Abstract
It is well documented that neuronal activity is required for the developmental segregation of retinal ganglion cell (RGC) synaptic connectivity with ON and OFF bipolar cells in mammalian retina. Our recent study showed that light deprivation preferentially blocked the developmental RGC dendritic redistribution from the center to sublamina a of the IPL. To determine whether OFF signals in the visual stimulation are required for OFF RGC dendritic development, the light evoked responses and dendritic stratification patterns of RGCs in Spastic mutant mice, in which the OFF signal transmission in the rod pathway is largely blocked due to a reduction of glycine receptor (GlyR) expression, were quantitatively studied at different ages and rearing conditions. The dendritic distribution in the IPL of these mice was indistinguishable from wild type controls at the age of P12. However, the adult Spastic mutants had altered RGC light evoked synaptic inputs from ON and OFF pathways, which could not be mimicked by pharmacologically blocking of glycinergic synaptic transmission on age-matched wild type animals. Spastic mutation also blocked the developmental redistribution of RGC dendrites from the center to the sublamina a of the IPL, which mimicked the effects induced by light deprivation on wild type animals. Moreover, light deprivation of the Spastic mutants had no additional impacts on the RGC dendritic distribution and light response patterns. We interpret these results as that visual stimulation regulates the maturation of RGC synaptic activity and connectivity primarily through GlyR-mediated synaptic transmission.
Keywords: Ganglion cell dendrites, synaptic plasticity, glycine receptor, synaptic activity, visual experience
Introduction
Retinal ganglion cells (RGCs) carry different aspects of visual signals from retina to visual cortex through separated parallel synaptic pathways. In the retina, the separation of these synaptic pathways relays on the precise synaptic connections between RGCs and bipolar cells (BCs) at distinct stratum of the inner plexiform layer (IPL) of the retina. One of the most extensively studied examples of these parallel synaptic pathways is the separation of the increment and decrement of luminance signals into ON and OFF pathways (Hartline, 1938). The separation of ON and OFF pathways starts at the synapses between photoreceptors and BCs. In mammalian retina, light stimulation hyperpolarizes the membrane potentials of photoreceptors and decreases the synaptic release of glutamate from these cells. Glutamate released from photoreceptors activates ionotropic glutamate receptors on cone OFF BCs and depolarizes their membrane potentials. Glutamate activates metabotropic glutamate receptors on cone ON BCs and rod BCs and thereby hyperpolarizes the membrane potentials of these cells. This sign reversing and conversing action of glutamate on the ON and OFF BCs, respectively, separates the increment and decrement luminance signals into ON and OFF pathways. At the level of synaptic inputs to RGCs the separation of ON and OFF pathways is preserved by the specific synaptic connections between BCs and RGCs in distinct layers of the IPL. All ON RGCs only synapse with ON BCs in the sublamina b of the IPL and all OFF RGC only synapse with OFF BCs in the sublamina a of the IPL (Famiglietti and Kolb, 1976; Nelson et al., 1978). When RGCs ramify their dendrites in both sublaminae, they synapse with both ON and OFF BCs and signal both the onset and termination of light (Amthor et al., 1984). The rod BCs do not directly synapse with RGCs. Instead, they synapse with a group of interneurons, AII amacrine cells (ACs), and depolarize these cells when the light is on. The latter then depolarizes cone ON BCs through gap junction connections and hyperpolarizes cone OFF BCs and OFF RGCs by releasing an inhibitory neurotransmitter, glycine, on these cells (Strettoi et al., 1992, 1994; Bloomfield and Dacheux, 2001; Tsukamoto, 2001). Therefore, the separation of rod-driven ON and OFF signals starts at the connections between AII ACs and cone BCs.
