Abstract
A method for iron-catalyzed deprotonative alkylation of arene C-H bonds by alkyl iodides and bromides has been developed. In the presence of an amide base, both primary and secondary alkyl halides can be coupled with furans, thiophenes, pyridine derivatives, and some electron-withdrawing-group containing arenes.
Direct arylation of heterocycle and directing-group containing arene sp2 C-H bonds has resulted in many synthetically useful procedures.1 However, transition-metal-catalyzed alkylation of C-H bonds is not common and the need for new methodology still exists. Several methods have been developed for converting an sp2 C-H bond to a C(sp2)-C(sp3) functionality. An industrially important Friedel-Crafts alkylation suffers from carbocation isomerization, polyalkylation, and regioselectivity problems that may limit its synthetic applicability.2 More recent methodology involves directing-group-containing arene and heterocycle alkylation by alkenes that employs ruthenium, rhodium, or cobalt catalysis. While regioselectivity is excellent with respect to the arene coupling component, the scope of alkenes that can be employed is often limited by double bond isomerization.3 The third method employs an alkyl halide coupling partner. Pioneering reports by Tremont and Liebeskind describe palladium-promoted alkylation of anilides and imines.4 More recently, methods for heterocycle and benzoic acid alkylation under ruthenium and palladium catalysis have been developed.5 A rare example that employs abundant first-row transition metal catalysis was recently reported by Knochel. An iron amide base was used to deprotonate a variety of ester and-/or fluorine-containing arenes. Trace nickel-catalyzed coupling with alkyl halides subsequently afforded the alkylated aromatic species.6 While iron-catalyzed Grignard alkylation reactions have been developed by Fürster and Nakamura,7 iron-catalyzed deprotonative alkylation of arene C-H bonds has not been reported.
We have recently developed a method for copper-catalyzed arylation of acidic arene sp2 C-H bonds.8 An in situ deprotonation/transmetalation is followed by the reaction with an aryl halide to afford a biaryl (Scheme 1). Both Li and Cu bases are competent metalating agents under the reaction conditions.8c Unfortunately, most alkylations attempted were not successful. Only benzylation afforded product in modest yield.8b A combination of deprotonation with an iron-catalyzed alkylation7 would allow for the development of a method for arene regioselective direct alkylation.
Scheme 1.
Arene deprotonative functionalization
We report here a method for iron-catalyzed deprotonative alkylation of furan, thiophene, pyridine, as well as ester- and cyano group-containing arene derivatives. Both primary and secondary alkyl halide electrophiles can be employed.
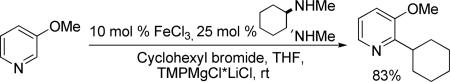
A short optimization showed that iron-catalyzed cross-coupling of 2-lithiobenzothiophene with cyclohexyl bromide is inefficient (<20% yield), and that the corresponding Grignard reagent couples with improved yields. The best ligands were found to be HMTA/TMEDTA9 and trans-N,N’-dimethylcyclohexane-1,2-diamine10 with the latter affording slightly higher yields. Iron(III) chloride catalyst was shown to be superior to other Fe sources. The optimized conditions involve TMPMgCl*LiCl base, 10 mol% FeCl3, 25 mol% trans N,N’ 1,2-dimethylcyclohexane-1,2-diamine ligand, and THF solvent at room temperature.
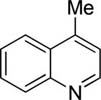
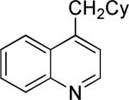
The scope with respect to arene coupling component is shown in Table 1. Five-membered heterocycles such as benzofuran (entry 1), 2-methylthiophene (entry 2), and benzothiophene (entry 3) can be coupled with cyclohexyl bromide in moderate to good yields. Various pyridines are also reactive. Pyridine can be cyclohexylated in a reasonable yield (entry 4). 3-Methoxy and 3-fluoropyridines react at 2-position (entries 5 and 6), in contrast to our previous copper-catalyzed arylation methodology where arylation at 4-position was observed.8b Isoquinoline is alkylated at the 1-position (entry 7) while 4-methylquinoline reacts at the methyl group (entry 8), presumably due to high acidity of benzylic sp3 protons. Arenes such as 1-cyano-3-methoxybenzene and 4-fluorobenzoic acid ethyl ester are also reactive (entries 9 and 10). In some cases, cheaper dicyclohexylamide base can be used (entries 3 and 8). For several arenes, addition of zinc amide base11 is beneficial. Interestingly, several substrates that were shown to be efficiently arylated under copper catalysis were unreactive in the alkylation. Alkylation is not successful for relatively acidic substrates such as tetrafluoroanisole, 3,5-dichloropyridine, and N-butyl-1,2,4-triazole. It appears that there is both lower and upper limit of C-H bond acidity that is acceptable for alkylation.12
Table 1.
Alkylation scope with respect to arenea
Cy = cyclohexyl. Substrate (1.3-3 equiv), cyclohexyl bromide (1 equiv), FeCl3 (5-10 mol %), ligand (13-25 mol %), TMPMgCl*LiCl (1.6-2.6 equiv), THF, rt.
Cy2NMgCl*LiCl base.
Benzothiophene (1 equiv), CyBr (2 equiv).
TMP2Zn*2MgCl2*LiCl/TMPMgCl*LiCl mixed base.
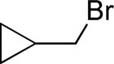
The scope with respect to alkyl halides is presented in Table 2. Primary alkyl iodides and bromides are reactive (entries 1 and 2). Some functionality, such as alkene (entry 3), ω-chloride (entry 4), and trifluoromethyl (entry 5), is tolerated. If cyclopropylmethyl bromide is employed (entry 6), ring-opening product is obtained, signifying possible radical intermediacy.7e Other secondary alkyl halides such as cyclopentyl bromide (entry 7) also afford product in a good yield.
Table 2.
Alkylation scope with respect to halidea
 | |||
|---|---|---|---|
| entry | alkyl halide | product | yield, % |
| 1 | n-C7H15Br |

