Summary
The BRCA1 gene product plays numerous roles in regulating genome integrity. Its ability to participate in the assembly of various super-molecular complexes in response to DNA damage has been extensively studied, however much less is understood about BRCA1’s direct role as a gene-specific transcriptional co-regulator. BRCA1 loss or mutation is highly associated with hereditary breast and ovarian cancer and numerous reports show that altered levels of BRCA1 expression are frequently found in sporadic forms of breast cancer, suggesting that control of BRCA1 transcription may also play a significant role in tumorigenesis. In this report we provide evidence of a stunning linkage between BRCA1’s role as a transcriptional co-regulator and the control of its expression via an auto-regulatory transcriptional loop. BRCA1 assembles with complexes containing E2F-1 and RB to form a repressive multi-component transcriptional complex that inhibits BRCA1 promoter transcription. This complex is disrupted by genotoxic stress resulting in the displacement of BRCA1 protein from the BRCA1 promoter and subsequent up-regulation of BRCA1 transcription. Cells depleted of BRCA1 in vivo show up-regulation of BRCA1 transcripts while cells over-expressing BRCA1 show BRCA1 transcript down-regulation. Tandem chromatin immmuno-precipitation studies demonstate that BRCA1 is regulated by a dynamic co-regulatory complex containing BRCA1, E2F1 and Rb at the BRCA1 promoter that is disrupted by DNA damaging agents to increase its transcription. These findings define a novel transcriptional mechanism in which BRCA1 expression is controlled by an autoregulated homeostasis that selectively titrates its levels to maintain genome integrity in response to genotoxic insult.
Keywords: BRCA1, cell cycle, DNA damage, transcription
Introduction
Loss or mutation of the BRCA1 gene is associated with nearly 5% all breast cancers and greater than 80% of all cases of hereditary ovarian/breast cancer syndromes (1). Cells carrying absent or mutated alleles of BRCA1 show many features characteristic of reduced genome stability including impaired cell cycle checkpoints, reduced efficiency in homologous recombination and defective DNA repair following genotoxic insults (2). Efforts to elucidate this genomic “caretaker” function have led to the identification of multiple roles for BRCA1 in a variety of cellular processes including cell cycle checkpoint control, homologous recombination, centrosome replication, response to DNA damage and transcriptional control (3, 4). The major BRCA1 gene product is an 1863 amino acid protein containing multiple functional domains and protein interaction surfaces. An N-terminal ring finger domain dimerizes with BARD1 to provide E3 ubiquitin ligase activity. The central portion interacts with many factors involved in DNA repair. Two tandem C-terminal BRCT motifs interact with protein complexes that control transcription and mediate the DNA damage response (reviewed in (3–5)).
Though BRCA1 was originally predicted to function as a transcription factor when it was first discovered in 1994 (1), subsequent studies focused primarily on defining characteristics of BRCA1-containing complexes induced by DNA damage (3, 6). Evidence of a transcriptional role for BRCA1 was first provided by reporter assays using hybrid fusions of BRCA1 with the DNA binding domain of Gal4 (7, 8). BRCA1 was subsequently found to form direct complexes with the RNA polymerase II holoenzyme and a variety of transcription factors including p53, c-Myc, Stat1, c-jun, estrogen receptors, p300 , E2F, Rb, TRAPP220 and CtIP-CtBP (reviewed in (4, 5)). Several genes were later found to be regulated by BRCA1. Many of these genes regulate cell cycle progression and the response to DNA damage, including p21, p27, GADD45a, GADD153, DDB2, 14-3-3 sigma, hTERT, and several estrogen-responsive genes (reviewed in (9) and (5)). However, it remains unclear how many of these BRCA1-regulated genes are directly targeted through gene-specific BRCA1 recruitment, as has been shown for MAD2 and ANG1 (10, 11).
Reports that many cases of sporadic breast cancer show decreased expression of BRCA1 in the absence of BRCA1 mutation (12) and loss of BRCA1 expression is associated with higher grade non-inherited breast cancer (13–15) created significant interest in defining mechanisms of BRCA1 regulation at the level of transcription. BRCA1 transcription is regulated by a highly active bi-directional promoter (16, 17). Transcriptional regulation of the BRCA1 promoter is complex and modulated by multiple different components including ETS factor complexes, E2F factors, CREB, Rb, p53BP1, and the SWI/SNF complex (reviewed in (5) and (4))
In this report we describe a new and unexpected transcriptional target of BRCA1: the BRCA1 promoter. We show that BRCA1 binds directly to its own promoter and this association down regulates expression. Furthermore we show that this negative auto-regulation by BRCA1 is modulated in response to genotoxic stress through an intricate and dynamic assembly of transcription factors and transcriptional coactivators that tritates the level of BRCA1 in response to environmental stress. This is the first description of an auto-regulatory loop for BRCA1 gene regulation and provides a novel mechanistic framework to study the transcriptional regulation of other BRCA1-dependent genes.
Materials and Methods
Cell culture and transfection
Jurkat T-cells, HEK 293 LTV, PC3 and LNCaP prostate cancer cells were maintained in RPMI 1640 with 10% fetal bovine serum at 37°C in a humidified atmosphere of 5% CO2. UV treatment was performed by irradiating 1×108 Jurkat T-cells in a Stratalinker 800 (Stratagene) followed by incubation in fresh media for the indicated times. Doxorubicin hydrochloride (Sigma Chemical Co.) was prepared in DMSO. G418 concentration for selection (100 µg/ml) or maintenance (50 µg/ml) were determined performing a concentration curve in PC3 cells between 10 to 500 µg/ml. PC3 stable cell lines were generated transfecting with 10 µg of pcDNA3 BRCA1 wt or pcDNA3 empty vector by calcium phosphate method in a 100 mm plate. Twenty four hours post-transfection, G418 was added at the concentration for selection. After 10 days, single clones were amplified and BRCA1 expression was determined by Western blot and RT-qPCR. Clones with the highest BRCA1 expression were used. For PC3 with BRCA1 silenced expression, 100 mm plates were transfected with 10 µg of pKD or shRNA BRCA1 plasmids and 2 µg of pcDNA3 to introduce G418 resistance. Same G418 concentrations were used for selection and maintenance.
