Abstract
Objective
To examine whether childbearing is associated with increased visceral adiposity and whether the increase is proportionally larger than other depots.
Methods and Procedures
This prospective study examined changes in adiposity assessed via computed tomography (CT) and dual-energy X-ray absorptiometry among 122 premenopausal women (50 black, 72 white) examined in 1995–1996 and again in 2000–2001. During the 5-year interval, 14 women had one interim birth and 108 had no interim births. Multiple linear regression models estimated mean (95% confidence interval (CI)) 5-year changes in anthropometric and adiposity measures by interim births adjusted for age, race, and changes in total and subcutaneous adiposity.
Results
We found no significant differences between one interim birth and no interim births for 5-year changes in weight, BMI, total body fat, subcutaneous adipose tissue, or total abdominal adipose tissue. Visceral adipose tissue increased by 40 and 14% above initial levels for 1 birth and 0 birth groups, respectively. Having 1 birth vs. 0 births was associated with a greater increase in visceral adipose tissue of 18.0 cm2 (4.8, 31.2), P < 0.01; gain of 27.1 cm2 (14.5, 39.7) vs. 9.2 cm2 (4.8, 13.6), and a borderline greater increase in waist girth of 2.3 cm (0, 4.5), P = 0.05; gain of 6.3 cm (4.1, 8.5) vs. 4.0 cm (3.2, 4.8), controlling for gain in total body fat and covariates.
Discussion
Pregnancy may be associated with preferential accumulation of adipose tissue in the visceral compartment for similar gains in total body fat. Further investigation is needed to confirm these findings and determine whether excess visceral fat deposition with pregnancy adversely affects metabolic risk profiles among women.
INTRODUCTION
Central adiposity is of greater importance metabolically than that of overall obesity because intra-abdominal (visceral) fat is associated with obesity-related insulin resistance, cardiovascular disease, lower high-density lipoprotein cholesterol levels, and progression to type 2 diabetes, particularly among women (1–4). Moreover, a centralized distribution of adiposity increases disease risk, even among those not overweight (5). Visceral adipose tissue is more metabolically active and is thought to differ from subcutaneous fat in the production of adipocytokines that may regulate insulin sensitivity (6). Indeed, a larger waist circumference (a crude surrogate measure of visceral adiposity) during the early postpartum period has been associated cross-sectionally with higher risk of diabetes and cardiovascular disease among women with a history of gestational diabetes (7,8).
Previous studies have examined the association of parity with indirect measures of central adiposity either cross-sectionally or longitudinally (9,10). Lifetime parity has been associated with larger waist girth many years after childbearing has ceased (10,11). Moreover, prospective studies have observed substantial increases in waist girth with more interim births (9), that was proportionately larger than the absolute weight gain associated with childbearing. These data show that pregnancy preferentially promotes central obesity, an important risk factor for future metabolic diseases.
A few studies have used imaging methods to measure distribution of adipose tissue in women as related to pregnancy. One cross-sectional study using computed tomography (CT) found that higher parity was associated with higher intra-abdominal fat levels (12). A longitudinal study using magnetic resonance imaging examined 15 women before and after pregnancy, and found higher nonsubcutaneous fat deposition in the trunk than peripheral region at postpartum (13). During pregnancy, increased intra-abdominal visceral adipose tissue was observed as measured using ultrasonography (14).
The purpose of this study is to examine whether childbearing is associated with a greater increase in visceral adipose tissue, and whether this putative increase was proportionally larger than increases in abdominal subcutaneous adipose tissue or other adipose tissue compartments. We explored these relationships longitudinally in a small sample of women for whom CT measurements and dual-energy X-ray absorptiometry were obtained before and after pregnancy and compared them with measurements of nongravid women within the same 5-year interval.
METHODS AND PROCEDURES
The Coronary Artery Risk Development in Young Adults (CARDIA) study is a multicenter, longitudinal, observational study describing the development of risk factors for coronary heart disease in young black and white individuals. Descriptions of the study design, methodology, and cohort characteristics have been reported previously (15,16). The study population was recruited from four geographic areas: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. From 1985 to 1986, baseline data collection was accomplished on a total of 5,115 subjects (2,787 women) aged 18–30 years of which 52 and 48% were black and white, respectively. Baseline and follow-up examinations included a variety of physiologic and self-reported measures (15,17). The retention rates at follow-up examinations at years 10 and 15 after baseline included in this analysis were 79 and 74% of the surviving cohort (17).
