Abstract
The therapeutic efficacy of individual components of fish oils (FO) in various human inflammatory diseases still remains unresolved, possibly due to low levels of n-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) or lower ratio of DHA to EPA. Since FO enriched with DHA (FO-DHA) or EPA (FO-EPA) has become available recently, we investigated their efficacy on survival and inflammatory kidney disease in a well-established animal model of human Systemic Lupus Erythematosus (SLE). Results show for the first time that FO-DHA dramatically extends both the median (658 days) and maximal (848 days) lifespan of (NZB × NZW)F1 (B × W) mice. In contrast, FO-EPA fed mice had a median and maximal lifespan of ~384 and 500 days, respectively. Investigations into possible survival mechanisms revealed that FO-DHA (Vs. FO-EPA) lowers serum anti-dsDNA antibodies, IgG deposition in kidneys, and proteinuria. Further, FO-DHA lowered LPS-mediated increases in serum IL-18 levels and caspase-1-dependent cleavage of pro-IL-18 to mature IL-18 in kidneys. Moreover, FO-DHA suppressed LPS-mediated PI3K, Akt, and NF-κB activations in kidney. These data indicate that DHA, but not EPA, is the most potent n-3 fatty acid that suppresses glomerulonephritis and extends lifespan of SLE-prone short-lived B × W mice, possibly via inhibition of IL-18 induction and IL-18-dependent signaling.
INTRODUCTION
Systemic Lupus Erythematosus (SLE) is a prototypic systemic autoimmune disease characterized by heterogeneous clinical manifestations including skin rashes, joint pain, glomerulonephritis, thrombocytopenia, hemolytic anemia, atherosclerosis and central nervous system damage (1, 2). Autoantibody production is the major pathogenic mediator in SLE, (3) and a hallmark of the disease is the elevation in serum IgG antinuclear antibodies (4). The heterogeneous clinical manifestations in SLE appear to be associated with the production of different pathogenic autoantibodies, particularly to nuclear antigens and by an abnormal production of proinflammatory cytokines (5).
SLE is an autoimmune and chronic inflammatory disease. Interleukin (IL)-18 is a pro-inflammatory cytokine, and is synthesized as a non-glycosylated inactive precursor and converted to its biologically active form following cleavage by the cysteine protease caspase-1. Released mature IL-18 exerts its effects upon binding to its cognate receptor (IL-18R), a heterodimer comprised of an α and a β subunit. Its levels are increased in both human and animal models of SLE (5–9). In MRL/lpr mice, a positive correlation has been shown between elevated systemic and kidney IL-18 levels to disease severity (10, 11). This is further confirmed in MRL/lpr mice deficient in IL-18Rα. These mice had reduced levels of anti-double stranded (ds) DNA antibodies and no leukocyte infiltration in kidneys and lungs. Importantly, these mice failed to develop autoimmune kidney disease (12), suggesting that IL-18 plays a critical role in glomerulonephritis, and thus a potential therapeutic target.
Dietary interventions with long-chain polyunsaturated fatty acids profoundly influence both physiological processes as well as inflammatory diseases (13, 14). The omega-3 (n-3) fatty acids eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) exert anti-inflammatory effects in various diseases, including inflammatory bowel disease and rheumatoid arthritis (13). We previously showed that dietary supplementation with Menhaden FO (20–22% n-3 fatty acids) delays the onset of renal disease and extends lifespan of (NZB × NZW)F1 (B × W) mice (15–17). Interestingly, dietary supplementation of FO to SLE patients showed only a moderate beneficial effect on disease severity (18, 19). The FO used in those studies contained both EPA and DHA, but at lower levels. However, Robinson et al demonstrated that feeding EPA prolongs survival (20), and in synergy with DHA exerts anti-inflammatory effects, and alleviates renal disease in B × W mice (21). But the mechanisms still remain elusive. Since FO enriched with EPA or DHA has become available, we investigated for the first time the prolonged effects of FO enriched with DHA (FO-DHA) or EPA (FO-EPA) on kidney disease, and median and maximal lifespan of short-lived SLE-prone B × W mice.
MATERIALS AND METHODS
Animals and experimental diets
Weanling (NZB × NZW)F1 (B × W) female mice were purchased from Jackson Laboratories, Bar Harbor, ME. At 2-months of age, mice were switched to semi-purified diets containing 10% corn oil (CO, MP Biomedicals, Irvine, CA) as control oil and fish oils (FO)s enriched in either eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA): 1) 18/12 fish oil (FO-18/12) 2) 55/5 EPA-enriched FO and 3) 5/60 DHA-enriched FO (Ocean Nutrition, Nova Scotia, Canada). Fatty acid composition of diets is given in Table I. The study was carried out in 2 phases. In the first phase, survival, systemic anti-dsDNA antibodies, IgG deposition in kidneys, and proteinuria were studied. In the second phase, to emphasize the mechanisms of improved survival by DHA and LPS-evoked IL-18 signaling (22, 23), 5-mo-old mice were challenged with LPS (5 mg/kg body weight; intraperitoneally). PBS served as a vehicle control. Both serum and kidneys were collected after 4 h, and analyzed for immunologic, biochemical and molecular changes. All studies were approved by the Institutional Animal Care and Use Committee of University of Texas Health Science Center, San Antonio, Texas.
Table I.
Composition of semi-purified corn oil (CO) and fish oil (FO) diets enriched with eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA).
| Ingredientsa | CO | FO-18/12 | FO-EPA | FO-DHA |
|---|---|---|---|---|
| Casein | 14.00 | 14.00 | 14.00 | 14.00 |
| Corn starch | 42.43 | 42.43 | 42.43 | 42.43 |
| Dextronized corn starch | 14.50 | 14.50 | 14.50 | 14.50 |
| Sucrose | 9.00 | 9.00 | 9.00 | 9.00 |
| Cellulose | 5.00 | 5.00 | 5.00 | 5.00 |
| AIN-93 mineral mix | 3.50 | 3.50 | 3.50 | 3.50 |
| AIN-93 vitamin mix | 1.00 | 1.00 | 1.00 | 1.00 |
| l-cystine | 0.18 | 0.18 | 0.18 | 0.18 |
| Choline bitartrate | 0.25 | 0.25 | 0.25 | 0.25 |
| TBHQ | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin E | 0.04 | 0.04 | 0.04 | 0.04 |
| CO | 10 | 1 | 1 | 1 |
| FO b | 0 | 9 | 9 | 9 |
All diet ingredients were purchased from MP Biomedicals (Irvine, CA).
Fish oils (FO)s enriched in either eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA): 1) FO-18/12, 2) 55/5 EPA-enriched FO and 3) 5/60 DHA-enriched FO (Ocean Nutrition, Nova Scotia, Canada).
Serum fatty acid analysis
Fatty acid composition was analyzed by gas chromatography as described previously (24). Briefly, 100 μl serum were subjected to lipid extraction. Fatty acid methyl esters were derived by heating at 75°C for 1 h in 5% hydrochloric acid–methanol reagent. Fatty acid methyl esters were analyzed by gas chromatography using a fully automated HP5890A series II system equipped with a flame-ionization detector. Peaks of resolved fatty acids were identified by comparison with fatty acid standards (Matreya LLC, Pleasant Gap, PA), and area percentage for all resolved peaks was analyzed by using a HP 3396 series II integrator.