Plasmids and RNAi reagents
The pGL12 BRCA1 promoter luciferase reporter plasmid was previously described (18). The pGL35 BRCA1 luciferase reporter plasmid was generated by cloning a PCR amplified 900 bp fragment of the promoter upstream of the transcription start site into the HindIII site of pGL3 Basic (Promega). BRCA1 promoter specific primers used were FW 5' AGC AAG CTT AAC GAC CAC CCC ATT GAC TG 3' and REV 5' GCT AAG CTT TCC AGG AAG TCT CAG CGA GCT CA 3’. BRCA1 expression vectors (pcDNA3 BRCA1 and pcDNA3 BRCA1ΔBRCT) have been previously described (19). BARD1 expression vector (pcDNA3 BARD1) was from purchased from Origene Technologies. shRNA control and shRNA BRCA1 were from Upstate. siRNA control and siRNA BARD1 (AACAGUAACAUGUCCGAUGAAdTdT) were from Dharmacon, Inc. shRNA E2F1 A, shRNA E2F1 B and pGIPZ control plasmids were obtained from OpenBiosystems. Plasmids expressing E2F-1 (E132) and E2F-1 (1–363) were generously provided by Doron Ginsberg. pGL3.BRCA1luc full length (1312–1627), pGL3.BRCA1luc-M2 (E2F1 “A” mutation), pGL3.BRCA1luc-M3 (E2F1 “B” mutation) and pGL3.BRCA1luc-M23 (both sites mutated) was previously described and was generously provided by P.M. Glazer (20, 21).
Chromatin immuno-precipitation and immuno-precipitation
Methods, antibodies and primers used for ChIP are described in detail in the Supplemental Methods. The anti-BRCA1 antibody was affinity-purified from the sera of rabbits inoculated with a GST-fusion containing the BRCA1 amino acids 304–772. Immuno-precipitation and western blot analysis from isolated nuclear extracts was performed as previously described (22).
RNA isolation, and RT-qPCR
RNA isolation, qRT-PCR and primer sequences are described in detail in the Supplemental Materials. Results were normalized to ACTB (Actin B).
Immunoblot Analysis
Nuclear extract and western blot analysis as previously described (23, 24) and are described in detail in the supplemental materials and methods.
Results
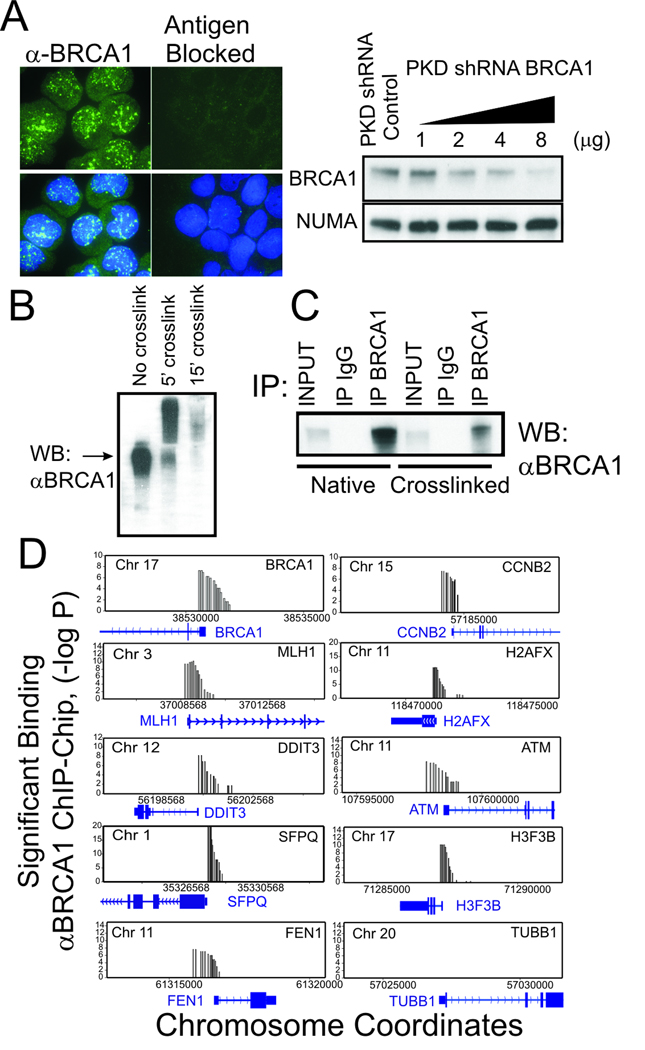
To profile BRCA1 interactions with the genome, we developed an affinity purified polyclonal antibodies against BRCA1 that selectively detects nuclear BRCA1 by immunofluorescence, immunoblot analysis, and immunoprecipitation from both native and formalin cross-linked nuclear extracts (Figs. 1A–C and Supplemenary Figure S1). Specificity of reactivity was demonstrated by antigen blockade of immunofluorescence (Fig. 1A) and gene depletion by RNA interference (Fig. 1B). This antibody was used in a genome-wide screen of 37,365 human proximal promoters by the combination of chromatin immunoprecipitation (ChIP) and micro-array technology (ChIP-chip) using NimbleGen (Roche) tiled proximal promoter arrays. In an initial screen, gene promoters showing a binding significance of p <0.000001 were selected for further examination (Fig. 1D). Not surprisingly, several of the genes identified by this genome location analysis screen (including CCNB2, MLH1, H2AFX, DDIT3, SFPQ, FEN1, and H3F3B) were previously reported to be regulated by BRCA1 in gene expression studies (5, 25, 26). Remarkably, one of the BRCA1-associated genes was the BRCA1 promoter itself. We focused on the intriguing possibility that BRCA1 could regulate its own expression for further analysis.
Figure 1. BRCA1 protein associates with the promoter regions of BRCA1 and multiple other genes.
(A) Characterization of affinity purified antibodies against BRCA1. (left) by Immunofluorescence staining of Jurkat T-cells before and after antigen blockade. α-BRCA1: Green; DAPI DNA staining: Blue (right). Characterization of α-BRCA1 antibodies by immunoblot analysis of cells lysates from Jurkat T-cells depleted of BRCA1 by transfection with control and increasing amounts of BRCA1 shRNA producing plasmid. NuMA antibody was used as loading control. (B) Immunoblot characterization of α-BRCA1 antibody reactivity against formalin cross-linked BRCA1 protein in Jurkat cell lysates. (C) Characterization of α-BRCA1 antibodies by immunoprecipitation of native and formalin cross-linked cell lysates compared to non-specific IgG. Following immunoprecipitation, formalin cross-links were reversed prior to SDS PAGE and immunoblot analysis. Input indicates signal from 10% of lysate. (D) BRCA1 ChIP profile of BRCA1 and other genes that show significant association (p ≤0.000001) with BRCA1 by ChIP-chip analysis using the Nimblegen HG17 tiled proximal promoter array (HG17 array 2005-04-18_min_promoter_set) compared to non-specific control (TUBB1). Results are the average of two independent biological replicates. Y-axis is shown in −log (P) scale and X-axis shows coordinates of the indicated chromosomes.
