Abstract
OBJECTIVE
We sought to determine whether childbearing increases incidence of type 2 diabetes after accounting for preconception glycemia and gestational glucose intolerance.
RESEARCH DESIGN AND METHODS
A prospective, biracial cohort was examined up to five times during 1985–2006 in the multicenter, U.S. population–based Coronary Artery Risk Development in Young Adults Study. The analysis included 2,408 women (1,226 black and 1,182 white) aged 18–30 years who were free of diabetes and had a fasting plasma glucose (FPG) <126 mg/dl at baseline. Incident diabetes was diagnosed by self-report, diabetes medication use, FPG ≥126 mg/dl, and/or plasma glucose ≥200 mg/dl after a 2-h oral glucose load. Time-dependent interim birth groups were those with zero and those with one or more births with or without gestational diabetes mellitus (GDM), stratified by baseline parity. Complementary log-log models estimated relative hazards of incident diabetes by interim births adjusted for age, race, family history of diabetes, and baseline covariates (FPG, BMI, education, smoking, and physical activity).
RESULTS
Of 193 incident diabetes cases in 42,782 person-years (4.5 cases/1,000 person-years), 84 (44%) had one or more interim births. Among nulliparas at baseline, incident rates per 1,000 person-years were 3.2 (95% CI 2.4–4.1) for those with no births, 2.9 (1.8 –3.9) for one or more births without GDM, and 18.4 (10.9 –25.9) for one or more births with GDM; adjusted relative hazards (95% CI) were 0.9 (0.6 –1.4) for one or more births without GDM and 3.8 (2.2– 6.6) for one or more births with GDM versus no births.
CONCLUSIONS
Childbearing did not elevate diabetes incidence among those with normal glucose tolerance during pregnancy (without GDM). GDM conferred the highest risk of developing diabetes independent of family history of diabetes and preconception glycemia and obesity.
Evidence that childbearing is associated with future development of type 2 diabetes in women remains conflicting (1–12). Both nulliparity and multiparity have been associated with higher fasting glucose and insulin levels independent of body size among nondiabetic women (6–8,13,14). In early cross-sectional and retrospective studies, grand multiparity (five or more births) was associated with higher rates of diabetes in women aged >45 years, unadjusted for age, body size, or socioeconomic status (1,2). In later population-based cross-sectional studies controlling for age, obesity, and socioeconomic status, the association between lifetime parity and prevalent diabetes was direct in three (9–11) and null in three (3,4,6). Two studies of indigenous groups with high rates of type 2 diabetes reported inverse associations (8,12). In a prospective study of 113,000 white women aged 30–55 years, the direct association between lifetime parity and incidence of self-reported diabetes was abolished after adjustment for age and obesity, with minimal confounding by family history of diabetes (5).
Two limitations of all studies are that preconception glycemia was not measured and prevalence of glucose intolerance during pregnancy was unknown. Women who develop gestational diabetes mellitus (GDM) have an elevated risk of developing type 2 diabetes (15–18), with subsequent weight gain and pregnancies apparently contributing to future risk (18). Residual confounding by GDM status and preconception hyperglycemia would tend to overestimate the association. Conversely, obesity, insulin resistance, and/or polycystic ovarian syndrome may cause infertility (i.e., nulliparity), which would tend to underestimate the association.
Conflicting evidence for an association between childbearing and development of diabetes may result from several factors. First, temporality of diabetes onset relative to pregnancy cannot be ascertained from previous studies, whether cross-sectional or prospective in design, because preconception glycemia had not been measured to rule out overt diabetes before pregnancy. Second, overweight status, which is in the causal pathway to type 2 diabetes and is twice as likely after a first birth compared with no births (19), was ascertained subsequent to childbearing years in the majority of subjects. Last, GDM status was not available in previous studies, except one that excluded women with GDM (5), because universal screening was not performed until the mid-1980s.
We prospectively investigated the natural course of childbearing in a biracial (black and white), population-based cohort of 2,408 U.S. women aged 18–30 years who were free of diabetes and normoglycemic at baseline in 1985–1986 and had measurements of fasting glycemia and/or glucose tolerance during 20 years of follow-up. We estimated population-based incidence rates (IRs) of type 2 diabetes across interim birth groups by GDM status and parity at baseline. The study sought to determine whether having one or more births versus none was associated with higher incidence of type 2 diabetes independent of family history of diabetes, race, preconception (baseline) fasting glycemia, obesity, age, sociodemographic and behavioral attributes, and GDM status. Finally, we assessed whether changes in physical activity and weight attenuated the associations.
RESEARCH DESIGN AND METHODS
The Coronary Artery Risk Development in Young Adults (CARDIA) Study is a multicenter, observational, population-based, longitudinal cohort study designed to describe the development of risk for coronary heart disease in young black and white men and women (20,21). Participants were recruited from four geographic areas: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. In 1985–1986, 5,115 subjects (2,787 women; 53% black) aged 18–30 years were enrolled. Retention rates were 86, 81, 79, 74, and 72% of the surviving cohort at 5, 7, 10, 15, and 20 years after baseline, respectively (22,23).
Sample selection criteria
Of 2,787 women, we excluded those at baseline who had type 1 or type 2 diabetes and/or fasting plasma glucose (FPG) ≥126 mg/dl (n = 41), had a hysterectomy or removal of both ovaries (n = 24), were currently pregnant (n = 7), or were missing FPG, sociodemographic, or behavioral covariates (n = 127). We also excluded women who had not attended at least one follow-up exam (n = 180) in years 5, 7, 10, 15, or 20. Women excluded were more likely to be smokers, unmarried, less educated, and of black race and to have a higher BMI than the analytic sample of 2,408 women (1,226 black and 1,182 white; 86% of the original cohort). FPG measurements were obtained at baseline and follow-up at years 7, 10, 15, and 20. For the five exams, FPG measurements were available for 1,083 (45%) subjects for all five exams, 613 (25%) for four exams, 367 (15%) for three exams, and 227 (9%) for two exams. Institutional review boards at each participating study center approved the study. Written informed consent was obtained from subjects for all study procedures.