IgG deposition in kidneys
Kidney tissues were snap-frozen in Optimal Cutting Temperature Compound (Miles Scientific, Naperville, IL) and sectioned (4 μm thick). To examine IgG deposits within renal glomeruli, the sections were incubated with FITC-conjugated goat anti-mouse IgG Ab (Serotec, Oxford, UK). Fluorescence intensity within glomerular capillary walls was scored on a scale of 0–3 (0, none; 1, weak; 2, moderate; 3, strong). At least 10 glomeruli per section were analyzed by two independent investigators in a blinded fashion, and scored.
Proteinuria
Proteinuria was assessed using chemstrips (Roche Diagnostic, Indianapolis, IN). In this semi-quantitative system, trace corresponds to <30 mg/ml, 1+ to 30–100 mg/dl, 2+ to 100–500 mg/dl, and 3+ to >500 mg/dl. Consistent with the criteria applied in previous studies of murine lupus, proteinuria >100 mg/dl (≥2+) was interpreted as an evidence of significant end-stage renal disease.
Anti-dsDNA antibodies
Serum anti-dsDNA antibody titers were assessed as previously described using a solid-phase ELISA (16).
Serum IL-18 levels
Serum IL-18 levels were quantified by ELISA (Bender MedSystems Inc, Burlingame, CA). The sensitivity of the assay is 10.0 pg/ml.
Caspase-1 activity
Caspase-1 activity in kidney homogenates was determined by the caspase-1/ICE colorimetric Protease assay kit (BioVision Research Products, Mountain View, CA, USA). The assay is based on the spectrophotometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the labeled substrate YVAD-pNA. The pNA light emission was quantified spectrophotometrically at 405 nm, and the results were expressed in fold-increase from controls.
Pro and mature IL-18 levels
IL-18 protein levels were quantified by Western blotting using antibodies specific for pro (R & D Systems, USA) and mature (Santa Cruz Biotechnology, Inc. USA) forms of IL-18.
Measurement of PI3-kinase
Phosphatidylinositol 3-kinase (PI3K) lipid kinase assays were performed as described previously (25) using p85 immunoprecipitates.
Akt levels and Akt kinase activity
We used two independent but complimentary methods to quantify activation of Akt; immunoblotting using whole cell homogenates and activation-specific antibodies, and immune-complex kinase assays using a commercially available non-radioactive Akt kinase assay kit (Cell signaling Technology, Inc., Danvers, MA). The assay is based on Akt-induced phosphorylation (Ser21/9) of glycogen synthase kinase-3 (GSK-3).
NF-κB activation
NF-κB DNA binding activity was analyzed by electrophoretic mobility shift assay (EMSA) using nuclear protein extracts and double stranded consensus (sense, 5′-AGT TGA GGG GAC TTT CCC AGG C-3′) or mutant (sense, 5′-AGT TGA GGC GAC TTT CCC AGG C-3′) NF-κB oligonucleotides (Santa Cruz Biotechnology Inc., USA). Nuclear p65 levels were quantified by Western blotting (Cell Signaling Technology, Inc). Actin served as a loading control.
Statistical Analysis
Data are expressed as mean ± SEM. Results were analyzed by ANOVA followed by Newman-Keuls test using Graph Prism 4 software (GraphPad, San Diego, CA) and p < 0.05 was considered statistically significant. Survival data were analyzed by Logrank followed by Chi square test.
RESULTS
Delayed onset of kidney disease and maximal lifespan in FO-DHA mice
We have previously demonstrated that Menhaden FO attenuates kidney disease and moderately extends lifespan of B × W mice (26). We now investigated whether enriching FO with DHA (60% DHA, 5% EPA) or EPA (5% DHA, 55% EPA) will further extend lifespan and delay progression of renal disease (Figure 1). Female B × W mice were fed regular FO (18% EPA, 12% DHA; FO-18/12), FO-DHA, and FO-EPA. Corn oil (CO) that contains neither DHA nor EPA served as a control. Results show that the median life span of CO-fed control animals was 372 days, and FO-18/12 moderately extended median life span to 414 days. In contrast, FO-DHA significantly increased median lifespan to 658 days. Interestingly, FO-EPA had minimal effect on lifespan (384 days), and was comparable to that of CO-fed mice. Similarly, maximal lifespan was significantly higher nearly doubled in FO-DHA fed mice (848 days) compared to FO-EPA (500 days), FO-18/12 (539 days), and CO-fed (444 days) mice. These results indicate that DHA, but not EPA-enriched FO, significantly extends both median and maximal life span of the short-lived B × W mice.
Figure 1.
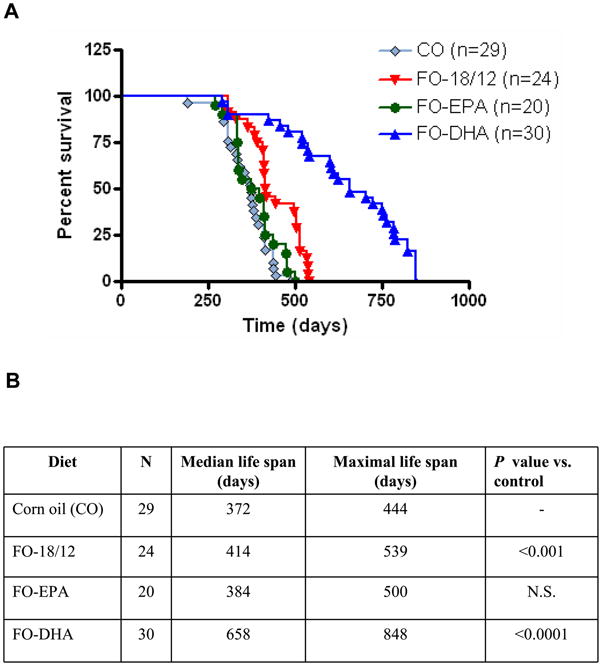
Effect of CO, FO-18/12, FO-EPA and FO-DHA diets on median and maximal lifespan of short-lived SLE-prone (NZB × NZW) F1 female mice. At 2-months of age, mice were switched to semi-purified diets containing 10% corn oil (control), and fish oils (FO) enriched in eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA): 1) 18/12 fish oil (FO-18/12) 2) 55/5 EPA-enriched FO (FO-EPA) and 3) 5/60 DHA-enriched FO (FO-DHA). Survival rate was monitored from 2 months onwards. Our results show that (A) FO-DHA significantly increases lifespan of (NZB × NZW)F1 mice compared to FO-EPA and CO control. (B) Both maximum and median survival rates were also increased in FO-DHA fed mice compared to FO-EPA fed mice. Values are expressed as percentage of mice in each dietary group alive. Results were analyzed by Logrank followed by Chi square test.