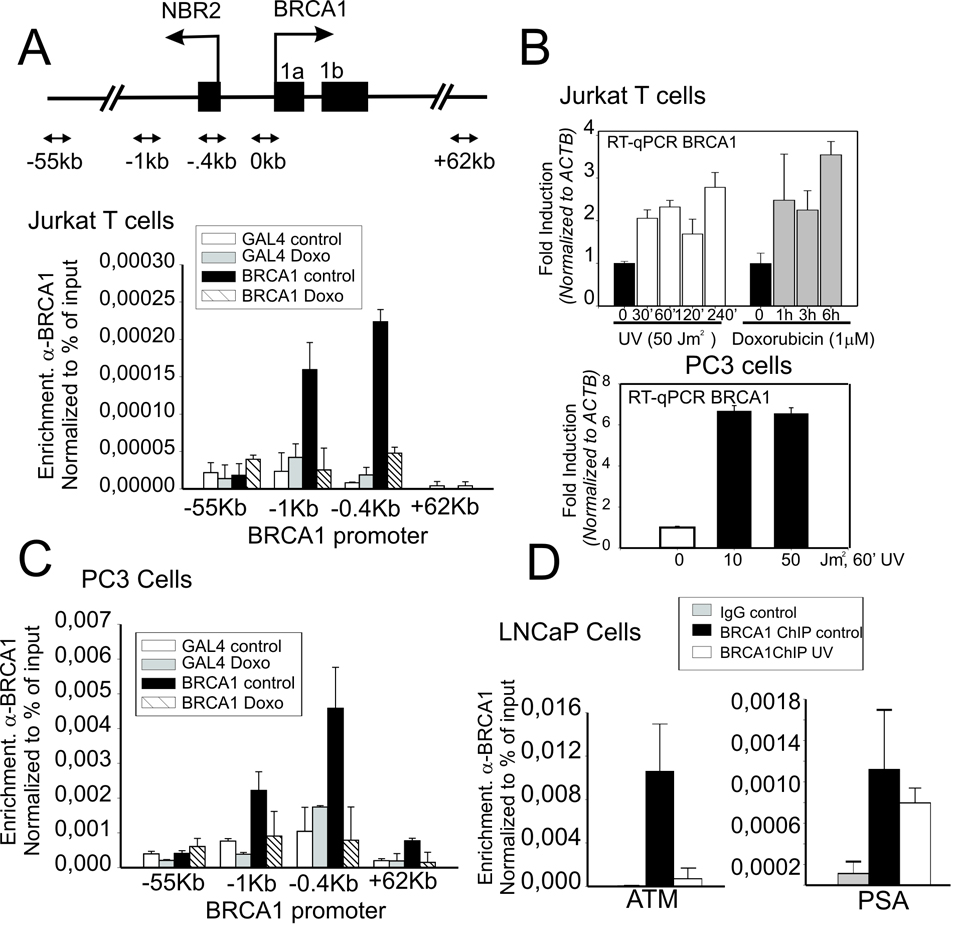
When compared to non-specific Gal4 antibody, a peak of BRCA1 binding to the proximal promoter region of the BRCA1 gene was readily detected by α-BRCA1 ChIP and quantitative real-time PCR (Fig. 2A). Interestingly this binding was disrupted by 3 h treatment with the DNA damage inducing agent doxorubicin (Fig. 2A) Most notably, loss of BRCA1 from the BRCA1 promoter correlated with an increase in BRCA1 transcription suggesting that BRCA1 may function as a negative regulator of its own transcription (Fig. 2B). This mode of BRCA1 regulation is not cell type specific since it is observed in the PC3 prostate cancer cell line (Figs. 2B–C). BRCA1 binding is readily detected at multiple gene promoters in different cell types. Similar to Jurkat, significant binding by BRCA1 is also demonstrated in LNCaP prostate cells at the ATM and PSA promoters (Fig. 2D).
Figure 2. BRCA1 protein association with the BRCA1 promoter and BRCA1 expression is regulated by genotoxic stress.
(A) Inset above, schematic diagram of BRCA1 promoter show divergent NBR2 gene and relative positions of primers set used in this study. ChIP analysis at the BRCA1 promoter using BRCA1-specific or non-specific control IgG (α-Gal4) in untreated Jurkat T-cells or cells treated 3h with doxorubicin (1µM). Shown is enrichment at positions of the BRCA1 locus relative to the transcription start site presented as percent recovery of input. (B) Jurkat T cells or PC3 cells were treated with either UV or doxorubicin (1µM) and harvested at the indicated times prior to RNA isolation. Mature RNA message was determined by RT-qPCR normalized to ACTB. (C) ChIP analysis of BRCA1 binding to the BRCA1 promoter in PC3 human prostate cell lines at indicated positions relative to the TSS in untreated and Doxorubin treated cells (1µM, 24 h). IgG against yeast Gal4 α-Gal4 was used as a non-specific binding control. (D) ChIP analysis of BRCA1 binding to the ATM and PSA promoters in LNCaP cells untreated or after UV treatment. In all cases (1A, C and D) mean enrichment and error shown correspond to the average and standard deviation of two independent experiments.
