Abstract
Rationale
In heart, Ca2+ entering myocytes via CaV1.2 channels controls essential functions including, excitation-contraction (EC) coupling, action potential duration (APD), and gene expression. RGK GTPases potently inhibit CaV1.2 channels, an effect that may figure prominently in cardiac Ca2+ homeostasis under physiological and disease conditions.
Objective
To define the mechanisms and molecular determinants underlying Rem GTPase inhibition of CaV1.2 channels in heart, and determine whether such inhibited channels can be pharmacologically rescued.
Methods and Results
Over-expressing Rem in adult guinea pig heart cells dramatically depresses L-type calcium current (ICa,L; ~90% inhibition) and moderately reduces maximum gating charge (Qmax; 33%), without appreciably diminishing the physical number of channels in the membrane. Rem-inhibited CaV1.2 channels were supra-modulated by BAY K 8644 (10-fold increase) compared to control channels (3-fold increase). However, Rem prevented protein kinase A (PKA)-mediated up-regulation of ICa,L, an effect achieved without disrupting the sympathetic signaling cascade since PKA modulation of IKS remained intact. In accord with its functional impact on ICa,L, Rem selectively prevented PKA-, but not BAY K 8644-induced prolongation of the cardiac APD. A GTP-binding-deficient Rem[T94N] mutant was functionally inert with respect to ICa,L inhibition. A chimeric construct, Rem265-H, featuring a swap of the Rem C-terminus tail for the analogous domain from H-Ras, inhibited ICa,L and Qmax to the same extent as wild type Rem despite lacking the capacity to autonomously localize to the sarcolemma.
Conclusions
Rem predominantly inhibits ICa,L in heart by arresting surface CaV1.2 channels in a low open probability (Po) gating mode, rather than by interfering with channel trafficking. Moreover, Rem-inhibited CaV1.2 channels can be selectively rescued by BAY K 8644 but not PKA-dependent phosphorylation. Contrary to findings in reconstituted systems, Rem-induced ablation of cardiac ICa,L requires GTP-binding, but not membrane-targeting of the nucleotide binding domain (NBD). These findings provide a different perspective on the molecular mechanisms and structural determinants underlying RGK GTPase inhibition of CaV1.2 channels in heart, and suggest new (patho)physiological dimensions of this crosstalk.
Keywords: L-type calcium channels, Rem, RGK GTPase, cardiac myocytes
Introduction
In heart, Ca2+ influx via L-type (CaV1.2) calcium channels links electrical excitation to contraction (E-C coupling); controls the action potential duration (APD) and myocyte excitability; and regulates gene expression1, 2. Dysregulation of cardiac CaV1.2 channels results in life-threatening atrial and ventricular arrhythmias, and may contribute to contractile dysfunction in heart failure3, 4. Cardiac CaV1.2 channels are subject to pharmacological and physiological modulation by various drugs and intracellular proteins, respectively5. Such modulation constitutes a primary mechanism for physiological regulation of the heartbeat, and is also an important therapeutic target for cardiovascular diseases including angina and arrhythmias6.
Rad/Rem/Rem2/Gem/Kir (RGK) GTPases belong to the Ras superfamily of monomeric G-proteins7. Like the prototypical Ras, RGK proteins contain a nucleotide binding domain (NBD) that can cycle between GTP- and GDP-bound states. However, these proteins also have unique features including long N- and C-terminus extensions, and non-conservative substitutions of residues important for nucleotide binding and hydrolysis compared to Ras8–11. All four RGK proteins potently inhibit high-voltage-activated CaV channels by associating with auxiliary CaVβ subunits12, 13. Distinct RGK GTPases are expressed in heart and changes in their levels occur under different pathological conditions. For example, expression of Rem in ventricular myocytes is down-regulated during conditions mimicking inflammation8; Gem is up-regulated in the failing human heart14; and Rad knock-out mice develop cardiac hypertrophy15. Owing to their strong impact on CaV1.2 channels, RGK GTPases may profoundly regulate Ca2+ signaling in heart under both physiological and disease states.
This study focuses on two fundamental questions: (1) How do RGK GTPases inhibit whole-cell ICa,L in heart?; and (2) Can RGK GTPase-inhibited channels be functionally rescued by pharmacological or physiological molecules? Over-expressing Gem or Rad in heart dramatically inhibits ICa,L16, 17. It has been suggested that RGK proteins inhibit ICa,L in heart by interfering with channel trafficking, thereby reducing the surface density of cardiac CaV1.2 channels. However, in other cell types it has been demonstrated that RGK proteins can inhibit ICa without reducing the surface density of channels18, 19. In HEK 293 cells, Rem inhibits recombinant CaV1.2 channels using three separate mechanisms: by reducing surface density of channels, accomplished through enhancing dynamin-dependent endocytosis; by immobilizing CaV channel voltage sensors; and by arresting channels that remain on the surface in a low-Po gating mode20. Whether RGK GTPases utilize a similar multiplicity of mechanisms to inhibit CaV1.2 channels in heart has not been explored. Ambiguity also surrounds the role played by key structural features of RGK GTPases in blocking ICa,L in heart. For example, GTP binding to the NBD is dispensable for Rem inhibition of CaV1-2 channels reconstituted in HEK 293 cells20. However, in heart a GTP-binding-deficient Rad mutant displays a dominant negative effect that results in enhancement of ICa,L17. It is unknown whether these variations reflect genuine differences among distinct RGK GTPases, or whether they are cell type-specific. Similarly, in some systems, membrane targeting of the NBD is necessary and sufficient for Rem inhibition of ICa,L18, 21, 22. Whether this rule holds true in heart is unclear. Finally, it is unknown whether RGK GTPase-inhibited CaV1.2 channels are irretrievably lost or whether they can be acutely rescued by agents that normally up-regulate ICa,L in heart. Discovering molecules that can functionally rescue RGK GTPase-inhibited CaV1.2 channels may not only refine understanding of the mechanisms underlying this modulation, but also have potential therapeutic applications by reversing cardiac dysfunction arising from RGK GTPase-mediated block of cardiac ICa,L.
Here, we find that over-expressing Rem in isolated guinea pig ventricular myocytes markedly inhibits ICa,L (90% reduction) and more modestly decreases Qmax (33% reduction), without appreciably decreasing the surface density of channels. Contrary to results in reconstituted systems, GTP binding to the NBD was necessary for Rem inhibition of ICa,L in heart. However, membrane targeting of the NBD was neither necessary nor sufficient for blocking cardiac CaV1.2 channels. Rem-inhibited CaV1.2 channels could be selectively up-regulated by BAY K 8644 but were insensitive to the physiological sympathetic β-adrenergic signaling pathway. The results offer a new perspective on the mechanisms of RGK GTPase inhibition of cardiac ICa,L, and reveal an intriguing crosstalk between two opposing signaling pathways that converge on CaV1.2 channels in the heart.
