Abstract
Morphine and methadone are both high affinity, potent mu opioid peptide (MOP) receptor analgesics. Here we compared the antinociceptive potencies of these two drugs when administered subcutaneously (s.c.), intrathecally (i.t.) or intracerebroventricularly (i.c.v.) in both rat and mouse using the tail-flick assay. We found that both morphine and methadone were potently antinociceptive when the drugs were administered s.c., showing comparable AD50 values in both species. However, the antinociception produced by methadone, when it was administered centrally, was much weaker than that produced by centrally-administered morphine. Specifically, the AD50 value for methadone antinociception was greater than 30-fold higher at both the i.t. and i.c.v. site in mouse, and not measurable in rat. Naloxone methiodide (NLX-M), a peripherally-restricted antagonist, was used to further examine the relative contribution of central versus peripheral sites to morphine and methadone antinociception. NLX-M, when administered s.c., blocked the antinociceptive effect of either systemically- or centrally-administered methadone, but had little effect on the antinociception produced by centrally-administered morphine. Furthermore, centrally-administered NLX-M significantly blocked antinociception produced by centrally-administered morphine, but not that produced by centrally-administered methadone. Together, these results suggest that methadone antinociception is significantly dependent on an action of the drug at peripheral sites, and could provide novel insight into the neural mechanisms that distinguish morphine versus methadone antinociception.
Keywords: antinociception, methadone, morphine, periphery, opioid receptor
Introduction
Although significant progress has been made in understanding the molecular, cellular and neuronal mechanisms underlying the perception and control of pain, chronic pain continues to produce severe distress and disrupt the quality of life for many people. The vast majority of non-malignant chronic pain patients report that their pain is not effectively treated [7,33]. Indeed, the options available for the treatment of chronic pain have diminished, rather than expanded, in recent years following the removal of several non-steroidal anti-inflammatory drugs from the market due to adverse side-effects. Among current clinical treatments, morphine and its derivatives are widely used analgesics. However, long-term use of morphine is often accompanied by analgesic tolerance, life-threatening side-effects, and has well-documented abuse potential. Therefore, a search for alternative or different analgesics, especially for the treatment of chronic pain, is the subject of intense investigation.
Methadone is a synthetic opioid best-known for its utility as a treatment for opioid addiction. In addition, methadone is a potent analgesic and is often used for the treatment of chronic malignant pain [4,13,24], most often in the context of opioid rotation [2]. Methadone differs from morphine in many aspects, most relevantly, for humans, in the different pharmacokinetics of drug metabolism. Intriguingly, although morphine and methadone have similar analgesic potency when administered systemically in animal models, methadone is a poor antinociceptive agent compared to morphine when the drug is administered i.c.v. [27,28,32]. These data suggest that the mechanism, or molecular target, for methadone-mediated analgesia could be different from that of morphine. The present study was undertaken to compare the analgesic properties of methadone and morphine in rats and mice to better elucidate the mechanisms by which these drugs alleviate pain.
Methods and Materials
Animals
Male CD-1 mice weighing 20-30 grams and Sprague–Dawley rats weighing 250-380 grams (Charles River) were used. Animals were maintained under a standard 12/12-h light/dark cycle (lights on at 07:00 h), with ambient temperature set at 20–22°C. Mice were housed in groups of 5 and rats were housed singly. All protocols were approved by the Institutional Animal Care and Use Committee of Gallo clinic and Research Center at University of California San Francisco and are in accordance with the National Institutes of Health guidelines for care and use of laboratory animals.
Stereotaxic surgeries
Rat i.c.v. catheter implantation
Animals were anesthetized with 2-3% isoflurane in oxygen via a gas mask. A stainless steel guide cannula (26-gauge; Plastics One, Roanoke, VA) was implanted stereotaxically (Model 902, Kopf Instruments, Tujunga, CA) into the left lateral ventricle under isoflurane anesthesia using the following coordinates: 1 mm anterior to the bregma suture, 1.3 mm lateral to the sagittal suture, and 3.6 mm from the top of the skull, based on the atlas of Paxinos and Watson [26]. The cannula was secured to the skull by three anchor screws with dental cement. The wounds were left unclosed and lidocaine ointment was applied to wound. Tylenol (80 mg/200 ml H2O) was present in drinking water for the first 2 days and animals were allowed at least one week to recover from surgery prior to any experimental procedure.
Rat periaqueductal grey (PAG) catheter implantation
Animals were anesthetized with 2-3% isoflurane in oxygen via a gas mask. A stainless steel guide cannula (26-gauge; Plastics One, Roanoke, VA) was implanted stereotaxically (Model 902, Kopf Instruments, Tujunga, CA) under isoflurane anesthesia into ventrolateral PAG using the following coordinates: 1.4 mm rostral to the lambda suture, 0.75 mm lateral to the sagittal suture, 5 mm from the top of the skull, based on the atlas of Paxinos and Watson [26]. The cannula was secured to the skull by two anchor screws with dental cement.
The wounds were left unclosed and lidocaine ointment was applied to wound. Tylenol (80 mg/200 ml H2O) was present in drinking water for the first 2 days and animals were allowed at least one week to recover from surgery prior to any experimental procedure.
Drug injections
For all the injections, animals were under light isoflurane anesthesia. Animals recovered from anesthesia within 2 minutes and were tested at 15 and 30 minutes.
I.c.v. injection in rat
Drugs were administered through a 32-gauge injection cannula inserted into and extending 0.2 mm beyond the tip of the guide cannula in a volume of 5 μl. Injections were performed over one minute and the cannula remained in place an additional 20 seconds to minimize backflow of the drug up the cannula track.
PAG injection in rat
Drugs were administered through a 32-gauge injection cannula inserted into and extending 2 mm beyond the tip of the guide cannula in a volume of 0.5 μl. Injections were performed over one minute and the cannula remained in place an additional 20 seconds to minimize backflow of the drug up the cannula track.
I.t. injection in rat
A 25 gauge needle was connected to a 25-μl Hamilton gastight syringe and inserted into the tissues of the dorsal aspects at the lumbar level, perpendicular to the vertebral column [25]. A volume of 5 μl/animal was used.
