Abstract
Purpose
human embryonic stem cells (hESCs) which express a reporter gene consistently during all phases of differentiation would be valuable for basic research on cell transplantation. In this study, we describe karyotypically-abnormal variant hESCs, BGO1V2-EFG, which express hrGFP driven by the EF1 promoter.
Methods
BGO1V2-EFG cells were analyzed by using immunocytochemistry, single cell-based confocal image, and in vitro differentiation, including dopaminergic differentiation.
Results
Undifferentiated BGO1V2-EFG cells expressed pluripotent ESC markers and retained the ability to differentiate into cell types of all three germ layers. BGO1V2-EFG cells maintained stable and robust hrGFP expression in vitro in the undifferentiated state and during differentiation. The EF1 promoter retained activity during dopaminergic differentiation, as 76% of tyrosine hydroxlase (TH)-positive cells co-expressed hrGFP by confocal analysis. Treated with sodium butyrate (0.02 mM to 2.0 mM), an inhibitor of histone deacetylase (HDAC), during differentiation did not affect hrGFP expression, although TH expression was reduced by higher concentrations of sodium butyrate.
Conclusion
BGO1V2-EFG cells maintain stable and robust hrGFP expression in the undifferentiated state and during neural differentiation. Especially, the EF1 promoter was effective in driving hrGFP expression during dopaminergic differentiation. BGO1V2-EFG cells may be useful for transplantation studies in Parkinson disease animal models.
Keywords: Human embryonic stem cells, EF1-hrGFP reporter gene, nucleofection, BGO1V2-EFG
1. Introduction
Embryonic stem cells (ESCs) are derived from inner cell mass of preimplantation embryos and retain the ability to differentiate into cells of various lineages in culture (Amit et al., 2000; Thomson et al., 1998; Zhang et al., 2001). Human embryonic stem cells (hESCs) are invaluable for the studies of gene expression, cellular differentiation and signal transduction, and may eventually provide a source of cells for cellular replacement therapies in various brain disorders, such as Parkinson's disease. Efficient introduction of exogenous genetic elements into the cell genome would be of benefit for the development of hESC-derived cells for various applications.
Transgenic hESC lines which express reporter genes such as GFP, or alternative fluorescent proteins such as EGFP or hrGFP, under the control of various promoters have became a valuable research tool. Such cells can be used for monitoring gene expression in live cells during various stages of differentiation, as well as cell survival and migration following transplantation (Costa et al., 2005; Eiges et al., 2001; Kim et al., 2005; Tomishima et al., 2007; Xia et al., 2008). Various promoters, including cellular polypeptide chain elongation factor 1 alpha (EF1), cytomegalovirus (CMV), rous sarcoma virus (RSV) and β-actin promoters have been used to drive green fluorescent protein (GFP) expression in hESC as well as various other cell types. None of the commonly used promoters are, however, known to be active in hESC when they have undergone terminal differentiation to dopamine (DA) neurons. In contrast to these promoters, a specific promoter for the DA phenotype, such as the tyrosine hydroxlase (TH) promoter (Yoshizaki et al., 2004; Hedlund et al., 2007), would presumably drive GFP expression in differentiated DA neurons, but would not drive GFP expression in undifferentiated cells. A promoter which could be used to express GFP in both differentiated neurons and at all intermediate stages of differentiation would be extremely useful, particularly for neural transplantation studies.
It has become clear in recent years that major factors which regulate gene transcription are the related phenomena of histone acetlyation and DNA methylation. Methylated DNA recruits histone deacetylases (HDACs). When DNA is associated with de-acetylated histones, transcription is repressed. This process is reversible, so that HDAC inhibitors, such as sodium butyrate, can result in acetylated histones and allow for transcription under conditions where transcription in normally repressed. In the case of transduced genes, especially where expression is under the control of viral promoters, it appears that histone deacetylation is a major factor which represses gene transcription (Chen et al., 1997; Choi et al., 2005; Jaalouk et al., 2006). In the previous study, we found that both CMV and EF1 promoters were effective in driving reporter expression in undifferentiated mouse ES cells (mESCs) and differentiated neural progenitors, but not dopaminergic neurons (Zeng et al., 2003). Therefore it is possible that some of the limitations in expression of transduced genes, such as the loss of expression of a reporter gene in mES differentiated dopaminergic neurons may involve histone deacetylation, rather than variations in promoter efficacy per se.
There are also recent reports that expression of the tyrosine hydroxylase gene is regulated by histone deacetylation (Kim et al., 2003; Patel et al., 2005). One recent report, in fact, suggests that physiological concentrations of butyrate, which can be produced in the gut, can regulate the tyrosine hydroxylase promoter (Patel et al., 2005), but not those of the other catecholamine synthetic enzymes phenylethanolamine N-methyltransferase or dopamine-beta-hydroxylase. We therefore determined whether expression of either the GFP reporter or TH gene is altered by sodium butyrate treatment.
Based on our previous studies, the variant hES cell lines show not only normal differentiation and pluripotency, but are also relatively easier to maintain in culture, recover rapidly from frozen preparations, and retain the property of differentiating reliably into dopaminergic neurons (Vazin et al., 2008a; Zeng et al., 2004a). In the present study, we therefore examine reporter expression in variant hESCs using the EF1 promoter. This study includes: 1) establishment of a stable hES cell line with the reporter gene, humanized renilla green fluorescent protein (hrGFP) driven by human cellular polypeptide chain elongation factor 1 alpha (EF1) promoter to provide a tool for monitoring cell pluripotency and differentiation at defined stages. 2) Observation of the relative strength and activity of the EF1 promoter in the undifferentiated state and during dopaminergic differentiation. 3) Investigation whether sodium butyrate affects hrGFP and TH gene expression during dopaminergic differentiation.