Data collection methods
Participants were asked to fast for at least 8 h before each examination, and fasting blood samples were drawn in the morning (24). Procedures followed in collection and storage of plasma samples and laboratory quality control procedures have previously been reported in detail (24). FPG was determined at Linco Research using the hexokinase-ultraviolet method (25).
Definition of cases of incident diabetes
Incident cases of diabetes after baseline were identified by elevated FPG levels in years 7, 10, 15, or 20 and current use of diabetes medications (insulin or oral hypoglycemics) or self-report of diabetes outside of pregnancy in years 5, 7, 10, 15, or 20. We administered 2-h 75-g oral glucose tolerance tests to 1,229 and 1,398 women in years 10 and 20, respectively. We defined incident diabetes cases by 1997 American Diabetes Association criteria (26) as FPG ≥126 mg/dl and/or 2-h plasma glucose ≥200 mg/dl after a 75-g oral glucose load. FPG levels became elevated (≥126 mg/dl) for 101 women during the 20-year follow-up period. Fifteen women had abnormal 2-h post– glucose load tests in year 10 and 31 women in year 20. For diabetes cases identified by self-report only (also reporting a pregnancy with diabetes since the previous exam), we conservatively classified their pregnancies as without GDM (n = 9) because we could not determine whether diagnosis of diabetes had occurred before or after these pregnancies.
Incident diabetes cases identified among nonpregnant women were primarily type 2 diabetes based on the epidemiology of diabetes. Of 269 women currently pregnant or lactating at one or more follow-up exams, only one, who was lactating at year 7, was classified as an incident diabetes case (FPG 297 mg/dl). Otherwise, 223 (83%) attended at least one subsequent exam in the nonpregnant or nonlactating state with all FPG values <126 mg/dl (range 57–112 mg/dl). Among women with and without subsequent exams in the nonpregnant or nonlactating state, the percentages with GDM were similar (10.8 vs. 10.9%, respectively).
Baseline parity and time-dependent interim birth groups by GDM status
Participants were asked at each exam about whether they were currently pregnant or breastfeeding and about number of pregnancies, including abortions, miscarriages, and live or stillbirths; the duration of each gestation; and the dates of deliveries. We classified pregnancies ending in miscarriages, abortions, and/or those <20 weeks’ gestation as pregnancy losses and those ≥20 weeks’ as interim births. The number of interim births was defined as the cumulative number of births since baseline within each specific time interval ending in years 5, 7, 10, 15, and 20. At each exam, women were asked whether they ever had diabetes and whether they only had diabetes during their pregnancies. In year 10, women reported whether they had diabetes during each previous pregnancy (before and after baseline).
Parity groups at baseline were defined as nulliparous (no live births >20 weeks’ gestation) and parous (one or more live births >20 weeks’ gestation) before baseline. We classified women into time-dependent interim birth groups—no births and one or more interim births—and by ever having a birth with GDM. Women transitioned from the baseline parity groups (nulliparous or parous) through each specific time interval into interim birth groups. A change from having none to one or more interim births was maintained through the end of follow-up. GDM status was maintained for future time intervals regardless of whether there was a subsequent birth with GDM.
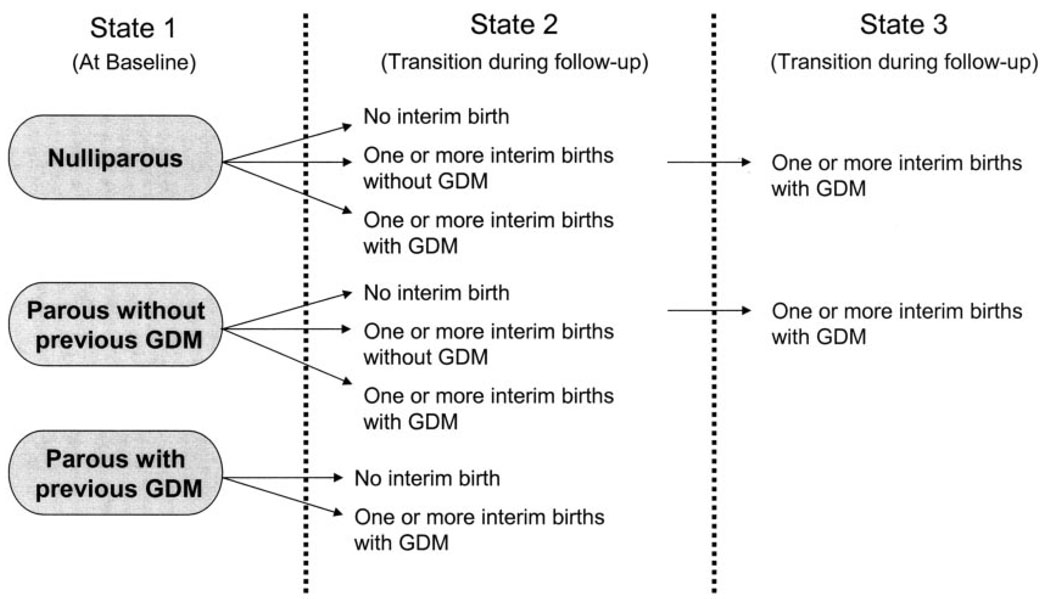
Interim birth groups irrespective of baseline parity include the following: having no interim births without GDM, no interim births with GDM (before baseline), one or more interim births without GDM, or one or more interim births with GDM. We further subdivided these groups by baseline parity (Fig. 1). Nulliparas transitioned within three groups: having no interim births (referent), one or more interim births without GDM, and one or more interim births with GDM. Women parous at baseline transitioned within two groups: those with zero or one or more interim births without GDM and those with zero or one or more interim births with GDM. We combined those with zero and one or more interim births for women parous at baseline because diabetes incidence rates were similar.