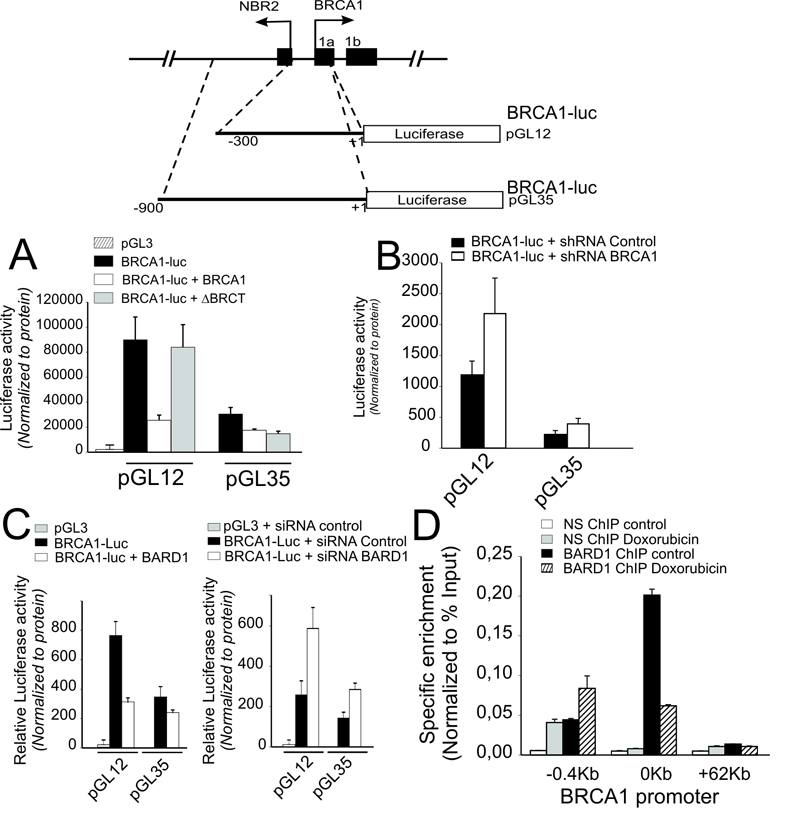
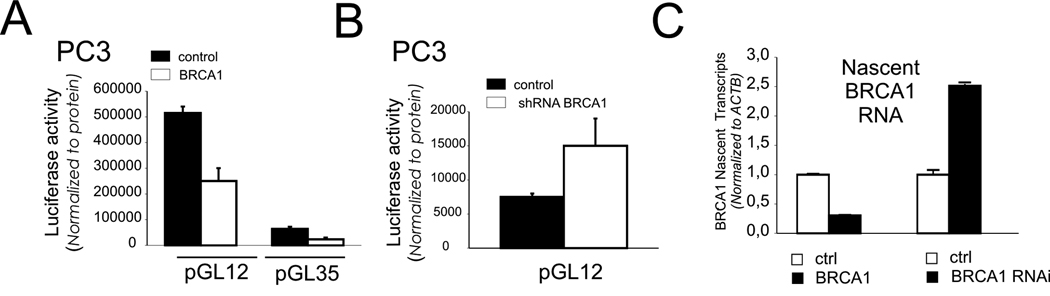
The region within 300 base pairs (bp) upstream of the BRCA1 TSS has high transcriptional activity as previously described (16) (Fig. 3A). This activity is significantly repressed by BRCA1 over-expression; however, a construct lacking the C-terminal BRCT domain showed reduced inhibitory activity (Fig 3A). Similar to previous studies (16), reporter constructs containing additional sequence from −300 to −900 bp showed reduced transcriptional activity (Fig 3A). Nonetheless, this activity is also repressed by BRCA1 over-expression though this repression shows little requirement for the C-terminal BRCA1 repeat domain (Fig. 3A). In sharp contrast, BRCA1 depletion by BRCA1 specific shRNA increased transcription from both fragments of the BRCA1 regulatory region (Fig. 3B). Similar results were obtained in response to enforced or depleted expression of the BRCA1 dimerization partner, BARD1 (Fig. 3C). Also, like BRCA1, BARD1 is enriched at the BRCA1 promoter but displaced by pretreatment with doxorubicin (Fig. 3D). Furthermore, stable over-expression or depletion of BRCA1 in the PC3 prostate cell line causes transcriptional repression and up-regulation of the BRCA1 promoter respectively (Fig. 4A and B). Most notably however, cells that stably over-express BRCA1 show down-regulation of endogenous nascent BRCA1 RNA expression while cells that have stable depletion of BRCA1 expression show up-regulation of nascent BRCA1 RNA transcripts.
Figure 3. BRCA1 and BARD1 negatively regulate transcription from the BRCA1 promoter.
(A) Jurkat T cells were cotransfected with luciferase reporter constructs (see schematic above) spanning the indicated lengths of regulatory sequences upstream of the BRCA1 TSS (pGL12 or pGL35) in combination with either: BRCA1 wt (pcDNA3 BRCA1), BRCA1 C-terminal mutant (pcDNA3 ΔBRCT) or empty (pcDNA3) expression vectors. (B) Jurkat T-cells were cotransfected with the indicated BRCA1 promoter reporters and either shRNA BRCA1 or control shRNA with scrambled sequence expression vectors. (C) Jurkat T-cells were cotransfected with the indicated BRCA1 promoter reporters and either wild type BARD1 (BARD1) or empty expression vectors or BARD1 siRNA or control duplex siRNA oligonucleotides. Error bars show the standard error of the mean from at least 3 independent transfections. (D) ChIP analysis of BARD1 enrichment at the BRCA1 promoter with and without pretreatment with 1 µM doxorubicin. Error bars indicate the standard error of the mean derived from 2 independent biological replicates.
Figure 4. Reciprocal regulation of BRCA1 promoter activation and transcription in PC3 prostate cell lines by stable over-expression or depletion of BRCA1 protein.
(A) PC3 cells stably transfected with control empty vector (ctrl) or BRCA1 expression vector (BRCA1) were transiently transfected with BRCA1-luc reporter constructs (pGL12 or pGL35). (B) PC3 cells stably expressing shRNA BRCA1 or control shRNA with scrambled sequence shRNA expression vectors were transfected with BRCA1-luc reporter (pGL12). Luciferase activity was normalized to protein. Error bars show the standard error of the mean from 3 independent transfections. (C) PC3 prostate cancer cells that stably over-expressing BRCA1 as in (A) were analyzed for nascent BRCA1 RNA levels (unspliced transcript) by qRT-PCR and compared to cells stably expressing empty vector (ctrl). Similarly, cells stably depleted of BRCA1 as in (B) were analyzed for nascent BRCA1 RNA levels compared to cells stably producing shRNA containing a scrambled sequence (ctrl).