Sample selection criteria
To assess the impact of childbearing on changes in visceral adipose tissue, we used data from the two consecutive CARDIA examinations in which body composition measures were available from ancillary studies. The purpose of the ancillary studies carried out in 1995–1996 and in 2000–2001 at CARDIA centers in Birmingham, Alabama, and Oakland, California was to examine gender and black–white differences in body composition during a 5-year interval. There were 398 participants who attended for the 1995–1996 (year 10) CARDIA examination and volunteered for the ancillary study of body composition conducted at Birmingham, Alabama (79 men and 78 women) and Oakland, California (116 men and 125 women) sites (18). Of the 203 women, 161 attended the subsequent 2000–2001 (year 15) CARDIA examination and participated in the ancillary study: 65 from Alabama and 96 from California sites. All body composition and anthropometric measurements at year 10 and year 15 were available for 154 women who were nonpregnant at both visits. Furthermore, we excluded women if they had a hysterectomy or removal of both ovaries and/or reported being postmenopausal at year 10 (n = 8) or year 15 (n = 17). We also excluded women with no interim births if they had any pregnancies (n = 6) and those who had more than one live birth (n = 1) during the 5-year interval. The analytic sample included 122 CARDIA women (50 black and 72 white) who were either not pregnant (0 interim birth) or delivered a singleton birth only once (1 interim birth) during the 5-year interval. Institutional Review Boards at each participating study center approved the study. We obtained written informed consent from subjects for all study procedures.
Data collection methods
A description of the methodology used to recruit subjects and perform data collection is detailed elsewhere (15,16). We obtained measurements of weight, height, and waist circumference (waist girth) at each examination according to standardized protocol described previously (19). Certified technicians obtained anthropometric measurements. They measured body weight to the nearest 0.2 kg using a calibrated balance beam scale of participants wearing light clothing, and height (without shoes) to the nearest 0.5 cm using a vertical ruler. Technicians also measured waist circumference to the nearest 0.5 cm at the minimal abdominal girth (20). We computed BMI as weight in kilograms divided by squared height in meters. The anthropometric measures are weight, waist girth, and BMI at examinations in years 10 and 15.
Body composition measures included abdominal adipose tissue compartments (total, subcutaneous, and visceral) and total body fat at examinations in years 10 and 15. We measured total body fat by dual-energy X-ray absorptiometry using a QDR-2000 scanner (Hologic, Waltham, MA) in the enhanced total-body-array scanning mode at each site. Procedures on performing CT scans to obtain adipose tissue compartment measures (measured as a cross-sectional area in cm2) have been published previously in detail elsewhere (18). In brief, technicians performed CT scans using a 9800 CT scanner (General Electric Medical Systems, Milwaukee, WI) at Oakland site and using either a 9800 CT scanner or a GE High Speed Advantage System scanner (General Electric Medical Systems) at Alabama site with a 10-mm cut at the L4–L5 disc space. They transferred the images to the central reading center at the University of Colorado Health Sciences Center on optical disks or tape storage media for analysis. We analyzed abdominal adipose tissue regions and estimated visceral and subcutaneous adipose tissue levels using techniques described previously (21,22). The central reading center developed the analysis programs using intermediate density lipoprotein (RSI, Boulder, CO). We used a traveling phantom to ensure compatibility between the sites, and standardized measures across the two sites.
Women reported pregnancies and births at each examination. Participants were asked whether they were currently pregnant, number of times they had been pregnant including abortions, miscarriages, and live- or stillbirths since the previous examination, duration of gestation, and dates of delivery. We calculated time (months) from delivery to the year 15 examination for each interim birth.
We collected sociodemographic and behavioral data (cigarette smoking, oral contraceptive use, and physical activity) at each examination using self- and interviewer-administered questionnaires. We categorized cigarette smoking as: never, former, or current, and calculated cigarette smoking in pack-years. We categorized self-reported oral contraceptive use as never, past, or current. We assessed physical activity levels using the interviewer-administered CARDIA Physical Activity History described previously (23).
Interim birth groups
All women included in the analytic sample were not pregnant at examinations in years 10 or 15. Of these women, we classified those who reported no pregnancies or births during the 5-year interval in the no interim birth group (0 births). We defined an interim birth as delivery of a singleton, live birth of longer than 20 weeks gestation that was conceived after year 10 (baseline) and delivered before the year 15 examination. Women in our sample had no more than one interim birth (1 birth).
Definition of changes in body fat and anthropometric measures
For this analysis, the year 10 examination measurements served as the preconception levels for women with one interim birth between 1995 and 2001 and as initial levels for the referent group of women with no interim births during the same interval. We calculated changes in measures of total body fat, abdominal adipose tissue compartments (total, subcutaneous, and visceral), and anthropometry (weight, BMI, and waist girth) by subtracting the measurement in year 10 (baseline) from the measurement in year 15.