FIG. 1.
Description of transitions from baseline panty groups into the interim birth groups during follow-up. Figure shows possible sequences of joint parity and GDM status (ignoring which exam transition). In terms of sequential states, the only allowable transitions are from state 1 to state 2 and from state 2 to state 3. State 1 is always at baseline, states 2 and 3 always during follow-up. Each transition can occur at any examination, except that GDM is an absorbing state (always henceforth classified as GDM after first having had it, even though a subsequent birth might be free of GDM) and nulliparous or no interim birth does not allow GDM.
We validated self-report of GDM among 165 women for whom laboratory data were abstracted from medical records for 200 births between baseline and year 10. Sensitivity for classification as ever having GDM was 100% (20 of 20), and specificity was 92% (134 of 145).
Definition of family history of diabetes
Positive family history of diabetes was defined as report of one or more first-degree relatives (i.e., father, mother, or siblings) with diabetes at one or more exams in years 0, 5, and 10. The variable was fixed and covered any family history of diabetes during follow-up.
Other covariates
Certified technicians obtained weight, height, and waist circumference (waist girth) measurements according to a standardized protocol (27). Body weight was measured to the nearest 0.2 kg using a calibrated balance-beam scale in participants wearing light clothing. Height (without shoes) was measured to the nearest 0.5 cm using a vertical ruler. Waist circumference was measured to the nearest 0.5 cm at the minimal abdominal girth (28). BMI was calculated as weight in kilograms divided by the square of height in meters (29).
Sociodemographic and behavioral data (medication use, alcohol intake [milliliters per day], cigarette smoking, education, employment status, marital status, oral contraceptive use, and physical activity) were collected at each exam using self- and interviewer-administered questionnaires. We categorized variables as follows: smoking (never, former, or current), years of education (≤12, 13–15, or ≥16), marital status (never married, widowed, divorced or separated, or married), employment (full-time, part-time, or not employed outside the home), oral conceptive use (never, past, or current), and alcohol intake (0, 1–15, 15–30, and >30 ml/day). Assessments of daily physical activity were obtained using the interviewer-administered CARDIA Physical Activity History assessment at each exam (30). Physical activity scores were divided into quartiles because of skewedness of the data (data not shown). Dietary intake during the previous month was assessed using the CARDIA Dietary History assessment, administered by a trained interviewer at baseline (31). We examined daily dietary intakes of energy (kilojoules per day); total fat, protein, and carbohydrate (percentages of kilocalories) and total fiber (grams per day per 100 kilocalories).
Statistical methods
Preliminary analyses involved description of race and baseline and follow-up characteristics by diabetes case status. Baseline characteristics included age, parity, FPG, BMI, height, waist girth, dietary intake (grams fiber per day per 100 kilocalories, percentage of kilocalories in carbohydrates, and percentage of kilocalories in fat), alcohol intake, cigarette smoking, education, marital status, employment status, physical activity, and oral conceptive use. Follow-up characteristics included family history of diabetes and GDM status. χ2 tests were used to assess associations of diabetes case status across categories of race, sociodemographics, behaviors, family history of diabetes, and GDM status. Multiple linear regression methods (ANOVA) were used to assess baseline differences in continuous variables among interim birth groups by diabetes case status. P values were obtained from two-sided tests (significance <0.05). Wilcoxon’s rank-sum test was used to assess differences in alcohol intake categories and physical activity quartiles.
We calculated the cumulative incidence of diabetes within each time interval (0 –5, >5–7, >7–10, >10–15, and >15–20 years) by dividing new cases of diabetes during each interval by the number of women at risk of diabetes at the end of the interval. We estimated the crude IRs and 95% CIs by dividing new cases of diabetes by the person-time for individuals observed. IRs for interim birth groups were stratified by baseline parity groups and family history of diabetes.
Because diabetes status was only determined at the CARDIA exams, the exact time of diabetes onset for a woman free of diabetes and diagnosed at a subsequent exam is unknown. We accounted for interval-censored data using the method of Prentice and Gloeckler (32) to provide point and interval estimates of the relative hazard (RH) of diabetes associated with exposure. These estimates were obtained in the context of a generalized linear model for binary outcome, with a complementary log-log link function. Relative hazard ratios for incidence of diabetes were estimated for time-dependent interim birth groups, with nulliparas as the referent group.
We estimated RHs for interim birth groups from multivariate models. We examined potential confounders, race, age, study center, baseline covariates (BMI, FPG, waist girth, behavioral, and sociodemographic), and family history of diabetes. We also examined pregnancy losses, smoking, and oral conceptive use as time-dependent potential confounders. Covariates were introduced into the regression models in specified order by type of potential confounder—biological, sociodemographic, or behavioral—based on a priori hypotheses. We examined race, BMI, family history of diabetes, and smoking as effect modifiers in the association between interim births and incidence of diabetes through introduction of corresponding cross-product terms. Potential mediators of this association (changes in weight, waist girth, and physical activity from baseline to the end of follow-up) were also examined.
From these analyses, multivariate-adjusted models were formed by forward stepwise addition of covariates. We added family history of diabetes, baseline FPG, and then race, age, and baseline BMI. The fully adjusted model included these covariates plus baseline education, smoking, and physical activity. In subsequent models, we separately added time-dependent changes in physical activity, weight gain, and waist girth.