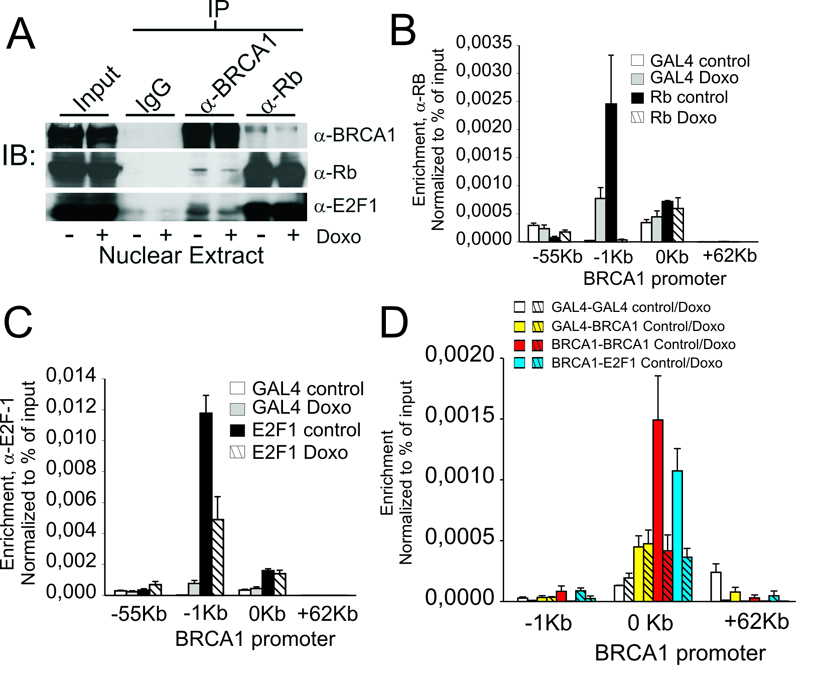
Though BRCA1 does not bind DNA specifically, prior studies have suggested that BRCA1 may be tethered directly or indirectly to sequence-specific DNA binding proteins. One potential interaction involves the retinoblastoma protein (Rb) which forms dynamic repressor complexes with the E2F family of transcription factors and is readily detected in complexes with BRCA1 by immunoprecipitation (19, 27). In addition, the E2F family of proteins are well known to bind to both the BRCA1 promoter (20, 28) and BRCA1 (29) and therefore may serve to recruit Rb/BRCA1 complexes to the BRCA1 promoter. As shown by BRCA1 and Rb immuno-precipitation in Fig 5A, a small population of Rb in the nuclear extracts of Jurkat cells is associated with BRCA1 and these levels decrease in cells treated with doxorubicin. Similarly, BRCA1 complexes containing E2F-1 show detectable binding above background in BRCA1 immuno-precipitates although this is a small population compared to the amount of E2F-1 associated with Rb (Fig. 5A). Similarly, ChIP analysis shows that both E2F-1 and Rb are associated with BRCA1 promoter under basal conditions and are significantly decreased in cells treated with doxorubicin (Figs. 5B–C). To further demonstrate the linkage between E2F-1 assembly at the BRCA1 promoter and BRCA1, tandem ChIP was performed at the BRCA1 promoter to sequentially enrich for BRCA1-associated complexes (Fig. 5D). Compared to non-specific control (α-Gal4), primary ChIP precipitates of BRCA1 promoter-containing complexes, isolated with α-BRCA1 antibodies, showed a significant retention of E2F-1 associated BRCA1 promoter sequences and this enrichment was dramatically decreased following genotoxic stress (Fig 5D). These findings further support the existence of a dynamic assembly of complexes containing Rb, E2F-1 and BRCA1 at the BRCA1 promoter and their displacement and/or disruption by genotoxic stress.
Figure 5. BRCA1 forms a negative regulatory complex with E2F-1 and Rb at the BRCA1 promoter that is disrupted by genotoxic stress.
(A) Co-immuno-precipitation of BRCA, Rb and E2F-1 using antibodies against non-specific IgG, α-Rb and α-BRCA1 antibodies. (B) ChIP-qPCR analysis of Rb enrichment at the indicated positions of the BRCA1 promoter region in untreated Jurkat T cells and cells treated 3 h with 1 µM doxorubicin compared to non-specific control (α-Gal4). (C) α-E2F-1 ChIP-qPCR analysis of E2F-1 enrichment at the indicated positions of the BRCA1 promoter region in untreated Jurkat T cells and cells treated 3 h with 1 µM doxorubicin compared to non-specific control (α-Gal4). (D) Untreated Jurkat cells or cells treated with 1 µM doxorubicin were analyzed by tandem BRCA1/E2F-1 ChIP using combinations of first ChIP with either non-specific antibody (α-Gal4), or α-BRCA1 antibody followed by Re-ChIP of the immunoprecipitated complexes with α-Gal4, α-BRCA1 or α-E2F-1. Mean enrichment and error shown correspond to the average and standard deviation of the mean of at least two independent experiments.
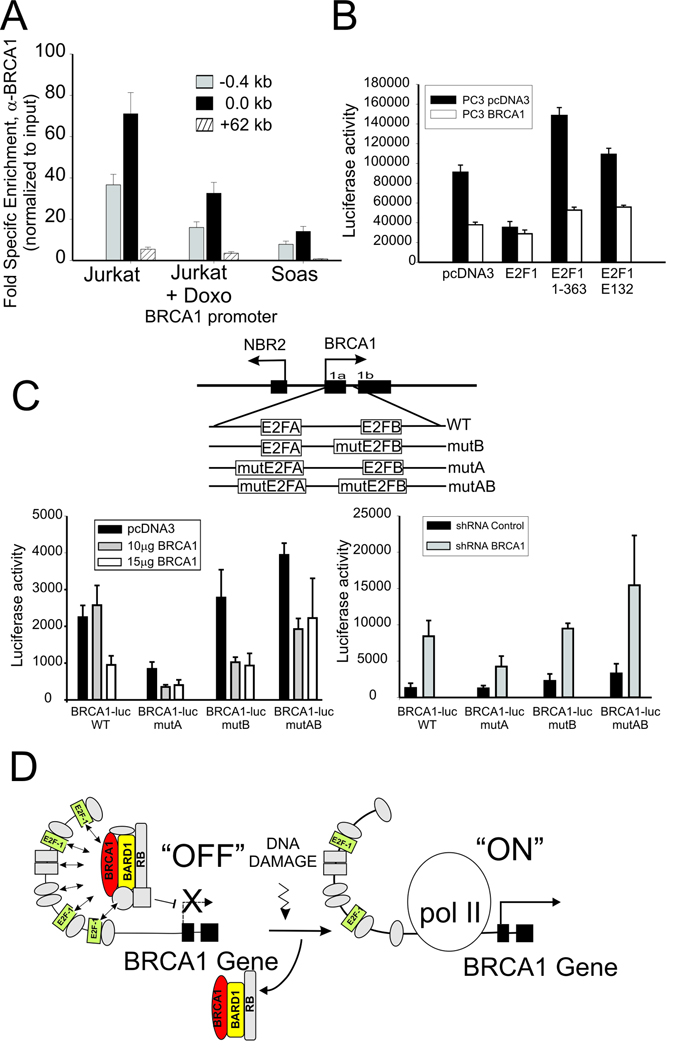
Several studies have reported a complex regulation of the BRCA1 promoter by members of E2F and Rb factors which is likely to be determined by the relative abundance of the factors (20, 21, 30). This is demonstrated by the significantly reduced basal formation of BRCA1 containing complexes at the BRCA1 promoter in the Soas cell line, which does not express Rb (Fig. 6A). In addition, transient expression of dominant interfering mutants of E2F1 relieves BRCA1 dependent repression of the BRCA1 promoter in PC3 cells. As shown in Fig. 6B, co-transfection of mutant containing only the DNA binding domain of E2F-1(aa 1–363) and, to a lesser extent, a DNA binding defective point mutant (E132) (31, 32) results in increased BRCA1 promoter activity compared to control. Notably, this effect is blunted in PC3 cells that stably over-express BRCA1 (Fig. 6B). Prior studies have shown that the manner in which E2F and Rb factors associate with the BRCA1 is significantly complex and differentially regulated by two separate E2F binding sites in the BRCA1 promoter region (20, 21). Reporter assays using BRCA1 promoter reporter constructs in which one or both of the E2F sites is mutated (21) demonstrates that the ability of BRCA1 to regulate the BRCA1 promoter is significantly influenced by the integrity of these sites (Figure 6C). Mutation of the most distal site (E2FA) results in decreased transcriptional activity suggesting this site may play a more positive role in activity of the BRCA1 promoter while mutation of the more proximal E2F site (E2FB) results in a slight increase in activity (Fig. 6C, left). Both single mutants are repressed by BRCA1 over-expression (Fig. 6C, left) and induced by BRCA1 depletion (Fig. 6C, right); however mutation of both sites results in the largest increase in BRCA1 promoter activity, the most resistance to BRCA1 overexpression (Fig. 6C, left) and the largest increase in promoter activity following BRCA1 depletion (Fig. 6C, right). The finding that BRCA1 promoter repression by BRCA1 overexpression and BRCA1 promoter induction by BRCA1 depletion persists following mutation of both sites suggests that other proteins or sites in addition to the two E2F binding sites are likely to be involved in recruitment of BRCA1 to the BRCA1 promoter.