Statistical analysis
Preliminary analyses involved examination of each measure of adiposity for the interim birth groups to detect influential data points, and description of the baseline (1995–1996, year 10) characteristics. We used χ2-tests to assess associations with baseline demographic and behavioral categorical variables (race, cigarette smoking status, oral contraceptive use, parity, gravidity) within each interim birth group and between groups. We used the Fisher exact χ2-test for categories with fewer than five subjects. We used t-test statistics to assess differences between interim birth groups in baseline (year 10) age, BMI, waist girth, weight, height, and body fat compartment measures. We used the Kruskal–Wallis test to assess differences in physical activity and smoking (pack-year) at year 10 between interim birth groups due to skewedness in the distributions. We obtained P values from two-sided tests (significance <0.05).
We used multiple linear regression to examine unadjusted and adjusted differences in mean changes in weight, waist girth, BMI, total body fat, and visceral, subcutaneous, and total abdominal adipose tissue between the interim birth groups. We adjusted changes in anthropometric or body adipose tissue measures (dependent variable) for the baseline (year 10) measurement of the dependent variable, race, and age (Model 1) to control for potential confounding. Next, we adjusted Model 1 for change in total body fat (except for the change in total body fat model) by stepwise addition. For visceral adipose tissue change models, we examined the difference in the mean change in visceral adipose tissue between the 0 interim birth and 1 interim birth groups after adjusting for all above-mentioned covariates (Model 1), and in another model added change in total body fat to Model 1 (e.g., Model 1+ change in total body fat). Next, we substituted change in subcutaneous adipose tissue for the change in total body fat, and constructed two additional separate models for change in visceral adipose tissue. Changes in total body fat were added separately to Model 1 for each dependent variable to determine whether the changes in total abdominal, visceral, and subcutaneous adipose tissue associated with an interim birth were proportionally larger than the overall increase in body fat.
Age and race were included in all models because of a priori evidence of associations with visceral adipose tissue. We did not include potential confounders, oral contraceptive use, parity, BMI, physical activity, and cigarette smoking, in the final models because of the small sample size. We examined these covariables in the multivariable models and found no evidence of influence on model coefficients for interim birth groups with the dependent variables: changes in body weight, waist girth, total body fat, total abdominal adipose tissue, visceral adipose tissue, or subcutaneous adipose tissue.
RESULTS
Of the 122 women in the analysis, 108 had no births, and 14 had one birth between the two examinations (1 interim birth). There were 41% black and 59% white women with a mean age in 1995–1996 (baseline, year 10) of 35.6 years (range 29–41). The median time from delivery of an interim birth to the year 15 examination was 28.5 months (range 2.6–49). At year 10, none of the sociodemographic, anthropometric, reproductive, or behavioral characteristics differed between these two groups, except that women with one interim birth were slightly younger (Table 1). At year 10, the interim birth groups had similar proportions of nulliparas, but the proportion of nulligravidas was slightly higher for the 0 interim birth group.
Table 1.
Baseline (1995–1996) characteristics for 0 interim birth and 1 interim birth groups
| Baseline (1995–1996) characteristics |
0 interim birth n = 108 |
1 interim birth n = 14 |
P value |
|---|---|---|---|
| n (%) | |||
| Race: black | 44 (40.7) | 6 (42.9) | 0.88 |
| Parity (nulliparous) | 52 (48.1) | 7 (50.0) | 0.43 |
| Gravidity (nulligravida) | 36 (33.3) | 3 (21.4) | 0.54 |
| Smoker (current) | 14 (13.0) | 1 (7.1) | 1.00 |
| Oral contraceptive use (current) |
13 (12.0) | 1 (7.1) | 0.19 |
| Mean (s.d.) | |||
| Age (years) | 35.8 (3.5) | 34.0 (3.1) | 0.06 |
| BMI (kg/m2) | 26.8 (6.1) | 27.6 (5.9) | 0.64 |
| Waist girth (cm) | 80.0 (12.6) | 81.9 (13.0) | 0.60 |
| Weight (kg) | 72.8 (16.1) | 73.9 (14.1) | 0.80 |
| Total body fat (kg) | 28.0 (12.1) | 30.1 (11.9) | 0.56 |
| Visceral adipose tissue (cm2) |
64.6 (34.5) | 68.3 (48.0) | 0.71 |
| Subcutaneous adipose tissue (cm2) |
324 (168) | 363 (176) | 0.41 |
| Total abdominal adipose tissue (cm2) |
388 (193) | 431 (209) | 0.44 |
| Median (interquartile range)a | |||
| Physical activity | 239.0 (250.5) | 262.0 (312.0) | 0.75 |
| Smoking pack-years | 0.0 (1.6) | 0.0 (0.5) | 0.77 |
OCs, oral contraceptives.
Test via Kruskal–Wallis test.