Materials and Methods
Detailed Methods are presented in the Supplemental Material section.
Construction of adenoviral vectors
Adenoviral vectors were generated using the AdEasy XL system (Stratagene, La Jolla, CA, USA) according to the manufacturer’s directions. Adenoviral amplification and purification were as previously described23, 24.
Cell culture and transfection
Primary cultures of adult guinea pig heart cells were prepared as previously described25. Adult male Hartley guinea pigs were killed with an overdose of isoflurane in accordance with the guidelines of the Columbia University Animal Care and Use Committee. Hearts were excised, ventricular myocytes isolated by enzymatic digestion using a Langendorff perfusion apparatus and cultured on laminin-coated coverslips. Myocytes were infected with adenovirus 3–5 h after initial plating.
Immunofluorescence detection of CaV1.2 channels
Isolated heart cells plated on glass-bottom 35 mm MatTek dishes were infected with adenovirus. After 24 h, cells were fixed with 2% paraformaldehyde in PBS and washed with 0.1 mol/L glycine-PBS. Fixed myocytes were permeabilized with 0.2% Triton X-100 in PBS followed by a 1 h incubation in blocking solution (3% goat serum/1% bovine serum albumin/0.1% Triton X-100). Cells were then incubated with anti-Cav1.2 antibody (1:1000 rabbit polyclonal) in blocking media for 1 h at room temperature. Cells were then washed and exposed to Alexa Fluor 594-conjugated goat anti-rabbit IgG (H+L) (1:400, Invitrogen Molecular Probes, Carlsbad, CA, USA) for 1 h at room temperature in blocking solution. Immunofluorescence detection was performed using a Leica TCS SP2 AOBS MP confocal microscope system (Leica Microsystems, Heidelberg, Germany). Data acquisition and analysis were done using Leica LCS imaging software.
Electrophysiology
Whole-cell and gating currents were recorded from cultured ventricular myocytes as previously described23.
Statistics
Pooled data are presented as means ± s.e.m., and P values were calculated using Student’s two-tailed t test; P < 0.05 was considered significant.
Results
Rem inhibition of CaV1.2 channels in adult guinea pig heart cells
We assessed the impact of Rem on endogenous CaV1.2 channels in cultured adult guinea pig ventricular myocytes using adenovirus-mediated somatic gene transfer. Control, uninfected myocytes displayed robust whole-cell CaV1.2 currents that activated at a threshold of −40 mV and peaked at 0 mV (Fig. 1, A–C). Myocytes infected with adenovirus coding for CFP-Rem exhibited CFP fluorescence in the sarcolemma, transverse tubules (t-tubules), and nuclei (Fig. 1D; Supplemental Data, Online Figure I). Cells expressing CFP-Rem demonstrated a marked decrease in whole-cell CaV1.2 current amplitude across all test pulse voltages (Fig. 1, D–F), as well as a 10-mV depolarizing shift in the voltage-dependence of channel activation (Supplemental Data, Online Table I). At the 0-mV voltage step, peak current density (Ipeak) was reduced by ~90% compared to control uninfected cells (Ipeak = 2.67 ± 0.35 pA/pF, n = 7, for CFP-Rem-expressing cells; Ipeak = 18.82 ± 2.31 pA/pF, n = 7, for control cells; P = 0.0007; Supplemental Data, Online Table I). It has been established that RGK GTPases target to the plasma membrane using electrostatic and hydrophobic interactions involving amino acid residues in the distal C-terminus26. In HEK 293 cells, deleting the distal Rem C-terminus extension (generating Rem265; Fig. 1G, top) eliminates Rem targeting to the plasma membrane, and prevents its capacity to block ICa13, 22. Similarly, adult guinea pig myocytes infected with YFP-Rem265 adenovirus displayed YFP fluorescence throughout the cytosol and nuclei, without any evident targeting to the sarcolemma or t-tubule network (Fig. 1G, bottom; Supplemental Data, Online Figure I). Most importantly, myocytes expressing YFP-Rem265 exhibited robust whole-cell CaV1.2 currents that were essentially the same as those recorded from uninfected cells with respect to amplitude and voltage-dependent properties (Fig. 1, H and I; Ipeak at 0 mV = 21.69 ± 1.93 pA/pF, n = 6; P = 0.36 compared to uninfected myocytes; Supplemental Data, Online Table I). Due to its inertness in blocking ICa,L, we used cells expressing YFP-Rem265 as effective controls in subsequent experiments.
Fig. 1. Rem inhibits CaV1.2 channels in cultured adult cardiac myocytes.
(A) Top, voltage protocol. Bottom, grayscale image of a cultured guinea pig ventricular myocyte. (B) Exemplar whole-cell CaV1.2 channel currents from a cultured myocyte. (C) Population peak current density versus voltage (Ipeak–V) relationship for uninfected myocytes (■, n = 7 for each point). (D) Top, schematic of CFP-Rem depicting the NBD (hexagon) appended by N- and C-termini extensions. Zigzag line represents the membrane-targeting domain in the distal C-terminus. Bottom, confocal image of a myocyte infected with CFP-Rem adenovirus. (E) Exemplar whole-cell currents from a myocyte expressing CFP-Rem. (F) Population Ipeak–V relationship for CFP-Rem myocytes (○, n = 7). The control data is reproduced (gray trace) to facilitate visual comparison. (G–I) Data for myocytes expressing YFP-Rem265. Same format as D–F.
These results establish that over-expressing Rem in guinea pig ventricular myocytes dramatically decreases ICa,L, in agreement with previous observations made using Gem and Rad16, 17. The marked inhibition of ICa,L suggests that in this experimental paradigm most CaV1.2 channels are modified by Rem. This fact greatly simplifies and justifies the use of this experimental system to probe the mechanistic bases of RGK GTPase inhibition of CaV1.2 channels in heart.
Impact of Rem on Qmax and surface density of CaV1.2 channels in heart
In HEK 293 cells, Rem inhibits recombinant CaV1.2 channels by three distinct mechanisms20. It has been suggested that Gem and Rad inhibit cardiac ICa,L by disrupting channel trafficking and, thus, reducing CaV1.2 channel surface density16, 17. However, whether RGK GTPases employ multiple mechanisms to inhibit CaV1.2 channels in heart has not been rigorously studied. To address this deficit, we next investigated the impact of Rem on gating charge and sub-cellular localization of CaV1.2 channels in heart cells (Fig. 2).
Fig. 2. Impact of Rem on CaV1.2 channel gating charge and surface density.