Figure 6. BRCA1 assembles at the BRCA1 promoter in association with E2F-1 and Rb complexes.
(A) BRCA1 enrichment by ChIP at BRCA1 promoter of untreated and Doxorubicin treated Jurkat cells compared to untreated Rb-deficient Saos cells normalized to nonspecific IgG. Error bar indicates standard error and mean from 2 independent experiments. (B) PC3 prostate cancer cells stably over-expressing BRCA1 (PC3 BRCA1) or empty vector (PC3 pcDNA3) were co-transfected with the BRCA1-luc reporter plasmid (pGL12) and wild type or the indicated mutant E2F-1 expression vectors (see text). Error bars show the standard error of the mean from 3 independent transfections. (C) BRCA1 regulation of the BRCA1 promoter depends on the E2F binding sites. BRCA1 was over-expressed (left) or depleted (right) in cells transfected with reporter plasmid containing wild type or mutated E2F transcription factor binding sites within the BRCA1 promoter sequences (see schematic diagram above). Result are the mean and standard error from 3 independent transfections. (D) Hypothetical schematic diagram depicting BRCA1 negative autoregulation by a complex containing BRCA1, E2F-1 and Rb at the BRCA1 promoter, which is up-regulated by displacement/disruption of this complex at the promoter to allow BRCA1 transcription in response to genotoxic stress. Note that E2F-1 complexes are rearranged but not completely lost from the promoter.
Discussion
Several groups have made significant contributions to our understanding of the regulation of the BRCA1 promoter in response to a variety of environmental conditions ranging from genotoxic insult to hypoxia (21, 33–36). The general consensus from these studies is that BRCA1 is regulated by a highly dynamic promoter that adapts rapidly to cellular conditions. Interestingly, several groups have examined the influence of genotoxic stress on BRCA1 expression with conflicting results. Previous studies showed that genotoxic stress repressed BRCA1 expression and demonstrated a significant role for p53 in the repression (33, 34). Subsequently it was shown that though BRCA1 expression decreases several hours following DNA damage, there is an initial rapid increase in expression within minutes following exposure to genotoxic stress with a subsequent p53-mediated feedback relay that quenches BRCA1 transcription (35, 36). Others have found that activation and repression could be seen in the same system depending on the time and dose of genotoxic agents (37). Interestingly, BRCA1 expression also stabilizes p53 which likely contributes to the quenching of its expression (38). The intersection of these feed-back loops suggests that BRCA1 participates in an intricate autoregulatory interplay following DNA damage (See schematic, Fig 6). In the early stages, levels of BRCA1 are elevated to allow it to perform its “caretaker” function by participating in DNA repair. Under these conditions, BRCA1 would be released from its promoter and recruited to sites of DNA repair allowing the now de-repressed BRCA1 promoter to increase BRCA1 transcription so that BRCA1 protein, consumed in the repair process, can be replaced. Should repair be insufficient in the ensuing hours and the DNA damage signal persist, increased expression of p53, stabilized by BRCA1, would activates its p53 “gatekeeper” function to shut down transcription of BRCA1 and other genes and initiate programmed cell death. It should be noted that p53 is not absolutely required for this entire process since the reduction in BRCA1 expression can occur in p53 deficient cells (33).
In retrospect a role for BRCA1 in regulation of its own expression could have been predicted. BRCA1 has been shown previously to interact with Rb and members of the E2F family of transcription factors (29). Rb, Rb-related proteins, and E2F factors have all been found to bind to the BRCA1 promoter (20, 28, 30, 39). Thus a role for BRCA1 in an auto-regulatory assembly at its own promoter responsive to environmental changes is an attractive hypothesis.
The role of Rb/E2F in the regulation of BRCA1 transcription during cell cycle progression and in response to DNA damage has yet to be fully explored. Though exposure to DNA damaging agents can ultimately disrupt normal cell cycle progession, the time periods used for the treatment of cells with genotoxic agents (1–3h) in this study are much too short to influence the cell cycle (see Supplementary Fig. S2) and therefore the observed effects are due predominantly to the immediate stress response from genotoxic insult. The expression profiles that correlate with BRCA1 release following DNA damage are likely to reflect a complex integration of multiple events linked to changes in covalent modification and protein-protein interactions involving E2F-1, Rb, and BRCA1 at the BRCA1 promoter. Presently the actual nature of the disruption of the E2F-1/Rb/ BRCA1 assembly at the BRCA1 promoter following genotoxic stress remains to be precisely defined. The work of Glazer and colleagues suggest that there may be an intricate rearrangement of the interaction of multiple E2F family members with two distinct E2F binding sites at the BRCA1 promoter in response environmental challenge (20, 21). Interacting components includes E2F-1 and E2F-4 in addition to the Rb related factors p107 and p130. Thus E2F-1 binding at the BRCA1 promoter is probably multivalent and may change, but may not be absent from the promoter, following DNA damage (Fig. 6) and is consistent with the observation that E2F-1 plays a role in both the activation and repression of BRCA1 promoter activity through its interaction with Rb. Furthermore, this interplay may be highly influenced by tissue specific ratios of individual Rb-related and E2F-related factors (Fig. 6A) (20, 21). The intricacy of this complex may also explain why peak ChIP enrichment for the BRCA1, E2F-1, and Rb components of these complexes spans a distance between 0 and −1kb relative to the transcription start site (Fig. 2 and 5).