2. Materials and Methods
2.1. Cell Culture
The BGO1 hESC line was obtained from BresaGen, Inc (Athens, GA) and cultured as described previously (Zeng et al., 2004b). Briefly, hESCs were maintained on inactivated mouse embryonic fibroblast (MEF) feeder cells in Dulbecco's modified Eagle's medium/Ham's F12 (DMEM/F12, 1:1) supplemented with 20% knockout serum replacement (KSR), 0.1 mM nonessential amino acids, 2 mM L-glutamine, 50 μg/ml Penn-Strep (all from Invitrogen,), 0.1 mM β-mercaotoethanol (Millipore), and 4 ng/ml basic fibroblast growth factor (bFGF, Sigma). Cells were routinely passaged at 1:3 by treatment with collagenase IV (1 mg/ml, Invitrogen). The karyotype of BGO1 cells was initially normal, but converted to trisomy 17 at passage 68 (47, XY, +17) (Vazin et al., 2008a). The trisomy 17 cells, designated BGO1V2, were used for hES cell transfection.
2.2. Plasmid construction
For cloning pEF1/hrGFP, first hrGFP was excised by NotI and EcoRV from phrGFP (Stratagene) and ligated into the NotI and XbaI sites (both the insert and the vector were blunt before ligation) of pRC/RSV (Invitrogen). The EF1 promoter was amplified by polymerase chain reaction (PCR) from pDrive-rEF.RUTM (Invivogen) using primers with a BglII site at the 5’ end and a NotI at the 3’ end. The sequences of the primers were 5’-CGGCAGTCTGGAGCCGAGAGTAATTCATACAAAAG-3’ and 5’-GCGCGCGGCCGCTGGCTTGGATCTGTAACGGCGCAG-3’. The PCR products were then digested with BglII and NotI, and ligated into the BglII and NotI digested pRSV/hrGFP plasmid after gel purification of the promoterless backbone, so that the RSV promoter was replaced by the EF1 promoter. The promoter was sequenced and there were no mutations.
2.3. Transfection and establishment of transgenic cell line
All reagents were prepared for nucleofector transfection as described in the mouse ES cell nucleofector kit (Amaxa Biosystems). For stable transfection, cells were dissociated with collagenase (1 mg/ml) to small clusters of approximately 50-100 μm in diameter. For each nucleofection, an estimated 1×106 cells was mixed with 4.0 μg of linearized DNA and resuspended in 100 μl of nucleofector solution. Cells were then transferred to an electroporation cuvette and exposed to the Amaxa Nucleofector with program A23 according to the manufacturer's instructions. Individual green colonies were manually picked up after 10-14 days of selection with medium containing neomycin at a concentration of 200 μg /ml, and expanded for further analysis. Transgenic ES cells were maintained in 100 μg/ml neomycin on Neo-resistant MEF. Neomycin was removed during neural induction. After transfection, the BGO1V2 cells were designated BGO1V2-EFG.
2.4. In Vitro differentiation via EB formation
Differentiation via embryoid bodies (EB) formation was performed as previously described (Zeng et al., 2004c). Briefly, BGO1V2-EFG cells were dissociated into small clumps using collagenase IV (Sigma-Aldrich) and grown as floating spheres (EB) in hESC medium without bFGF for 4 days. Then, cells were plated on poly-L-lysine/ Fibronectin-coated plate and further cultured until used for immunocytochemistry.
2.5. Neural Differentiation of hESC
Neural differentiation of BGO1V2-EFG was induced by the mouse stromal cell line PA6 followed by additional differentiation in the absence of feeder cells as described previously (Vazin et al., 2008b; Zeng et al., 2004b). The BGO1V2-EFG cells were cultured on PA6 feeder cells to form colonies in Glasgow minimum essential media (Invitrogen) supplemented with 10% KSR (Invitrogen), 1 mM pyruvate (Sigma), 0.1 mM nonessential amino acids, and 0.1 mM – mercaptoethanol. After 10 days, the ES cells were dissociated from the PA6 feeder layer using papain (Worthington) according to the manufacturer's instructions. Then 50,000 cells /4-cm2 dish were seeded on poly-L-lysine/Laminin-coated plates. For single cell-based confocal imaging, dissociated cells were seeded on 18 mm coated coverslips in 12-well plates. The ES cells were further cultured with differentiation medium containing sonic hedgehog (SHH, 40 ng/ml, R & D Systems); FGF8 (40 ng/ml, Biosource) and glial cell line-derived neurotrophic factor (GDNF, 20 ng/ml, R & D Systems,) for an additional 18 to 20 days, during which half of the medium was changed every 2 to 4 days.
2.6. Treatment of hES cells with sodium butyrate
The cells were treated with different concentrations of the histone deacetylase inhibitor sodium butyrate (0.02 to 2.0 mM) for 48 hr at two stages of cell differentiation. The early stage was at day 4 after dissociation from PA6 cells, while the later stage was 16 days after dissociation from PA6 cells.
2.7. Immunocytochemistry and confocal laser scanning microscopy
Immunocytochemistry was performed as described previously (Zeng et al., 2004b). Antibodies used included octamer-binding transcription factor (OCT-3/4, [Santa Cruz Biotechnology]; 1:100), SSEA4 (Developmental Studies Hybridoma Bank; University of Iowa; 1:50), α-fetoprotein (AFP, [Sigma]; 1:100), nestin (BD Bioscience; 1:500); MAP2 (BD Bioscience 1: 500): βIII-tubulin (Pomega; 1:500), glial fibrillary acid protein (GFAP, [Dako; 1: 2000), tyrosine hydroxlase (TH, [Pel Freez]. 1:500), and smooth muscle actin (SMA, [Sigma], 1:200), The localization of antigens was visualized by using appropriate fluorescent-labeled secondary antibodies (Alexa fluor 594 or 488; Molecular Probes; 1:500). In randomly selected wells, the primary antibody was replaced with normal goat serum, in which case no specific immunoreactivity was seen. Images were viewed under a Zeiss Axiovert 200M fluorescent microscope.
For confocal imaging, cells growing on coverslips were first fixed with paraformaldehyde and processed for immunostaining. Coverslips were mounted on the slides with anti-fading media (Molecular Probes) and analyzed using the UltraView confocal system (PerkinElmer).
