antibody did not recognize α1(XI)Npp. Inset depicts the results of Scatchard analysis of binding. Bovine serum albumin was used as a control for nonspecific interaction (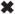 ). Error bars indicate ± S.E.
). Error bars indicate ± S.E.
Specificity of α1(XI)Npp Interaction: Co-precipitation of Endogenous and Recombinant α1(XI)Npp
Bovine chondrocytes maintained in pellet culture were used as a source of cartilage extracellular matrix proteins. Extracts were prepared from 10-day pellets using 1 m NaCl in Tris buffer in the absence of denaturants. These extracts were incubated with recombinant α1(XI)Npp bound to agarose beads. Samples were analyzed by SDS-polyacrylamide gel electrophoresis and subsequent immunoblot (Fig. 5). As a control, endogenous α2(XI)Npp was analyzed for its ability to bind to recombinant α1(XI)Npp under the same conditions. Using an α2(XI)-specific antibody, α2(XI)Npp was found primarily in the free fraction, whereas bovine α1(XI)Npp bound to the recombinant α1(XI)Npp-agarose beads (Fig. 5).
Fig.5. Specificity of α1(XI)Npp interaction by co-precipitation of endogenous α1(XI)Npp with recombinant α1(XI)Npp-nickel-agarose.
Proteins were extracted from chondrocyte pellet cultures in 1 m NaCl-containing buffer. Buffer conditions were changed by dialysis to reduce the salt concentration to 150 mm NaCl, and extract was incubated with α1(XI)Npp-bound nickel-agarose beads. Proteins were resolved by SDS-PAGE 12% polyacrylamide gel and analyzed by immunoblot using an antibody to α1(XI)Npp and an antibody to α2(XI)Npp. Lanes 1 and 3, material not bound to α1(XI)Npp-bound nickel-agarose beads. Lanes 2 and 4, material bound to α1(XI)Npp-bound nickel-agarose beads. Lanes 1 and 2, immunoblot with α2(XI)Npp antibody. Lanes 3 and 4, immunoblot with α1(XI)Npp antibody. Lane 5, molecular weight markers. Arrows on left-hand side indicate position of the α1(XI)Npp and the α2(XI)Npp.
Specificity of Antibody for the Endogenous α1(XI)Npp
The ability of the α1(XI)Npp antibody to discriminate between endogenous and recombinant α1(XI)Npp was demonstrated by immunoblot and nickel affinity blot (Fig. 6). Samples of increasing amounts of recombinant α1(XI)Npp were loaded onto an SDS gel and subsequently transferred to polyvinylidene difluoride membrane. Nickel-NTA-alkaline phosphatase conjugate was used to detect recombinant protein from a sample that was three orders of magnitude more dilute than the concentration needed for detection by the polyclonal antibody to α1(XI)Npp. Location of the epitope site within the amino propeptide and differences in amino acid sequence among the peptide antigen, endogenous bovine sequence, and the sequence within the recombinant protein are demonstrated in Fig. 6C.
Interaction in Chondrocyte Pellet Culture System Resulted in a Stable Association
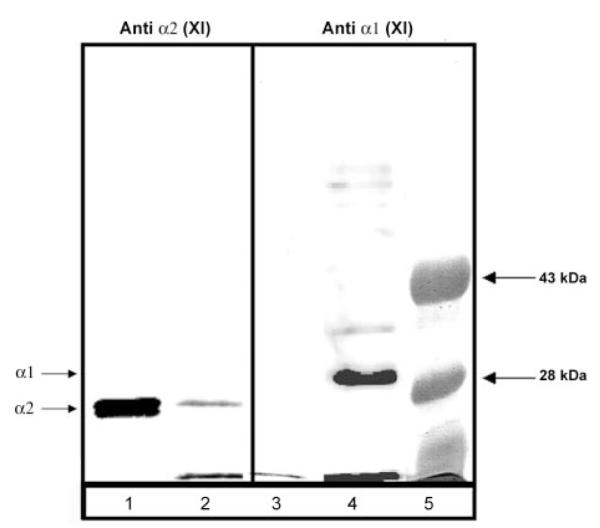
Recombinant α1(XI)Npp was converted from a protein that migrated as an ~30-kDa protein to one that had an apparent molecular mass of ~60 kDa under denaturing and reducing conditions when incubated for 24 h with bovine chondrocytes maintained in pellet culture. Both the 30- and 60-kDa bands were detectable via the His6 tag by Nickel affinity blot (Fig. 7). Using the α1(XI)Npp antibody that can discriminate between recombinant and endogenous bovine α1(XI)Npp, the 60-kDa band was also recognized by this antibody, suggesting the formation of a dimer of α1(XI)Npp monomers, one contributed by the endogenous bovine α1(XI) and the other by the added recombinant α1(XI)Npp. In addition, a band with an apparent molecular mass of 35 kDa was identified as immunologically related to endogenous α1(XI)Npp. The