As measured by dual-energy X-ray absorptiometry, the mean (95% confidence interval (CI)) increase in total body fat (kg) did not differ by interim births; the gain was 3.7 kg (2.5, 4.9) for 0 interim birth and 3.2 kg (0, 6.4) for 1 interim birth groups; P = 0.79 (Table 2). Adjustment for year 10 measures, age, and race widened the difference in means to 1.0 kg (−4.5, 2.4) between the groups (3.7 kg (2.5, 4.9) vs. 2.7 (−0.5, 5.9)), but it remained statistically nonsignificant. The 5-year mean change in weight and in BMI did not differ between the interim birth groups (Table 2). There was a borderline greater mean increase in waist girth of 2.3 cm (0, 4.5) for 1 birth vs. 0 births; an increase of 6.3 cm (4.1, 8.5) for 1 interim birth and 4.0 cm (3.2, 4.8) for 0 interim birth groups (P = 0.05) after adjusting for race, age, and change in total body fat.
Table 2.
Number of interim births and 5-year changes in anthropometry and total body fat
| Change in weight (kg) | Change in waist girth (cm) | Change in BMI (kg/m2) | Change in total body fat (kg) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |||||||||||||
| 0 interim birth |
1 interim birth |
Difference | 0 interim birth |
1 interim birth |
Difference | 0 interim birth |
1 interim birth |
Difference | 0 interim birth |
1 interim birth |
Difference | |||||
| Models | n = 108 | n = 14 | 1 vs. 0 births |
P value | n = 108 | n = 14 | 1 vs. 0 births |
P value | n = 108 | n = 14 | 1 vs. 0 births |
P value | n = 108 | n = 14 | 1 vs. 0 births |
P value |
| Unadjusted | 3.8 (2.4, 5.2) |
4.4 (0.8, 8.0) |
0.5 (−3.3, 4.3) |
0.78 | 4.1 (2.9, 5.3) |
6.1 (2.9, 9.3) |
2.0 (−1.3, 5.3) |
0.23 | 1.5 (1.1, 1.9) |
1.8 (0.4, 3.2) |
0.2 (−1.2, 1.7) |
0.73 | 3.7 (2.5, 4.9) |
3.2 (0, 6.4) |
−0.5 (−3.9, 3.0) |
0.79 |
| Model 1 | 3.9 (2.7, 5.1) |
3.7 (0.1, 7.3) |
−0.2 (−4.0, 3.6) |
0.92 | 4.1 (2.9, 5.3) |
5.7 (2.5, 8.9) |
1.5 (−1.8, 4.9) |
0.37 | 1.5 (1.1, 1.9) |
1.6 (0.2, 3.0) |
0 (−1.4, 1.5) |
0.98 | 3.7 (2.5, 4.9) |
2.7 (−0.5, 5.9) |
−1.0 (−4.5, 2.4) |
0.55 |
| Model 1 + change in total body fat |
3.8 (3.2, 4.4) |
4.6 (2.8, 6.4) |
0.8 (−1.1, 2.7) |
0.40 | 4.0 (3.2, 4.8) |
6.3 (4.1, 8.5) |
2.3 (0, 4.5) |
0.05 | 1.5 (1.3, 1.7) |
1.9 (1.1, 2.7) |
0.4 (−0.4, 1.2) |
0.37 | — | — | — | — |
Model 1 is adjusted for race, age, and dependent variable measurement at baseline (1995–1996, year 10).
CI, confidence interval.
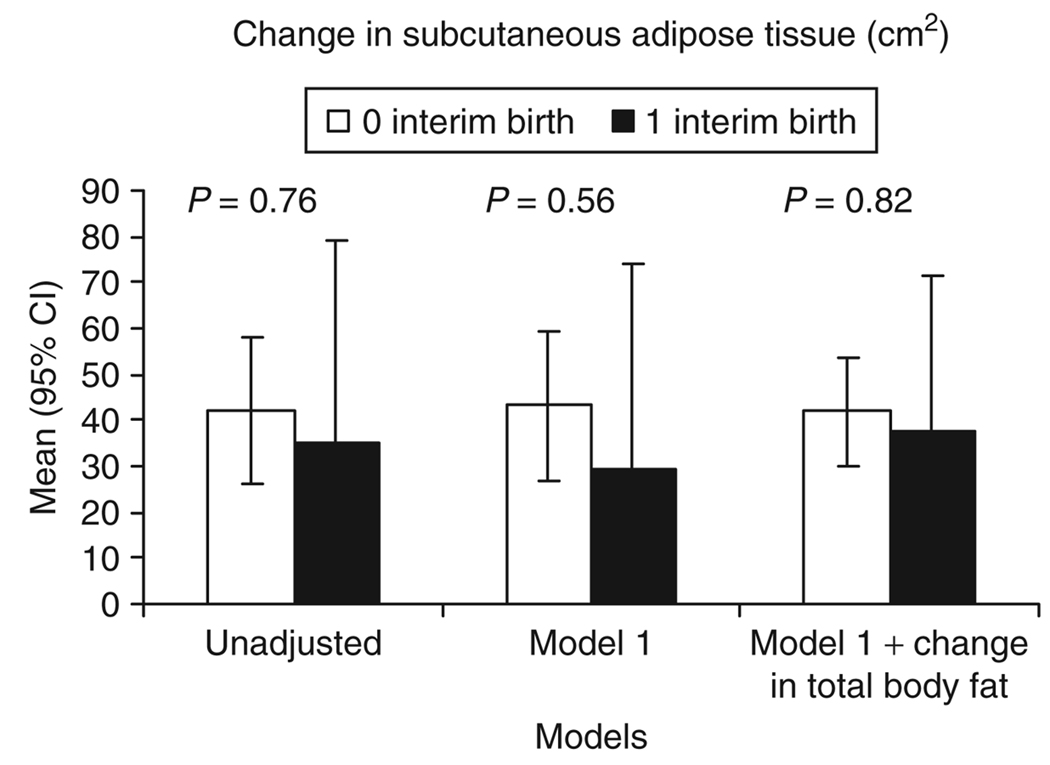