2.8. Statistical analysis
All in vitro experiments were conducted independently three times. Statistical analysis was performed in GraphPad Instat (V 3.05). Data were analyzed by one way-ANOVA with post-hoc tests by Tukey multiple comparisons. P values of <0.05 were considered to be statistically significant.
3. Results
3.1. Generation of the hrGFP-expressing hESCs
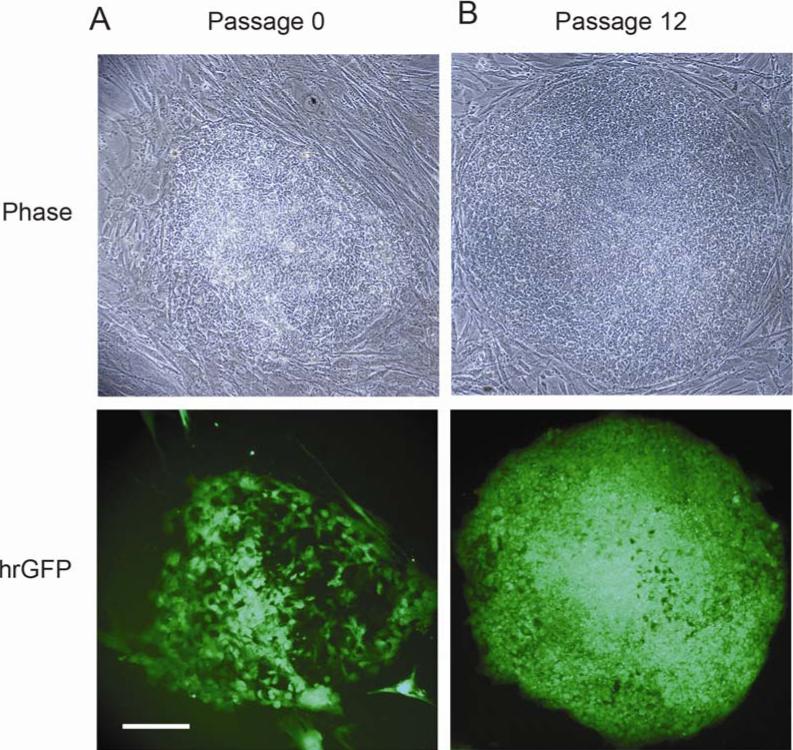
A number of stable G418-resistant transgenic ES cell colonies were generated after nucleofection with the EF1/hrGFP plasmid. Figure 1A shows phase contrast and fluorescence views of one of hrGFP-positive colony at passage 0. The EF1 promoter directed strong hrGFP expression and an almost homogeneous expression pattern in vitro over 3 months under neomycin selection as shown in Figure 1B. This expression was also maintained in frozen and thawed cells, which were kept in liquid nitrogen for over one year. The percentage of cells expressing hrGFP was evaluated by counting hrGFP+ cells within colonies normalized to total cells (DAPI) (staining not shown). The percentage of cells expressing hrGFP in the undifferentiated state with G418 selection was 98.3 ± 1.0%.
Fig. 1.
Expression of hrGFP in hESC transfected with the plasmid pEF1-hrGFP. (A) At passage 0, one hrGFP-positive colony is shown after 10 days of neomycin selection with phase contrast (upper frame) and fluorescence microscopy (lower frame). (B) At passage 12, hESCs were kept in culture for three months under neomycin selection. The EF1 promoter directed strong hrGFP expression with an almost homogeneous expression pattern. Scale bar = 100 μm.
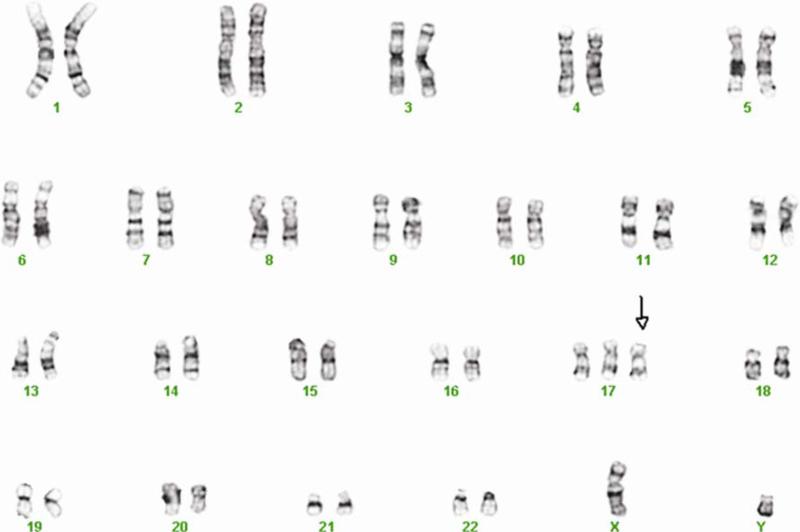
3.2. Karyotype of BGO1V2-EFG cells
Karyotype abnormalities may develop in hESC after extended maintenance in vitro (Buzzard et al., 2004; Draper et al., 2004), especially after genetic modification. The parental BG01 hESC line is male (XY) and has a normal complement of 46 chromosomes. The cells used for nucleofection, named BGO1V2 (Vazin et al., 2008a), exhibited a karyotype of 47 chromosomes (XY, +17) at passage 68. The transgenic cells retained the same abnormal karyotype at passage 7, which was used for the present experiments (Fig. 2).
Fig. 2.
Karyotype of BGO1V2-EFG cells. Karyotype of BGO1V2-EFG was detected by standard G banding. The cells retained the same abnormal karyotype as BGO1V2, the cells used for nucleofection (47, XY, +17, passage 7).