nature of this band is currently unknown. Stability of the 60-kDa band was investigated by subjecting samples to prolonged incubation in the presence of 250 mm imidazole, EDTA, or β-mercaptoethanol at 90 °C with no change observed in migration on SDS gels. The generation of the 60-kDa band during 24 h in bovine pellet culture was not inhibited by inclusion of β-aminopropionitrile fumarate to inhibit lysine-derived covalent cross-linking or by inclusion of putrescine to inhibit transglutaminase in the culture (data not shown). To rule out a possible interaction mediated by the His6 tag present on the recombinant protein, recombinant α1(XI)Npp was generated without a His6 tag, by removing the carboxyl domain from the recombinant α1(XI) amino-terminal domain isoform by enzymatic proteolysis with BMP-1 (Fig. 7, lanes 6 and 7). The resulting recombinant α1(XI)Npp was included in chondrocyte cell culture with similar results (Fig. 7).
Fig.7. Incubation of recombinant α1(XI)Npp with bovine chondrocytes in pellet culture.
A, conversion of monomer to dimer by covalent interaction between endogenous bovine α1(XI)Npp and recombinant α1(XI)Npp. Lanes 1–3, nickel affinity blot; lanes 4–7, immunoblot with antibody to endogenous α1(XI)Npp. Lane 1, purified recombinant α1(XI)Npp containing a His6 tag migrated with an approximate size of 30 kDa on an SDS (11.5%) polyacrylamide gel after 24-h incubation at 37 °C in tissue culture medium in the absence of cells; lanes 2 and 4, no proteins were detected by nickel-NTA-alkaline phosphatase from control cell pellet cultures incubated in the absence of recombinant α1(XI)Npp for 24 h; however, endogenous α1(XI)Npp was detected; lanes 3 and 5, material extracted (in 1 m NaCl-containing buffer) from cell pellet cultures, incubated in the presence of 10 μg/ml recombinant α1(XI)Npp, contained the 30-kDa recombinant protein and some of the endogenous α1(XI)Npp (apparent molecular mass, 30 kDa) and in addition contained larger proteins including a 35-kDa protein and a protein with an apparent molecular mass of 60 kDa, which was recognized by nickel-NTA-alkaline phosphatase (lane 3) and the endogenous-specific α1(XI)Npp antibody (lane 5). Lane 6, material extracted (in 1 m NaCl-containing buffer) from cell pellet cultures incubated in the presence of 1 μg/ml recombinant α1(XI)Npp without a His6 tag. The 60-kDa band is present as well as a minor band present at 30 kDa. Lane 7, material extracted (in 1 m NaCl-containing buffer) from cell pellet cultures incubated in the presence of 10 μg/ml recombinant α1(XI)Npp without a His6 tag. The 60-kDa band is present as well as the 30-kDa band and a minor band, which migrates with an apparent molecular mass of 35 kDa.
DISCUSSION
The α1 chain of type XI collagen exists as a set of isoforms that arise by alternative splicing of the primary RNA transcript. The portion encoding the variable region gives rise to biochemically unique domains. However, common to all isoforms is the amino propeptide, the distal globular 233 amino acids, which is ultimately proteolytically removed during the process of extracellular matrix assembly and fibril formation. Although the amino propeptide domain is thought to play a role in the regulation of fibril diameter, the molecular mechanism of this event is not fully understood.
A homotypic interaction was detected among the α1(XI)Npp domains. The possibility of such an interaction was supported by the use of multi-angle laser light scattering by which purified recombinant Npp was found to exist in equilibrium between monomer and dimer. Yeast-two hybrid system also supported the possibility that an interaction between two α1(XI)Npp domains could exist.