(A) Exemplar gating currents from myocytes expressing YFP-Rem265 or CFP-Rem. (B) Population ON gating charge versus voltage (QON–V) relationship for Rem265- (□, n = 8 for each point) and Rem-expressing (●, n = 7 for each point) myocytes. * P < 0.05. (C) Representative confocal images of CaV1.2 immunofluorescence in heart cells expressing YFP-Rem265 or CFP-Rem. All fluorescence images were collected at the same gain setting of the microscope. (D) Average CaV1.2 immunofluorescence staining intensity measurements (n = 4 for each condition). Control bar represents analyses of fluorescence images obtained when primary antibody was pre-incubated with the antigen peptide.
To isolate CaV1.2 channel gating currents we used a holding potential of −50 mV and blocked ionic currents with 2 mmol/L CdCl2/0.1 mmol/L LaCl323. Comparing exemplar ON gating currents between Rem265- and Rem-expressing myocytes indicated that Rem reduced, but did not eliminate this gating parameter (Fig. 2A). This impression was confirmed in population gating charge versus voltage (Q–V) data: myocytes expressing Rem displayed a 33% reduction in maximal gating charge (Qmax) compared to control cells expressing Rem265 (Fig. 2B; Qmax = 8.47 ± 0.56 fC/pF, n = 8 for Rem265; Qmax = 5.78 ± 0.41 fC/pF, n = 7, for Rem-expressing myocytes, P = 0.002).
We used immunofluorescence to examine the impact of Rem on CaV1.2 channel sub-cellular localization in heart cells (Fig. 2C). In myocytes expressing YFP-Rem265, CaV1.2 channel staining presented as punctate clusters organized along z-lines (Fig. 2C, top row). Cells expressing CFP-Rem displayed a CaV1.2 channel staining pattern similar to control cells (Fig. 2C, bottom row). The preferential targeting of CFP-Rem to the sarcolemma and t-tubules made it possible to explicitly confirm that CaV1.2 channel staining was present at the cell surface (Fig. 2C). Controls in which CaV1.2 antibody was pre-incubated with immunizing antigen displayed low fluorescence, ruling out non-specific staining (Supplemental Data, Online Figure II). Quantifying CaV1.2 immunofluorescence staining across a number of cells indicated no difference between myocytes expressing Rem265 or Rem (Fig. 2D), in agreement with the impression gathered from visual inspection. These data argue against diminished trafficking of CaV1.2 channels as a dominant mechanism for Rem-induced decrease in cardiac ICa,L.
Thus, under conditions where Rem severely depresses ICa,L in heart, most CaV1.2 channels remain in the cardiac sarcolemma. In light of this, the moderate reduction in Qmax suggests that Rem partially immobilizes the voltage sensors of sarcolemmal CaV1.2 channels. The data also suggest that Rem-modified CaV1.2 channels in the sarcolemma are either electrically silent or populate a low-Po gating mode.
Selective pharmacological rescue of Rem-inhibited CaV1.2 channels in heart
The notion that Rem-modified CaV1.2 channels remain in the sarcolemma suggested it might be possible to acutely rescue such inhibited channels pharmacologically. We investigated this possibility using the CaV1 channel agonist BAY K 8644. Control myocytes expressing Rem265 showed a rapid and robust increase in current amplitude in response to 1 μmol/L BAY K 8644 at all voltages (Fig. 3, A–C). On average, control cells exhibited a three-fold increase in currents evoked with a 0 mV test pulse (Fig. 3G). Remarkably, cells expressing Rem also responded to 1 μmol/L BAY K 8644 with a large and rapid increase in ICa,L at all voltages (Fig. 3, D–F). In fact, the average fold-increase in ICa,L was substantially larger for Rem-expressing myocytes compared to control cells (Fig. 3G; fold increase = 3.18 ± 0.46, n = 6 for control YFP-Rem265-expressing cells; fold increase = 9.42 ± 1.05, n = 6 for CFP-Rem, P < 0.005). Similar results were obtained using the CaV channel agonist FPL-64176 (Supplemental Data, Online Figure III). Importantly, in the presence of BAY K 8644, the whole-cell current density recorded from cells expressing Rem265 or Rem was not significantly different (Fig. 3H), consolidating the idea that the majority of Rem-inhibited channels remained on the surface sarcolemma. BAY K 8644 did not increase Qmax in either YFP-Rem265- or CFP-Rem-expressing cells (Supplemental Data, Online Figure IV), eliminating recovery of Qmax as a potential mechanism to explain the larger effect of BAY K 8644 on cells expressing Rem compared to control.
Fig. 3. Functional rescue of Rem-inhibited channels by BAY K 8644.
(A) Exemplar CaV1.2 channel currents from control YFP-Rem265 myocytes before and after exposure to 1 μmol/L BAY K 8644. (B) Diary plot of BAY K 8644-induced up-regulation of exemplar control CaV1.2 channel currents. (C) Ipeak–V relationships before (▲) and after BAY K 8644 ( ) for the exemplar control cell. (D–F) Data for myocytes expressing CFP-Rem. Same format as A–C. (G) Average BAY K 8644-induced fold-increase in CaV1.2 channel amplitude in Rem265- and Rem-expressing myocytes. * P < 0.05 compared to Rem265-expressing myocytes. (H) Impact of BAY K 8644 on absolute Ipeak in Rem265- and Rem-expressing myocytes.
) for the exemplar control cell. (D–F) Data for myocytes expressing CFP-Rem. Same format as A–C. (G) Average BAY K 8644-induced fold-increase in CaV1.2 channel amplitude in Rem265- and Rem-expressing myocytes. * P < 0.05 compared to Rem265-expressing myocytes. (H) Impact of BAY K 8644 on absolute Ipeak in Rem265- and Rem-expressing myocytes.