In most mammals, these synaptic pathways mature postnatally. Previous studies demonstrated that synaptic transmission from BCs plays an important role in the maturation of RGC synaptic connectivity during postnatal development (Bodnarenko and Chalupa, 1993; Bodnarenko et al., 1995; 1999). Blockage of visual activity retarded the developmental segregation of RGCs into ON and OFF synaptic pathways (Tian and Copenhagen, 2003). However, little is known about the synaptic mechanisms, with which visual signals regulate RGC synaptic connectivity. Our recent study showed that light deprivation preferentially blocked RGC dendritic redistribution from the center to sublamina a (OFF sublamina) of the IPL (Xu and Tian, 2007). Similarly, a previous report showed that long-term treatment of cat’s eyes with 2-amino-4-phosphonobutyrate (APB), which mimic the effect of constant glutamate release from photoreceptors in darkness on the metabotropic glutamate receptor subtype 6 (mGluR6) located on the dendritic terminals of rod and ON cone BCs, significantly reduced the number of α RGCs ramifying in the sublamina a and increased the number of multistratified α cells (Deplano et al., 2004). These results demonstrated that the dendritic distributions of RGCs to the sublaminae a and b of the IPL are differentially regulated by visual stimulation or mGluR6-mediated synaptic activity. Therefore, the synaptic structure, which differentiates the modulation of RGC dendrites into ON and OFF pathways by light stimulation, would have to be located between BCs and RGCs. From the morphological point of view, the most likely structure for this differential modulation would be the synaptic connections between AII ACs and cone BCs, where AII ACs depolarize ON cone BCs through gap junctions and hyperpolarize OFF cone BCs through glycinergic synaptic transmission. If this were the case, long-term blockade of glycinergic synaptic transmission would retard the developmental redistribution of RGC dendrites into sublamina a of the IPL as that induced by light deprivation and APB treatment.
The goal of this study is to determine the roles of glycine receptor (GlyR)-mediated synaptic transmission in the maturation of RGC synaptic activity and connectivity by examining the synaptic inputs and dendritic distribution of RGCs in Spastic mice. Spastic mice have a mutation of the gene encoding β subunit of GlyR and thus have a reduction of glycine receptor expression for more than 80% in the nervous system as well as in the retina (White and Heller, 1982; Kingsmore et al., 1994; Pinto et al., 1994). Functionally, the surround OFF responses of ON-center and ON-OFF RGCs were abolished in these mice (Stone and Pinto, 1992), suggesting Spastic mutation largely blocked the OFF signal transmission in the rod pathway. We found that mutation of β subunit of GlyR had minimum effect on the development of outer retina, including photoreceptors and BCs. The dendritic stratification pattern of RGCs of immature Spastic mice was indistinguishable from that of age-matched wild type controls before eye opening. However, the patterns of RGC dendritic stratification of young adult wild type and Spastic mice raised under cyclic light/dark conditions are significantly different. The dendritic stratification pattern of RGCs of Spastic mice mimicked the dendritic stratification pattern of RGCs of wild type mice raised in constant darkness. Light deprivation has no additional effect on the RGC dendritic distribution of Spastic mice. Thus, we concluded that GlyR-mediated synaptic transmission after eye opening plays a critical role in the maturation of RGC synaptic connectivity in mouse retina.
Materials and Methods
Animals
Transgenic mice expressing Yellow Fluorescent Protein (YFP) in RGCs controlled by Thy1 promoter (Feng et al., 2000, H line) and Spastic mutant mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Thy1-YFP/Spastic mice were generated by cross Thy1-YFP mice with Spastic mice in our laboratory. All the procedures of rearing of mice in either cyclic light/dark conditions or constant darkness were the same as the preceding paper described (Xu and Tian, 2007). The handling and maintenance of animals and tissue preparation met the NIH guidelines and were approved by Yale University Committees of animal research.