Freshly isolated chondrocytes were maintained as pellet cultures under conditions that allow the maintenance of the chondrocyte phenotype and reestablishment of a characteristic cartilage extracellular matrix. The newly synthesized matrix constituents were used as a source of cartilage matrix candidates for this study. An interaction between recombinant rat α1(XI)Npp and endogenous bovine α1(XI)Npp was detected by co-precipitation. The interaction resulted in a stable and apparently covalent cross-link. The interaction was neither a result of lysine-derived cross-linking nor transglutaminase-mediated cross-links as β-aminopropionitrile fumarate or putrescine did not inhibit the cross-link. The interaction was also not mediated by the His6 tag present on the recombinant protein. Noncollagenous domains of other collagens have been reported to form stable interactions including type X collagen (21–23), type IV collagen (24, 25), type IX collagen (26), and type VIII collagen (27). However, no such interaction has been described for any of the other fibrillar collagens and no such interactions have been described between propeptide domains. In the case of types IV and X, the interactions were hydrophobic in nature rather than due to covalent bond formation. In the case of the type XI α1 Npp dimer, the stable interaction takes place only in the presence of living cells because incubation of purified Npp does not result in the appearance of higher molecular weight bands on subsequent SDS gels, neither does the incubation of mixtures of recombinant isoforms of α1(XI) amino-terminal domain that contains any of the possible variable domains adjacent to the Npp domain (data not shown). This interaction may because of hydrophobic interaction or may be the result of covalent bond formation between the two Npp subunits. Data from the light scattering experiments do not support a hydrophobic interaction, because a decrease in average molecular weight was observed with increasing ionic strength of the buffer. If the stable interaction is the result of the formation of a covalent bond, it is unknown what enzyme is responsible at this time.
Type XI collagen serves primarily a regulatory function in collagen fibrillogenesis. The interaction between α1(XI)Npp domains demonstrated in this study may be coupled to enzymatic events including covalent cross-linking of amino propeptide domains and proteolytic cleavage to remove these domains from collagen fibrils. Such an interaction could contribute to our understanding of 1) initial nucleation of collagen fibrils, 2) lateral fusion of preexisting thin fibrils, and 3) increase in diameter because of accretion of individual collagen molecules to the surface of a growing collagen fibril.
The role of type XI collagen in limitation of lateral growth of collagen fibrils is well established, although the molecular mechanism is not fully understood. The α1(XI)Npp has been localized to the surface of thin collagen fibrils, it is relatively long-lived at the surface, and it is clear from the molecular dimensions that the α1(XI)Npp, the variable region, and the minor helix would not be accommodated within the gap region and would therefore sterically hinder further accretion of type II collagen molecules to the surface of a growing fibril. An interaction between Npp domains that favor proteolytic removal of globular domains from the surface may facilitate lateral growth of collagen fibrils.
Acknowledgments
We thank Dr. Lin Randall and Traci Topping for assistance with the size exclusion chromatography/multi-angle light scattering experiments.
Footnotes
This work was supported by the Arthritis Foundation, the Gerlinger Foundation, National Institutes of Health Grants R01AR47985, K02AR48672, and P20RR16454-Biomedical Research Infrastructure Network for Idaho (BRIN).
The abbreviations used are: BMP, bone morphogenetic protein; NTA, nitrilotriacetic acid; α1(XI)NTD, α1(XI) amino-terminal domain containing the variable region; Npp, amino propeptide; ELISA, enzyme-linked immunosorbent assay.