I.c.v. injection in mouse
The injection was performed as previously described [9]. Drugs were administered through a 27 gauge BD*Yale* reusable hypodermic needle, connected to a 50 μl Hamilton gastight syringe with a repeating dispenser. The needle was inserted into the lateral ventricle of mouse brain through the skull. A volume of 5 μl/animal was used.
I.t. injection in mouse
A 30 gauge needle was used and the insertions were made at the lumbar level with an angle of approximate 20° to the vertebral column[12]. A volume of 5 μl/animal was used.
S.c. injection
Drugs were administered in a volume of 10 ml/kg for mouse and 1.0 ml/kg for rat.
Drugs
Methadone hydrochloride, morphine sulfate, naloxone hydrochloride (NLX) and naloxone methiodide (NLX-M) were purchased from Sigma (St. Louis, MO) and dissolved in physiological saline.
Antinociception assay
Antinociception was determined using the radiant-heat tail-flick assay. The intensity of the light was adjusted so that baseline tail-flick latencies ranged 1.8 to 3.0 seconds for mice and 2.0 to 3.5 seconds for rats. A cutoff time of 6 seconds (mouse) or 7 seconds (rat) was set, to minimize damage to the tail. Animals were tested for antinociception 15 or 30 min following drug administration. Data are displayed as the “maximum possible effect” (%MPE): 100% x [(drug response time - basal response time)/(cut-off time - basal response time)]. AD50 values (doses producing an approximate 50% antinociceptive response) were calculated from a dose-response curve. The dose-response curves were generated using separate groups of animals for each of three drug doses for each route. Each group consisted of at least 10 animals for mice and at least 5 animals for rats. Differences in antinociception were determined using a Student’s t test.
Radioligand binding
Mice were killed by cervical dislocation and rats were decapitated with a guillotine. Whole brains were quickly removed, weighed, and homogenized in ice-cold 50 mM Tris buffer (pH 7.4). Homogenates were centrifuged at 15,000xg for 15 min, the supernatant discarded, and the pellet resuspended in the same buffer and centrifuged again at 15,000xg. The pellet was resuspended and incubated in the same buffer for 30 min at 25°C, centrifuged a third time at 15,000xg, and finally resuspended in phosphate buffer (50 mM, pH 7.2). 50 μg of the membrane protein prepared as above, 5 nM [3H]-DAMGO, and 0.1- 10,000 nM methadone or morphine in a final volume of 200 μl was incubated in a 96-well cell culture at room temperature for 1.5 h to generate competition binding curves. Reactions were terminated by transferring the preparation to 96-well Unifil plate GF/B glass fiber filters with rapid filtration under vacuum, followed by three washes with 200 μl cold 50 mM Tris-HCl buffer pH 7.4. Bound radioactivity was determined by liquid scintillation spectrophotometry after overnight extraction of the filters in Microscint scintillation fluid. Protein was determined using the Bradford method with reagents purchased from Bio-Rad (Richmond, CA). Receptor binding competition curves were generated using nonlinear regression and analyzed using Prism (version 4.03, GraphPad software, San Diego, CA), which calculated IC50 values and standard errors.
Rat brain [35S]GTPγS binding
Autoradiographic determination of drug-stimulated [35S]GTPγS binding was performed using methods previously described [11,35]. Rats were decapitated with a guillotine, brains were quickly removed and frozen in isopentane on dry ice and stored at −80°C. Coronal sections (16 μm) were cut on a cryostat at −20°C, thaw-mounted onto gelatin-subbed slides, dried, desiccated at −80°C and stored until use. Sections were preincubated in assay buffer (50 mmol/l Tris-HCl, 3 mmol/l MgCl, 0.2 mmol/l EGTA, 100 mmol/l NaCl, pH 7.4) at room temperature for 10 min and then incubated with 2 mmol/l GDP in assay buffer for 10 min at room temperature. Sections were then incubated for 90 min at room temperature in assay buffer containing [35S]GTPγS (0.05 nmol/l) and 2 mmol/l GDP, with and without the agonist morphine or methadone (5 μmol/l). After incubation, slides were rinsed twice in cold 50 mmol/l Tris-HCl buffer, pH 7.4, and once in deionized water, dried and exposed to Kodak BioMax MS films for 3 days. Relative binding intensities were quantified using Scion Image software comparing unstimulated and stimulated sections. The atlas of Paxinos and Watson [26] was used to determine the regional neuroanatomy of the rat brain for these sections. Data are expressed as percent stimulation, where percent stimulation = [(stimulated– basal)/basal] X 100 and means ± SEM.