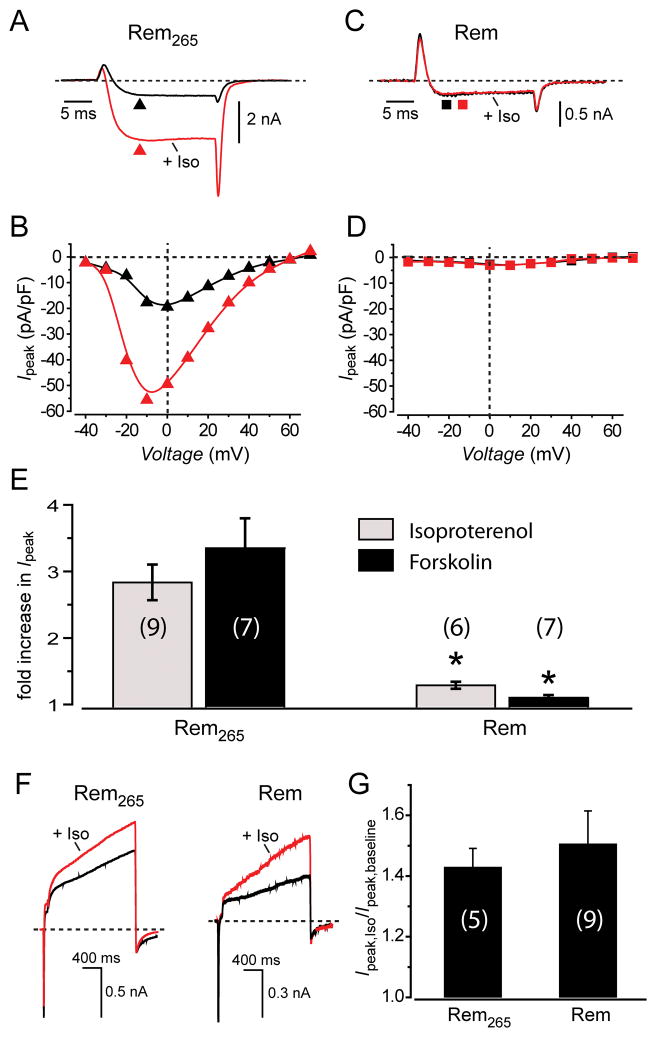
The pharmacological rescue of Rem-inhibited currents was intriguing and gave reason to wonder whether physiological up-regulators of CaV1.2 channels could similarly nullify Rem block of ICa,L. In heart, activation of β-adrenergic receptors strongly enhances ICa,L via the Gsα–cAMP–PKA signal transduction cascade27, 28. In control myocytes expressing Rem265, exposure to 1 μmol/L isoproterenol rapidly increased ICa,L across all voltages (Fig. 4, A and B). On average, 1 μmol/L isoproterenol resulted in a nearly three-fold increase in ICa,L, similar to the magnitude of response obtained with 1 μmol/L BAY K 8644 (Fig. 4E). Surprisingly, in sharp contrast to our observations with BAY K 8644, isoproterenol did not increase ICa,L at any voltage in cells expressing CFP-Rem (Fig. 4, C–E). The same qualitative result was obtained when 1 μmol/L forskolin was used to activate the PKA pathway downstream of the β-adrenergic receptor, at the level of adenylyl cyclase (Fig. 4E). One possible explanation for the ineffectiveness of the PKA pathway to up-regulate Rem-inhibited CaV1.2 channels is that Rem may have an unrecognized ability to eliminate or disrupt this signal transduction cascade in cells. We explored this possibility by evaluating β-adrenergic regulation of IKS in control and Rem-expressing myocytes. Under both conditions, 1 μmol/L isoproterenol increased IKS to the same extent (Fig. 4, F and G), demonstrating the β-adrenergic receptor signaling pathway remained intact in myocytes expressing Rem. Recently, it has been reported that Rad similarly inhibits β-adrenergic regulation of ICa,L in heart29, suggesting this may be a general property of all RGK GTPases.
Fig. 4. Rem ablates PKA modulation of CaV1.2 channels.
(A) Exemplar CaV1.2 channel currents from control Rem265-expressing myocytes before and after exposure to 1 μmol/L isoproterenol. (B) Ipeak–V relationships before (▲) and after ( ) isoproterenol for the exemplar cell. (C and D) Data for a cell expressing CFP-Rem. Same format as A and B. (E) Bar chart showing average impact of isoproterenol and forskolin on fold-increase in CaV1.2 channel current amplitude in Rem265- and Rem-expressing myocytes. * P < 0.05 compared to Rem265-expressing myocytes. (F) Exemplar IKS currents from Rem265-(left) and Rem-expressing (right) myocytes before and after exposure to 1 μmol/L isoproterenol. (G) Bar chart showing the average isoproterenol-induced fold-increase in IKS current amplitude in control Rem265-and Rem-expressing myocytes.
) isoproterenol for the exemplar cell. (C and D) Data for a cell expressing CFP-Rem. Same format as A and B. (E) Bar chart showing average impact of isoproterenol and forskolin on fold-increase in CaV1.2 channel current amplitude in Rem265- and Rem-expressing myocytes. * P < 0.05 compared to Rem265-expressing myocytes. (F) Exemplar IKS currents from Rem265-(left) and Rem-expressing (right) myocytes before and after exposure to 1 μmol/L isoproterenol. (G) Bar chart showing the average isoproterenol-induced fold-increase in IKS current amplitude in control Rem265-and Rem-expressing myocytes.
CaV1.2 channels prominently control the cardiac action potential duration (APD), and also provide the trigger Ca2+ for Ca2+-induced Ca2+ release that underlies cardiac EC coupling. Therefore, it might be expected that the divergent effects of BAY K 8644 and forskolin on Rem-inhibited CaV1.2 channels in heart would translate into sharply differing effects of these two agents on cardiac excitability and contractility. To directly investigate this we compared the impact of BAY K 8644 and forskolin on action potentials and Ca2+ transients in myocytes expressing Rem265 and Rem, respectively (Fig. 5). Cells expressing Rem displayed a significantly shortened basal APD compared to control cells expressing Rem265 (APD50 = 139.3 ± 17.3 ms, n = 6 for Rem-expressing myocytes versus APD50 = 241.9 ± 35.6 ms, n = 5 for Rem265 myocytes, P < 0.05, two-tailed unpaired t test). In myocytes expressing Rem265, both BAY K 8644 and forskolin markedly increased the APD by three-fold (Fig. 5, A and C). By contrast, in myocytes expressing Rem, the APD response to forskolin was essentially abolished whereas the response to BAY K 8644 remained intact (Fig. 5, B and C). Similarly, both BAY K 8644 and forskolin significantly increased the amplitude of evoked Ca2+ transients in cells expressing mCherry-tagged Rem265 (Fig. 5D; Supplemental Data, Online Figure V), whereas in cells expressing Rem, the response to forskolin was selectively and significantly muted (Fig. 5E; Supplemental Data, Online Figure V). These results mirror the differential impact of BAY K 8644 and forskolin on ICa,L in Rem265- and Rem-expressing myocytes (Figs. 3 and 4).
Fig. 5. Differential impact of Bay K 8644 and forskolin on action potentials and Ca2+ transients in Rem265- and Rem-expressing myocytes.
(A) Exemplar action potentials from control Rem265-expressing myocytes before and after exposure to1 μmol/L Bay K 8644 (red) or 1 μmol/L forskolin (blue). (B) Data for a cell expressing CFP-Rem. Same format as A. (C) Bar chart showing average impact of Bay K 8644 and forskolin on fold-increase in action potential duration in Rem265- and Rem-expressing myocytes. * P < 0.05 compared to Rem265-expressing myocytes. (D, E) Confocal line scan images and waveforms showing impact of BAY K 8644 and forskolin on fluo-4-reported Ca2+ transients in myocytes expressing mCherry-tagged Rem265 and Rem, respectively.