|
82 |
| 2 | n-C8H17I |

|
78 |
| 3 | 5-Bromopentene |

|
85 |
| 4 | 1-Chloro-6-iodohexane |

|
57 |
| 5 | 1-Bromo-4,4,4-trifluorobutane |

|
68 |
| 6 |
|

|
62 |
| 7 |

|

|
81 |
3-Methoxypyridine (1.4-1.8 equiv), halide (1 equiv), FeCl3 (10 mol %), ligand (25 mol %), TMPMgCl*LiCl (1.5-2.0 equiv), THF, rt.
The identity of metalating reagent was determined by subjecting benzothiophene to deprotonation by either TMPMgCl*LiCl or Knochels’ TMP2Fe*2MgCl2*4LiCl reagent6a followed by quench with D2O. The magnesium base is more efficient thus showing that it is the most likely deprotonating agent (Scheme 2). At longer reaction times (t>10 min) extensive decomposition of benzothiophene was observed if TMP2Fe*2MgCl2*4LiCl base was employed. It was also shown that the coupling reaction is rapid and 64% conversion to 2-cyclohexylbenzothiophene is observed in 10 min.14
Scheme 2.
Deprotonation
Control experiments were run to determine if trace of another transition metal catalyzes the dimerization (Scheme 3).15 With reagent grade or ultra-pure FeCl3 nearly identical results were obtained showing that reactivity by contaminants is unlikely. If iron salt was omitted, no product was obtained.
Scheme 3.
Control experiments
In conclusion, we have developed a method for iron-catalyzed deprotonative alkylation of arene C-H bonds by alkyl iodides and bromides. Both primary and secondary alkyl halides can be coupled with furans, thiophenes, pyridine derivatives, and some electron-withdrawing-group containing arenes.
Supplementary Material
Acknowledgment
We thank the Welch Foundation (Grant No. E-1571), NIGMS (Grant No. R01GM077635), A. P. Sloan Foundation, Camille and Henry Dreyfus Foundation, and Norman Hackerman Advanced Research Program for supporting this research.
Footnotes
Supporting Information Available: Experimental procedures and characterization data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Selected reviews: Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2010;110:624. doi: 10.1021/cr900005n.Ackermann L, Vicente R, Kapdi AR. Angew. Chem., Int. Ed. 2009;48:9792. doi: 10.1002/anie.200902996.Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem., Int. Ed. 2009;48:5094. doi: 10.1002/anie.200806273.Seregin IV, Gevorgyan V. Chem. Soc. Rev. 2007;36:1173. doi: 10.1039/b606984n.Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e.Daugulis O, Do H-Q, Shabashov D. Acc. Chem. Res. 2009;42:1074. doi: 10.1021/ar9000058.Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174. doi: 10.1021/cr0509760.
- 2.Roberts RM, Khalaf AA. Friedel Crafts Alkylation Chemistry: A Century of Discovery. Wiley-Interscience; New York: 1984. [Google Scholar]
- 3.a Murai S, Kakiuchi F, Sekine S, Tanaka Y, Kamatani A, Sonoda M, Chatani N. Nature. 1993;366:529. [Google Scholar]; b Lenges CP, Brookhart M. J. Am. Chem. Soc. 1999;121:6616. [Google Scholar]; c Lewis JC, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2007;129:5332. doi: 10.1021/ja070388z. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Lail M, Arrowood BN, Gunnoe TB. J. Am. Chem. Soc. 2003;125:7506. doi: 10.1021/ja035076k. [DOI] [PubMed] [Google Scholar]
- 4.a Tremont SJ, Rahman HU. J. Am. Chem. Soc. 1984;106:5759. [Google Scholar]; b McCallum JS, Gasdaska JR, Liebeskind LS, Tremont SJ. Tetrahedron Lett. 1989;30:4085. [Google Scholar]