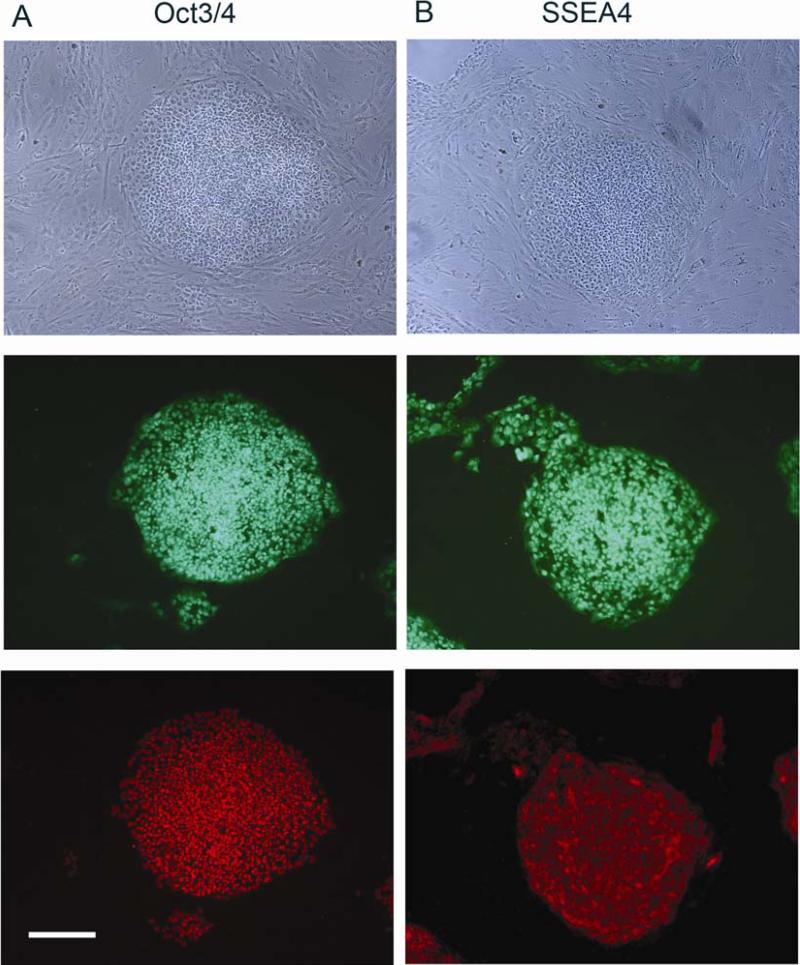
3.3. BGO1V2-EFG cells retain hESC properties
Similar to the parental BGO1 and also BGO1V2 cells, undifferentiated BGO1V2-EFG cells maintained expression of two characteristic ES markers, Oct3/4 (Fig. 3A) and SSEA4 (Fig. 3B). When BGO1V2-EFG cells were kept culture in vitro and propagated for 20 passages (over 5 months), expression of the pluripotency markers was maintained and cell growth rate was not changed, as compared to the original BGO1V2 cell line (data not shown).
Fig. 3.
BGO1V2-EFG cells maintain Oct3/4 and SSEA 4 expression for 3 months in vitro after transfection. Phase contrast (top frame), fluorescence detection of hrGFP (middle frame, green) and Oct3/4 immunoreactivity (lower frame, red in A) or SSEA4 immunoreactivity (lower frame, red in B). Scale bar = 200 μm.
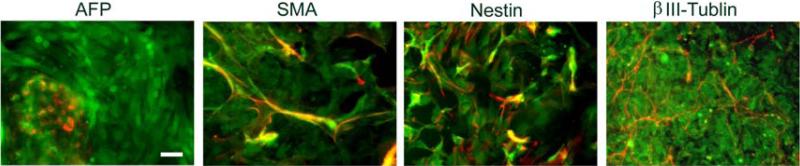
3.4. BGO1V2-EFG cells retain the ability of differentiating into cell types from all three germ layers
The capacity of BGO1V2-EFG to differentiate into ectodermal, mesodermal and endodermal derivatives was evaluated. Figure 4 shows that hrGFP fluorescence and expression of AFP (endodermal marker), SMA (mesodermal marker), β-tubulin III and nestin (both ectodermal markers) were found in differentiated BGO1V2-EFG cells derived by EB formation. Therefore, BGO1V2-EFG cells retain the ability to differentiate into cell types representing all three germ layers, a property consistent with pluripotency.
Fig. 4.
In vitro differentiation of BGO1V2-EFG via EB formation. Differentiation of BGOV2-EFG cells was initiated by forming EBs in the absence of MEFs and bFGF. Positive staining for AFP, SMA, nestin and βIII-tublin was identified (red), indicating that BGOV2-EFG cells can differentiate to express markers of ectoderm, mesoderm and endoderm. Scale bar = 50μm.