The 5-year mean changes in subcutaneous adipose tissue and total abdominal adipose tissue did not differ significantly (Table 3, Figure 1 and Figure 2) between the two groups in unadjusted or fully adjusted models. However, the gain in total abdominal adipose tissue was 13 cm2 (−27.0, 53.2) greater (Table 3) for 1 birth vs. 0 births (mean change (95% CI) of 64.3 cm2 (26.3, 102.3) for 1 interim birth and 51.2 cm2 (37.6, 64.8) for 0 interim birth groups). The 5-year mean change in visceral adipose tissue was 15.1 cm2 greater for 1 birth vs. 0 births in the adjusted model 1 (Table 3). This difference was accentuated after adjustment for change in subcutaneous adipose tissue (17.5 cm2 (3.6, 31.5); P = 0.014) or change in total body fat (18.0 cm2 (4.8, 31.2); P < 0.01). The 95% CIs did not overlap between the two groups for models adjusted for change in total body fat or change in total abdominal adipose tissue. The mean (95% CI) increase in visceral adipose tissue for the 1 interim birth group of 27.1 cm2 (14.5, 39.7) represents a 40% increase above the initial mean level at baseline (1995–1996). The mean (95% CI) increase in visceral adipose tissue of 9.2 cm2 (4.8, 13.6) for the 0 interim birth group is an increase of 14% over the same time interval. In adjusted models, visceral adipose tissue gain was proportionately greater than subcutaneous adipose tissue or total body fat gain for women who bore a child vs. those who did not (Figure 3). The change in visceral adipose tissue was uncorrelated with the time from delivery until the year 15 exam (2000–2001).
Table 3.
Unadjusted and multivariable adjusted mean (95% CI) for 5-year changes in abdominal adipose tissue compartments by number of interim births
| Change in visceral adipose tissue (cm2) | Change in subcutaneous adipose tissue (cm2) | Change in total abdominal adipose tissue (cm2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |||||||||||
| 0 interim birth | 1 interim birth | Difference | 0 interim birth | 1 interim birth | Difference | 0 interim birth | 1 interim birth | Difference | |||||
| Models | n = 108 | n = 14 | 1 vs. 0 births |
P value |
n = 108 | n = 14 | 1 vs. 0 births |
P value |
n = 108 | n = 14 | 1 vs. 0 births |
P value |
|
| Unadjusted | 9.4 (4.0, 14.8) |
25.1 (9.9, 40.3) |
15.7 (−0.2, 31.7) |
0.054 | 42.3 (26.3, 58.3) |
35.1 (−9.1, 79.3) |
−7.3 (−53.7, 39.2) |
0.76 | 51.8 (32.6, 71.0) |
60.2 (6.8, 113.6) |
8.5 (−47.8, 64.7) |
0.77 | |
| Model 1 | 9.5 (4.1, 14.9) |
24.6 (9.2, 40.0) |
15.1 (−1.2, 31.3) |
0.069 | 43.1 (27.1, 59.1) |
29.1 (−15.7, 73.9) |
−14.0 (−61.3, 33.2) |
0.56 | 52.7 (33.3, 72.1) |
53.0 (−1.4, 107.4) |
0.3 (−57.0, 57.6) |
0.99 | |
| Model 1 + change in total body fat |
9.2 (4.8, 13.6) |
27.1 (14.5, 39.7) |
18.0 (4.8, 31.2) |
0.008 | 42.0 (30.2, 53.8) |
37.8 (4.4, 71.2) |
−4.2 (−39.4, 31.1) |
0.82 | 51.2 (37.6, 64.8) |
64.3 (26.3, 102.3) |
13.1 (−27.0, 53.2) |
0.52 | |
| Model 1 + change in subcutaneous adipose tissue |
9.2 (4.6, 13.8) |
26.7 (13.5, 39.9) |
17.5 (3.6, 31.5) |
0.014 | — | — | — | — | — | — | — | — | |
Model 1 is adjusted for race, age, and dependent variable measurement at baseline (1995–1996, Year 10). Model 1 + = includes all covariates and the addition of each of the changes in fat compartments separately.