RESULTS
Among 2,408 women in our sample followed for 42,782 person-years, we identified 193 incident cases of diabetes, yielding an IR of 4.5/1,000 person-years. Among incident cases, 39 were ascertained by self-report only, 43 by diabetes medication use with or without diabetes self-report, and 111 by FPG ≥126 mg/dl and/or a 2-h plasma glucose ≥ 200 mg/dl after a 75-g oral glucose load. Incident diabetes cases (Table 1) were characterized by black race, family history of diabetes, and GDM. At baseline, diabetes case subjects were less educated and had higher parity, BMI, FPG, and waist girth. Moreover, they also consumed more energy from carbohydrate and less alcohol and were less physically active.
TABLE 1.
Baseline characteristics, race, family history of diabetes, and GDM status by incident diabetes
| Variable | Incident diabetes cases | Noncases | P |
|---|---|---|---|
| n | 193 | 2,215 | — |
| Black race | 142 (74) | 1,084 (49) | <0.001 |
| Baseline | |||
| Parity (nulliparous) | 105 (54) | 1448 (65) | 0.002 |
| Education (high school or less) | 89 (46) | 795 (36) | <0.001 |
| Oral contraceptive use (current) | 41 (21) | 573 (26) | 0.009 |
| Marital status (married) | 57 (30) | 527 (24) | 0.19 |
| Smoker (current) | 65 (34) | 619 (28) | 0.23 |
| Age (years) | 25.3 ± 3.7 | 24.9 ± 3.7 | 0.10 |
| BMI (kg/m2) | 29.7 ± 7.4 | 24 ± 5.2 | <0.001 |
| Waist girth (cm) | 85.2 ± 16.4 | 73 ± 10.3 | <0.001 |
| FPG (mg/dl) | 83.7 ± 9.7 | 79.6 ± 7.6 | <0.001 |
| Percent kcal as carbohydrate | 48.5 ± 7.9 | 46.9 ± 7.4 | 0.007 |
| Percent kcal as fat | 37 ± 6.3 | 37.4 ± 6.2 | 0.42 |
| Alcohol intake (ml/day)* | 0.0 (7.3) | 2.4 ± 9.6 | 0.01 |
| Physical activity score* | 223.0 (280.0) | 292.0 ± 322.0 | <0.001 |
| During follow-up | |||
| Family history of diabetes | 114 (59) | 604 (27) | <0.001 |
| GDM status (ever) including baseline | 43 (22) | 130 (6) | <0.001 |
| GDM status for interim births only | 32 (17) | 117 (5) | <0.001 |
Data are n (%) or means ± SD unless otherwise indicated.
Median (interquartile range), Kruskal-Wallis test.
In our sample, 1,218 women (50% black and 50% white) had one or more interim births, and 149 (12%) had at least one birth with GDM. Of the 193 diabetes cases, 84 (44%) had one or more interim births. Time from last birth until the end of follow-up averaged 119 months (range 3.9 –240) for diabetes case subjects vs. 123 months (2.5–236) for noncase subjects. Among nulliparas at baseline, crude IRs per 1,000 person-years were 3.2 (95% CI 2.4–4.1) for those with no births, 2.9 (1.8 –3.9) for one or more births without GDM, and 18.4 (10.9 –25.9) for one or more births with GDM. Among paras at baseline, IRs were 4.9 (3.8–6.1) for those with zero or one or more births without GDM and 17.9 (10.0 –25.8) for zero or one or more births with GDM.
Incidence of diabetes increased across all interim birth groups during 20 years (Table 2). The largest number of cases occurred between years 15 and 20 compared with earlier intervals. Cumulative incidence of diabetes among individuals at risk in these intervals ranged from 0.2 to 6.2% for no births without GDM, 4.2 to 38.5% for having no births with GDM (before baseline), 0 to 3.4% for one or more births without GDM, and 0 to 16.8% for one or more births with GDM.
TABLE 2.
New cases of diabetes divided by the number of individuals at risk for each interval after baseline by interim birth groups stratified by GDM status
| New cases of diabetes/individuals at risk (exam year) |
|||||
|---|---|---|---|---|---|
| Interim birth groups | Year 5 | Year 7 | Year 10 | Year 15 | Year 20 |
| 0 births without GDM | 3/1,748 (0.2) | 8/1,518 (0.5) | 18/1,254 (1.4) | 17/1,010 (1.7) | 53/859 (6.2) |
| 0 births with GDM (before baseline) | 1/24 (4.2) | 1/23 (4.4) | 2/20 (10.0) | 1/18 (5.6) | 5/13 (38.5) |
| ≥1 birth without GDM | 0/581 (0.0) | 4/715 (0.6) | 9/858 (1.1) | 10/904 (1.1) | 28/820 (3.4) |
| ≥1 birth with GDM | 0/55 (0.0) | 1/87 (1.2) | 6/115 (5.2) | 7/126 (5.6) | 19/113 (16.8) |
| Total | 4/2,408 (0.2) | 14/2,343 (0.6) | 35/2,247 (1.6) | 35/2,058 (1.7) | 105/1,805 (5.8) |
Data n/N (%), where n = new cases of diabetes and N = individuals at risk. FPG was not available at year 5. 2-h OGTTs were administered at years 10 and 20.
We examined the crude IR of diabetes for interim birth groups by family history of diabetes and parity at baseline (Table 3). Rates are similar for women nulliparous and parous at baseline by family history of diabetes and GDM status. The rates were lowest for nulliparas with one or more interim births without GDM and no family history of diabetes, intermediate for those with one or more interim births without GDM and a family history of diabetes, and highest for those with one or more interim births with GDM and a family history of diabetes. Crude IRs were 20/1,000 person-years among women with only one GDM birth and 18/1,000 person-years among women with one or more births before or subsequent to a GDM birth.
TABLE 3.