Rb interacts with a common set of factors involved in transcriptional repression including CtIP, CtBP, RbAP46/48 (19, 27, 40). These components are also known to associate with certain histone deacetylases and chromatin remodelers. Detailed analyses of the acute changes in chromatin structure and covalent modification at the BRCA1 promoter in response to genotoxic insult will also be important future objectives. Though the E2F transcription factors are primary candidates in BRCA1 recruitment the possibility of a role for other sequence-specific DNA binding factors such as CREB, ZBRK1 and Ets must be considered. This is particularly important since site specific alteration of the two characterized E2F sites does not completely abolish BRCA1 regulation of the BRCA1 promoter (Fig. 6C). The recent finding that BRCA1 can repress transcription by ubiquitylation of pre-initiation complex components suggests that genotoxic stress may induce dynamic changes in protein ubiquitylation at the BRCA1 promoter via BRCA1’s E3-ligase activity in complex with BARD1 (41). Future experiments will have to assess if and how such modifications occur at the BRCA1 promoter in vivo.
Earlier clues that BRCA1 may have a regulatory role in its own expression come from mouse embryonic tissue expression studies in which exon 11 has been deleted. Deletion of exon 11 produces a gene product with significantly reduced nuclear localization (42). RNA isolated from this tissue show a greater than two fold increase in transcription from the mutant BRCA1 alleles, consistent with loss of repression due to impaired nuclear entry of BRCA1 protein (43) and is highly consistent with the BRCA1 gene depletion data presented in this study (Fig. 3–4).
Our current understanding of the role of BRCA1 as a regulator of transcription is still in its infancy. The list of direct transcriptional targets of BRCA1 now includes BRCA1 itself but still remains quite small despite the implications from several gene expression studies. Expanded efforts to identify those genes that represent direct targets of BRCA1 will be of central importance in improving our understanding of the function of BRCA1 as a transcriptional co-regulator.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, the National Cancer Institute and the National Institute on Aging
Reference List
- 1.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 2.Shen SX, Weaver Z, Xu X, et al. A targeted disruption of the murine Brca1 gene causes gamma-irradiation hypersensitivity and genetic instability. Oncogene. 1998;17:3115–3124. doi: 10.1038/sj.onc.1202243. [DOI] [PubMed] [Google Scholar]
- 3.Deng CX. BRCA1: cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Res. 2006;34:1416–1426. doi: 10.1093/nar/gkl010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen EM, Fan S, Ma Y. BRCA1 regulation of transcription. Cancer Lett. 2006;236:175–185. doi: 10.1016/j.canlet.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 5.Mullan PB, Quinn JE, Harkin DP. The role of BRCA1 in transcriptional regulation and cell cycle control. Oncogene. 2006;25:5854–5863. doi: 10.1038/sj.onc.1209872. [DOI] [PubMed] [Google Scholar]
- 6.Zhong Q, Chen CF, Li S, et al. Association of BRCA1 with the hRad50-hMre11-p95 complex and the DNA damage response. Science. 1999;285:747–750. doi: 10.1126/science.285.5428.747. [DOI] [PubMed] [Google Scholar]
- 7.Monteiro AN, August A, Hanafusa H. Evidence for a transcriptional activation function of BRCA1 C-terminal region. Proc Natl Acad Sci U S A. 1996;93:13595–13599. doi: 10.1073/pnas.93.24.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman MS, Verma IM. Transcriptional activation by BRCA1. Nature. 1996;382:678–679. doi: 10.1038/382678a0. [DOI] [PubMed] [Google Scholar]
- 9.El-Deiry WS. Transactivation of repair genes by BRCA1. Cancer Biol Ther. 2002;1:490–491. doi: 10.4161/cbt.1.5.162. [DOI] [PubMed] [Google Scholar]
- 10.Wang RH, Yu H, Deng CX. A requirement for breast-cancer-associated gene 1 (BRCA1) in the spindle checkpoint. Proc Natl Acad Sci U S A. 2004;101:17108–17113. doi: 10.1073/pnas.0407585101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuta S, Wang JM, Wei S, et al. Removal of BRCA1/CtIP/ZBRK1 repressor complex on ANG1 promoter leads to accelerated mammary tumor growth contributed by prominent vasculature. Cancer Cell. 2006;10:13–24. doi: 10.1016/j.ccr.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Thompson ME, Jensen RA, Obermiller PS, Page DL, Holt JT. Decreased expression of BRCA1 accelerates growth and is often present during sporadic breast cancer progression. Nat Genet. 1995;9:444–450. doi: 10.1038/ng0495-444. [DOI] [PubMed] [Google Scholar]
- 13.Wilson CA, Ramos L, Villasenor MR, et al. Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nat Genet. 1999;21:236–240. doi: 10.1038/6029. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q, Sakurai T, Mori I, et al. Prognostic significance of BRCA1 expression in Japanese sporadic breast carcinomas. Cancer. 2001;92:54–60. doi: 10.1002/1097-0142(20010701)92:1<54::aid-cncr1291>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Ding SL, Sheu LF, Yu JC, et al. Abnormality of the DNA double-strand-break checkpoint/repair genes, ATM, BRCA1 and TP53, in breast cancer is related to tumour grade. Br J Cancer. 2004;90:1995–2001. doi: 10.1038/sj.bjc.6601804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu CF, Chambers JA, Solomon E. Complex regulation of the BRCA1 gene. J Biol Chem. 1997;272:20994–20997. doi: 10.1074/jbc.272.34.20994. [DOI] [PubMed] [Google Scholar]
- 17.Suen TC, Goss PE. Transcription of BRCA1 is dependent on the formation of a specific protein-DNA complex on the minimal BRCA1 Bi-directional promoter. J Biol Chem. 1999;274:31297–31304. doi: 10.1074/jbc.274.44.31297. [DOI] [PubMed] [Google Scholar]
- 18.Baker KM, Wei G, Schaffner AE, Ostrowski MC. Ets-2 and components of mammalian SWI/SNF form a repressor complex that negatively regulates the BRCA1 promoter. J Biol Chem. 2003;278:17876–17884. doi: 10.1074/jbc.M209480200. [DOI] [PubMed] [Google Scholar]