Overall these results demonstrate the novel finding that Rem inhibited CaV1.2 channels can be selectively pharmacologically rescued. Furthermore, taken together, the data provide the new insight that Rem-modified CaV1.2 channels in the sarcolemma are arrested in a low-Po state, rather than being completely electrically silent.
Role of GTP binding and membrane localization in Rem inhibition of cardiac ICa,L
We next examined the role played by GTP binding and the Rem C-terminus in Rem-mediated inhibition of ICa,L and the loss of sympathetic regulation. To examine the role of GTP binding, we introduced a T94N point mutation into the Rem NBD that is analogous to a mutation which locks Ras in a GDP-bound state.30 When expressed in heart cells, CFP-Rem[T94N] was targeted to the surface sarcolemma, t-tubules, and nucleus (Fig. 6A, top, Supplemental Data, Online Figure I) similar to the sub-cellular distribution observed with CFP-Rem (Fig. 1). However, heart cells expressing CFP-Rem[T94N] displayed robust ICa,L that was indistinguishable from control (Fig. 6, A and B). Furthermore, cells expressing CFP-Rem[T94N] responded robustly to both BAY K 8644 and to PKA activation with 1 μM forskolin (Fig. 6, C–F). These results differ from data obtained in HEK 293 cells, where Rem[T94N] effectively ablates ICa,L. Our data also differ somewhat from a report that the analogous Rad[S105N] exerts a dominant negative effect when expressed in guinea pig and murine cardiac myocytes, resulting in increased ICa,L16. By contrast, we did not observe a dominant negative function of CFP-Rem[T94N], which in this context simply behaves as if it is functionally inert with respect to CaV1.2 channel modulation.
Fig. 6. A putative GTP-binding-deficient Rem[T94N] mutant is functionally inert in heart.
(A) Top, confocal image showing sub-cellular localization of CFP-Rem[T94N] in a guinea pig ventricular myocyte. Bottom, exemplar whole-cell CaV1.2 channel currents from a myocyte expressing CFP-Rem[T94N]. (B) Population Ipeak–V relationship for myocytes expressing CFP-Rem[T94N] (■, n = 6 for each point). Data from uninfected (gray trace) and Rem-expressing cells (cyan trace) are reproduced from Fig. 1 to facilitate visual comparison. (C) Top, exemplar CaV1.2 channel currents from a myocyte expressing CFP-Rem[T94N] before (black trace) and after (red trace) exposure to 1 μM BAY K 8644. Bottom, diary plot showing time course of the forskolin-mediated increase in ICa,L. (D) Ipeak–V relationship for myocyte expressing CFP-Rem[T94N] before (■) and after ( ) exposure to 1 μM BAY K 8644. (E,F) Impact of forskolin on ICa,L in myocytes expressing CFP-Rem[T94N]. Same format as C and D.
) exposure to 1 μM BAY K 8644. (E,F) Impact of forskolin on ICa,L in myocytes expressing CFP-Rem[T94N]. Same format as C and D.
Previous work has identified the distal C-terminus as a locus critical to the CaV channel blocking function of RGK GTPases18, 21, 22. Consistent with observations in other cells, deleting the distal Rem C-terminus eliminates the capacity to block ICa,L in heart (Fig. 1). Previous studies have correlated this functional output of the distal C-terminus with its role in autonomously targeting Rem to the plasma membrane. We investigated whether the essential role of the distal Rem C-terminus could be substituted by the analogous domain from H-Ras (Fig. 7A), which in most cell types functions as a robust membrane targeting module. Unexpectedly, in heart cells, CFP-Rem265-H was not targeted to the sarcolemma (Fig. 7B), in sharp contrast to its membrane localization in other cell types (Supplemental Data, Online Figure VI). Nevertheless, CFP-Rem265-H depressed cardiac ICa,L and CaV1.2 channel Qmax to the same extent as wild-type Rem (Fig. 7, B–D). Moreover, the remnant current was robustly up-regulated by BAY K 8644 (Fig. 7E) but almost completely insensitive to PKA activation (Fig. 7F). Consistent with these findings, the APD in cells expressing CFP-Rem265-H was largely unaffected by forskolin, but significantly prolonged by BAY K 8644 (Fig. 7G). Overall, these data provide the surprising insight that in heart cells membrane targeting of the Rem NBD is neither sufficient (Fig. 6) nor necessary (Fig. 7) to reconstitute ICa,L inhibition. This finding is opposite to conclusions based on studies from recombinant channels reconstituted in heterologous expression systems.
Fig. 7. Functional impact of swapping the Rem C-terminus with the analogous domain from H-Ras.
(A) Schematic showing structural landmarks and C-terminus sequences of Rem and two derivatives. (B) Top, confocal image showing sub-cellular localization of CFP-Rem265-H in a guinea pig ventricular myocyte. Bottom, exemplar whole-cell CaV1.2 channel currents from a myocyte expressing CFP-Rem265-H. (C) Population Ipeak–V relationship for myocytes expressing CFP-Rem265-H (▲, n = 6 for each point). Data from uninfected (gray trace) and Rem-expressing cells (cyan trace) are reproduced from Fig. 1 to facilitate visual comparison. (D) Population ON gating charge versus voltage (QON–V) relationship for isochronal Rem265- (□, n = 8 for each point) and Rem265-H-expressing (●, n = 7 for each point) myocytes. * P < 0.05 compared to Rem265-expressing myocytes. (E) Impact of BAY K 8644 on ICa,L in myocyte expressing Rem265-H. (F) Lack of effect of forskolin on ICa,L in Rem265-H myocyte. (G) Impact of BAY K 8644 (red trace) and forskolin (blue) on action potential from a Rem265-H-expressing myocyte.
Discussion
In this work we have examined the properties of Rem-inhibited CaV1.2 channels in adult heart cells. Our findings differ from prevailing views of the mechanisms underlying RGK GTPase-mediated decrease in cardiac ICa,L and suggest new (patho)physiological ramifications for this potent form of CaV1.2 channel modulation. The results also suggest that RGK GTPases may potentially be exploited as novel tools to probe outstanding biophysical questions related to sympathetic modulation of CaV1.2 channels. We discuss these aspects of our work in relation to previous studies.