3.5. Characteristics of BGO1V2-EFG during differentiation
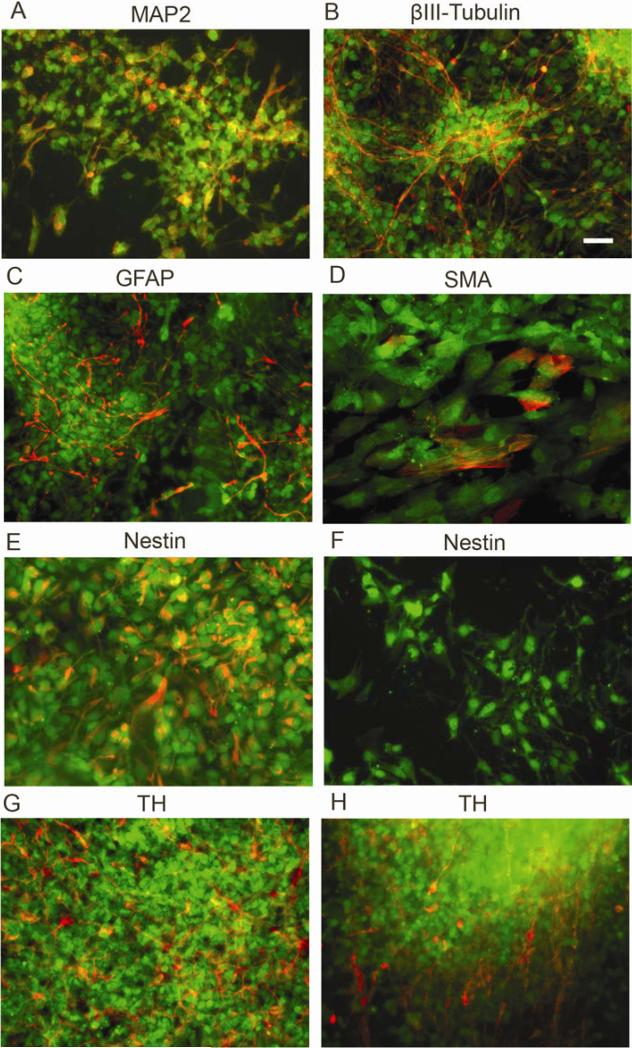
Characteristics of BGO1V2-EFG during differentiation were examined by immunocytochemistry. Eighteen days after removal from the PA6 cell feeder layer, most of the differentiated hESCs had neural characteristics, staining positively with neuron subtype-specific markers as indicated in Figure 5. Some cells, both inside and in the periphery of colonies, expressed the neuronal markers MAP2 (Fig. 5A), and βIII-tubulin (Fig. 5B). GFAP+ astrocytes were also found in the most of the outgrowth areas as well as within colonies (Fig. 5C). Very few smooth muscle actin (SMA)-positive cells were detected after differentiation (Fig. 5D). Cells expressing the neural precursor marker nestin were found inside of colonies (Fig. 5E), but rarely in outgrowth areas (Fig. 5F). TH+ cells were found both inside and in the periphery of colonies (Fig. 5, G,H). The percentage of TH+ neurons as a fraction of the total cells was 24.7 ± 1.7 % (n=1000; 3 repeats). Therefore, BGO1V2-EFG cells were able to differentiate into neural and dopaminergic cells in a manner similar to the parental BGO1 and also BGO1V2 cells.
Fig. 5.
Characterization of cellular phenotypes present in BGO1V2-EFG cells after 18 days of differentiation following removal from the PA6 feeder cells. (A) MAP2; (B) βIII-tubulin; (C) GFAP; (D) SMA; (E, F) nestin; (G, H) TH. Staining for all markers is shown in red while hrGFP expression is shown in green. Scale bar = 50μm.
3.6. EF1 activity is retained in TH+ cells during dopaminergic differentiation
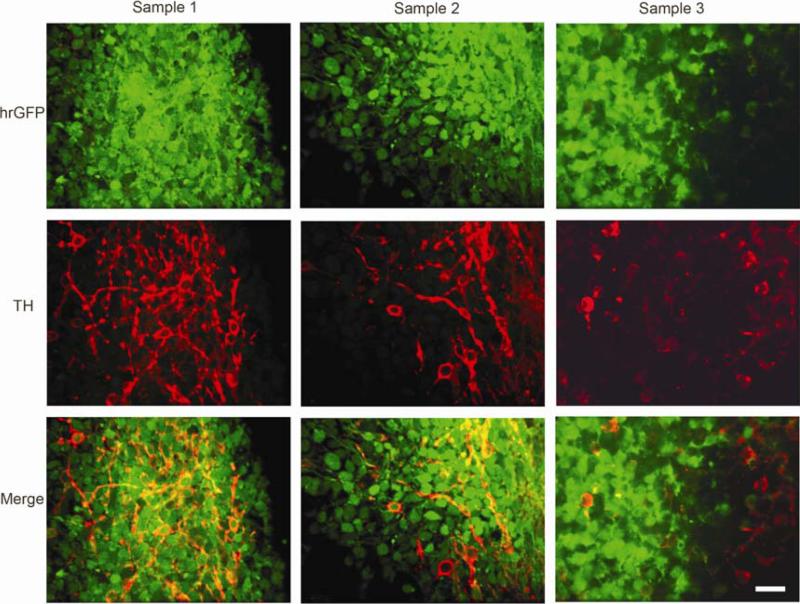
BGO1V2-EFG cells retained uniform and robust hrGFP expression after in vitro differentiation. hrGFP expression was not only stable during cell differentiation, but it also appeared to even be intensified in a population of cells. Whether EF1 activity is retained in TH+ cells during dopaminergic differentiation was one of critical questions in this study. Figure 6 shows three different views by confocal imaging. The percentage of TH+ cells co-expression hrGFP was 76 ± 1.5% (N = 1000; 3 repeats).
Fig. 6.
The EF1 promoter retains activity in hESC during dopaminergic differentiation. Co-localization of TH and hrGFP in individual cells during cell differentiation was examined by using single cell-based confocal imaging. The experiments were performed in triplicate wells, and repeated three times. Most TH-positive cells were co-expressed hrGFP (yellow), while some TH-positive cells were negative for hrGFP. Scale bar = 50μm.
3.7. Effects of sodium butyrate during dopaminergic differentiation
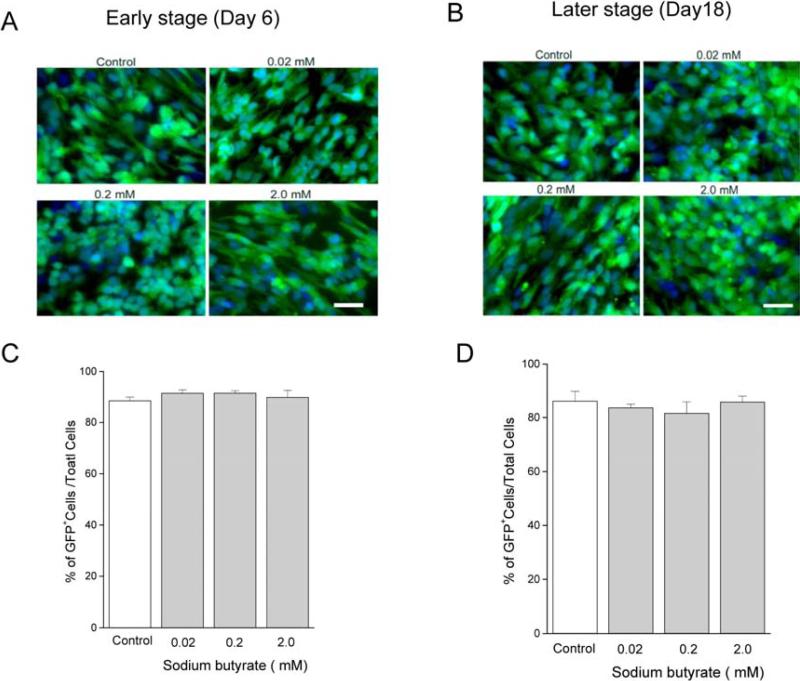
To test the possibility that transgene expression was limited by promoter methylation, hESCs were treated with sodium butyrate (0.02 mM to 2.0 mM) on days 4 to 6 (early stage) or days 16 to 18 (later stage) after dissociation from the PA6 cells. The percentage of hrGFP-positive cells was evaluated for at least 1000 total DAPI+ cells counted for each condition (Fig.7, A,B). hrGFP+ expression was not affected by sodium butyrate at either the early stage, or at the later stage of differentiation (Fig. 7, C,D). Also, there was no apparent effect of sodium butyrate on the intensity of hrGFP fluorescence within individual cells.