Serum fatty acid profile
In order to verify whether dietary oils influence serum fatty acid profile, we analyzed serum for PUFA by gas chromatography (24). FO-fed mice exhibited higher levels of n-3 FA as compared to CO-fed mice. While there was no difference in 20:5n-3 (EPA) levels between the fish oils, DHA-enriched FO fed mice showed the highest incorporation of 22:6n-3 (DHA). 18:2n-6 (LA) was incorporated to lower extent in EPA-enriched FO and DHA-enriched FO fed mice as compared to FO-18/12 fed mice. In mice fed DHA-enriched FO, 20:n-6 (AA) was incorporated to the lowest extent followed by FO-18/12 FO and FO-EPA. Total n-3 FA content was highest and total n-6 FA content was lowest in FO-DHA fed mice compared to other FO and CO diet fed mice. Consequently, n-6/n-3 FA ratio was lowest in FO-DHA fed mice (Table II).
Table II.
Profiles of polyunsaturated n-6 and n-3 fatty acids in serum of (NZB × NZW)F1 mice fed with specialized corn oil (CO) and fish oil (FO) diets enriched with eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA).
| Fatty acids | CO | FO-18/12 | FO-EPA | FO-DHA |
|---|---|---|---|---|
| 18:2n-6 | 30.33±0.70a | 13.69±0.31b | 9.79±0.49c | 8.85±0.37c |
| 20:3n-6 | 0.28±0.03 | 0.27±0.00 | nd | nd |
| 20:4n-6 | 19.52±0.36a | 9.59±0.25c | 12.51±0.48b | 5.21±0.22d |
| 20:5n-3 | nd | 20.17±1.33 | 22.69±1.17 | 20.76±0.41 |
| 22:5n-6 | 0.34±0.12 | nd | nd | nd |
| 22:5n-3 | nd | 0.39±0.02a | 0.96±0.15b | 0.70±0.07b |
| 22:6n-3 | 3.33±0.40a | 12.55±0.28c | 9.89±0.49b | 23.89±0.74d |
| PUFA | 53.79±0.74 | 56.48±0.56 | 55.84±0.92 | 59.40±0.62 |
| n-3 FA | 3.33±0.40a | 33.11±1.20b | 33.54±1.01b | 45.34±0.66c |
| n-6 FA | 50.46±0.48a | 23.37±0.64b | 22.30±0.24b | 14.06±0.57c |
| n-6/n-3 | 15.85±1.90 | 0.71±0.05 | 0.67±0.03 | 0.31±0.02 |
CO, corn oil; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FA, fatty acids; FO, fish oil; nd – not detected; PUFA, polyunsaturated fatty acids. Total lipids of (NZB × NZW)F1 mice serum were extracted, methylated and subjected to analysis by gas chromatography. The values (% of total fatty acids) are means of three independent measurements ± SEM. n=5. Significant difference (P<0.05) is indicated with different alphabets analyzed by ANOVA followed by Newman-Keuls test. Ratio of n-6/n-3 fatty acids is expressed as (18:2n-6 + 20:3n-6 +20:4n-6 +22:5n-6)/(20:5n-3 + 22:5n-3 + 22:6n-3).
Serum anti-dsDNA antibodies are reduced in FO-DHA fed mice
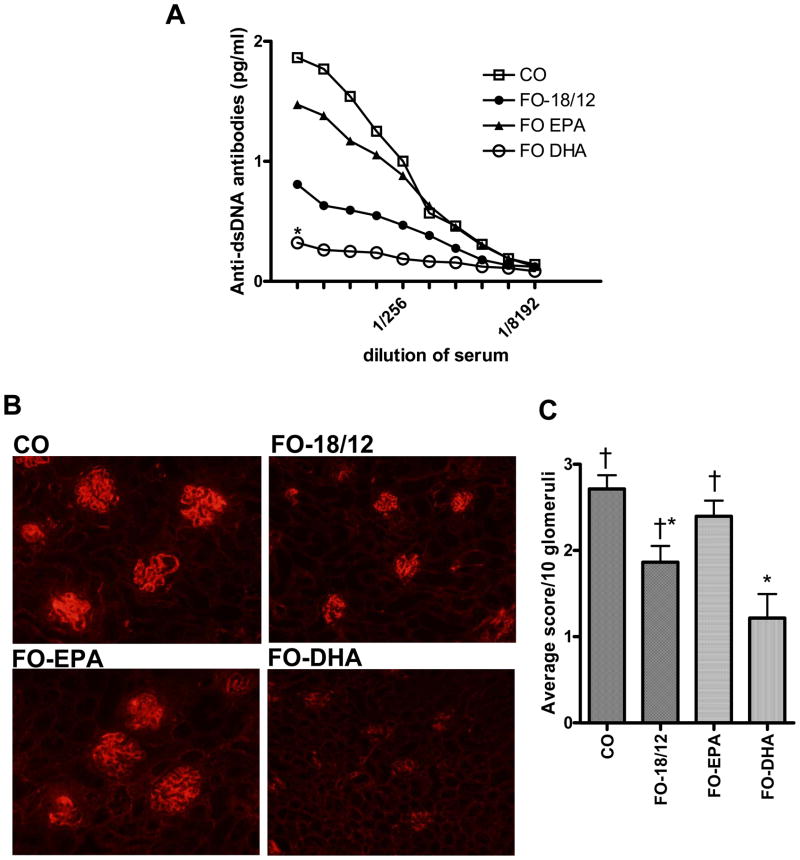
Anti-dsDNA antibodies are implicated in the pathogenesis of SLE. FO-18/12 significantly lowered the serum anti-dsDNA antibody titer as compared to CO-fed mice (Figure. 2A). These effects were more pronounced in FO-DHA fed mice. In contrast, FO-EPA failed to significantly modulate anti-dsDNA antibody titer, and the levels were comparable to that seen in CO-fed mice, indicating that DHA, but not EPA, significantly lowers systemic anti-dsDNA antibodies in SLE-prone B × W mice (Figure 2A).
Figure 2. Effect of CO, FO-18/12, FO-EPA and FO-DHA diets on serum anti-dsDNA antibodies and IgG deposition in kidneys of (NZB × NZW)F1 female mice.
At 2-months of age, female (NZB × NZW)F1 mice were fed semi-purified diets containing 10% corn oil (control), and fish oils (FO) enriched in eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA): 1) 18/12 fish oil (FO-18/12), 2) 55/5 EPA-enriched FO (FO-EPA) and 3) 5/60 DHA-enriched FO (FO-DHA). At 5-months of age, mice were challenged with LPS intraperitonelly, and serum anti-dsDNA antibodies were quantified by ELISA. Histological evaluation of IgG deposition was scored on a scale of 0–3 (0, none; 1, weak; 2, moderate; 3, strong) based on fluorescence intensity within glomerular capillary walls (10 glomeruli/mouse and 6 mice/group). (A) FO-DHA fed mice exhibit significantly lower anti-dsDNA antibodies in serum (P<0.01, ANOVA). (B) Representative photomicrographs of fluorescent stained kidneys indicate lower IgG deposition in FO-DHA fed mice compared to FO-EPA and CO fed mice. (C) Histological evaluation of kidney demonstrates significantly decreased IgG deposition in FO-DHA fed mice compared to CO, FO-18/12 and FO-EPA fed mice. Different signs (*†, † and *) indicates significant differences (P< 0.05; ANOVA followed by Newman-Keuls test).