Crude IRs of diabetes and 95% CIs by baseline parity groups and family history of diabetes
| No family history of diabetes |
Family history of diabetes |
|||
|---|---|---|---|---|
| n/1,000 person-years | IR (95% CI) | n/1,000 person-years | IR (95% CI) | |
| Nulliparous at baseline | ||||
| 0 births | 25/12,337 | 2.0 (1.2–2.8) | 28/4,165 | 6.7 (4.2–9.2) |
| ≥1 birth without GDM | 9/7,294 | 1.2 (0.4–2.0) | 20/2,835 | 7.1 (4.0–10.1) |
| ≥1 birth with GDM | 10/747 | 13.4 (5.1–21.7) | 13/506 | 25.7 (11.7–39.7) |
| Parous at baseline | ||||
| 0 or ≥1 birth without GDM | 28/9,223 | 3.0 (1.9–4.2) | 40/4,558 | 8.8 (6.1–11.5) |
| 0 or ≥1 birth with GDM | 7/556 | 12.6 (3.3–21.9) | 13/561 | 23.2 (10.6–35.8) |
n = number of incident cases of diabetes.
RHs for diabetes were highest among women who ever had a GDM pregnancy whether before baseline or in an interim birth (Table 4). Adjustment for family history of diabetes attenuated RHs of diabetes among interim birth groups. Inclusion of baseline FPG minimally attenuated the RHs. Control for confounding by race, age, and baseline BMI moderately strengthened RHs of diabetes to 3.5 among nulliparas at baseline who had one or more interim births with GDM. In the fully adjusted model, compared with those who had no interim births, diabetes risk was nearly fourfold higher among nulliparas at baseline who had one or more interim births with GDM. Having one or more interim births without GDM was not associated with risk of diabetes. Among women parous at baseline, fully adjusted RHs for diabetes was similar to those among nulliparas at baseline, although weaker; diabetes risk was about threefold higher for those ever having a birth with GDM (zero or one or more interim births with GDM) and not associated with having zero or one or more interim births for those without GDM. Exclusion of 39 incident diabetes cases identified by self-report only strengthened associations in nulliparas at baseline; RH for diabetes was 5.1 (95 CI% 2.9 –9.1) for having one or more births with GDM and 1.0 (0.6 –1.6) for one or more births without GDM compared with no births.
TABLE 4.
Unadjusted and multivariable-adjusted RH (95% CI) for diabetes among interim birth groups by GDM status and stratified by baseline parity groups
| Interim birth groups stratified by baseline parity groups |
n | Unadjusted | Model 1 | Model 2 | Model 3 | Model 4 (fully adjusted) |
Model 4 + physical activity change(mediator) |
Model 4 + weight change (mediator) |
Model 4 + waist girth change (mediator) |
|---|---|---|---|---|---|---|---|---|---|
| Nulliparous at baseline | |||||||||
| 0 births (referent) | 737 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| ≥1 birth without GDM | 720 | 0.7 (0.5–1.1) | 0.7 (0.5–1.1) | 0.7 (0.5–1.1) | 0.9 (0.6–1.3) | 0.9 (0.6–1.4) | 0.8 (0.5–1.3) | 0.8 (0.5–1.3) | 0.8 (0.5–1.2) |
| ≥1 birth with GDM | 96 | 3.7 (2.2–6.4) | 3.1 (1.8–5.4) | 3.0 (1.8–5.2) | 3.5 (2.0–6.0) | 3.8 (2.2–6.6) | 3.7 (2.1–6.4) | 3.8 (2.2–6.6) | 3.4 (2.0–6.0) |
| Parous at baseline | |||||||||
| 0 or ≥1 birth without GDM | 778 | 1.4 (1.0–1.9) | 1.2 (0.9–1.8) | 1.2 (0.9–1.8) | 0.9 (0.6–1.3) | 0.8 (0.5–1.2) | 0.8 (0.5–1.1) | 0.8 (0.5–1.1) | 0.8 (0.5–1.1) |
| 0 or ≥1 birth with GDM | 77 | 4.8 (2.9–8.2) | 3.7 (2.2–6.3) | 3.5 (2.0–5.9) | 3.2 (1.9–5.5) | 2.9 (1.7–5.0) | 2.7 (1.5–4.7) | 2.7 (1.6–4.7) | 2.6 (1.5–4.5) |
Model 1 is adjusted for family history of diabetes; model 2 for family history of diabetes plus baseline FPG; model 3 for all of the above plus baseline BMI, age, and race; and model 4 (fully adjusted) for all of the above plus baseline covariates (education, smoking, and physical activity).
There was no evidence of effect modification by race, BMI, smoking, or family history of diabetes in the association of interim births and incidence of diabetes (all interaction P values >0.30). Family history of diabetes conferred a more than twofold higher risk of diabetes (RH 2.4 [95% CI 1.8 –3.2]) independent of number of interim births and other covariates.
Compared with women with no births, having one or more births resulted in greater weight gain (P < 0.05) and increased waist girth (P < 0.001). Mean ± SD weight gain was 12.9 ± 14.1, 14.3 ± 13.5, and 15.9 ± 13.1 kg and waist girth increase was 11.9 ± 11.4, 14.0 ± 10.8, and 16.4 ± 9.8 cm among women having no interim births, one or more interim births without GDM, and one or more interim births with GDM, respectively. Despite the greater weight gain and waist girth increase in women who gave birth, controlling for changes in body weight had minimal impact, while adding increased waist girth modestly attenuated the associations among nulliparas at baseline. Change in physical activity added separately to these models had little influence on RH for diabetes.
DISCUSSION
Key strengths of this prospective study are the verification that women were free of diabetes at baseline based on actual measurements of FPG, as well as the collection of multiple FPG measurements at 3- to 7-year intervals and 2-h oral glucose tolerance test results 10 and 20 years later to identify incident cases of diabetes. Moreover, these same measurements were available for a referent group of nulliparous women during the same interval. Finally, we validated GDM status and examined risks for this group separately. Our study overcomes weaknesses of previous studies that may have led to inconsistent findings (1–12) by controlling for preconception glycemia and obesity, preserving the temporality of pregnancy and diabetes, and estimating the risks separately for women with and without gestational diabetes.