Fig. 7.
hrGFP expression was not influenced by sodium butyrate treatment during differentiation. BGO1V2-EFG cells were incubated with sodium butyrate (0.02, 0.2, and 2 mM) for 48 hr at an early stage and at a later stage of cell differentiation as described in Methods. The effect of sodium butyrate on hrGFP expression was evaluated by counting hrGFP+ cells (green) vs total cells (DAPI, blue) at the early stage (A) or at the later stage of differentiation (B). hrGFP expression was not influenced by sodium butyrate treatment at either the early stage (C) or the later stage (D) of differentiation. Scale bar = 50 μm.
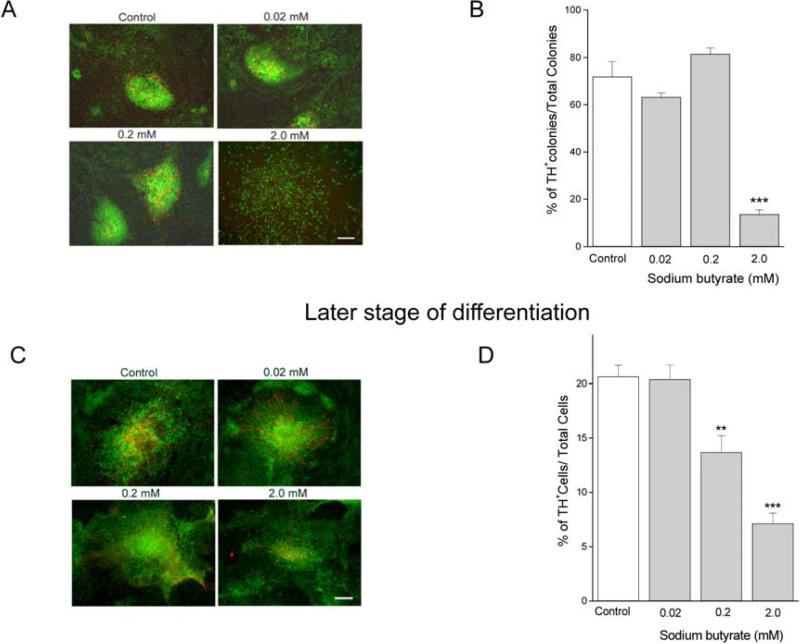
The effect of sodium butyrate on TH gene expression was examined by immunocytochemistry. At the early stages of differentiation, TH+ cells were mainly found inside of neural rosette-like colonies. The lower concentrations of sodium butyrate did not produce any apparent changes in colony morphology. With 2.0 mM of sodium butyrate, the cells in neural rosette-like colonies were more spread out (Fig. 8A). The numbers of neural rosette-like colonies containing TH+ cells was significantly decreased by the 2.0 mM concentration of sodium butyrate (Fig. 8B). At the later stage, TH expression was markedly reduced in cells incubated with either the 0.2 or 2.0 mM concentrations of sodium butyrate (Fig. 8C).
Fig. 8.
TH expression was reduced by higher concentrations of sodium butyrate during dopaminergic differentiation. The effect of sodium butyrate on TH expression was determined by immunocytochemistry. (A) The morphology of colonies and neural-like rosettes was changed, and TH immunoreactivity (red) was reduced, by 2 mM of sodium butyrate treatment at the early stage of differentiation. (B) The number of colonies containing TH-positive cells was decreased significantly, as compared to the control group, *** indicates ρ< 0.001. (C) TH expression was reduced by 0.2 and 2.0 mM sodium butyrate treatment at the later stage of differentiation. (D) The reduction in TH expression by 0.2 and 2.0 mM concentrations of sodium butyrate was statistically significant. ** and *** indicate ρ< 0.01 and ρ< 0.001 respectively. Data shown are means ± SEM. Scale bar = 200μm.
4. Discussion
A stable preparation of transgenic hESCs, BGO1V2-EFG, is described, in which the fluorescent protein hrGFP driven by EF1 promoter is expressed after nucleofection and subsequent neomycin selection. The cells maintained proliferative potential for prolonged periods in culture and exhibited an almost homogeneous hrGFP expression pattern in the undifferentiated state. The EF1 promoter was able to drive robust hrGFP expression at all stages of cell differentiation, including cells differentiated to a dopaminergic phenotype.
A hESC line which can produce a high-yield of midbrain DA neurons while expressing a reporter gene would be a potentially valuable tool for studies of neural transplantation. In the present study a recently developed electroporation technique, nucleofection (Siemen et al., 2005) was used to generate transgenic hESCs. This technique facilitates direct transport of DNA into the nucleus of various cell types, including embryonic and adult stem cells (Lakshmipathy et al., 2004). The EF1 promoter used here was previously reported to be effective in undifferentiated mouse ES cells (mESCs) and neural progenitors, but not dopaminergic neurons (Zeng et al., 2003). In contrast, we observed that the EF1 promoter was active in neurally differentiated cells derived from hESC, especially in TH+ neurons. Confocal analysis confirmed that approximately 76% of differentiated TH+ neurons co-expressed hrGFP.