IgG deposition and proteinuria are decreased in kidneys of FO-DHA fed mice
Elevated proteinuia and deposition of IgG in glomeruli are characteristic features of renal disease in B × W mice (27). When compared to CO-fed mice, histological evaluation of IgG deposition in kidneys (Figure 2B) and proteinuria levels (Table III) were both significantly decreased in FO-18/12 and FO-DHA fed mice, but to a greater extent in the latter group. There was, however, no difference in IgG deposition between CO and FO-EPA fed mice, indicating that DHA, but not EPA, potently down regulates IgG deposition in kidneys of SLE-prone B × W mice (Figure 2C).
Table III.
Proteinuria of (NZB × NZW)F1 mice fed with specialized corn oil (CO) and fish oil (FO) diets enriched with eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA).
| Groups (diet) | Age (Months) | Number of mice | Proteinuria Levels1 | |||
|---|---|---|---|---|---|---|
| Trace (<30 mg/dl) | + (30–100 mg/dl) | ++ (100–500 mg/dl) | +++ (>500 mg/dl) | |||
| CO | 4 | 15 | 3 | 0 | 0 | 0 |
| 6 | 13 | 7 | 2 | 1 | 0 | |
| 8 | 10 | 3 | 0 | 7 | 0 | |
| 10 | 5 | 0 | 0 | 4 | 1 | |
| 12 | 4 | 0 | 0 | 1 | 3 | |
| FO-18/12 | 4 | 15 | 4 | 0 | 0 | 0 |
| 6 | 15 | 4 | 1 | 0 | 0 | |
| 8* | 13 | 5 | 2 | 0 | 0 | |
| 10* | 11 | 6 | 1 | 1 | 0 | |
| 12 | 8 | 0 | 6 | 2 | 0 | |
| FO-EPA | 4 | 15 | 2 | 0 | 0 | 0 |
| 6 | 12 | 4 | 2 | 0 | 0 | |
| 8* | 7 | 5 | 1 | 1 | 0 | |
| 10* | 6 | 0 | 4 | 1 | 1 | |
| 12 | 6 | 0 | 1 | 2 | 2 | |
| FO-DHA | 4 | 15 | 1 | 0 | 0 | 0 |
| 6 | 14 | 2 | 0 | 0 | 0 | |
| 8 | 14 | 2 | 0 | 0 | 0 | |
| 10*† | 13 | 1 | 1 | 0 | 0 | |
| 12*† | 11 | 1 | 1 | 1 | 0 | |
At 2-months of age, mice were switched to semi-purified diets containing 10% corn oil (control), and fish oils (FO) enriched in eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA): 1) 18/12 fish oil (FO-18/12) 2) 55/5 EPA-enriched FO (FO-EPA), and 3) 5/60 DHA-enriched FO (FO-DHA).
Proteinuria was determined using Chemstrips (Roche Diagnostic Corporation, Indianapolis, Inc.) (n=15).
Different signs (* and *†) indicates significant difference in proteinuria levels of FO-18/12, FO-EPA, FO-DHA fed mice compared to CO fed mice measured by ANOVA.
Serum IL-18 levels are lowered in FO-DHA fed mice
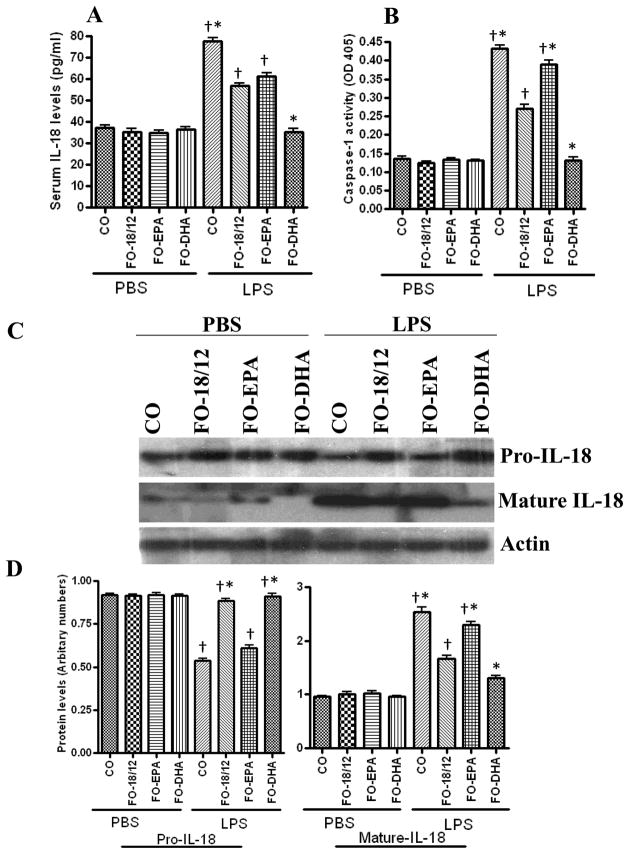
IL-18 plays a causal role in SLE (5), and LPS is potent inducer of IL-18 (22). Therefore, we investigated serum IL-18 levels following LPS challenge. Results in Figure 3A show that FO-18/12 and FO-DHA both significantly lowered LPS-induced IL-18 in serum as compared to CO-fed mice. Once again, FO-EPA failed to modulate LPS-mediated IL-18 expression, indicating that FO-DHA significantly lowers pro-inflammatory IL-18 levels in serum of SLE-prone B × W mice (Figure 3A).
Figure 3. Effect of CO, FO-18/12, FO-EPA and FO-DHA diets on LPS-induced serum IL-18 levels, kidney caspase-1 activity, and pro/mature IL-18 expression in (NZB × NZW)F1 female mice.
At 2-months of age, mice were switched to semi-purified diets containing 10% corn oil (control), and fish oils (FO) enriched in eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA): 1) 18/12 fish oil (FO-18/12) 2) 55/5 EPA-enriched FO (FO-EPA) and 3) 5/60 DHA-enriched FO (FO-DHA). At 5 months of age, mice (n=5) were challenged with LPS intraperitonelly to evaluate the IL-18 expression profile in serum and kidney. PBS served as a solvent control. (A) FO-DHA fed mice significantly lowered LPS-induced serum IL-18 levels compared to CO, FO-18/12, FO-EPA and FO-DHA fed mice. (B) LPS-induced caspase-1 activity in kidneys was significantly decreased in FO-DHA fed mice compared to CO, FO-18/12 and FO-EPA fed mice. (C) FO-DHA fed mice expressed lower levels of LPS-induced mature IL-18 in kidneys compared to CO, FO-18/12 and FO-EPA fed mice. (D) Densitometric analysis of pro IL-18 and mature IL-18 after LPS-injection in mice. Results are representative of two independent experiments. Data are expressed as mean ± SEM. Results were analyzed by ANOVA followed by Newman-Keuls test. *LPS-treated FO-DHA fed mice showed significantly (P<0.05) decreased pro IL-18 levels, Caspase-1 activity and mature-IL-18 expression compared to CO, FO-18/12 and FO-EPA fed mice. Different signs (*†, † and *) indicate a significant difference (P<0.05, ANOVA followed by Newman-Keuls test) in LPS-injected groups. Similar sign assigned groups indicates no significant difference between the groups.