Primary antibodies
Antibody against Rhodopsin was raised by hyperimmunization of Balb/c mice with unfixed rat retinal photoreceptor outer segment membrane preparation and recognize Rhodopsin N terminal 7–10 amino acids. This antibody had been tested immunohistochemically with rat retina, revealing a specific labeling of inner and outer segments of rods but not cones (Barnstable, 1980; Hicks and Barnstable, 1987). Polyclonal antibodies against blue opsin (Chemicon International, Cat. No. AB5407, Temecula CA) and red/green opsin (Chemicon International, Cat. No. AB5405) were initially raised in rabbit with recombinant human blue opsin to against the last 42 amino acids at the C terminus and red/green opsin to against the last 38 amino acids at the C terminus, respectively (Wang et al., 1992). These antibodies were tested by immunoflourescent staining of transiently transfected tissue culture cells expressing recombinant human cone pigments (Merbs and Nathans, 1992). Each was observed to stain cells transfected with the corresponding cDNA clone but not untransfected cells. They were also found to label the outer segments and cell membrane of specific types of cones in human, mouse and ground squirrel retina (Wang et al., 1992; Li and DeVries, 2004; Otani et al., 2004; Roberts et al., 2005). Antibody directed toward calretinin was purchased from Chemicon International (Cat. No. AB1550). This polyclonal antiserum was raised in goat against purified recombinant rat calretinin and has been characterized previously in rat brain by western blotting, recognizing a band at 29–30 kD (Winsky et al., 1996). Sheep polyclonal anti-tyrosine hydroxylase (anti-TH, Chemicon International, Cat. No. AB1542) was raised in sheep against pheochromocytoma tyrosine hydroxylase (Haycock and Waymire, 1982). This antibody staining a single band of 60kD molecular weight in PC12 cells (manufacturer’s technical information). Rabbit polyclonal anti-tyrosine hydroxylase (anti-TH, Chemicon International, Cat. No. AB152) was raised in rabbit against denatured tyrosine hydroxylase from rat pheochromocytoma. This antibody recognizes a single band of 60kD molecular weight (manufacturer’s technical information). Antibody against calcium binding protein 5 (CaBP5), a generous gift from Dr. Haeseleer Francoise, University of Washington, was raised in New Zealand white rabbits against bacterially expressed CaBP5. This antibody has been characterized previously by western blotting in bovine retina (recognize a band of molecular mass of about 16 kD) and immunocytochemistry in mouse, bovine, baboon, and human retina (Haeseleer et al., 2000; Ghosh et al., 2004). Polyclonal rabbit antibody against GFP conjugated with Alexa Flour 488 was purchased from molecular probes (Molecular Probes, Inc., Eugene, OR, Cat. No. A21311). This antibody was raised against GFP isolated directly from Aequorea Victoria and has been characterized by immunocytochemistry in granule cells (Overstreet-Wadiche et al., 2006), olfactory sensory neurons (Lèvai and Strotmann, 2003) and hipocampal neurons that expressing GFP (Huang et al., 2005). Table 1 summarized the antigen, host, source and specificity of each antibody used in this study.
TABLE 1.
Primary antibodies
| Antibody (Dilution) | Antigen | Host species | Source (Catalog No.) | Specificity (References) |
|---|---|---|---|---|
| Rhodopsin (1:10) | Membrane preparation from adult rat retina | mouse | Chemicon (MAB5316) | Specifically label rod cell body, inner and outer segment (Barnstable, 1980; Hick and Barnstable, 1987) |
| B-Opsin (1:300) | recombinant human blue opsin | Rabbit | Chemicon (AB5407) | Specifically label blue cone inner and outer segment (Li and DeVries, 2004; Roberts et al., 2005) |
| M/L Opsin (1:300) | recombinant human red/green opsin | Rabbit | Chemicon (AB5405) | Specifically label red/green cone inner and outer segment (Wang et al., 1992; Otani et al., 2004) |
| Calretinin (1:1000) | purified recombinant rat calretinin | Goat | Chemicon (AB1550) | Single 29–30kD band on rat brain extract by western blotting (Winsky et al., 1996) |
| TH (1:200) | Pheochromocytoma tyrosine hydroxylase | Sheep | Chemicon (AB1542) | Single band of 60kD molecular weight by western blotting in PC12 cells (Haycock and Waymire, 1982) |
| TH (1:400) | denatured TH from rat pheochromocytoma | Rabbit | Chemicon (AB152) | Recognize 60 kD single band of tyrosine hydroxylase protein by western blotting (Warnecke et al., 2005) |
| CaBP5 (1:200) | Bacterial expressed CaBP5 | Rabbit | gift from Dr. Haeseleer Francoise | Specifically label bipolar cells in mouse, bovine and human retina (Haeseleer et al., 2000; Ghosh et al., 2004) |
| GFP (1:500) | GFP isolated from Aequorea Victoria | Rabbit | Molecular Probes (A21311) | Characterized on Granule, olfactory and hipocampal GFP expressing neurons (Overstreet-Wadiche et al., 2006; Levai and Strotmann, 2003; Huang et al., 2005) |
Multielectrode array (MEA) recordings and data analysis
The procedures of retina preparation, action potential recording and data analysis have been described previously in detail (Meister et al., 1994; Tian and Copenhagen, 2003). Briefly, mice were dark adapted for 30 minutes before euthanization. Retinas were isolated under infrared illumination in oxygenated extracellular solution, which contained (in mM) NaCl 124, KCl 2.5, CaCl2 2, MgCl2 2, NaH2PO2 1.25, NaHCO3 26 and glucose 22 (pH 7.35 with 95% O2 and 5% CO2). Isolated retina was mounted on a piece of nitrocellulose filter paper (Millipore Corp., Bedford, MA), placed in the MEA-60 chamber with the ganglion cell layer facing the recording electrodes and continuously perfused with oxygenated extracellular solution at 34°C. The filter paper and the retina were held in position using a miniature manipulator so the retinas have stable and close contact with the electrode array.