Mechanisms and structural determinants of the RGK GTPase/CaV channel crosstalk in heart
We found that over-expressing Rem in heart cells markedly decreased ICa,L, similar to previous findings with Gem and Rad16. What is the mechanistic basis of this effect in heart? One prevailing hypothesis is that RGK GTPases inhibit ICa,L in heart cells by interfering with the trafficking of pore-forming α1C subunits to the sarcolemma, possibly by buffering endogenous CaVβ subunits, thereby reducing the surface density of CaV1.2 channels16, 17. In this scenario, the residual currents remaining in the presence of RGK GTPase expression represent the small fraction of wild-type (unbound) channels that make it to the cell surface (Fig. 8A; model I). Our results offer several lines of proof that argue against model I as a viable mechanism for Rem-mediated block of ICa,L in heart. First, contrary to the prediction of this model, we find no evidence that the surface density of CaV1.2 channels in heart cells is substantially diminished in the presence of Rem. The second line of evidence relates to the observed responses of Rem-inhibited CaV1.2 channels to BAY K 8644 and PKA activation. Model I predicts that the residual currents recorded in the presence of Rem would have the same magnitude of response to BAY K 8644 and PKA activation as channels from control cells (i.e. a similar fold increase in ICa,L). Instead, we observed that Rem-inhibited channels were supra-modulated by BAY K 8644 and insensitive to PKA activation compared to control channels. The results similarly argue against a model in which Rem-modulated channels remain on the surface but are completely electrically silent (Fig. 8A; model 2), since in this scenario the remnant current would still be provided by unmodified wild-type channels. Rather, the findings are consistent with the residual currents observed in Rem-expressing myocytes arising from Rem-bound low-Po channels on the cell surface (Fig. 8A; model 3). In this scenario, the enhanced fold increase in current amplitude in response to BAY K 8644 observed in Rem-modified channels is explained by the larger dynamic range available due to their lower basal Po (Fig. 8B). It is not clear from our data precisely how Rem arrests CaV1.2 channels in a low-Po mode in the sarcolemma. An attractive hypothesis is that this is related to the decrease in Qmax which, in the absence of a decrease in the physical number of surface channels, suggests a Rem-induced partial immobilization of voltage sensors. Deciphering precisely how Rem reduces channel Po and Qmax remains an important challenge for the future.
Fig. 8. Conceptualization of mechanism underlying Rem inhibition of cardiac CaV1.2 channels.
(A) Schematic showing alternative models that could explain Rem inhibition of CaV1.2 channels in heart. Under control conditions a cadre of CaV1.2 channels are present on the cardiac cell surface and give rise to a robust ICa,L upon membrane depolarization. Over-expressing Rem or other RGK GTPases dramatically, but not completely, decreases ICa,L. There are three candidate mechanisms for this effect: model 1 – Rem reduces surface density of channels by disrupting trafficking; model 2 – Rem renders a sub-population of channels on the sarcolemma electrically silent; and (model 3) – Rem arrests virtually all surface channels in a low-Po gating mode. In models 1 and 2, the remnant current seen with Rem over-expression in myocytes represents a small fraction of unmodified wild-type channels. The pattern of responses of Rem-modified channels to BAY K 8644 and PKA activation indicate that the remnant current does not emanate from wild-type channels, thus discounting models 1 and 2. Our results are consistent model 3 being the dominant mechanism of Rem inhibition of ICa,L in heart. (B) Conceptualization of impact of Rem, BAY K 8644 and PKA activation on modal gating activity of CaV1.2 channels in heart.
Based on the assumption that Rem[T94N] is deficient in binding GTP, our results indicate that GTP binding to the NBD is necessary for Rem inhibition of CaV1.2 channels in heart. By contrast, several studies in other cell types have shown that GTP binding is dispensable for RGK block of CaV1-2 channels18, 22. This discrepancy appears to reflect a genuine difference among cell types since it has also been shown that GTP-binding-deficient Rad does not inhibit CaV1.2 channels in heart16. Another sharp distinction from previous studies concerns the role of membrane targeting in ICa inhibition. Studies on recombinant channels reconstituted in heterologous systems, or endogenous CaV2.2 channels in sympathetic cervical ganglion (SCG) neurons, have indicated that membrane targeting is necessary and/or sufficient for RGK GTPases to inhibit ICa18, 21, 22. By contrast, we find that in heart cells membrane targeting of the Rem NBD is neither necessary nor sufficient for blocking ICa,L. Overall, these distinctions emphasize the idea that RGK proteins use diverse mechanisms and determinants to inhibit ICa and that different cell types may utilize unique subsets of the available toolkit to achieve CaV channel block.
Surprisingly, Rem265-H was not targeted to the sarcolemma in heart cells. In other cell types, the H-Ras C-terminus is posttranslationally processed in a sequence of enzyme-catalysed steps that convert it into an effective membrane-targeting module31: farnesylation of the C-terminus CAAX prenylation motif at the cysteine residue followed by proteolysis of the AAX sequence; carboxymethylation of the farnesylated cysteine; and finally, palmitoylation of the two cysteine residues upstream of the farnesylated cysteine (Fig. 7A). The lack of membrane-targeting of Rem265-H suggests that one or more catalytic components of this pathway may be lacking in adult guinea pig heart cells.
(Patho)physiological implications of the RGK GTPase/CaV channel crosstalk in heart
RGK proteins are expressed in hearts from different species: mRNA for Rem has been found in rat and mouse cardiomyocytes8; Rad has been detected in mouse heart17; and, Gem expression has been characterized at the mRNA and protein levels in human and guinea pig heart14, 16, respectively. The powerful impact of RGK proteins on CaV channels suggest that under physiological conditions these proteins may play an integral role in maintaining Ca2+ homeostasis in cardiac myocytes. In heart, Ca2+ influx through CaV1.2 channels is a multi-purpose signal that controls various critical functions including: acting as the trigger for Ca2+-induced Ca2+ release that underlies cardiac EC coupling; adjusting the cardiac action potential duration; and regulating gene expression1, 2. By virtue of their crosstalk with CaV1.2 channels in heart, RGK GTPases could play a critical role in keeping these vital functions within physiological norms. One facet of RGK GTPases that could be useful in fulfilling such a homeostatic function relates to dynamic regulation of their expression, which in some systems has characteristics reminiscent of immediate early gene expression behavior10. While potential dynamism in RGK GTPase expression in heart has not been rigorously explored, there is one report that conditions mimicking inflammation acutely down-regulate Rem mRNA in heart8. Future experiments aimed at investigating how and whether the expression patterns of specific RGK proteins change under different conditions in heart will be important in defining their individual roles in maintaining cardiac Ca2+ homeostasis.