Results
The antinociceptive effects of methadone versus morphine in mouse and rat
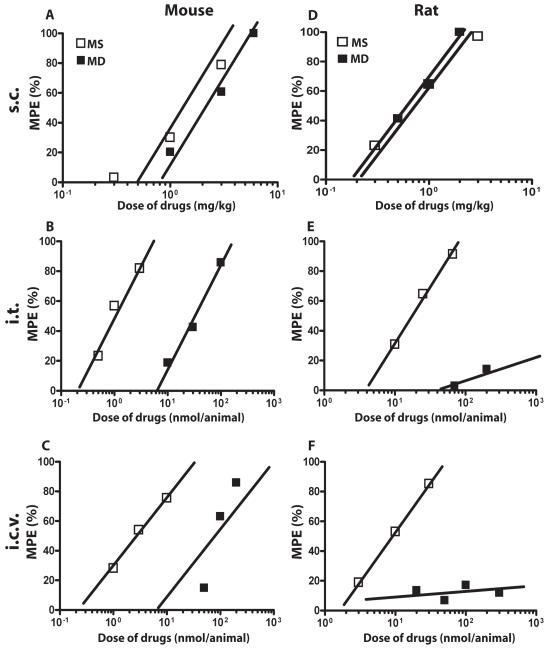
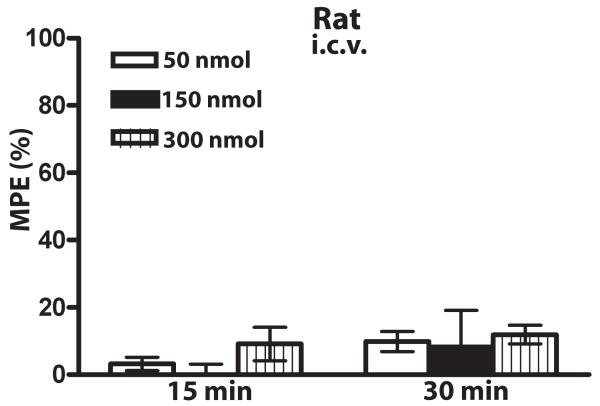
Methadone produced antinociception when given s.c. to mice with a potency similar to that of morphine (Fig. 1A). However, the dose-response to methadone compared to morphine was shifted significantly rightward when drug was administered centrally, either i.t. (Fig. 1B) or i.c.v. (Fig. 1C) (n=10-12 mice per group per dose). To examine whether the lack of potent antinociception by centrally-administered methadone in mice was species-specific, antinociceptive dose-responses to morphine and methadone were also examined in rat. As for mice, in rat, morphine and methadone showed similar potencies when drug was administered s.c. (Fig. 1D), while methadone produced little to no antinociception when it was administered either i.t. (Fig. 1E) or i.c.v. (Fig. 1F) (n=5-6 rats per group per dose). Thus, the potency of methadone, but not morphine, was significantly different depending on the route of drug administration (Table 1; ***p<0.001 i.c.v. methadone versus i.c.v. morphine in mouse, student’s t-test; ***p<0.001 i.t. methadone versus i.t. morphine in mouse, student’s t-test). This effect was not due to a faster onset and decline of methadone antinociception, as methadone also did not produce measurable antinociception in rat 15 minutes following i.c.v. administration (Fig. 2) (n=5-6 per group per dose).
Figure 1. Dose-response comparison of the antinociceptive effects of methadone and morphine administered s.c., i.t and i.c.v. in mouse and rat.
(A-C) Mouse antinociception. Morphine (open squares) induces potent antinociception in a dose-dependent manner when drug was administered s.c. (A), i.t. (B), or i.c.v. (C). Methadone (closed squares) induced potent antinociception when administered s.c. (A) but the dose response was dramatically right shifted when methadone was administered either i.t. (B) or i.c.v. (C). (D-F) Rat antinociception. Morphine (open squares) induces potent antinociception in a dose-dependent manner when drug was administered s.c. (D), i.t. (E), or i.c.v. (F). Methadone (closed squares) induced potent antinociception when administered s.c. (D), but no detectable antinociception when it was administered either i.t. (E) or i.c.v. (F). Ten to 12 mice were used for each dose for every route of administration and 5 to 6 rats were used for each dose for every route of administration. Antinociception was measured 30 min following drug administration using the tail-flick assay. MD, methadone; MS: morphine sulfate.
Table 1.
Antinociceptive effects of methadone and morphine with different routes of administration in rat and mouse
| Routes of administration |
Animals | AD50 ± S.E.M. | Ratio (methadone/morphine) |
|
|---|---|---|---|---|
| Methadone | Morphine | |||
| s.c. (mg/kg) | Rat | 0.6 ± 0.15 | 0.7 ± 0.17 | 0.86 |
| Mouse | 1.8 ± 0.3 | 1.0 ± 0.4 | 1.8 | |
| i.c.v. (nmol/animal) | Rat | ND# | 8.9 ± 2.1 | ND# |
| Mouse | 91.5 ± 6.7*** | 2.7 ± 1.2 | 33.9 | |
| i.t. (nmol/animal) | Rat | ND# | 17.3 ± 1.2 | ND# |
| Mouse | 32.1 ± 3.1*** | 1.0 ± 0.2 | 32.1 | |
p<0.001 compared to morphine group
ND: not able to determine
Figure 2. Lack of i.c.v. methadone antinociception in rats is not due to timing.
No significant methadone antinociception was observed when drug was given i.c.v. and antinociception was measured 15 or 30 min following drug administration using the tail flick assay. Data are expressed as the mean ± SEM. 5-6 rats were used at each dose. MD, methadone.
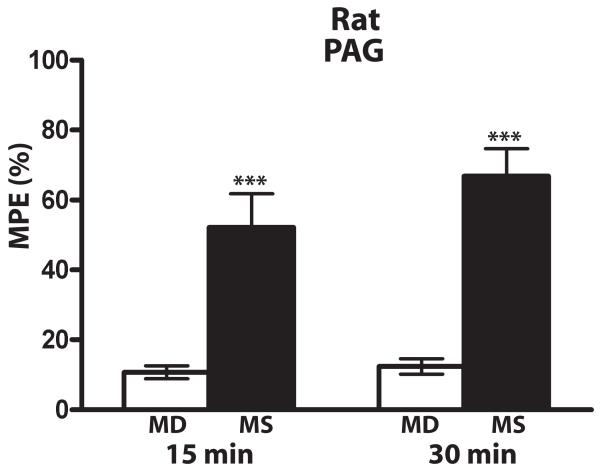
To examine whether the low antinociceptive potency of centrally-administered methadone was due to poor drug diffusion to antinociceptive regions, methadone was locally injected into the region of the PAG in rat. No significant antinociception was produced by methadone (20 nmol/rat) directed to the PAG (Fig. 3, white bars), while morphine did induce potent antinociception when given to the same rats through the same cannulae, even when a dose lower than methadone was administered (6 nmol/rat) (Fig. 3, black bars, n=15 rats per group). Postmortem histology was conducted on a subset of these animals which confirmed correct cannuala placement (data not shown), consistent with the robust morphine antinociception produced through these cannulae.
Figure 3. Comparison of morphine and methadone antinociception in rat PAG.
Morphine (6 nmol, black bars), but not methadone (20 nmol, white bars), produced significant antinociception when drug was administered directly to PAG in rat. *** p< 0.001, compared to methadone. Antinociception was measured 15 and 30 min following drug administration using the tail flick assay. The rats (n=15) received methadone then morphine through the same catheter one week later. Data are expressed as the mean ± SEM. MD, methadone; MS morphine sulfate.