REFERENCES
- 1.Eyre DE, Wu JJ. In: Structure and Function of Collagen Types. Mayne R, Burgeson RE, editors. Academic Press, Inc.; Orlando, FL: 1987. pp. 261–309. [Google Scholar]
- 2.Smith GN, Williams JM, Brandt KD. Collagen Relat. Res. 1987;7:17–25. doi: 10.1016/s0174-173x(87)80018-3. [DOI] [PubMed] [Google Scholar]
- 3.Mendler M, Eich-Bender SG, Vaughan L, Winterhalter K, Bruckner P. J. Cell Biol. 1989;108:191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Lacerda DA, Warman ML, Beier DR, Yoshioka H, Ninomiya Y, Oxford JT, Morris NP, Andrikopoulos K, Ramirez F, Wardell BB, Lifferth GD, Teuscher C, Woodward SR, Taylor BA, Seegmiller RE, Olsen BR. Cell. 1995;80:423–430. doi: 10.1016/0092-8674(95)90492-1. [DOI] [PubMed] [Google Scholar]
- 5.Eikenberry EF, Mendler M, Burgin R, Winterhalter KH, Bruckner P. In: Articular Cartilage and Osteoarthritis. Kuettner KE, Schleyerbach R, Peyron JG, Hascall VC, editors. Raven Press; New York: 1982. pp. 133–149. [Google Scholar]
- 6.Wu JJ, Eyre DR. J. Biol. Chem. 1995;270:18865–18870. doi: 10.1074/jbc.270.32.18865. [DOI] [PubMed] [Google Scholar]
- 7.Sussman MD, Ogle RC, Balian G. J. Orthop. Res. 1984;2:134–142. doi: 10.1002/jor.1100020204. [DOI] [PubMed] [Google Scholar]
- 8.Rousseau J-C, Farjanel J, Boutillon M-M, Hartmann D, van der Rest M, Moradi-Ameli M. J. Biol. Chem. 1996;271:23743–23748. doi: 10.1074/jbc.271.39.23743. [DOI] [PubMed] [Google Scholar]
- 9.Moradi-Ameli M, de Chassey B, Farjanel J, van der Rest M. Matrix Biol. 1998;17:393–396. doi: 10.1016/s0945-053x(98)90091-9. [DOI] [PubMed] [Google Scholar]
- 10.Medeck RJ, Sosa S, Morris N, Oxford JT. Biochem. J. 2003;376:361–368. doi: 10.1042/BJ20030894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oxford JT, Doege KJ, Morris NP. J. Biol. Chem. 1995;270:9478–9485. doi: 10.1074/jbc.270.16.9478. [DOI] [PubMed] [Google Scholar]
- 12.Imamura Y, Steiglitz BM, Greenspan DS. J. Biol. Chem. 1998;273:27511–27517. doi: 10.1074/jbc.273.42.27511. [DOI] [PubMed] [Google Scholar]
- 13.Moradi-Ameli M, Deleage G, Geourjon C, van der Rest M. Matrix Biol. 1994;3:233–239. doi: 10.1016/0945-053x(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 14.Gregory KE, Oxford JT, Chen Y, Gambee JE, Gygi SP, Aebersold R, Neame PJ, Mechling DE, Bachinger HP, Morris NP. J. Biol. Chem. 2000;275:11498–11506. doi: 10.1074/jbc.275.15.11498. [DOI] [PubMed] [Google Scholar]
- 15.Keene DR, Oxford JT, Morris NP. J. Histochem. Cytochem. 1995;10:967–979. doi: 10.1177/43.10.7560887. [DOI] [PubMed] [Google Scholar]
- 16.Thom JR, Morris NP. J. Biol. Chem. 1991;266:7262–7269. [PubMed] [Google Scholar]
- 17.Soni R, Carmichael JP, Murray JA. Curr. Genet. 1993;24:455–459. doi: 10.1007/BF00351857. [DOI] [PubMed] [Google Scholar]
- 18.Neame PJ, Young CN, Treep JT. J. Biol. Chem. 1990;265:20401–20408. [PubMed] [Google Scholar]
- 19.Davies GB, Oxford JT, Hausafus LC, Smoody BF, Morris NP. Dev. Dyn. 1998;213:12–26. doi: 10.1002/(SICI)1097-0177(199809)213:1<12::AID-AJA2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Ballock RT, Reddi AH. Cell Biol. 1994;126:1311–1318. doi: 10.1083/jcb.126.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barber RE, Kwan AP. Biochem. J. 1996;320:479–485. doi: 10.1042/bj3200479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frischholz S, Beier F, Girkontaite I, Wagner K, Poschl E, Turnay J, Mayer U, von der Mark K. J. Biol. Chem. 1998;273:4547–4555. doi: 10.1074/jbc.273.8.4547. [DOI] [PubMed] [Google Scholar]
- 23.Kwan APL, Cummings CE, Chapman JA, Grant ME. J. Cell Biol. 1991;114:597–605. doi: 10.1083/jcb.114.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timpl R, Wiedemann H, van Delden V, Furthmayr H, Kuhn K. Eur. J. Biochem. 1981;120:203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- 25.Yurchenco PD, Furthmayr H. Biochemistry. 1984;23:1839–1850. doi: 10.1021/bi00303a040. [DOI] [PubMed] [Google Scholar]
- 26.Douglas SP, Jenkins JM, Kadler KE. Matrix Biol. 1998;16:497–505. doi: 10.1016/s0945-053x(98)90020-8. [DOI] [PubMed] [Google Scholar]
- 27.Sawada H, Konomi H, Hirosawa K. J. Cell Biol. 1990;110:219–227. doi: 10.1083/jcb.110.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]