Our findings show that childbearing does not increase the incidence of diabetes as long as women never developed GDM; their risk was similar to that for nulliparous women. In fact, the trend was for a nonsignificant lower risk of diabetes among ever-parous women compared with women remaining nulliparous, which moved closer to 1 after adjustment for BMI, age and race. A small subset of nulliparas may have infertility secondary to insulin resistance and obesity, which could explain why the risk was slightly higher in this group. In a prior prospective study, the Nurses’ Health Study, BMI was primarily measured subsequent to childbearing years (5), which may have underestimated the association by ignoring the excess risk of overweight due to childbearing.
Second, having one or more GDM pregnancies conferred a fourfold higher risk of developing diabetes independent of preconception fasting glycemia, family history of diabetes, and other risk factors. The IR of diabetes was 300% higher for women with previous GDM compared with that for nulliparas or women who had one or more births without GDM. The validity of our estimates is strongest for primiparas during our study years because FPG levels helped rule out diabetes before pregnancies and preserved the temporality of the exposure (pregnancy) before specific risk factor changes and disease onset. Our study also found that development of diabetes was associated with increased central obesity, especially among women who reported GDM for an interim birth. Decreased physical activity and higher weight gain had a minimal impact on risk.
Healthy pregnancy is a temporary, diabetogenic state wherein hyperinsulinemia shifts fuel metabolism toward accentuated excursions in pre- and postprandial glycemia. Gestational hormones promote insulin resistance and pancreatic β-cell proliferation to achieve the 1.5 times higher insulin secretion needed to maintain maternal euglycemia (33,34). Failure of β-cells to meet the greater demands for insulin production results in gestational glucose intolerance. Usually, the metabolic profile returns to the normal preconception state shortly after delivery. In earlier studies of women unscreened for GDM, multiparas (five or more births) had higher plasma glucose and insulin levels and prevalence of diabetes. This evidence raised the possibility that repeated pregnancies had lasting adverse effects on glucose tolerance apart from obesity (6–8,13,14) and that β-cell function deteriorated to a threshold level, with intermittent demands for greater insulin production (11). In our cohort, among 40 (3%) women with four or more births within the 20-year period, 1 developed diabetes.
Women with a history of GDM comprise a high-risk group for future development of primarily type 2 diabetes. Based on current diagnostic criteria (26), it is estimated that 5–10% of women will be diagnosed with type 2 diabetes within 6 months of GDM delivery and that another 10–15% will develop type 2 diabetes within the subsequent 1–2 years (35–41). Among women with previous GDM, having a subsequent birth was associated with a threefold greater risk of developing type 2 diabetes, independent of weight gain in one study (18) but not another (42). Our sample of women with GDM and subsequent births was too small to adequately assess the association with number of births.
Limitations of our study include diabetes cases identified by a single elevated fasting and/or 2-h post– glucose load test result, 39 diabetes cases by self-report only, GDM pregnancies by self-report, and the variable time period from exams to conception. Although our study lacked prospective data on infertility, we controlled for family history of diabetes and baseline fasting glycemia to reduce confounding from nulliparity due to infertility. Infertility cases due to associated diagnoses (e.g., obesity, insulin resistance) are likely to be few and would bias our results toward the null.
Strengths of our study that enhance the validity of our findings are the high cohort retention rate over 20 years of follow-up (72%), the availability of FPG at baseline for 100% of subjects, and at least three measurements after baseline (3- to 7-year intervals) for 86% of the analytic sample. Thus, women in our cohort were likely to be free of diabetes before conception. Other strengths are measurements of preconception obesity and a variety of sociodemographic and behavioral attributes to examine potential confounding.
We conclude that pregnancy does not have an adverse impact on women’s future risk of diabetes, despite greater gains in overall and central adiposity (43). The fourfold higher risk of diabetes for an interim birth with GDM did not vary by family history of diabetes. Our findings provide evidence that pregnancy in which GDM develops may unmask a predisposition to develop type 2 diabetes after delivery rather than cause type 2 diabetes. However, we cannot completely rule out the possibility that pregnancy hastens development of type 2 diabetes. Underlying defects in glucose homeostasis are likely to contribute to GDM, the strongest predictor of future diabetes among women of childbearing age. Identification of risk profiles for women who are most susceptible to the physiological stress of pregnancy may guide development of screening protocols to target preconception, prenatal, and postpartum interventions in high-risk groups to prevent diabetes.
ACKNOWLEDGMENTS
This study was supported by contracts N01-HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050, and N01-HC-95095 from the National Heart, Lung, and Blood Institute; National Institute of Diabetes, Digestive and Kidney Diseases Career Development Award Grant K01 DK059944; and a research award from the American Diabetes Association.