Mature, but not pro-IL-18 expression, is lowered in kidneys of FO-DHA-fed mice
IL-18 is synthesized as a pro-form, and is cleaved by caspase-1 to a mature biologically active 18 kDa secreted form. Since FO-DHA significantly attenuated serum IL-18 levels (Figure 3A), we investigated whether decreased serum IL-18 levels reflect reduced IL-18 expression in the kidneys. Western blot analysis of kidney homogenates revealed detectable levels of pro-IL-18, and LPS administration failed to significantly modulate its expression. On the other hand, mature IL-18 was detected at low levels in vehicle (PBS)-treated animals, and was increased in all groups following LPS administration. However, LPS-mediated increase in mature IL-18 expression was attenuated in both FO-18/12 and FO-DHA groups, and once again, FO-DHA was the most potent. Since caspase-1 cleaves pro-IL-18 to mature IL-18, we further investigated whether decreased levels of mature IL-18 were due to reduced caspase-1 activity (Figure 3B). Indeed, FO-DHA was more potent in inhibiting LPS-mediated increase in caspase-1 activity. These results indicate that FO-DHA attenuates IL-18 expression by inhibiting caspase-1 activity and caspase-1-dependent pro-IL-18 to IL-18 processing (Figure 3C and 3D).
FO-DHA inhibits LPS-mediated PI3K, Akt and NF-κB activations in kidneys
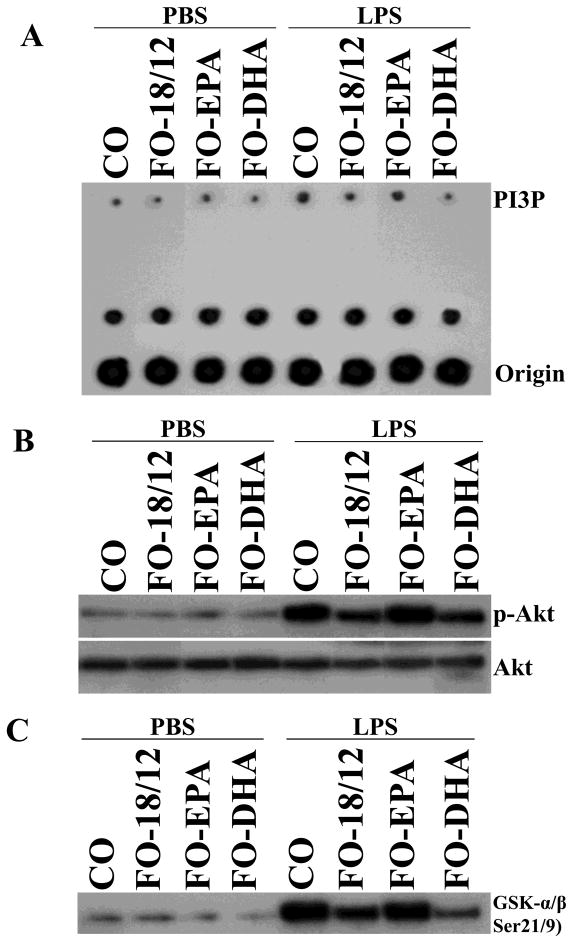
Since LPS signals via PI3K, we measured PI3P in kidneys of PBS and LPS-injected mice. While there was no difference in basal levels of PI3P, FO-18/12 and to a higher extent DHA-enriched FO prevented LPS-stimulated PI3P activation status as compared to CO feeding. There was no difference in PI3P activation between CO-fed and EPA-enriched FO-fed mice (Figure 4A) Akt is downstream of PI3K and its phosphorylation at Ser473 denotes activation. Similar to its inhibition of PI3K, a significant inhibition of phospho-Akt levels (Figure 4B) as well as Akt kinase activity (Figure 4C) were observed in FO-18/12 fed mice, and to a much higher extent in DHA-enriched FO-fed mice as compared to CO-fed mice. No inhibition was observed in EPA-enriched FO fed mice.
Figure 4.
Effect of CO, FO-18/12, FO-EPA and FO-DHA diets on PI3K/Akt kinase pathway in (NZB × NZW)F1 female mice. LPS challenged kidney PI3K and Akt activations were determined in mice fed with CO, FO-18/12, FO-EPA and FO-DHA for 3 months. PI3K activation was analyzed by PI3K lipid kinase assays using p85 immunoprecipitates. Akt activation was analyzed by immunoblotting using whole cell homogenates and activation-specific antibodies, and by using a commercially available kinase assay that quantifies Akt-induced glycogen synthase kinase-3 (GSK-3) phosphorylation at Ser21/9. (A) Our results indicated that FO-DHA diet significantly suppressed PI3K activation in kidneys of LPS-injected mice. (B) Western Blot showing significant inhibition of phospho-Akt in kidneys of LPS-injected FO-DHA fed mice. (C) Similarly, immune-complex kinase assays demonstrated significant inhibition of Akt kinase activity in kidneys of LPS-injected FO-DHA fed mice. Results are representative of three independent experiments.
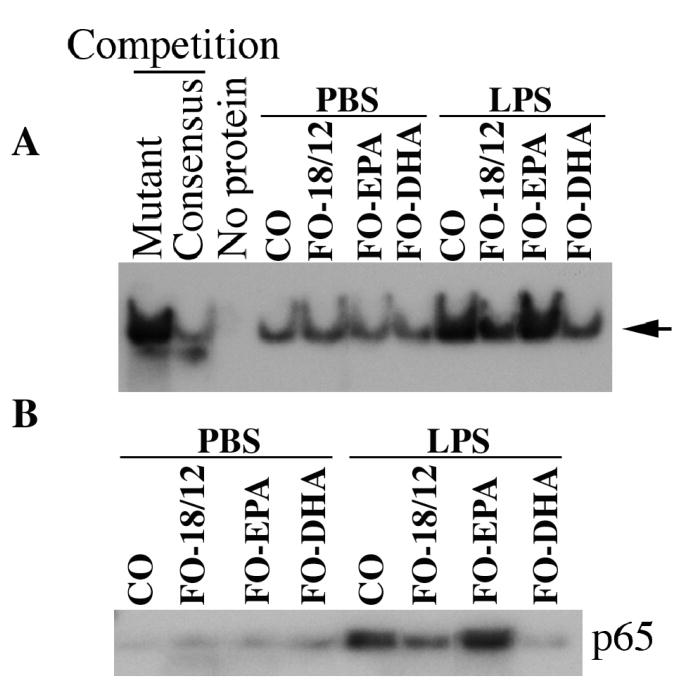
Since NF-κB is involved in IL-18 induction, and is downstream of PI3K and Akt, we next analyzed NF-κB DNA binding activity by EMSA using kidney nuclear extracts. We also analyzed nuclear translocation of NF-κB p65 by immunoblotting. Significant inhibition of NF-κB DNA binding activity and NF-κB p65 subunit nuclear translocation was observed in FO-18/12 fed mice and it was even higher in DHA-enriched FO-fed mice as compared to CO-fed mice. There was, however, no inhibition in EPA-enriched FO fed mice (Figure 5A and 5B).