CI, confidence interval.
Figure 1.
Unadjusted and adjusted mean (95% confidence interval (CI)) 5-year changes in subcutaneous adipose tissue (cm2) for interim birth groups. Model 1 is adjusted for race, age, and subcutaneous adipose tissue at baseline (1995–1996).
Figure 2.
Unadjusted and adjusted mean (95% confidence interval (CI)) 5-year changes in total abdominal adipose tissue (cm2) for interim birth groups. Model 1 is adjusted for race, age, and total abdominal adipose tissue at baseline (1995–1996).
Figure 3.
Unadjusted and adjusted mean (95% confidence interval (CI)) 5-year changes in visceral adipose tissue (cm2) for interim birth groups. Model 1 is adjusted for race, age, and visceral adipose tissue at baseline (1995–1996).
DISCUSSION
We found that childbearing was associated with a threefold greater increase in visceral fat deposition from preconception to postpartum compared to those not bearing children. Furthermore, this effect was not due to overall excess total body fat deposition, but is a greater accumulation of visceral fat relative to abdominal subcutaneous fat. The unique feature of the study is that longitudinal data were obtained before conception and after delivery to provide evidence that pregnancy precedes levels of visceral adipose tissue. The new and important finding of our study is that for women, childbearing may be an important contributor to the enlargement of the visceral fat depot. Therefore, one mechanism by which pregnancy may contribute to future disease risk is via adverse metabolic effects mediated by excess deposition of visceral fat. Visceral fat is not an inert storage depot; rather it is a metabolically active endocrine tissue, the clinical effects of which are largely uncharacterized. Visceral adipose tissue is thought to differ from subcutaneous fat in the production of adipocytokines that may regulate insulin sensitivity, (6) and ultimately influence the development of type 2 diabetes (24). Visceral adipose tissue increases among nonpregnant pre- and perimenopausal women (n = 30) averaged 32.1 cm2 (95% CI 14.5, 49.7) during a 7-year period, and was associated with a deterioration of glucose tolerance (4). However, changes from before conception to after delivery, or cross-sectionally by parity were not available. A cross-sectional study of 170 women aged 18–76 years found that intra-abdominal fat levels assessed by CT scans were positively correlated with higher parity (partial r = 0.18; P = 0.02) after controlling for total body fat and other confounders (12). During pregnancy, ~30% of gestational weight gain comprises fat, with deposition preferentially in the femoral and abdominal regions (25). In large, population-based, cross-sectional studies, multiparity has been consistently positively correlated with abdominal girth in women for whom childbearing ended many years earlier, and with larger waist-to-hip ratios in both pre- and postmenopausal women (11,26). Prospective studies have found that childbearing is associated with greater cumulative increases in waist-to-hip ratio (20) and waist girth during 5–10 years (9). Our previous CARDIA analyses showed that childbearing was associated with greater cumulative increases (2–3 cm per birth) in waist girth compared with nongravid women during a 10-year interval among both black and white women (9). Findings in this smaller sample of CARDIA women are similar, showing a borderline significantly greater increase in waist girth of 2.3 cm among women with one interim birth.
Fat distribution changes have been examined longitudinally during pregnancy or during 6 weeks to 6 months postpartum via skinfold thicknesses, a less accurate measure of body fat distribution compared to newer imaging techniques to quantify fat mass. In 47 pregnant women, central obesity had increased by 6 months postpartum, particularly if they were obese (27). In 557 healthy women, subcutaneous body fat was measured via skinfold thicknesses from before, during, and 6 weeks after pregnancy. Pregnant women gained high amounts of central body fat in the subscapular area, and after delivery this subscapular fat was mobilized (or reduced) lesser than the fat in the triceps and thigh regions within the first 6 weeks postpartum (28).
Few prospective data are available on direct imaging measures of body composition or adipose tissue compartments around pregnancy relative to nongravid women. One study (n = 15) using magnetic resonance imaging (tomographs) to measure longitudinal changes in subcutaneous and nonsubcutaneous adipose tissue before pregnancy and at four intervals postpartum found that 68% of gestational fat gain was deposited in the trunk and excess fat gain remaining at 1 year postpartum tended to be localized centrally (13). Another study found increases in visceral fat during pregnancy using ultrasonography (14). Studies have rarely examined preconception to postpartum changes in total body fat and regional fat distribution using imaging techniques, albeit for a small number of pregnant women.