Glossary
- CARDIA
Coronary Artery Risk Development in Young Adults
- FPG
fasting plasma glucose
- GDM
gestational diabetes mellitus
- IR
incidence rate
- RH
relative hazard
REFERENCES
- 1.Pyke DA. Parity and the incidence of diabetes. Lancet. 1956;270:818–820. doi: 10.1016/s0140-6736(56)91293-4. [DOI] [PubMed] [Google Scholar]
- 2.O’Sullivan JB, Gordon T. Childbearing and diabetes mellitus. United States-1960 –1962. Vital Health Stat. 1966;11:1–19. [PubMed] [Google Scholar]
- 3.Boyko EJ. The effect of parity on the later development of diabetes. N Engl J Med. 1990;322:1320. [PubMed] [Google Scholar]
- 4.Collins VR, Dowse GK, Zimmet PZ. Evidence against association between parity and NIDDM from five population groups. Diabetes Care. 1991;14:975–981. doi: 10.2337/diacare.14.11.975. [DOI] [PubMed] [Google Scholar]
- 5.Manson JE, Rimm EB, Colditz GA, Stampfer MJ, Willett WC, Arky RA, Rosner B, Hennekens CH, Speizer FE. Parity and incidence of non-insulin-dependent diabetes mellitus. Am J Med. 1992;93:13–18. doi: 10.1016/0002-9343(92)90674-z. [DOI] [PubMed] [Google Scholar]
- 6.Alderman BW, Marshall JA, Boyko EJ, Markham KA, Baxter J, Hamman RF. Reproductive history, glucose tolerance, and NIDDM in Hispanic and non-Hispanic white women: the San Luis Valley Diabetes Study. Diabetes Care. 1993;16:1557–1564. doi: 10.2337/diacare.16.12.1557. [DOI] [PubMed] [Google Scholar]
- 7.Cowan LD, Go OT, Howard BV, Devereux RB, Pettitt DJ, Fabsitz RR, Lee ET, Welty TK. Parity, postmenopausal estrogen use, and cardiovascular disease risk factors in American Indian women: the Strong Heart Study. J Womens Health. 1997;6:441–449. doi: 10.1089/jwh.1997.6.441. [DOI] [PubMed] [Google Scholar]
- 8.Charles MA, Pettitt DJ, McCance DR, Hanson RL, Bennett PH, Knowler WC. Gravidity, obesity, and non-insulin-dependent diabetes among Pima Indian women. Am J Med. 1994;97:250–255. doi: 10.1016/0002-9343(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 9.Dawson SI, Smith WC, Watson MS, Wilson BJ, Prescott GJ, Campbell D, Hannaford P. A cohort study of reproductive risk factors, weight and weight change and the development of diabetes mellitus. Diabetes Obes Metab. 2003;5:244–250. doi: 10.1046/j.1463-1326.2003.00269.x. [DOI] [PubMed] [Google Scholar]
- 10.Martin FI, Hopper JL, Dean B, Campbell DG, Hammond P. Glucose tolerance and mortality in diabetes mellitus in Maltese-born residents of Victoria. Med J Aust. 1984;141:93–97. doi: 10.5694/j.1326-5377.1984.tb132711.x. [DOI] [PubMed] [Google Scholar]
- 11.Kritz-Silverstein D, Barrett-Connor E, Wingard DL. The effect of parity on the later development of non-insulin-dependent diabetes mellitus or impaired glucose tolerance. N Engl J Med. 1989;321:1214–1219. doi: 10.1056/NEJM198911023211802. [DOI] [PubMed] [Google Scholar]
- 12.Hanley AJ, McKeown-Eyssen G, Harris SB, Hegele RA, Wolever TM, Kwan J, Zinman B. Association of parity with risk of type 2 diabetes and related metabolic disorders. Diabetes Care. 2002;25:690–695. doi: 10.2337/diacare.25.4.690. [DOI] [PubMed] [Google Scholar]
- 13.Humphries KH, Westendorp IC, Bots ML, Spinelli JJ, Carere RG, Hofman A, Witteman JC. Parity and carotid artery atherosclerosis in elderly women: the Rotterdam Study. Stroke. 2001;32:2259–2264. doi: 10.1161/hs1001.097224. [DOI] [PubMed] [Google Scholar]
- 14.Kritz-Silverstein D, Barrett-Connor E, Wingard DL, Friedlander NJ. Relation of pregnancy history to insulin levels in older, nondiabetic women. Am J Epidemiol. 1994;140:375–382. doi: 10.1093/oxfordjournals.aje.a117260. [DOI] [PubMed] [Google Scholar]
- 15.Metzger BE, Cho NH, Roston SM, Radvany R. Prepregnancy weight and antepartum insulin secretion predict glucose tolerance five years after gestational diabetes mellitus. Diabetes Care. 1993;16:1598–1605. doi: 10.2337/diacare.16.12.1598. [DOI] [PubMed] [Google Scholar]
- 16.Mestman JH, Anderson GV, Guadalupe V. Follow-up study of 360 subjects with abnormal carbohydrate metabolism during pregnancy. Obstet Gynecol. 1972;39:421–425. [PubMed] [Google Scholar]
- 17.O’Sullivan JB. Body weight and subsequent diabetes mellitus. JAMA. 1982;248:949–952. [PubMed] [Google Scholar]
- 18.Peters RK, Kjos SL, Xiang A, Buchanan TA. Long-term diabetogenic effect of single pregnancy in women with previous gestational diabetes mellitus. Lancet. 1996;347:227–230. doi: 10.1016/s0140-6736(96)90405-5. [DOI] [PubMed] [Google Scholar]
- 19.Gunderson EP, Quesenberry CP, Jr, Lewis CE, Tsai AL, Sternfeld B, Smith, West D, Sidney S. Development of overweight associated with childbearing depends on smoking habit: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Obes Res. 2004;12:2041–2053. doi: 10.1038/oby.2004.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cutter GR, Burke GL, Dyer AR, Friedman GD, Hilner JE, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Manolio TA. Cardiovascular risk factors in young adults: the CARDIA baseline monograph. Control Clin Trials. 1991;12:1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 21.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 22.Lewis CE, Jacobs DR, Jr, McCreath H, Kiefe CI, Schreiner PJ, Smith DE, Williams OD. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 2000;151:1172–1181. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 23.Steffen LM, Kroenke CH, Yu X, Pereira MA, Slattery ML, Van Horn L, Gross MD, Jacobs DR., Jr Associations of plant food, dairy product, and meat intakes with 15-y incidence of elevated blood pressure in young black and white adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2005;82:1169–1177. doi: 10.1093/ajcn/82.6.1169. [DOI] [PubMed] [Google Scholar]