Figure 5.
Effect of CO, FO-18/12, FO-EPA and FO-DHA diets on NF-κB activation. Female (NZB × NZW)F1 mice were fed with CO, FO-18/12, FO-EPA and FO-DHA for 3 months, and then challenged with LPS (5 mg/kg body weight, IP) for 4 hrs. Kidneys were harvested and analyzed for NF-κB DNA binding activity by EMSA using nuclear protein extracts (A). Activation of NF-κB was confirmed by analyzing nuclear p65 levels immunoblotting. Immunoblotting (B). Actin served as a loading control. The results show significant inhibition of LPS-mediated NF-κB DNA binding activity (A) and nuclear p65 translocation (B) in FO-DHA-fed mice.
DISCUSSION
We previously reported that n-3 FA (20–25% EPA+DHA) present in Menhaden FO extends median and maximal lifespan of short-lived lupus-prone B × W mice compared to a diet supplemented with n-6 FA-rich CO (15, 16, 28). Our subsequent studies demonstrated that a combination of n-3 FA and caloric restriction (CR) further extends the lifespan of B × W mice even more than n-6 FA fed ad libitum (AL) or with CR (29), suggesting that source of dietary fat (n-3 FA vs. n-6 FA) was an important determinant of disease progression and severity in B × W mice. While these findings are encouraging, there are obvious concerns that a dietary regimen of 30–40% CR may be impractical for SLE patients, and that FO with low EPA/DHA content would have only moderate beneficial effects in the same patients. Since EPA and DHA are the principal biologically active fatty acids in FO, and as their levels/ratio affect the anti-inflammatory effects, in the present study we investigated the efficacy of FO enriched with DHA (60% DHA/5% EPA; FO-DHA) or EPA (5% DHA/55% EPA; FO-EPA) on disease severity and longevity in B × W mice. Our results demonstrate for the first time that DHA is a potent inhibitor of autoantibody production, IL-18 expression and kidney disease, and that DHA significantly extends life span of short-lived B × W mice.
FO-DHA significantly attenuated serum and kidney IL-18 expression. IL-18 is a proinflammatory cytokine and its systemic levels are significantly elevated in SLE patients (5, 10). Similarly, SLE-prone MRL/lpr mice also express high levels of serum IL-18 (10). Further, B × W mice repeatedly exposed to LPS develop an early and accelerated form of lupus nephritis (23), with enhanced polyclonal B cell activation and persistence of exacerbated nephritis, even after LPS clearance (30). In the present study, we found that LPS treatment significantly increased serum IL-18 levels in both CO and FO-EPA fed mice. However, a significant inhibition in serum and kidney IL-18 levels was observed in FO-DHA and FO-18/12 fed mice (inhibitory activity: FO-DHA>FO-18/12>FO-EPA). Since caspase-1 cleaves pro-IL-18 to mature IL-18, and as caspase-1-activity was lower in the LPS-treated FO-DHA fed mice, our results suggest that lower levels of mature IL-18 in serum and kidneys were due to the reduced cleavage of pro-IL-18 to mature IL-18.
Since PI3 kinase plays a critical role in IL-18 induction as well as in IL-18 signaling (31, 32), we next analyzed the activation status of PI3K in kidneys following LPS treatment. Compared to vehicle-treated mice, a robust increase in PI3K activation was noted in kidneys from LPS injected CO and FO-EPA fed mice. In contrast, FO-DHA and FO-18/12 fed mice showed lower levels of PI3K-dependent PI3P levels. Of note, PI3Kγ is an important target for inhibition of glomerulonephritis and extension of lifespan in MRL/lpr mice (33). Since FO-DHA attenuated PI3K activation, and since inhibition of PI3Kγ was shown to prolong lifespan and decrease glomerulonephritis, our results also suggest that dietary supplementation of FO-DHA is a viable therapeutic strategy to ameliorate chronic inflammation (33). Because the serine/threonine kinase Akt/protein kinase B is one of the major downstream targetsof PI3K, we also analyzed total and phospho-Akt (Ser473) levels in kidney homogenates. Our results indicate that while total Akt remained similar in the kidneys of all four groups, phospho-Akt levels were reduced in FO-DHA and FO-18/12 fed mice. Once again FO-DHA was more potent in inhibiting Akt activation. These results were further confirmed by immune-complex kinase assays, which revealed reduced Akt kinase activity in FO-DHA fed mice, suggesting that FO-DHA potently inhibits Akt activation in kidneys in vivo. While DHA has been shown to inhibit LPS-induced Akt phosphorylation in RAW264.7 macrophages in vitro (34), our studies are the first to show that DHA, but not EPA, inhibits PI3K/Akt signaling in vivo in the kidneys of SLE-prone mice.
NF-κB is a ubiquitous stress-responsive transcription factor and plays a role in inflammation. Since NF-κB is a downstream mediator of PI3K and Akt pathways, and plays a role in IL-18 induction and signaling (31, 32), we analyzed NF-κB activation by EMSA and nuclear translocation of p65 by immunoblotting in kidneys following LPS treatment. Our results show a significant inhibition in LPS-mediated NF-κB DNA-binding activity in FO-DHA fed mice, followed by FO-18/12 fed mice. These results corroborate previous studies demonstrating inhibition of NF-κB activation by n-3 fatty acids both in vivo and in vitro (35–38). Our results also show that reduced levels of nuclear NF-κBp65 in kidneys of FO-DHA fed mice, suggesting that FO-DHA inhibits NF-κB activation by inhibiting nuclear translocation of p65. This finding is in agreement with a recent study which showed that DHA attenuates LPS-induced nuclear p65 levels in cultured human THP-1 macrophages, whereas EPA had no effect (36). Our study provides the first in vivo evidence that DHA is a potent inhibitor of NF-κB activation, and suggests that reduced NF-κB activation might be a contributing factor in the inhibition of LPS-induced renal disease in FO-DHA fed mice.
Activations of PI3K, Akt, and NF-κB play a role in both IL-18 induction and IL-18-dependent signaling. Importantly, our results show that FO-DHA is a potent inhibitor of these three critical players of inflammation, thus inhibiting perpetuation of inflammatory signaling during SLE. While we demonstrated that FO-DHA inhibits LPS-mediated NF-κBp65 nuclear translocation, recently it has also been shown that IκB degradation contributes to LPS-mediated NF-κB activation and IL-18 signaling (22, 39, 40), suggesting that the observed p65 nuclear translocation follows IκB degradation, and FO-DHA might activate NF-κB via classic IκB degradation and NF-κB activation in LPS-treated mice. Further, PI3K, which is now considered a potential therapeutic target in SLE (33, 41), has also been shown to be a target of DHA in neuronal cells, thus its inhibition improves their survival (42). Since, FO-DHA fed mice exhibited reduced levels of LPS-induced NF-κB activation, and serum and kidney IL-18 expression, it is reasonable to speculate that FO-DHA inhibits IL-18 expression by attenuating LPS-mediated P3K/Akt-dependent NF-κB activation. We will investigate this possibility in future in isolated mesangial cells treated with DHA and LPS. In addition, we will also investigate the origin of IL-18 in kidneys using in situ hybridization and immunohistochemistry.