Action potentials were simultaneously recorded from 60 channels with a multielectrode array having 10 μm diameter electrodes spaced 200 μm apart. A green LED (567 nm) was used to stimulate the retina with a 1s full field light at six different light intensities, which covered ~3 log units from 3.45 × 1010 photo/cm2/s (−3.11 log) to 4.42 × 1013 photo/cm2/s (0 log). The photon densities (photo/cm2/s) were converted to photoisomerization (Rh*) using an average collecting area of 0.5 (Field and Rieke, 2002). Responses were averaged from 15–50 recordings. Data were collected using a PC-based interface card and software (Multi Channel System MCS GmbH, Reutlingen, Genmany). The signals were filtered between 100 Hz and 3 kHz. Offline data analysis was carried out using Offline Sorter (Plexon Inc., Dallas, TX) and NeuroExplorer (Nex Technologies, Lexington, MA).
Electroretinogram (ERG) recordings and data analysis
The procedures of animal preparation, ERG recording and data analysis have been described previously in detail (Vistamehr and Tian, 2004). Animals were dark-adapted before experiments and anesthetized with Xylazine (13 mg/kg) and Ketamine (87 mg/kg). The pupils were dilated with Atropine (1%, Bausch and Lomb, Pharmaceuticals, Inc., Tampa, FL) and Phenylephrine HCl (Mydfrin 2.5%, Alcon Inc., Humacao, Puerto Rico). Proparacarine (0.5%, Alcon Inc., Humacao, Puerto Rico) was used before the contact electrodes were applied to the corneas. ERGs were evoked by 100 ms white flashes generated by LED arrays built in to a pair of miniature Ganzfield stimulators (EPIC-3000, LKC Technologies Inc., Gaithersburg, MD). Signals were band-pass filtered between 0.3 Hz to 500 Hz. ERGs were averaged from 5 single flashes for intensities between 0.008 cd × s/m2 and 0.8 cd × s/m2 and 3 single flashes for intensities between 2.5 cd × s/m2 and 25 cd × s/m2.
Preparation of retinal whole-mounts and retina sections for fluorescent imaging
The procedures for fluorescent immuno-labeling of YFP-expressing RGCs and dopaminergic ACs have been described previously in detail (Xu and Tian, 2007). In brief, retinas were isolated and fixed in 4% paraformaldehyde (PFA) in 0.01 M phosphate-buffered saline (PBS, pH 7.4) for 30 min at room temperature. Fixed retinas were washed 10 min × 3 in 0.01 M PBS and incubated in 30% sucrose at 4°C overnight. After blocked in 10% normal donkey serum, retinas were then incubated in a mixture of a rabbit polyclonal anti-GFP antibody conjugated with Alexa Fluor 488 (1:500) and a sheep polyclonal anti-TH (1:200) for 6 days at 4°C. A secondary antibody (donkey anti-sheep antibody) conjugated with Texas Red at 1:50 dilution was used to reveal the anti-TH binding site at 4°C overnight. Then retinas were flat mounted on Super-Frost Plus slides (Fisher Scientific, Pittsburgh, PA) with Vectashield (Vector Laboratories, Burlingame, CA).