In a previous study, we found significantly greater decrements in plasma high-density lipoprotein cholesterol of 3–4 mg/dl in women after their first birth compared with nongravid women that was independent of gains in body weight and waist girth measures (29). An important question for future research is to ascertain whether pregnancy-related changes in specific adipose tissue compartments (i.e., visceral fat) are associated with future chronic disease risks and specifically whether they explain the lower high-density lipoprotein cholesterol levels after a first birth.
Limitations of our study include its small sample size of women with one birth during the interval resulting in low power to detect main effects of interim birth group, and to assess the impact of a greater number of potential confounders and effect modification by race or initial parity. With this sample size, statistical tests are not robust to departures from model assumptions and influential data points, and therefore limited our ability to assess such departures via standard diagnostic tools. Also, there is variability in time intervals before and after delivery, that is proximity to delivery may result in greater retention of gestational weight gain. Strengths of this study are the availability of preconception measurements of metabolic risk factor levels, its prospective nature with adipose tissue and anthropometric measures before conception and after delivery, and control for behavioral attributes. Our nongravid reference group included only premenopausal women with fairly similar reproductive histories from within the same population. The analytic sample consists of CARDIA women who agreed to participate in the ancillary studies of body composition at examinations in years 10 and 15. Women were not queried about whether they planned future pregnancies. Hence, selection bias related to fecundity is unlikely and the sample reflects the natural history of childbearing within the larger population-based cohort.
A centralized distribution of adiposity is a risk factor for chronic disease even for those with a healthy weight (i.e., normal BMI). Indeed reports have shown that among those not overweight, a larger waist circumference or sagittal abdominal diameter (anthropometric estimates of visceral adiposity) is associated with a large increase in risk of cardiovascular disease and diabetes (4,30). Pregnancy may have an adverse effect on women’s future risk for cardiovascular and metabolic diseases through increases in visceral fat depots. Research is warranted to identify modifiable behaviors during the postpartum period, such as lactation, and their impact on reduction in abdominal and visceral fat accumulation.
Further investigation is needed to confirm these findings given our small sample, and to determine whether childbearing is associated with greater increases visceral fat deposition with each additional birth, and whether the adverse effects of pregnancy on metabolic risk profiles in women (i.e., lower high-density lipoprotein cholesterol levels) are related to increases in visceral adipose tissue levels.
ACKNOWLEDGMENTS
This work was supported by contracts # N01-HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050, and N01-HC-95095, from the National Heart, Lung, and Blood Institute, and by Career Development Award, 2 K01 DK059944 from the National Institute of Diabetes, Digestive and Kidney Diseases.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Albu JB, Murphy L, Frager DH, Johnson JA, Pi-Sunyer FX. Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes. 1997;46:456–462. doi: 10.2337/diab.46.3.456. [DOI] [PubMed] [Google Scholar]
- 2.Pascot A, Lemieux S, Lemieux I, et al. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care. 1999;22:1471–1478. doi: 10.2337/diacare.22.9.1471. [DOI] [PubMed] [Google Scholar]
- 3.Onat A, Avci GS, Barlan MM, et al. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int J Obes Relat Metab Disord. 2004;28:1018–1025. doi: 10.1038/sj.ijo.0802695. [DOI] [PubMed] [Google Scholar]
- 4.Lemieux S, Prud’homme D, Nadeau A, et al. Seven-year changes in body fat and visceral adipose tissue in women. Association with indexes of plasma glucose-insulin homeostasis. Diabetes Care. 1996;19:983–991. doi: 10.2337/diacare.19.9.983. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic disease. Obes Res. 1995;3:73–95. doi: 10.1002/j.1550-8528.1995.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 6.Altomonte J, Harbaran S, Richter A, Dong H. Fat depot-specific expression of adiponectin is impaired in Zucker fatty rats. Metabolism. 2003;52:958–963. doi: 10.1016/s0026-0495(03)00092-1. [DOI] [PubMed] [Google Scholar]
- 7.Pallardo LF, Herranz L, Martin-Vaquero P, et al. Impaired fasting glucose and impaired glucose tolerance in women with prior gestational diabetes are associated with a different cardiovascular profile. Diabetes Care. 2003;26:2318–2322. doi: 10.2337/diacare.26.8.2318. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan TA, Xiang A, Kjos SL, et al. Gestational diabetes: antepartum characteristics that predict postpartum glucose intolerance and type 2 diabetes in Latino women. Diabetes. 1998;47:1302–1310. doi: 10.2337/diab.47.8.1302. [DOI] [PubMed] [Google Scholar]