Beyond the putative physiological function of RGK proteins in heart, an intriguing concept is the potential role they may play in cardiac diseases including heart failure, cardiac arrhythmias, and hypertrophy. In human heart failure there is an ~3-fold elevation in Gem GTPase mRNA14. Our studies suggest that such increases in RGK GTPase expression could significantly impair cardiac function due to these proteins diminishing the Po of some sarcolemmal CaV1.2 channels. If severe enough, such 2+ functional elimination of CaV1.2 channels could contribute to the phenomenon of dyssynchronous Ca sparks observed in failing hearts32, 33. Further exploration of potential linkages between RGK proteins and cardiac diseases will be an important focus of future studies. Ultimately, should harmful effects of RGK GTPases in heart be confirmed, our results suggest that functional deficits may be overcome with the use of Ca2+ channel agonists but not by activating the sympathetic β-adrenergic pathway.
Potential use of Rem as a tool to explore biophysical mechanisms of CaV1.2 channel modulation
A fortuitous outcome from these studies concerns the potential to exploit Rem as a tool to probe outstanding questions concerning the mechanisms underlying CaV1.2 channel modulation by Ca2+ channel agonists and sympathetic activation. Previous single-channel studies have characterized distinct gating modes of cardiac CaV1.2 channels34, 35, including: mode 0a, which is characterized by brief infrequent openings; mode 1, which features frequent millisecond openings; and mode 2, wherein the channels display long openings with brief closures (Fig. 8B). In control channels, both BAY K 8644 and PKA increase the propensity for mode 2 openings from the dominant mode 1 gating mode (Fig. 8B)34, 35. Whether these two modulators employ common transduction events to achieve CaV1.2 channel enhancement is unknown. Although we have not directly measured single-channel events here, the whole-cell data indicate Rem arrests channels in a low-Po mode. We conceptualize this low-Po mode as being akin to the previously described mode 0a. The ability of Rem to selectively prevent PKA-mediated up-regulation, while preserving BAY K 8644 modulation (Fig. 8B), may provide a discriminating tool to probe the structural determinants leading to the two forms of CaV1.2 channel modulation.
Regarding PKA modulation of CaV1.2 channels, fundamental mechanistic questions remain unresolved despite intense study by many groups over almost two decades25, 36–39. For example, the precise phosphorylation site/s responsible for channel up-regulation remains unclear, and how this posttranslational modification is transduced into a functional effect on channel gating is unknown. The ability of Rem to block PKA-mediated enhancement of ICa,L at the level of CaV1.2 channels could provide a unique tool to gain new mechanistic insights into this physiologically important form of channel regulation. Potentially, Rem could nullify PKA modulation of ICa,L by either preventing phosphorylation of the functionally relevant residues or by directly opposing the conformational change involved in the transduction event. Future work focusing on these questions will likely shed new insights into the mechanisms underlying PKA modulation of cardiac CaV1.2 channels.
Novelty and Significance.
What Is Known?
RGK GTPases use multiple mechanisms to inhibit L-type calcium current (ICa,L) in reconstituted systems.
Over-expressing RGK GTPases in heart cells inhibits ICa,L.
What New Information Does This Article Contribute?
Rem GTPase inhibits ICa,L in heart cells by arresting calcium channels in a low open probability state, without changing the number of channels at the cell surface.
Rem-inhibited ICa,L in heart cells can be selectively rescued with BAYK 8644, but not PKA-dependent phosphorylation.
The structural determinants of Rem necessary and sufficient for ICa,L inhibition in heart differ from those in reconstituted systems.
Calcium ions entering via the L-type calcium channels in heart cells are essential for cardiac contractility and rhythm. Therefore, molecules that regulate L-type channel activity are critical determinants of the heartbeat under both physiological and disease conditions. RGK proteins are Ras-like GTPases that are the most powerful intracellular blockers of L-type calcium channels known. Though these proteins are found in heart, and their levels altered in cardiac diseases, the precise mechanisms by which they inhibit L-type calcium channels are unclear. Here we show that one RGK GTPase, Rem, inhibits L-type channels in heart cells by arresting them in a low open probability state, without changing the number of channels at the cell surface. Moreover, the Rem-inhibited channels can be completely rescued by L-type channel agonists, but not by the physiological sympathetic adrenergic pathway. Finally, we found that the structural determinants of Rem that are necessary and sufficient for L-type channel inhibition differ from those found in other cell preparations. The study has implications for the future design of novel calcium channel blockers, and suggests the RGK GTPase/L-type calcium channel signaling axis as a potential therapeutic target in cardiac diseases where enhancing L-type calcium current would be beneficial.
Supplementary Material
Acknowledgments
The authors thank Eun Sook Park and Ming Chen for technical assistance; Drs. Tingting Yang, LinLing He and Ademuyiwa Aromolaran for comments on the manuscript.
Sources of Funding
This work was supported by grants from the National Institutes of Health (RO1 HL069911 and RO1 HL084332) to H.M.C. H.M.C. is an Established Investigator of the American Heart Association.
Non-standard Abbreviations and Acronyms
- RGK GTPases
Rad/Rem/Rem2/Gem/Kir sub-family of Ras-like GTPases
- CaV
voltage-dependent calcium channels
- EC
excitation-contraction
- APD
action potential duration
- ICa,L
L-type calcium current
- Qmax
maximum gating charge
- PKA
protein kinase A
- IKS
the slow component of the delayed rectifier potassium current
- NBD
nucleotide binding domain
- Po
open probability
- CFP
cyan fluorescent protein
- YFP
yellow fluorescent protein
- Ipeak
peak current density
- t-tubule
transverse tubule
- Q-V
gating charge versus voltage
- SCG
sympathetic cervical ganglion
Footnotes
Disclosures
None.