- 19.Aprelikova ON, Fang BS, Meissner EG, et al. BRCA1-associated growth arrest is RB-dependent. Proc Natl Acad Sci U S A. 1999;96:11866–11871. doi: 10.1073/pnas.96.21.11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bindra RS, Glazer PM. Basal repression of BRCA1 by multiple E2Fs and pocket proteins at adjacent E2F sites. Cancer Biol Ther. 2006;5:1400–1407. doi: 10.4161/cbt.5.10.3454. [DOI] [PubMed] [Google Scholar]
- 21.Bindra RS, Gibson SL, Meng A, et al. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 2005;65:11597–11604. doi: 10.1158/0008-5472.CAN-05-2119. [DOI] [PubMed] [Google Scholar]
- 22.Yang Z, Yik JH, Chen R, et al. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell. 2005;19:535–545. doi: 10.1016/j.molcel.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhry S, Freebern WJ, Smith JL, Butscher WG, Haggerty CM, Gardner K. Cross-regulation of T cell growth factor expression by p53 and the Tax oncogene. J Immunol. 2002;169:6767–6778. doi: 10.4049/jimmunol.169.12.6767. [DOI] [PubMed] [Google Scholar]
- 24.Butscher WG, Powers C, Olive M, Vinson C, Gardner K. Coordinate transactivation of the interleukin-2 CD28 response element by c-Rel and ATF-1/CREB2. J Biol Chem. 1998;273:552–560. doi: 10.1074/jbc.273.1.552. [DOI] [PubMed] [Google Scholar]
- 25.Welcsh PL, Lee MK, Gonzalez-Hernandez RM, et al. BRCA1 transcriptionally regulates genes involved in breast tumorigenesis. Proc Natl Acad Sci U S A. 2002;99:7560–7565. doi: 10.1073/pnas.062181799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae I, Rih JK, Kim HJ, et al. BRCA1 regulates gene expression for orderly mitotic progression. Cell Cycle. 2005;4:1641–1666. doi: 10.4161/cc.4.11.2152. [DOI] [PubMed] [Google Scholar]
- 27.Yarden RI, Brody LC. BRCA1 interacts with components of the histone deacetylase complex. Proc Natl Acad Sci U S A. 1999;96:4983–4988. doi: 10.1073/pnas.96.9.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oberley MJ, Inman DR, Farnham PJ. E2F6 negatively regulates BRCA1 in human cancer cells without methylation of histone H3 on lysine 9. J Biol Chem. 2003;278:42466–42476. doi: 10.1074/jbc.M307733200. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Shao N, Ding QM, Cui J, Reddy ES, Rao VN. BRCA1 proteins are transported to the nucleus in the absence of serum and splice variants BRCA1a, BRCA1b are tyrosine phosphoproteins that associate with E2F, cyclins and cyclin dependent kinases. Oncogene. 1997;15:143–157. doi: 10.1038/sj.onc.1201252. [DOI] [PubMed] [Google Scholar]
- 30.Wang A, Schneider-Broussard R, Kumar AP, MacLeod MC, Johnson DG. Regulation of BRCA1 expression by the Rb-E2F pathway. J Biol Chem. 2000;275:4532–4536. doi: 10.1074/jbc.275.6.4532. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann F, Martelli F, Livingston DM, Wang Z. The retinoblastoma gene product protects E2F-1 from degradation by the ubiquitin-proteasome pathway. Genes Dev. 1996;10:2949–2959. doi: 10.1101/gad.10.23.2949. [DOI] [PubMed] [Google Scholar]
- 32.Halaban R, Cheng E, Zhang Y, Mandigo CE, Miglarese MR. Release of cell cycle constraints in mouse melanocytes by overexpressed mutant E2F1E132, but not by deletion of p16INK4A or p21WAF1/CIP1. Oncogene. 1998;16:2489–2501. doi: 10.1038/sj.onc.1201773. [DOI] [PubMed] [Google Scholar]
- 33.Andres JL, Fan S, Turkel GJ, et al. Regulation of BRCA1 and BRCA2 expression in human breast cancer cells by DNA-damaging agents. Oncogene. 1998;16:2229–2241. doi: 10.1038/sj.onc.1201752. [DOI] [PubMed] [Google Scholar]
- 34.Fan S, Twu NF, Wang JA, et al. Down-regulation of BRCA1 and BRCA2 in human ovarian cancer cells exposed to adriamycin and ultraviolet radiation. Int J Cancer. 1998;77:600–609. doi: 10.1002/(sici)1097-0215(19980812)77:4<600::aid-ijc21>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 35.MacLachlan TK, Dash BC, Dicker DT, El-Deiry WS. Repression of BRCA1 through a feedback loop involving p53. J Biol Chem. 2000;275:31869–31875. doi: 10.1074/jbc.M003338200. [DOI] [PubMed] [Google Scholar]
- 36.Clarkin CE, Zhang H, Weber BL. Kinetics of BRCA1 regulation in response to UVC radiation. Cell Mol Life Sci. 2000;57:1126–1134. doi: 10.1007/PL00000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang WW, Wang ZH, Zhu Y, Yang HT. E2F6 negatively regulates ultraviolet-induced apoptosis via modulation of BRCA1. Cell Death Differ. 2007;14:807–817. doi: 10.1038/sj.cdd.4402062. [DOI] [PubMed] [Google Scholar]
- 38.MacLachlan TK, Takimoto R, El-Deiry WS. BRCA1 directs a selective p53-dependent transcriptional response towards growth arrest and DNA repair targets. Mol Cell Biol. 2002;22:4280–4292. doi: 10.1128/MCB.22.12.4280-4292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cam H, Balciunaite E, Blais A, et al. A common set of gene regulatory networks links metabolism and growth inhibition. Mol Cell. 2004;16:399–411. doi: 10.1016/j.molcel.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 40.Yu X, Wu LC, Bowcock AM, Aronheim A, Baer R. The C-terminal (BRCT) domains of BRCA1 interact in vivo with CtIP, a protein implicated in the CtBP pathway of transcriptional repression. J Biol Chem. 1998;273:25388–25392. doi: 10.1074/jbc.273.39.25388. [DOI] [PubMed] [Google Scholar]
- 41.Horwitz AA, Affar eB, Heine GF, Shi Y, Parvin JD. A mechanism for transcriptional repression dependent on the BRCA1 E3 ubiquitin ligase. Proc Natl Acad Sci U S A. 2007;104:6614–6619. doi: 10.1073/pnas.0610481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thakur S, Zhang HB, Peng Y, et al. Localization of BRCA1 and a splice variant identifies the nuclear localization signal. Mol Cell Biol. 1997;17:444–452. doi: 10.1128/mcb.17.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu X, Qiao W, Linke SP, et al. Genetic interactions between tumor suppressors Brca1 and p53 in apoptosis, cell cycle and tumorigenesis. Nat Genet. 2001;28:266–271. doi: 10.1038/90108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.