Circulating autoantibodies to DNA is one of the hallmarks of SLE in humans (1) and B × W mice (3, 4). Anti-dsDNA antibodies form immune complexes and their deposition results in arthritis and nephritis. B × W mice on CO and FO-EPA diets exhibited higher anti-dsDNA antibody levels in serum and higher IgG deposition in the kidneys. In correlation with their survival data, FO-DHA fed mice had lower levels of serum anti-dsDNA antibody and kidney IgG deposition. It was previously reported that MRL/lpr mice show higher serum IgG1 and IgG2 anti-dsDNA antibodies and higher IgG deposition in kidneys following IL-18 administration (10). In contrast, lower total IgG and IgGa anti-dsDNA antibodies were reported in serum of MRL/lpr mice deficient in IL-18Rα mice (12). These mice also exhibited lower glomerular deposition of IgG, thus supporting the role of IL-18 as an important mediator of renal disease in SLE-prone mice, and inhibition of IL-18 and its signaling by DHA may alleviate clinical features of SLE. It should be pointed out that FO doses used in this study are relatively higher compared to human consumption via food or dietary supplementation. Our recent findings, using prescribed FO (LOVAZA™) 1% as a human equivalent dose in B × W mice also revealed significant decrease in proteinuria and improved survival compared to 1% placebo (unpublished data). Future clinical trials with purified DHA enriched FO or LOVAZA™ in lupus patients is warranted.
In summary, our studies demonstrate for the first time that FO enriched in DHA attenuates glomerulonephritis and significantly extends lifespan of short-lived SLE-prone B × W mice, compared to EPA enriched FO or FO with lower EPA+ DHA levels. This beneficial effect of DHA may be attributed to its anti-inflammatory activity through inhibition of IL-18 expression and IL-18-dependent signaling.
Acknowledgments
Grant support: NIH funding support 1R01AT004259-01 and 1R01AG030161-01A1 to Gabriel Fernandes, HL-86787 to Chandrasekar and the BiomedicalLaboratory Research & Development Service of the VA Office of Research and Development to Chandrasekar.
Abbreviations
- AA
arachidonic acid
- AL
ad libitum
- CR
calorie restriction
- CO
corn oil
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FA
fatty acids
- FO
fish oil
- IL-18
Interleukin-18
- GSK-3
Glycogen synthase kinase-3
- LA
linoleic acid
- LPS
lipopolysaccharide
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PBS
phosphate buffer saline
- SLE
systemic lupus erythematosus
- PI3K
Phosphatidylinositol 3-kinase
References
- 1.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 3.Helyer BJ, Howie JB. Renal disease associated with positive lupus erythematosus tests in a cross-bred strain of mice. Nature. 1963;197:197. doi: 10.1038/197197a0. [DOI] [PubMed] [Google Scholar]
- 4.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 5.Novick D, Elbirt D, Miller G, Dinarello CA, Rubinstein M, Sthoeger ZM. High circulating levels of free interleukin-18 in patients with active SLE in the presence of elevated levels of interleukin-18 binding protein. J Autoimmun. 2009 doi: 10.1016/j.jaut.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Nicoletti F, Di Marco R, Mangano K, Patti F, Reggio E, Nicoletti A, Bendtzen K, Reggio A. Increased serum levels of interleukin-18 in patients with multiple sclerosis. Neurology. 2001;57:342–344. doi: 10.1212/wnl.57.2.342. [DOI] [PubMed] [Google Scholar]
- 7.Gracie JA, Forsey RJ, Chan WL, Gilmour A, Leung BP, Greer MR, Kennedy K, Carter R, Wei XQ, Xu D, Field M, Foulis A, Liew FY, McInnes IB. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999;104:1393–1401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong CK, Li EK, Ho CY, Lam CW. Elevation of plasma interleukin-18 concentration is correlated with disease activity in systemic lupus erythematosus. Rheumatology (Oxford) 2000;39:1078–1081. doi: 10.1093/rheumatology/39.10.1078. [DOI] [PubMed] [Google Scholar]
- 9.Monteleone G, Trapasso F, Parrello T, Biancone L, Stella A, Iuliano R, Luzza F, Fusco A, Pallone F. Bioactive IL-18 expression is up-regulated in Crohn’s disease. J Immunol. 1999;163:143–147. [PubMed] [Google Scholar]
- 10.Esfandiari E, I, McInnes B, Lindop G, Huang FP, Field M, Komai-Koma M, Wei X, Liew FY. A proinflammatory role of IL-18 in the development of spontaneous autoimmune disease. J Immunol. 2001;167:5338–5347. doi: 10.4049/jimmunol.167.9.5338. [DOI] [PubMed] [Google Scholar]
- 11.Faust J, Menke J, Kriegsmann J, Kelley VR, Mayet WJ, Galle PR, Schwarting A. Correlation of renal tubular epithelial cell-derived interleukin-18 up-regulation with disease activity in MRL-Faslpr mice with autoimmune lupus nephritis. Arthritis Rheum. 2002;46:3083–3095. doi: 10.1002/art.10563. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita K, Yamagata T, Nozaki Y, Sugiyama M, Ikoma S, Funauchi M, Kanamaru A. Blockade of IL-18 receptor signaling delays the onset of autoimmune disease in MRL-Faslpr mice. J Immunol. 2004;173:5312–5318. doi: 10.4049/jimmunol.173.8.5312. [DOI] [PubMed] [Google Scholar]
- 13.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 14.Lavie CJ, Milani RV, Mehra MR, Ventura HO. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol. 2009;54:585–594. doi: 10.1016/j.jacc.2009.02.084. [DOI] [PubMed] [Google Scholar]
- 15.Chandrasekar B, Fernandes G. Decreased pro-inflammatory cytokines and increased antioxidant enzyme gene expression by omega-3 lipids in murine lupus nephritis. Biochem Biophys Res Commun. 1994;200:893–898. doi: 10.1006/bbrc.1994.1534. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes G, Bysani C, Venkatraman JT, Tomar V, Zhao W. Increased TGF-beta and decreased oncogene expression by omega-3 fatty acids in the spleen delays onset of autoimmune disease in B/W mice. J Immunol. 1994;152:5979–5987. [PubMed] [Google Scholar]
- 17.Fernandes G, Bhattacharya A, Rahman M, Zaman K, Banu J. Effects of n-3 fatty acids on autoimmunity and osteoporosis. Front Biosci. 2008;13:4015–4020. doi: 10.2741/2989. [DOI] [PubMed] [Google Scholar]