- 9.Gunderson EP, Murtaugh MA, Lewis CE, et al. Excess gains in weight and waist circumference associated with childbearing: The Coronary Artery Risk Development in Young Adults Study (CARDIA) Int J Obes Relat Metab Disord. 2004;28:525–535. doi: 10.1038/sj.ijo.0802551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Björkelund C, Lissner L, Andersson S, Lapidus L, Bengtsson C. Reproductive history in relation to relative weight and fat distribution. Int J Obes Relat Metab Disord. 1996;20:213–219. [PubMed] [Google Scholar]
- 11.Troisi RJ, Wolf AM, Mason JE, Klingler KM, Colditz GA. Relation of body fat distribution to reproductive factors in pre- and postmenopausal women. Obes Res. 1995;3:143–151. [PubMed] [Google Scholar]
- 12.Blaudeau TE, Hunter GR, Sirikul B. Intra-abdominal adipose tissue deposition and parity. Int J Obes (Lond) 2006;30:1119–1124. doi: 10.1038/sj.ijo.0803252. [DOI] [PubMed] [Google Scholar]
- 13.Sohlstrom A, Forsum E. Changes in adipose tissue volume and distribution during reproduction in Swedish women as assessed by magnetic resonance imaging. Am J Clin Nutr. 1995;61:287–295. doi: 10.1093/ajcn/61.2.287. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita T, Itoh M. Longitudinal variance of fat mass deposition during pregnancy evaluated by ultrasonography: the ratio of visceral fat to subcutaneous fat in the abdomen. Gynecol Obstet Invest. 2006;61:115–118. doi: 10.1159/000089456. [DOI] [PubMed] [Google Scholar]
- 15.Cutter GR, Burke GL, Dyer AR, et al. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials. 1991;12 Suppl 1:S1–S77. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 16.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 17.Lewis CE, Jacobs DR, Jr, McCreath H, et al. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 2000;151:1172–1181. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 18.Sidney S, Lewis CE, Hill JO, et al. Association of total and central adiposity measures with fasting insulin in a biracial population of young adults with normal glucose tolerance: the CARDIA study. Obes Res. 1999;7:265–272. doi: 10.1002/j.1550-8528.1999.tb00405.x. [DOI] [PubMed] [Google Scholar]
- 19.Lewis CE, Smith DE, Wallace DD, et al. Seven-year trends in body weight and associations with lifestyle and behavioral characteristics in black and white young adults: the CARDIA study. Am J Public Health. 1997;87:635–642. doi: 10.2105/ajph.87.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith DE, Lewis CE, Caveny JL, et al. Longitudinal changes in adiposity associated with pregnancy. The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. JAMA. 1994;271:1747–1751. [PubMed] [Google Scholar]
- 21.Kvist H, Chowdhury B, Grangard U, Tylen U, Sjostrom L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351–1361. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- 22.van der KK, Leenen R, Seidell JC, et al. Waist-hip ratio is a poor predictor of changes in visceral fat. Am J Clin Nutr. 1993;57:327–333. doi: 10.1093/ajcn/57.3.327. [DOI] [PubMed] [Google Scholar]
- 23.Anderssen N, Jacobs DR, Jr, Sidney S, et al. Change and secular trends in physical activity patterns in young adults: a seven-year longitudinal follow-up in the Coronary Artery Risk Development in Young Adults Study (CARDIA) Am J Epidemiol. 1996;143:351–362. doi: 10.1093/oxfordjournals.aje.a008749. [DOI] [PubMed] [Google Scholar]
- 24.Rondinone CM. Adipocyte-derived hormones, cytokines, and mediators. Endocrine. 2006;29:81–90. doi: 10.1385/endo:29:1:81. [DOI] [PubMed] [Google Scholar]
- 25.Hytten FE, Leitch I. The Physiology of Pregnancy. 2nd edn. Oxford, London, and Edinburgh, UK: Blackwell Scientific Publications; 1971. [Google Scholar]
- 26.Kaye SA, Folsom AR, Prineas RJ, Potter JD, Gapstur SM. The association of body fat distribution with lifestyle and reproductive factors in a population study of postmenopausal women. Int J Obes. 1990;14:583–591. [PubMed] [Google Scholar]
- 27.Soltani H, Fraser RB. A longitudinal study of maternal anthropometric changes in normal weight, overweight and obese women during pregnancy and postpartum. Br J Nutr. 2000;84:95–101. doi: 10.1017/s0007114500001276. [DOI] [PubMed] [Google Scholar]
- 28.Sidebottom AC, Brown JE, Jacobs DR., Jr Pregnancy-related changes in body fat. Eur J Obstet Gynecol Reprod Biol. 2001;94:216–223. doi: 10.1016/s0301-2115(00)00329-8. [DOI] [PubMed] [Google Scholar]
- 29.Gunderson EP, Lewis CE, Murtaugh MA, et al. Long-term plasma lipid changes associated with a first birth: the Coronary Artery Risk Development in Young Adults study. Am J Epidemiol. 2004;159:1028–1039. doi: 10.1093/aje/kwh146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pouliot MC, Despres JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]