The binding affinity and activation of G-protein by morphine and methadone in rat and mouse brain
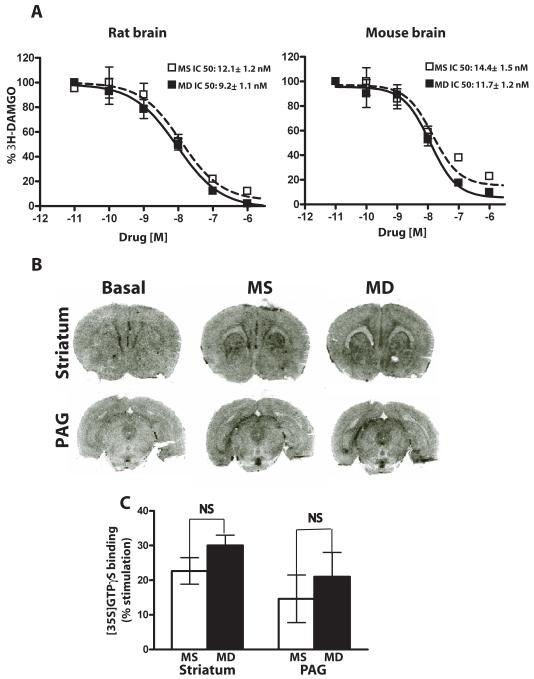
We next examined the possibility that the poor antinociceptive potency of centrally-administered methadone was due to a lower affinity or efficacy of methadone versus morphine to mu opioid peptide (MOP) receptors in the brain. Importantly, the affinity of methadone for the MOP receptor was not significantly different than that of morphine in both rat and mouse brain (Fig. 4A), as assessed by the ability of methadone and morphine to displace the MOP-R-selective agonist [D-Ala2, N-MePhe4, Gly5-ol]-enkephalin (DAMGO) (morphine IC50 12.1 ± 1.2 nM, methadone IC50 9.2 ± 1.1 nM in rat; morphine IC50 14.4 ± 1.5 nM, methadone IC50 11.7 ± 1.2 nM in mouse) (p>0.05 by student’s t-test; n=6 for both rat and mouse). Moreover, methadone was at least as effective as morphine at activating MOP receptors in several rat brain regions including striatum and PAG (Fig. 4B, C), as assessed by GTPγS binding on brain slices. No significant difference in binding was detected for morphine versus methadone (Fig. 4C; p>0.05, student’s t-test). However, there was a trend towards methadone being more active despite the lack of antinociception with methadone.
Figure 4. Biochemical assessment of methadone and morphine activity in rodent brain.
(A) Morphine (open squares) and methadone (closed squares) displacement of 3H-DAMGO from brain membranes prepared from rat (left panel) or mouse (right panel). The IC50values of morphine and methadone did not differ significantly in either rat or mouse. Data are the mean ± SEM values from two independent experiments with each performed in triplicate. (B) MOP receptor-G protein coupling was assessed by autoradiographic [35S]GTPγS binding in the absence (left sections) and presence of 5 μM of morphine (middle sections) or methadone (right sections) in both striatum and PAG. (C) Relative binding intensities in (B) were quantified using Scion Image software comparing unstimulated and stimulated sections. The atlas of Paxinos and Watson [26] was used to determine the regional neuroanatomy of the rat brain for these sections. Data are expressed as the mean % stimulation ± SEM, where % stimulation = [(stimulated–basal)/basal] X 100. Morphine and methadone showed similar efficacy in both striatum and PAG. N= 4 for PAG and 8 for striatum. N.S., not significant. MD, methadone; MS morphine sulfate.
Effects of a peripheral opioid receptor antagonist on methadone- and morphine-induced antinociception
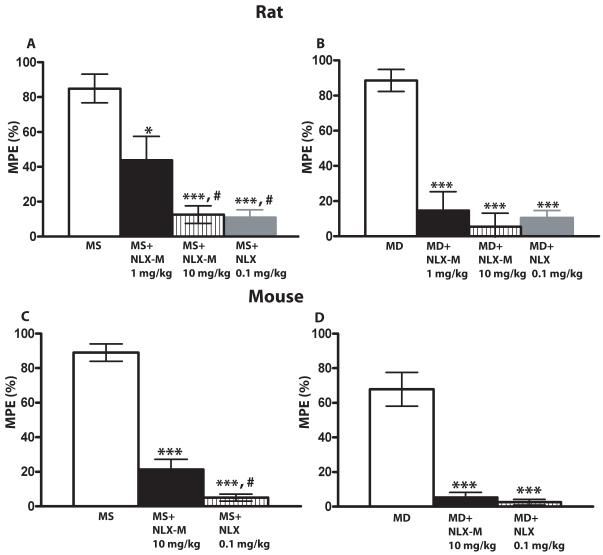
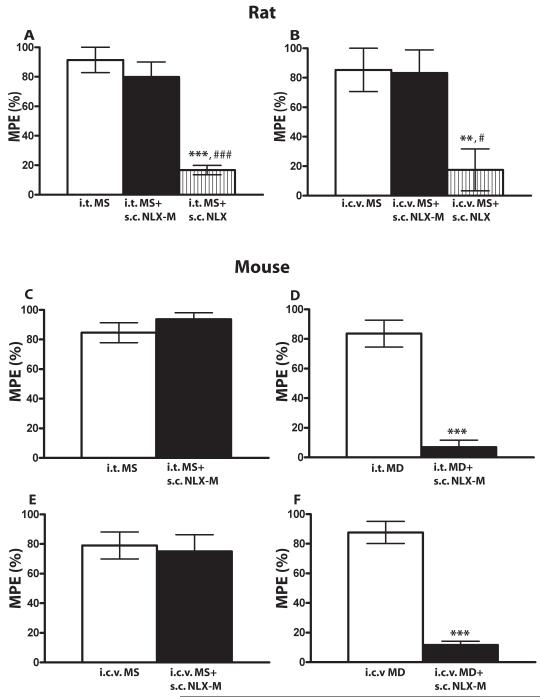
The discrepancy between the antinociceptive potency of methadone delivered systemically versus centrally could indicate that a significant component of methadone antinociception occurs at a peripheral site. To examine this possibility, we compared the effects of the blood-brain-barrier-permeant antagonist naloxone (NLX) and the effects of a peripherally-restricted opioid receptor antagonist, naloxone methiodide (NLX-M) [31] on morphine- and methadone-mediated antinociception. As expected, systemically administered NLX (0.1 mg/kg) efficiently blocked the antinociception produced by systemically-administered morphine and methadone in both rats (Fig. 5A, 5B, gray bars, n=6-10 per group) and mice (Fig. 5C, 5D, striped bars, n=8-13 per group) (*** p< 0.001, compared to morphine alone group, student’s t-test). However, systemically-administered NLX-M (1 mg/kg; 10 mg/kg) also significantly blocked the antinociceptive effect of both systemic morphine and systemic methadone in rat (Fig. 5A, 5B, black and striped bars, n=6-10 per group; *** p< 0.001, * p< 0.05 compared to morphine alone group, student’s t-test) and mouse (Fig. 5C, 5D, black bars, n=8-13 per group; *** p< 0.001 compared to morphine alone group, student’s t-test). NLX-M was less potent than NLX (# p< 0.05, compared to morphine plus NLX-M 1 