- 24.Bild DE, Jacobs DR, Liu K, Williams OD, Hilner JE, Perkins LL, Marcovina SM, Hulley SB. Seven-year trends in plasma low-density-lipoprotein-cholesterol in young adults: the CARDIA Study. Ann Epidemiol. 1996;6:235–245. doi: 10.1016/1047-2797(96)00005-1. [DOI] [PubMed] [Google Scholar]
- 25.Slein MW, Cori GT, Cori CF. A comparative study of hexokinase from yeast and animal tissues. J Biol Chem. 1950;186:763–780. [PubMed] [Google Scholar]
- 26.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 27.Lewis CE, Smith DE, Wallace DD, Williams OD, Bild DE, Jacobs DR., Jr Seven-year trends in body weight and associations with lifestyle and behavioral characteristics in black and white young adults: the CARDIA study. Am J Public Health. 1997;87:635–642. doi: 10.2105/ajph.87.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith DE, Lewis CE, Caveny JL, Perkins LL, Burke GL, Bild DE. Longitudinal changes in adiposity associated with pregnancy. The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. JAMA. 1994;271:1747–1751. [PubMed] [Google Scholar]
- 29.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Washington, D.C.: National Institutes of Health, National Heart, Lung, and Blood Institute; The Evidence Report. 1998
- 30.Anderssen N, Jacobs DR, Jr, Sidney S, Bild DE, Sternfeld B, Slattery ML, Hannan P. Change and secular trends in physical activity patterns in young adults: a seven-year longitudinal follow-up in the Coronary Artery Risk Development in Young Adults Study (CARDIA) Am J Epidemiol. 1996;143:351–362. doi: 10.1093/oxfordjournals.aje.a008749. [DOI] [PubMed] [Google Scholar]
- 31.McDonald A, Van Horn L, Slattery M, Hilner J, Bragg C, Caan B, Jacobs D, Jr, Liu K, Hubert H, Gernhofer N. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc. 1991;91:1104–1112. [PubMed] [Google Scholar]
- 32.Prentice R, Gloeckler L. Regression analysis of grouped survival data with application to breast cancer data. Biometrics. 1978;34:57–67. [PubMed] [Google Scholar]
- 33.Desoye G, Schweditsch MO, Pfeiffer KP, Zechner R, Kostner GM. Correlation of hormones with lipid and lipoprotein levels during normal pregnancy and postpartum. J Clin Endocrinol Metab. 1987;64:704–712. doi: 10.1210/jcem-64-4-704. [DOI] [PubMed] [Google Scholar]
- 34.Ledoux F, Genest J, Nowaczynski W, Kuchel O, Lebel M. Plasma progesterone and aldosterone in pregnancy. Can Med Assoc J. 1975;112:943–947. [PMC free article] [PubMed] [Google Scholar]
- 35.Pallardo F, Herranz L, Garcia-Ingelmo T, Grande C, Martin-Vaquero P, Janez M, Gonzalez A. Early postpartum metabolic assessment in women with prior gestational diabetes. Diabetes Care. 1999;22:1053–1058. doi: 10.2337/diacare.22.7.1053. [DOI] [PubMed] [Google Scholar]
- 36.Pallardo LF, Herranz L, Martin-Vaquero P, Garcia-Ingelmo T, Grande C, Janez M. Impaired fasting glucose and impaired glucose tolerance in women with prior gestational diabetes are associated with a different cardiovascular profile. Diabetes Care. 2003;26:2318–2322. doi: 10.2337/diacare.26.8.2318. [DOI] [PubMed] [Google Scholar]
- 37.Conway DL, Langer O. Effects of new criteria for type 2 diabetes on the rate of postpartum glucose intolerance in women with gestational diabetes. Am J Obstet Gynecol. 1999;181:610–614. doi: 10.1016/s0002-9378(99)70500-4. [DOI] [PubMed] [Google Scholar]
- 38.Schaefer-Graf UM, Buchanan TA, Xiang AH, Peters RK, Kjos SL. Clinical predictors for a high risk for the development of diabetes mellitus in the early puerperium in women with recent gestational diabetes mellitus. Am J Obstet Gynecol. 2002;186:751–756. doi: 10.1067/mob.2002.121895. [DOI] [PubMed] [Google Scholar]
- 39.Aberg AE, Jonsson EK, Eskilsson I, Landin-Olsson M, Frid AH. Predictive factors of developing diabetes mellitus in women with gestational diabetes. Acta Obstet Gynecol Scand. 2002;81:11–16. doi: 10.1046/j.0001-6349.2001.00000.x. [DOI] [PubMed] [Google Scholar]
- 40.Buchanan TA, Xiang AH, Kjos SL, Trigo E, Lee WP, Peters RK. Antepartum predictors of the development of type 2 diabetes in Latino women 11–26 months after pregnancies complicated by gestational diabetes. Diabetes. 1999;48:2430–2436. doi: 10.2337/diabetes.48.12.2430. [DOI] [PubMed] [Google Scholar]
- 41.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 42.Albareda M, Caballero A, Badell G, Piquer S, Ortiz A, De Leiva A, Corcoy R. Diabetes and abnormal glucose tolerance in women with previous gestational diabetes. Diabetes Care. 2003;26:1199–1205. doi: 10.2337/diacare.26.4.1199. [DOI] [PubMed] [Google Scholar]
- 43.Gunderson EP, Murtaugh MA, Lewis CE, Quesenberry CP, West DS, Sidney S. Excess gains in weight and waist circumference associated with childbearing: The Coronary Artery Risk Development in Young Adults Study (CARDIA) Int J Obes Relat Metab Disord. 2004;28:525–535. doi: 10.1038/sj.ijo.0802551. [DOI] [PMC free article] [PubMed] [Google Scholar]