The electroporation technique used was modified to employ small clusters of hESCs, rather than single cells, in order to increase transfection efficiency and to maintain survival and pluripotency of the hESCs. Thus, inhomogeneity of transfection efficiency could be part of the reason that the EF1 promoter was active in some, but not all of the cells during dopaminergic differentiation, even though the cells were kept in culture under G418 selection before neural induction. Approximately 10 to 20 percent of total cells were not hrGFP positive after 18 days of neural differentiation. This was consistent with the 24 % of total TH+ positive neurons that showed no co-expression of hrGFP.
Sodium butyrate can produce many effects on cultured mammalian cells, including inhibition of proliferation, induction of differentiation, and regulation of gene expression (Byrd and Alho, 1987). We were primarily interested in the effect of sodium butyrate on hrGFP expression during dopaminergic differentiation, because whether hrGFP expression was limited by histone deacetylation in the previous study on mESC (Zeng et al., 2003) is unknown. In the present study, we observed that there was no significant effect on hrGFP expression when hES cells were treated with various concentration of sodium butyrate, either at an early stage or at later stage of cell differentiation. This result suggests that hrGFP expression was not limited by histone deacetylation. The hESCs used in the present study as well as the previous study on mESCs (Zeng et al., 2003) consisted of a number of separate clonal lines with different integration sites, indicating that integration site is not critical. Therefore, the lack of the effect of sodium butyrate on hrGFP expression suggests that the EF1 promoter is more effective in DA-differentiated hESC, as compared to mESC.
We also observed that higher concentrations of sodium butyrate affected both cell differentiation and TH expression, at either an early stage or at a later stage of cell differentiation. As a gut fermentation product, sodium butyrate may have effects on cells in vivo. Previous studies reported that sodium butyrate stimulates endogenous TH and ppENK expression (Parab et al., 2007), and that sodium butyrate does not influence transcription of the TH gene, but rather inhibits the expression of TH gene by destabilizing TH mRNA (Arányi et al., 2007). The mechanism of reduction of TH expression by sodium butyrate was not explored in the present study.
Human neural stem cells (hNSC) can integrate, migrate, and differentiate when transplanted into the developing or young adult rodent and primate brain (Ourednik et al., 2001; Tabar et al., 2005). The principal aim of the present study was to establish stable hESCs expressing the reporter gene, hrGFP, to facilitate the discrimination of transplanted hESCs from host cells. Thus, BGO1V2-EFG cells may be useful as a tool for monitoring cell survival and development in cell transplantation. In our preliminary experiments, we used BGO1V2-EFG-derived cells at an early stage of differentiation for transplantation. Although we detected small numbers of surviving hrGFP-labeled ES-derived cells in the rat striatum 3 weeks after transplantation (Y. Wang and B. Harvey; data not shown), most hESCs were not well developed in rat brain over the very short period of differentiation which was studied. The current study also found that numerous nestin+ cells remained in hESCs cultured in vitro for an additional 18 days after dissociation from the PA6 cells, as shown in Figure 5E. Therefore, future long-term in vivo experimentation and behavioral studies would be required to determine the potential for hrGFP labeled hESCs to differentiate towards a functional dopaminergic lineage after transplantation.
In the present study, we have established a stably-transfected population of hESCs, BGO1V2-EFG, which express hrGFP as a reporter gene driven by the EF1 promoter. The cells maintain stable and robust hrGFP expression in the undifferentiated state and during neural differentiation. The transfected ES cells retain both pluripotency and the ability to differentiate. Especially, these hESC cells which express hrGFP driven by the EF1 promoter exhibit reporter activity during dopaminergic differentiation. Thus, BGO1V2-EFG cells, carrying the hrGFP reporter gene, may be a valuable tool for transplantation studies, especially in Parkinson disease animal models.
Acknowledgments
Research was supported by the IRP of NIDA, NIH, DHHS.
References
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227(2):271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- Arányi T, Sarkis C, Berrard S, Sardin K, Siron V, Khalfallah O, et al. Sodium butyrate modifies the stabilizing complexes of tyrosine hydroxylase mRNA. Biochem Biophys Res Commun. 2007;359(1):15–19. doi: 10.1016/j.bbrc.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Buzzard JJ, Gough NM, Crook JM, Colman A. Karyotype of human ES cells during extended culture. Nat Biotechnol. 2004;22(4):381–382. doi: 10.1038/nbt0404-381. [DOI] [PubMed] [Google Scholar]
- Byrd JC, Alho H. Differentiation of PC12 pheochromocytoma cells by sodium butyrate. Brain Res. 1987;428(1):151–155. doi: 10.1016/0165-3806(87)90096-4. [DOI] [PubMed] [Google Scholar]
- Chen WY, Bailey EC, McCune SL, Dong JY, Townes TM. Reactivation of silenced, virally transduced genes by inhibitors of histone deacetylase. Proc Natl Acad Sci U S A. 1997;94(11):5798–5803. doi: 10.1073/pnas.94.11.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KH, Basma H, Singh J, Cheng PW. Activation of CMV promoter-controlled glycosyltransferase and beta -galactosidase glycogenes by butyrate, tricostatin A, and 5-aza-2'-deoxycytidine. Glycoconj J. 2005;22(1-2):63–69. doi: 10.1007/s10719-005-0326-1. [DOI] [PubMed] [Google Scholar]