mg/kg group in rat and morphine plus NLX-M 10 mg/kg group in mouse, student’s t-test), which may be due, at least in part to its lower affinity at the MOR [21]. These data suggest that either NLX-M was not peripherally restricted (and thus inhibited central MOP-Rs) or peripheral MOP-Rs contribute to both morphine and methadone-mediated antinociception. We thought it was unlikely that NLX-M was not peripherally restricted since the doses we utilized were the same or lower than those used in previous studies [20,22]. Nevertheless, to differentiate between these possibilities, we administered morphine or methadone centrally and NLX-M systemically. In rat (n=5-6 per group), systemic NLX-M (10 mg/kg) did not inhibit antinociception produced by either i.t.- or i.c.v-administered morphine (Fig. 6A, 6B), while NLX (1 mg/kg) did. In mice (n=7-12 per group), systemic NLX-M also had no effect on antinociception produced by i.t. (Fig. 6C) or i.c.v. (Fig. 6E) morphine. These data strongly suggest that, even at this high dose, NLX-M does not cross the blood brain barrier to inhibit MOP-Rs. In contrast, systemically administered NLX-M (10 mg/kg) did inhibit both i.t.- and i.c.v.-mediated methadone antinociception (Fig 6D, 6F, ** p< 0.01 and *** p< 0.001 compared to morphine or methadone alone group; ### p<0.001 compared to morphine plus NLX-M group, student’s t-test).
Figure 5. The antagonist effects of systemic NLX-M and NLX on systemic morphine and methadone antinociception in rat (A, B) and mouse (C, D).
(A) Rats received 2 mg/kg of s.c. morphine alone (white bar), or morphine plus s.c. NLX-M (1 mg/kg, black bar), morphine plus s.c. NLX-M (10 mg/kg, striped bar) or s.c. NLX (0.1 mg/kg, gray bar). (B) Rats received 1 mg/kg s.c. methadone alone (white bar), or methadone plus s.c. NLX-M (1 mg/kg, black bar), methadone plus s.c. NLX-M (10 mg/kg, striped bar) or s.c. NLX (0.1 mg/kg, gray bar). (C) Mice received 3 mg/kg s.c. morphine alone (white bar), or morphine plus s.c. NLX-M (10 mg/kg, black bar) or plus s.c. NLX (0.1 mg/kg, striped bar). (D) Mice received 3 mg/kg s.c. methadone alone (white bar), or methadone plus s.c. NLX-M (10 mg/kg, black bar) or methadone plus s.c. NLX (0.1 mg/kg, striped bar). In all cases, antagonist was administered simultaneously with morphine or methadone and antinociception was measured 30 min later using the tail-flick assay. Data are expressed as the mean ± SEM. N= 6-10 per group for rat and 8-13 for mouse. *** p< 0.001, * p< 0.05 compared to morphine alone group; # p< 0.05, compared to morphine plus NLX-M 1 mg/kg group in rat and morphine plus NLX-M 10 mg/kg group in mouse. MD, methadone; MS morphine sulfate.
Figure 6. The antagonist effects of systemic NLX-M on centrally-administered morphine and methadone antinociception in rat (A, B) and mouse (C-F).
(A) Rats received 66 nmol of i.t. morphine alone (white bar), or morphine plus s.c. NLX-M (10 mg/kg, black bar) or s.c. NLX (10 mg/kg, striped bar). (B) Rats received 30 nmol of i.c.v. morphine alone (white bar), or morphine plus s.c. NLX-M (10 mg/kg, black bar) or s.c. NLX (10 mg/kg, striped bar). (C) Mice received 3 nmol of i.t. morphine alone (white bar), or morphine plus s.c. NLX-M (10 mg/kg, black bar). (D) Mice received 100 nmol of i.t. methadone alone (white bar), or methadone plus s.c. NLX-M (10 mg/kg, black bar). (E) Mice received 10 nmol of i.c.v. morphine alone (white bar), or morphine plus s.c. NLX-M (10 mg/kg, black bar). (F) Mice received 200 nmol of i.c.v. methadone alone (white bar), or methadone plus s.c. NLX-M (10 mg/kg, black bar). The antagonist was administered immediately following morphine or methadone treatment and antinociception was measured 30 min later using the tail-flick assay. Data are expressed as the mean ± SEM. N= 5-6 for rat and 7-12 for mouse. ** p< 0.01 and *** p< 0.001 compared to morphine or methadone alone group; ### p<0.001 compared to morphine plus NLX-M group. MD, methadone; MS morphine sulfate.