References
- 1.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 2.Molkentin JD. Dichotomy of Ca2+ in the heart: contraction versus intracellular signaling. J Clin Invest. 2006;116:623–626. doi: 10.1172/JCI27824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bito V, Heinzel FR, Biesmans L, Antoons G, Sipido KR. Crosstalk between L-type Ca2+ channels and the sarcoplasmic reticulum: alterations during cardiac remodelling. Cardiovasc Res. 2008;77:315–324. doi: 10.1093/cvr/cvm063. [DOI] [PubMed] [Google Scholar]
- 4.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 5.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 6.Triggle DJ. Calcium channel antagonists: clinical uses--past, present and future. Biochem Pharmacol. 2007;74:1–9. doi: 10.1016/j.bcp.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;250:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finlin BS, Andres DA. Rem is a new member of the Rad- and Gem/Kir Ras-related GTP-binding protein family repressed by lipopolysaccharide stimulation. J Biol Chem. 1997;272:21982–21988. doi: 10.1074/jbc.272.35.21982. [DOI] [PubMed] [Google Scholar]
- 9.Finlin BS, Shao H, Kadono-Okuda K, Guo N, Andres DA. Rem2, a new member of the Rem/Rad/Gem/Kir family of Ras-related GTPases. Biochem J. 2000;347:223–231. [PMC free article] [PubMed] [Google Scholar]
- 10.Maguire J, Santoro T, Jensen P, Siebenlist U, Yewdell J, Kelly K. Gem: an induced, immediate early protein belonging to the Ras family. Science. 1994;265:241–244. doi: 10.1126/science.7912851. [DOI] [PubMed] [Google Scholar]
- 11.Reynet C, Kahn CR. Rad: a member of the Ras family overexpressed in muscle of type II diabetic humans. Science. 1993;262:1441–1444. doi: 10.1126/science.8248782. [DOI] [PubMed] [Google Scholar]
- 12.Beguin P, Nagashima K, Gonoi T, Shibasaki T, Takahashi K, Kashima Y, Ozaki N, Geering K, Iwanaga T, Seino S. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature. 2001;411:701–706. doi: 10.1038/35079621. [DOI] [PubMed] [Google Scholar]
- 13.Finlin BS, Crump SM, Satin J, Andres DA. Regulation of voltage-gated calcium channel activity by the Rem and Rad GTPases. Proc Natl Acad Sci U S A. 2003;100:14469–14474. doi: 10.1073/pnas.2437756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan FL, Moravec CS, Li J, Apperson-Hansen C, McCarthy PM, Young JB, Bond M. The gene expression fingerprint of human heart failure. Proc Natl Acad Sci U S A. 2002;99:11387–11392. doi: 10.1073/pnas.162370099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang L, Zhang J, Tseng YH, Xie CQ, Ilany J, Bruning JC, Sun Z, Zhu X, Cui T, Youker KA, Yang Q, Day SM, Kahn CR, Chen YE. Rad GTPase deficiency leads to cardiac hypertrophy. Circulation. 2007;116:2976–2983. doi: 10.1161/CIRCULATIONAHA.107.707257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murata M, Cingolani E, McDonald AD, Donahue JK, Marban E. Creation of a genetic calcium channel blocker by targeted gem gene transfer in the heart. Circ Res. 2004;95:398–405. doi: 10.1161/01.RES.0000138449.85324.c5. [DOI] [PubMed] [Google Scholar]
- 17.Yada H, Murata M, Shimoda K, Yuasa S, Kawaguchi H, Ieda M, Adachi T, Ogawa S, Fukuda K. Dominant negative suppression of Rad leads to QT prolongation and causes ventricular arrhythmias via modulation of L-type Ca2+ channels in the heart. Circ Res. 2007;101:69–77. doi: 10.1161/CIRCRESAHA.106.146399. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Puhl HL, 3rd, Niu SL, Mitchell DC, Ikeda SR. Expression of Rem2, an RGK family small GTPase, reduces N-type calcium current without affecting channel surface density. J Neurosci. 2005;25:9762–9772. doi: 10.1523/JNEUROSCI.3111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finlin BS, Mosley AL, Crump SM, Correll RN, Ozcan S, Satin J, Andres DA. Regulation of L-type Ca2+ channel activity and insulin secretion by the Rem2 GTPase. J Biol Chem. 2005;280:41864–41871. doi: 10.1074/jbc.M414261200. [DOI] [PubMed] [Google Scholar]
- 20.Yang T, Xu X, Kernan T, Wu V, Colecraft H. Rem inhibits recombinant CaV1.2 channels using multiple mechanisms that require distinct configurations of the GTPase. J Physiol. 2010;588:1665–1681. doi: 10.1113/jphysiol.2010.187203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Correll RN, Pang C, Finlin BS, Dailey AM, Satin J, Andres DA. Plasma membrane targeting is essential for Rem-mediated Ca2+ channel inhibition. J Biol Chem. 2007;282:28431–28440. doi: 10.1074/jbc.M706176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang T, Suhail Y, Dalton S, Kernan T, Colecraft HM. Genetically encoded molecules for inducibly inactivating CaV channels. Nat Chem Biol. 2007;3:795–804. doi: 10.1038/nchembio.2007.42. [DOI] [PubMed] [Google Scholar]
- 23.Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, Yegnasubramanian V, Alvania RS, Johns DC, Marban E, Yue DT. Novel functional properties of Ca2+ channel beta subunits revealed by their expression in adult rat heart cells. J Physiol. 2002;541:435–452. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML. Construction of adenovirus vectors through Cre-lox recombination. J Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miriyala J, Nguyen T, Yue DT, Colecraft HM. Role of CaVbeta subunits, and lack of functional reserve, in protein kinase A modulation of cardiac CaV1.2 channels. Circ Res. 2008;102:e54–64. doi: 10.1161/CIRCRESAHA.108.171736. [DOI] [PubMed] [Google Scholar]
- 26.Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 28.Reuter H, Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977;264:49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang G, Zhu X, Xie W, Han P, Li K, Sun Z, Wang Y, Chen C, Song R, Cao C, Zhang J, Wu C, Liu J, Cheng H. Rad as a novel regulator of excitation-contraction coupling and beta-adrenergic signaling in heart. Circ Res. 2010;106:317–327. doi: 10.1161/CIRCRESAHA.109.208272. [DOI] [PubMed] [Google Scholar]
- 30.Feig LA. Tools of the trade: use of dominant-inhibitory mutants of Ras-family GTPases. Nat Cell Biol. 1999;1:E25–27. doi: 10.1038/10018. [DOI] [PubMed] [Google Scholar]
- 31.Henis YI, Hancock JF, Prior IA. Ras acylation, compartmentalization and signaling nanoclusters. Mol Membr Biol. 2009;26:80–92. doi: 10.1080/09687680802649582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Litwin SE, Zhang D, Bridge JH. Dyssynchronous Ca2+ sparks in myocytes from infarcted hearts. Circ Res. 2000;87:1040–1047. doi: 10.1161/01.res.87.11.1040. [DOI] [PubMed] [Google Scholar]
- 33.Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci U S A. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hess P, Lansman JB, Tsien RW. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984;311:538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- 35.Yue DT, Herzig S, Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci U S A. 1990;87:753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao T, Yatani A, Dell’Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 37.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Reyes E, Yuan W, Wei X, Bers DM. Regulation of the cloned L-type cardiac calcium channel by cyclic-AMP-dependent protein kinase. FEBS Lett. 1994;342:119–123. doi: 10.1016/0014-5793(94)80484-2. [DOI] [PubMed] [Google Scholar]
- 39.Zong X, Schreieck J, Mehrke G, Welling A, Schuster A, Bosse E, Flockerzi V, Hofmann F. On the regulation of the expressed L-type calcium channel by cAMP-dependent phosphorylation. Pflugers Arch. 1995;430:340–347. doi: 10.1007/BF00373908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.