- 5.Fagnou K, Lapointe D. Org. Lett. 2009;11:4160. doi: 10.1021/ol901689q.Zhang Y-H, Shi B-F, Yu J-Q. Angew. Chem., Int. Ed. 2009;48:6097. doi: 10.1002/anie.200902262.Ackermann L, Novák P, Vicente R, Hofmann N. Angew. Chem., Int. Ed. 2009;48:6045. doi: 10.1002/anie.200902458.Mukai T, Hirano K, Satoh T, Miura M. Org. Lett. 2010;12:1360. doi: 10.1021/ol1002576.Yue W, Li Y, Jiang W, Zhen Y, Wang Z. Org. Lett. 2009;11:5430. doi: 10.1021/ol9023198. Other alkylation methods: Shabashov D, Daugulis O. J. Am. Chem. Soc. 2010;132:3965. doi: 10.1021/ja910900p.Catellani M, Motti E, Della Ca’ N. Acc. Chem. Res. 2008;41:1512. doi: 10.1021/ar800040u.Rudolph A, Rackelmann N, Lautens M. Angew. Chem., Int. Ed. 2007;46:1485. doi: 10.1002/anie.200603888.Chen X, Li J-J, Hao X-S, Goodhue CE, Yu J-Q. J. Am. Chem. Soc. 2006;128:78. doi: 10.1021/ja0570943.Deng G, Li C-J. Org. Lett. 2009;11:1171. doi: 10.1021/ol900070x.
- 6.a Wunderlich SH, Knochel P. Angew. Chem., Int. Ed. 2009;48:9717. doi: 10.1002/anie.200905196. [DOI] [PubMed] [Google Scholar]; b Vechorkin O, Proust V, Hu X. Angew. Chem., Int. Ed. 2010;49:3061. doi: 10.1002/anie.200907040. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura M, Matsuo K, Ito S, Nakamura E. J. Am. Chem. Soc. 2004;126:3686. doi: 10.1021/ja049744t.Martin R, Fürstner A. Angew. Chem., Int. Ed. 2004;43:3955. doi: 10.1002/anie.200460504.Czaplik WA, Mayer M, von Wangelin AJ. Angew. Chem., Int. Ed. 2009;48:607. doi: 10.1002/anie.200804434.Tamura M, Kochi JK. J. Am. Chem. Soc. 1971;93:1487.Fürstner A, Martin R, Krause H, Seidel G, Goddard R, Lehmann CW. J. Am. Chem. Soc. 2008;130:8773. doi: 10.1021/ja801466t.Nagano T, Hayashi T. Org. Lett. 2004;6:1297. doi: 10.1021/ol049779y. Review: Sherry BD, Fürstner A. Acc. Chem. Res. 2008;41:1500. doi: 10.1021/ar800039x.
- 8.Do H-Q, Daugulis O. J. Am. Chem. Soc. 2007;129:12404. doi: 10.1021/ja075802+.Do H-Q, Daugulis O. J. Am. Chem. Soc. 2008;130:1128. doi: 10.1021/ja077862l.Do H-Q, Khan RMK, Daugulis O. J. Am. Chem. Soc. 2008;130:15185. doi: 10.1021/ja805688p.Yotphan S, Bergman RG, Ellman JA. Org. Lett. 2009;11:1511. doi: 10.1021/ol900103a. Fe catalysis: Norinder J, Matsumoto A, Yoshikai N, Nakamura E. J. Am. Chem. Soc. 2008;130:5858. doi: 10.1021/ja800818b.
- 9.Cahiez G, Habiak V, Duplais C, Moyeux A. Angew. Chem., Int. Ed. 2007;46:4364. doi: 10.1002/anie.200700742. [DOI] [PubMed] [Google Scholar]
- 10.Klapars A, Huang X, Buchwald SL. J. Am. Chem. Soc. 2002;124:7421. doi: 10.1021/ja0260465. [DOI] [PubMed] [Google Scholar]
- 11.a Wunderlich SH, Knochel P. Angew. Chem. Int. Ed. 2007;46:7685. doi: 10.1002/anie.200701984. [DOI] [PubMed] [Google Scholar]; b Krasovskiy A, Krasovskaya V, Knochel P. Angew. Chem., Int. Ed. 2006;45:2958. doi: 10.1002/anie.200504024. [DOI] [PubMed] [Google Scholar]
- 12.a Shen K, Fu Y, Li J-N, Liu L, Guo Q-X. Tetrahedron. 2007;63:1568. [Google Scholar]; b Bordwell FG. Acc. Chem. Res. 1988;21:456. [Google Scholar]
- 13.A reviewer asked us to evaluate several other alkyl halides for the alkylation. We have checked the reactivity of benzyl bromide with 3-methoxypyridine and obtained 28% isolated yield of 2-benzyl-3-methoxypyridine. Allyl bromide and t-butyl 2-bromoisobutyrate did not afford significant conversion to the desired alkylation products. It was also suggested to check the reactivity of toluene derivatives. Attempted reaction of mesitylene with cyclohexyl bromide afforded no alkylation product. Please see Supporting Information for details.
- 14.Please see Supporting Information for details.
- 15.a Buchwald SL, Bolm C. Angew. Chem., Int. Ed. 2009;48:5586. doi: 10.1002/anie.200902237. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Arvela RK, Leadbeater NE, Sangi MS, Williams VA, Granados P, Singer RD. J. Org. Chem. 2005;70:161. doi: 10.1021/jo048531j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.