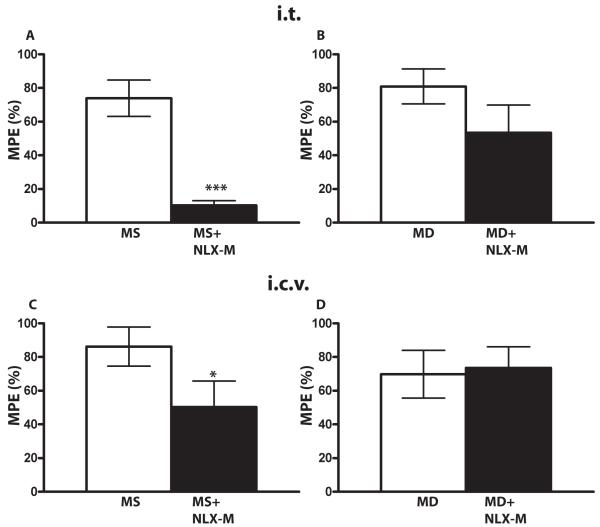
This result indicates that a peripheral component is essential for methadone-mediated antinociception even when the drug is administered to central sites. Alternatively, it was possible that NLX-M was more potent at inhibiting methadone antinociception (i.e., a small amount may be crossing the blood brain barrier and inhibiting methadone-mediated, but not morphine-mediated, antinociception). To address this question, we compared the potency of NLX-M for antagonizing methadone versus morphine antinociception in mice at central sites when the agonist and antagonist were co-administered centrally. NLX-M significantly inhibited the antinociception mediated by morphine at both i.t. (Fig. 7A) and i.c.v. (Fig. 7C) sites, while methadone-mediated antinociception was not significantly affected at either site (Fig. 7B, 7D, n=6-9 per group; * p< 0.05 and *** p< 0.001 compared to morphine alone group, student’s t-test). Hence, NLX-M is not more potent at inhibiting methadone- versus morphine-mediated antinociception. Rather, these data suggest that, even when given centrally, methadone activates peripheral receptors, inaccessible to centrally-administered NLX-M, to promote antinociception.
Figure 7. The antagonist effects of NLX-M on morphine or methadone antinociception in mouse when both were given centrally.
(A) Mice received 3 nmol of i.t. morphine alone (white bar), or morphine plus i.t. NLX-M (1 nmol, black bar). (B) Mice received 100 nmol of i.t. methadone alone (white bar), or methadone plus NLX-M (1 nmol, black bar). (C) Mice received 10 nmol of i.c.v. morphine alone (white bar), or morphine plus i.c.v. NLX-M (1 nmol, black bar). (D) Mice received 200 nmol of i.c.v. methadone alone (white bar), or methadone plus i.c.v. NLX-M (1 nmol, black bar). NLX-M was administered simultaneously with morphine or methadone at the i.t. or i.c.v. site. Antinociception was measured 30 min later using the tail-flick assay. Data are expressed as the mean ± SEM. N= 6-9 animals per group. * p< 0.05 and *** p< 0.001 compared to morphine alone group. MD, methadone; MS morphine sulfate.
Taken together, these data suggest that, while there is a peripheral component to morphine antinociception (see Fig. 5A, 5C), as has been reported previously [18], effective morphine antinociception can be achieved through activation of only central sites (see Fig. 6). Importantly, these data also suggest that methadone antinociception is strictly dependent on the peripheral component, as all antinociception was abolished by NLX-M, even when methadone was administered centrally (Fig. 6D, 6F).
Discussion
The goal of this study was to understand the mechanisms underlying methadone-mediated antinociception. Here we found that antinociception by methadone appears to require a peripheral site of action. This conclusion is supported by our findings that naloxone methiodide, a peripherally-restricted opioid antagonist, not only almost completely blocked the antinociceptive effect of systemically-administered methadone (Fig. 5B, 5D), but also significantly antagonized the effect of centrally administered methadone (Fig. 6D, 6F).
It was unexpected, and very surprising, that a peripheral site of action plays such a crucial role in methadone-mediated antinociception in both rat and mouse given that it is generally-accepted that the central nervous system is the predominant site for opioid-induced analgesic effects [8]. However, increasing evidence suggests that peripheral sites can also play a role in opioid analgesia [3,6,17-19,29,34]. For example, King et al. have shown that a significant portion of opioid drug can be transported by P-glycoprotein and redistributed to peripheral tissues following i.c.v. injection. Importantly, the antinociceptive effect of central opioids was significantly reduced if this transport system was disrupted either by antisense in rat or by gene knock-out in mouse [17]. In addition, a peripheral effect of opioids appears to play a significant analgesic role in inflammatory pain in both animal models and human clinical situations, possibly through local inflammation-induced activation of opioid production and concomitant release of endogenous opioids from immune cells [14,36]. Interestingly, another recent study has shown that a peripheral action plays a paramount role in cannabinoid-mediated antinociception, as well [1]. The mechanism underlying a peripheral contribution to antinociception remains to be elucidated. Peripheral opioid receptors could be critical mediators of pain perception due to an integration of multiple antinociceptive circuits -- a hypothesis that has been the focus of many studies [18,30,37]. Alternatively, peripheral production of active metabolites could contribute to antinociception, in particular for agonists such as morphine that have active metabolites. However, no active metabolites of methadone have been described.
The phenomenon that methadone is a weak analgesic when given centrally has been previously reported [27,32]. This effect has often been attributed to methadone’s high lipophilicity, which was hypothesized to result in selective distribution of methadone to white matter as opposed to gray matter when the drug was administered either i.t .or i.c.v. [23] or altered pharmacokinetic properties. While methadone’s lipophilicity may contribute to its lower potency when centrally administered, it does not fully explain this phenomenon. Other highly lipophilic opioids, such as sufentanil, are able to produce potent antinociception in mice when administered i.c.v. [28]. In addition, here we show that methadone is ineffective as an antinociceptive agent even when it is directly administered to the PAG, a brain region important for MOP-R effects on pain transmission (Fig. 3). The pharmacokinetic properties of methadone are indeed different from morphine. Nevertheless, the brain concentration of methadone required to produce antinociception is substantially higher than the concentration of morphine required for the same effect [15], suggesting that differences in pharmacokinetics alone can not explain the poor antinociception of central versus systemic methadone.