- 18.Duffy EM, Meenagh GK, McMillan SA, Strain JJ, Hannigan BM, Bell AL. The clinical effect of dietary supplementation with omega-3 fish oils and/or copper in systemic lupus erythematosus. J Rheumatol. 2004;31:1551–1556. [PubMed] [Google Scholar]
- 19.Clark WF, Parbtani A, Huff MW, Reid B, Holub BJ, Falardeau P. Omega-3 fatty acid dietary supplementation in systemic lupus erythematosus. Kidney Int. 1989;36:653–660. doi: 10.1038/ki.1989.242. [DOI] [PubMed] [Google Scholar]
- 20.Prickett JD, Robinson DR, Steinberg AD. Dietary enrichment with the polyunsaturated fatty acid eicosapentaenoic acid prevents proteinuria and prolongs survival in NZB × NZW F1 mice. J Clin Invest. 1981;68:556–559. doi: 10.1172/JCI110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson DR, Xu LL, Tateno S, Guo M, Colvin RB. Suppression of autoimmune disease by dietary n-3 fatty acids. J Lipid Res. 1993;34:1435–1444. [PubMed] [Google Scholar]
- 22.Shui HA, Ka SM, Wu WM, Lin YF, Hou YC, Su LC, Chen A. LPS-evoked IL-18 expression in mesangial cells plays a role in accelerating lupus nephritis. Rheumatology (Oxford) 2007;46:1277–1284. doi: 10.1093/rheumatology/kem136. [DOI] [PubMed] [Google Scholar]
- 23.Cavallo T, Granholm NA. Bacterial lipopolysaccharide transforms mesangial into proliferative lupus nephritis without interfering with processing of pathogenic immune complexes in NZB/W mice. Am J Pathol. 1990;137:971–978. [PMC free article] [PubMed] [Google Scholar]
- 24.Bhattacharya A, Sun D, Rahman M, Fernandes G. Different ratios of eicosapentaenoic and docosahexaenoic omega-3 fatty acids in commercial fish oils differentially alter pro-inflammatory cytokines in peritoneal macrophages from C57BL/6 female mice. J Nutr Biochem. 2007;18:23–30. doi: 10.1016/j.jnutbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Chandrasekar B, Bysani S, Mummidi S. CXCL16 signals via Gi, phosphatidylinositol 3-kinase, Akt, I kappa B kinase, and nuclear factor-kappa B and induces cell-cell adhesion and aortic smooth muscle cell proliferation. J Biol Chem. 2004;279:3188–3196. doi: 10.1074/jbc.M311660200. [DOI] [PubMed] [Google Scholar]
- 26.Chandrasekar B, Troyer DA, Venkatraman JT, Fernandes G. Dietary omega-3 lipids delay the onset and progression of autoimmune lupus nephritis by inhibiting transforming growth factor beta mRNA and protein expression. J Autoimmun. 1995;8:381–393. doi: 10.1006/jaut.1995.0030. [DOI] [PubMed] [Google Scholar]
- 27.Muthukumar AR, Jolly CA, Zaman K, Fernandes G. Calorie restriction decreases proinflammatory cytokines and polymeric Ig receptor expression in the submandibular glands of autoimmune prone (NZB × NZW)F1 mice. Journal of clinical immunology. 2000;20:354–361. doi: 10.1023/a:1006620130114. [DOI] [PubMed] [Google Scholar]
- 28.Venkatraman JT, Chandrasekar B, Kim JD, Fernandes G. Effects of n-3 and n-6 fatty acids on the activities and expression of hepatic antioxidant enzymes in autoimmune-prone NZBxNZW F1 mice. Lipids. 1994;29:561–568. doi: 10.1007/BF02536628. [DOI] [PubMed] [Google Scholar]
- 29.Jolly CA, Muthukumar A, Avula CP, Troyer D, Fernandes G. Life span is prolonged in food-restricted autoimmune-prone (NZB × NZW)F(1) mice fed a diet enriched with (n-3) fatty acids. J Nutr. 2001;131:2753–2760. doi: 10.1093/jn/131.10.2753. [DOI] [PubMed] [Google Scholar]
- 30.Granholm NA, Cavallo T. Long-lasting effects of bacterial lipopolysaccharide promote progression of lupus nephritis in NZB/W mice. Lupus. 1994;3:507–514. doi: 10.1177/096120339400300614. [DOI] [PubMed] [Google Scholar]
- 31.Morel JC, Park CC, Kumar P, Koch AE. Interleukin-18 induces rheumatoid arthritis synovial fibroblast CXC chemokine production through NFkappaB activation. Lab Invest. 2001;81:1371–1383. doi: 10.1038/labinvest.3780351. [DOI] [PubMed] [Google Scholar]
- 32.Morel JC, Park CC, Woods JM, Koch AE. A novel role for interleukin-18 in adhesion molecule induction through NF kappa B and phosphatidylinositol (PI) 3-kinase-dependent signal transduction pathways. J Biol Chem. 2001;276:37069–37075. doi: 10.1074/jbc.M103574200. [DOI] [PubMed] [Google Scholar]
- 33.Barber DF, Bartolome A, Hernandez C, Flores JM, Redondo C, Fernandez-Arias C, Camps M, Ruckle T, Schwarz MK, Rodriguez S, Martinez AC, Balomenos D, Rommel C, Carrera AC. PI3Kgamma inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med. 2005;11:933–935. doi: 10.1038/nm1291. [DOI] [PubMed] [Google Scholar]
- 34.Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 35.Sun D, Krishnan A, Zaman K, Lawrence R, Bhattacharya A, Fernandes G. Dietary n-3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J Bone Miner Res. 2003;18:1206–1216. doi: 10.1359/jbmr.2003.18.7.1206. [DOI] [PubMed] [Google Scholar]
- 36.Weldon SM, Mullen AC, Loscher CE, Hurley LA, Roche HM. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J Nutr Biochem. 2006 doi: 10.1016/j.jnutbio.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Theuer J, Shagdarsuren E, Muller DN, Kaergel E, Honeck H, Park JK, Fiebeler A, Dechend R, Haller H, Luft FC, Schunck WH. Inducible NOS inhibition, eicosapentaenoic acid supplementation, and angiotensin II-induced renal damage. Kidney Int. 2005;67:248–258. doi: 10.1111/j.1523-1755.2005.00075.x. [DOI] [PubMed] [Google Scholar]
- 38.Komatsu W, Ishihara K, Murata M, Saito H, Shinohara K. Docosahexaenoic acid suppresses nitric oxide production and inducible nitric oxide synthase expression in interferon-gamma plus lipopolysaccharide-stimulated murine macrophages by inhibiting the oxidative stress. Free Radic Biol Med. 2003;34:1006–1016. doi: 10.1016/s0891-5849(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 39.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73:213–224. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 40.Cheng J, Imanishi H, Morisaki H, Liu W, Nakamura H, Morisaki T, Hada T. Recombinant HBsAg inhibits LPS-induced COX-2 expression and IL-18 production by interfering with the NFkappaB pathway in a human monocytic cell line, THP-1. J Hepatol. 2005;43:465–471. doi: 10.1016/j.jhep.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 41.Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta. 2008;1784:159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci U S A. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]