- Costa M, Dottori M, Ng E, Hawes SM, Sourris K, Jamshidi P, et al. The hESC line Envy expresses high levels of GFP in all differentiated progeny. Nat Methods. 2005;2(4):259–260. doi: 10.1038/nmeth748. [DOI] [PubMed] [Google Scholar]
- Draper JS, Smith K, Gokhale P, Moore HD, Maltby E, Johnson J, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22(1):53–54. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- Eiges R, Schuldiner M, Drukker M, Yanuka O, Itskovitz-Eldor J, Benvenisty N. Establishment of human embryonic stem cell-transfected clones carrying a marker for undifferentiated cells. Curr Biol. 2001;11(7):514–518. doi: 10.1016/s0960-9822(01)00144-0. [DOI] [PubMed] [Google Scholar]
- Hedlund E, Pruszak J, Ferree A, Viñuela A, Hong S, Isacson O, et al. Selection of embryonic stem cell-derived enhanced green fluorescent protein-positive dopamine neurons using the tyrosine hydroxylase promoter is confounded by reporter gene expression in immature cell populations. Stem Cells. 2007;25(5):1126–1135. doi: 10.1634/stemcells.2006-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaalouk DE, Crosato M, Brodt P, Galipeau J. Inhibition of histone deacetylation in 293GPG packaging cell line improves the production of self-inactivating MLV-derived retroviral vectors. Virol J. 2006;3:27. doi: 10.1186/1743-422X-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Park JS, Hong SJ, Woo MS, Kim SY, Kim KS. Regulation of the tyrosine hydroxylase gene promoter by histone deacetylase inhibitors. Biochem Biophys Res Commun. 2003;312(4):950–957. doi: 10.1016/j.bbrc.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Kim JH, Do HJ, Choi SJ, Cho HJ, Park KH, Yang HM, et al. Efficient gene delivery in differentiated human embryonic stem cells. Exp Mol Med. 2005;37(1):36–44. doi: 10.1038/emm.2005.5. [DOI] [PubMed] [Google Scholar]
- Lakshmipathy U, Pelacho B, Sudo K, Linehan JL, Coucouvanis E, Kaufman DS, et al. Efficient transfection of embryonic and adult stem cells. Stem Cells. 2004;22(4):531–543. doi: 10.1634/stemcells.22-4-531. [DOI] [PubMed] [Google Scholar]
- Ourednik V, Ourednik J, Flax JD, Zawada WM, Hutt C, Yang C, et al. Segregation of human neural stem cells in the developing primate forebrain. Science. 2001;293(5536):1820–1824. doi: 10.1126/science.1060580. [DOI] [PubMed] [Google Scholar]
- Parab S, Nankova BB, La Gamma EF. Differential regulation of the tyrosine hydroxylase and enkephalin neuropeptide transmitter genes in rat PC12 cells by short chain fatty acids: concentration-dependent effects on transcription and RNA stability. Brain Res. 2007;1132(1):42–50. doi: 10.1016/j.brainres.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Patel P, Nankova BB, LaGamma EF. Butyrate, a gut-derived environmental signal, regulates tyrosine hydroxylase gene expression via a novel promoter element. Brain Res Dev Brain Res. 2005;160(1):53–62. doi: 10.1016/j.devbrainres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Siemen H, Nix M, Endl E, Koch P, Itskovitz-Eldor J, Brüstle O. Nucleofection of human embryonic stem cells. Stem Cells Dev. 2005;14(4):378–383. doi: 10.1089/scd.2005.14.378. [DOI] [PubMed] [Google Scholar]
- Tabar V, Panagiotakos G, Greenberg ED, Chan BK, Sadelain M, Gutin PH, et al. Migration and differentiation of neural precursors derived from human embryonic stem cells in the rat brain. Nat Biotechnol. 2005;23(5):601–606. doi: 10.1038/nbt1088. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tomishima MJ, Hadjantonakis AK, Gong S, Studer L. Production of green fluorescent protein transgenic embryonic stem cells using the GENSAT bacterial artificial chromosome library. Stem Cells. 2007;25(1):39–45. doi: 10.1634/stemcells.2006-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazin T, Chen J, Spivak CE, Amable R, Gabitzsch E, Lee CT, et al. Dopaminergic neurons derived from BG01V2, a variant of human embryonic stem cell line BG01. Restor Neurol Neurosci. 2008a;26(6):447–458. [PMC free article] [PubMed] [Google Scholar]
- Vazin T, Chen J, Lee CT, Amable R, Freed WJ. Assessment of stromal-derived inducing activity in the generation of dopaminergic neurons from human embryonic stem cells. Stem Cells. 2008b;26(6):1517–1525. doi: 10.1634/stemcells.2008-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Ayala M, Thiede BR, Zhang SC. In vitro- and in vivo-induced transgene expression in human embryonic stem cells and derivatives. Stem Cells. 2008;26(2):525–533. doi: 10.1634/stemcells.2007-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki T, Inaji M, Kouike H, Shimazaki T, Sawamoto K, Ando K, et al. Isolation and transplantation of dopaminergic neurons generated from mouse embryonic stem cells. Neurosci Lett. 2004;363(1):33–37. doi: 10.1016/j.neulet.2004.03.074. [DOI] [PubMed] [Google Scholar]
- Zeng X, Chen J, Sanchez JF, Coggiano M, Dillon-Carter O, Petersen J, et al. Stable expression of hrGFP by mouse embryonic stem cells: Promoter activity in the undifferentiated state and during dopaminergic neural differentiation. Stem Cells. 2003;21(6):647–653. doi: 10.1634/stemcells.21-6-647. [DOI] [PubMed] [Google Scholar]
- Zeng X, Chen J, Liu Y, Luo Y, Schulz TC, Robins AJ, et al. BG01V: A variant human embryonic stem cell line which exhibits rapid growth after passaging and reliable dopaminergic differentiation. Restor Neurol Neurosci. 2004a;22(6):421–428. [PubMed] [Google Scholar]
- Zeng X, Cai J, Chen J, Luo Y, You ZB, Fotter E, et al. Dopaminergic differentiation of human embryonic stem cells. Stem Cells. 2004b;22(6):925–940. doi: 10.1634/stemcells.22-6-925. [DOI] [PubMed] [Google Scholar]
- Zeng X, Miura T, Luo Y, Bhattacharya B, Condie B, Chen J, et al. Properties of pluripotent human embryonic stem cells BG01 and BG02. Stem Cells. 2004c;22(3):292–312. doi: 10.1634/stemcells.22-3-292. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19(12):1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]