Although we propose here that methadone mediates a substantial portion of its antinociception via peripheral sites, this does not mean that some of the other effects of methadone, such as respiratory suppression and reward, are not mediated by central receptors. Indeed, we have shown that methadone has a similar binding affinity for and activation efficacy at the MOP receptors as morphine in rodent brain (Fig. 4). There are several possibilities that could explain why central sites could be required for methadone reward, or prevention of opiate withdrawal but not for antinociception. For example, chronic morphine or heroin use could alter the affinity of methadone at MORs. In addition, methadone could be accessing both nociceptive and antinociceptive targets centrally. Chronic morphine or heroin use could, therefore, also alter the relative proportion of nociceptive and antinociceptive methadone targets. Importantly, methadone does not appear to antagonize morphine antinociception when the drugs are coadministered centrally [10]. It will be important in future studies to determine the relative importance of peripheral and central methadone for reward and the suppression of withdrawal signs.
Despite growing evidence that opioid analgesia can be mediated, at least in part, by receptors in the periphery, whether this phenomenon is ligand-specific had not been previously explored. In the present study, we found that a peripheral effect contributes to antinociception produced by both methadone and morphine since antinociception by both drugs was significantly decreased by systemically administered NLX-M (Fig. 5). However, our observations that systematically-administered NLX-M also significantly reduced the antinociception produced by centrally-administered methadone (Fig. 6D, 6F), but not morphine (Fig. 6A-C, 6E), clearly distinguishes these two opioids. This is not the first demonstration of a difference in activity between morphine and methadone. For example, CXBK mice are insensitive to the antinociceptive effects of s.c. morphine but retain antinociceptive responsiveness to methadone [5]. The molecular mechanism mediating these agonist-selective antinociceptive effects in CXBK mice has not been fully elucidated, although alterations in the splice variants of the MOP receptor (see for example [5]) and alterations in delta opioid receptor function have been implicated [16].
In conclusion, the present study demonstrates that peripheral opioid receptors serve an essential role in mediating methadone antinociception. This observation could have significant clinical relevance, especially under conditions of inflammatory or neuropathic pain, both of which involve central and peripheral opioid receptors.
Perspective: Methadone is often used as an alternative for pain management. The present study shows that a peripheral action plays a crucial role in methadone antinociception. This finding could have significant clinical relevance for the use of methadone versus morphine for the treatment of certain types of pain.
Supplementary Material
Acknowledgements
We thank Johan Enquist for critical reading of the manuscript, and Amy Chang for her valuable suggestions. This study was supported by NIDA grants R01DA015232 and R01DA19958 (to J.L.W.) and by funds provided by the state of California for medical research on alcohol and substance abuse through the University of California, San Francisco (to J.L.W.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–9. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Benitez-Rosario MA, Feria M, Salinas-Martin A, Martinez-Castillo LP, Martin-Ortega JJ. Opioid switching from transdermal fentanyl to oral methadone in patients with cancer pain. Cancer. 2004;101:2866–73. doi: 10.1002/cncr.20712. [DOI] [PubMed] [Google Scholar]
- [3].Bileviciute-Ljungar I, Spetea M, Guo Y, Schutz J, Windisch P, Schmidhammer H. Peripherally mediated antinociception of the mu-opioid receptor agonist 2-[(4,5alpha-epoxy-3-hydroxy-14beta-methoxy-17-methylmorphinan-6beta-yl)am ino]acetic acid (HS-731) after subcutaneous and oral administration in rats with carrageenan-induced hindpaw inflammation. J Pharmacol Exp Ther. 2006;317:220–7. doi: 10.1124/jpet.105.096032. [DOI] [PubMed] [Google Scholar]
- [4].Bruera E, Sweeney C. Palliative care models: international perspective. J Palliat Med. 2002;5:319–27. doi: 10.1089/109662102753641331. [DOI] [PubMed] [Google Scholar]
- [5].Chang A, Emmel DW, Rossi GC, Pasternak GW. Methadone analgesia in morphine-insensitive CXBK mice. Eur J Pharmacol. 1998;351:189–91. doi: 10.1016/s0014-2999(98)00366-5. [DOI] [PubMed] [Google Scholar]
- [6].DeHaven-Hudkins DL. Peripherally restricted opioid drugs: advances and retreats. Curr Opin Anaesthesiol. 2003;16:541–5. doi: 10.1097/00001503-200310000-00016. [DOI] [PubMed] [Google Scholar]
- [7].Dionne RA, Witter J. NIH-FDA Analgesic Drug Development Workshop: translating scientific advances into improved pain relief. Clin J Pain. 2003;19:139–47. doi: 10.1097/00002508-200305000-00001. [DOI] [PubMed] [Google Scholar]
- [8].Fields HL. Understanding how opioids contribute to reward and analgesia. Reg Anesth Pain Med. 2007;32:242–6. doi: 10.1016/j.rapm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- [9].Haley TJ, McCormick WG. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br J Pharmacol Chemother. 1957;12:12–5. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].He L, Whistler JL. An opiate cocktail that reduces morphine tolerance and dependence. Curr Biol. 2005;15:1028–33. doi: 10.1016/j.cub.2005.04.052. [DOI] [PubMed] [Google Scholar]
- [11].He L, Whistler JL. The biochemical analysis of methadone modulation on morphine-induced tolerance and dependence in the rat brain. Pharmacology. 2007;79:193–202. doi: 10.1159/000100893. [DOI] [PubMed] [Google Scholar]
- [12].Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–6. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- [13].Inturrisi CE, Portenoy RK, Max MB, Colburn WA, Foley KM. Pharmacokinetic-pharmacodynamic relationships of methadone infusions in patients with cancer pain. Clin Pharmacol Ther. 1990;47:565–77. doi: 10.1038/clpt.1990.77. [DOI] [PubMed] [Google Scholar]
- [14].Janson W, Stein C. Peripheral opioid analgesia. Curr Pharm Biotechnol. 2003;4:270–4. doi: 10.2174/1389201033489766. [DOI] [PubMed] [Google Scholar]
- [15].Kalvass JC, Olson ER, Cassidy MP, Selley DE, Pollack GM. Pharmacokinetics and pharmacodynamics of seven opioids in P-glycoprotein-competent mice: assessment of unbound brain EC50,u and correlation of in vitro, preclinical, and clinical data. J Pharmacol Exp Ther. 2007;323:346–55. doi: 10.1124/jpet.107.119560. [DOI] [PubMed] [Google Scholar]
- [16].Kest B, Beczkowska I, Franklin SO, Lee CE, Mogil JS, Inturrisi CE. Differences in delta opioid receptor antinociception, binding, and mRNA levels between BALB/c and CXBK mice. Brain Res. 1998;805:131–7. doi: 10.1016/s0006-8993(98)00696-9. [DOI] [PubMed] [Google Scholar]
- [17].King M, Su W, Chang A, Zuckerman A, Pasternak GW. Transport of opioids from the brain to the periphery by P-glycoprotein: peripheral actions of central drugs. Nat Neurosci. 2001;4:268–74. doi: 10.1038/85115. [DOI] [PubMed] [Google Scholar]
- [18].Kolesnikov YA, Jain S, Wilson R, Pasternak GW. Peripheral morphine analgesia: synergy with central sites and a target of morphine tolerance. J Pharmacol Exp Ther. 1996;279:502–6. [PubMed] [Google Scholar]
- [19].Labuz D, Mousa SA, Schafer M, Stein C, Machelska H. Relative contribution of peripheral versus central opioid receptors to antinociception. Brain Res. 2007;1160:30–8. doi: 10.1016/j.brainres.2007.05.049. [DOI] [PubMed] [Google Scholar]
- [20].Lewanowitsch T, Irvine RJ. Naloxone methiodide reverses opioid-induced respiratory depression and analgesia without withdrawal. Eur J Pharmacol. 2002;445:61–7. doi: 10.1016/s0014-2999(02)01715-6. [DOI] [PubMed] [Google Scholar]
- [21].Lewanowitsch T, Irvine RJ. Naloxone and its quaternary derivative, naloxone methiodide, have differing affinities for mu, delta, and kappa opioid receptors in mouse brain homogenates. Brain Res. 2003;964:302–5. doi: 10.1016/s0006-8993(02)04117-3. [DOI] [PubMed] [Google Scholar]
- [22].Lewanowitsch T, Miller JH, Irvine RJ. Reversal of morphine, methadone and heroin induced effects in mice by naloxone methiodide. Life Sci. 2006;78:682–8. doi: 10.1016/j.lfs.2005.05.062. [DOI] [PubMed] [Google Scholar]
- [23].McQuay HJ, Sullivan AF, Smallman K, Dickenson AH. Intrathecal opioids, potency and lipophilicity. Pain. 1989;36:111–5. doi: 10.1016/0304-3959(89)90118-8. [DOI] [PubMed] [Google Scholar]
- [24].Mercadante S, Casuccio A, Agnello A, Barresi L. Methadone response in advanced cancer patients with pain followed at home. J Pain Symptom Manage. 1999;18:188–92. doi: 10.1016/s0885-3924(99)00048-2. [DOI] [PubMed] [Google Scholar]
- [25].Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- [26].Paxinos G, Watson C. The rat brain, in stereotaxic coordinates. 2nd edn. Academic Press; Sydney: 2005. edn. [Google Scholar]
- [27].Rady JJ, Portoghese PS, Fujimo JM. Methadone and heroin antinociception: predominant delta-opioid-receptor responses in methadone-tolerant mice. Jpn J Pharmacol. 2002;88:319–31. doi: 10.1254/jjp.88.319. [DOI] [PubMed] [Google Scholar]
- [28].Raffa RB, Martinez RP. The ‘glibenclamide-shift’ of centrally-acting antinociceptive agents in mice. Brain Res. 1995;677:277–82. doi: 10.1016/0006-8993(95)00164-l. [DOI] [PubMed] [Google Scholar]
- [29].Reichert JA, Daughters RS, Rivard R, Simone DA. Peripheral and preemptive opioid antinociception in a mouse visceral pain model. Pain. 2001;89:221–7. doi: 10.1016/s0304-3959(00)00365-1. [DOI] [PubMed] [Google Scholar]
- [30].Roerig SC, Fujimoto JM. Multiplicative interaction between intrathecally and intracerebroventricularly administered mu opioid agonists but limited interactions between delta and kappa agonists for antinociception in mice. J Pharmacol Exp Ther. 1989;249:762–8. [PubMed] [Google Scholar]
- [31].Rohde DS, McKay WR, Chang DS, Abbadie C, Basbaum AI. The contribution of supraspinal, peripheral and intrinsic spinal circuits to the pattern and magnitude of Fos-like immunoreactivity in the lumbar spinal cord of the rat withdrawing from morphine. Neuroscience. 1997;80:599–612. doi: 10.1016/s0306-4522(97)00096-1. [DOI] [PubMed] [Google Scholar]
- [32].Sanchez-Blazquez P, Gomez-Serranillos P, Garzon J. Agonists determine the pattern of G-protein activation in mu-opioid receptor-mediated supraspinal analgesia. Brain Res Bull. 2001;54:229–35. doi: 10.1016/s0361-9230(00)00448-2. [DOI] [PubMed] [Google Scholar]
- [33].Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci. 2002;5(Suppl):1062–7. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- [34].Shannon HE, Lutz EA. Comparison of the peripheral and central effects of the opioid agonists loperamide and morphine in the formalin test in rats. Neuropharmacology. 2002;42:253–61. doi: 10.1016/s0028-3908(01)00173-3. [DOI] [PubMed] [Google Scholar]
- [35].Sim LJ, Selley DE, Childers SR. In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5′-[gamma-[35S]thio]-triphosphate binding. Proc Natl Acad Sci U S A. 1995;92:7242–6. doi: 10.1073/pnas.92.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat Med. 2003;9:1003–8. doi: 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- [37].Yeung JC, Rudy TA. Multiplicative interaction between narcotic agonisms expressed at spinal and supraspinal sites of antinociceptive action as revealed by concurrent intrathecal and intracerebroventricular injections of morphine. J Pharmacol Exp Ther. 1980;215:633–42. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.