For retina section preparation, the whole eyes were removed and fixed in 4% PFA for 2 hours, embedded in OCT (Sakura Finetek USA, Inc. Torrance, CA), sectioned vertically at 12 μm thickness on a freezing microtome (Bright Instrument Company LTD, Huntingdon, Cambs, England) and collected on Super-Frost Plus slides. Mouse monoclonal antibody against Rhodopsin (RET-P1, 1:10), rabbit polyclonal antibodies against red/green Opsin (1:300) and blue Opsin (1:300) were used to label rods, red/green cones and blue cones, respectively, and goat anti-mouse with Texa red conjugated antibody (1:100) and goat anti-rabbit with Rhodamine conjugated antibody (1:100) were used as the secondary antibodies, respectively. A rabbit antibody against CaBP5 (1:200), a goat anti-calretinin antibody (1:1000,) and a rabbit anti-TH antibody (1:400) were used to label BCs and ACs, respectively. The immuno-labeling follows the same protocol described above except FITC conjugated donkey anti-rabbit (1:50) and Rhodamine red conjugated donkey anti-goat (1:100) secondary antibodies were used to reveal the anti-CaBP5, anti-calretinin and anti-TH antibodies, respectively. All secondary antibodies used in this study were purchased form Jackson Immuno Research Laboratories (West Grove, PA).
Confocal laser scanning microscopy
The procedure for confocal laser scanning microscopy has been described previously in detail (Xu and Tian, 2007). Briefly, fluorescent images were collected using a dual-channel Olympus FV5-PSU microscope (Optical Analysis Corporation, Nashua, NH) with a PlanApo 60× oil lens (numerical aperture: 1.4). Image stacks of YFP-expressing RGCs in whole mount retinas were collected with confocal aperture #2 (100 μm pinhole) at z-step intervals of 0.5 μm. Software IPLab (Scanalystics, Inc., Fairfax, VA) was used to calculate pixel intensity of images in Z-stacks. The dendritic stratification of each RGC was characterized by their ramification depth (peak dendritic location) and thickness (dendritic width) in the IPL using software Igor (WaveMetrics, Lake Oswego, OR). The IPL was defined as 0–100 % from the border of inner nuclear layer to the border of ganglion cell layer. Multiple images of immuno-labeled retinal sections were taken at a step of 0.1 μm. Several optical sections were assembled to achieve the final image. The brightness and contrast of the final images were adjusted using Adobe Photoshop 5.5 (Adobe Systems Inc., San Jose, CA).
Statistical analysis
Kolmogorov-Smirnov (K-S) test was used to determine the difference of the cumulative distributions and Student t-test was used to examine the difference between two means using software StatView (Abacus Concepts, Berkeley, CA). Data were all presented as mean ± SEM in the text and figures. The distributions of peak dendritic locations in histograms were fitted with multiple Gauss distribution using software Origin (OriginLab Corporation, Northampton, MA).
Results
We first examined the impact of mutation of GlyR β subunit on the structure and function of photoreceptors, BCs and ACs. We then characterized the functional changes of RGC synaptic inputs from ON and OFF synaptic pathways induced by GlyR mutation. Third, we determined the roles of GlyR-mediated synaptic transmission on the development of RGC synaptic connectivity by profiling RGC dendritic distribution in the IPL of developing retina. Finally, we tested the relationship between the GlyR-mediated synaptic transmission and light evoked synaptic activity in the regulation of retinal synaptic circuitry maturation.
Glycine receptor β subunit mutation in Spastic mice caused minimum structural and functional changes in photoreceptors and BCs
We first determined whether mutation of GlyR β subunit disturb the development of photoreceptors and BCs by examining the structure and function of these cells using immunohistochemical labeling and ERG measurements. Rods, M/L- and S-cones of Spastic retinas were labeled using antibodies against Rhodopsin, M/L Opsin and S Opsin, respectively, and compared with that of age-matched wild type controls. In 15 out of 17 mice tested in this study, no detectable difference was found for the labeling patterns of Rhodopsin, M/L Opsin and S-Opsin expression neurons between Spastic mutants (Fig. 1B) and age-matched wild type controls (Fig. 1A). An antibody against calcium binding protein 5 (CaBP5) was used to label several subtypes of BCs. In the IPL, CaBP5 labeled three distinct bands of BC axonal terminals in both Spastic mutants (Fig 1D, left) and wild type controls (Fig 1C, left). The innermost CaBP5 immunoreactive band (single arrow head) represents the axonal terminals of rod BCs. The middle band (single arrow) represents the axonal terminals of type 5 ON cone BC axon terminals and the outermost one (double arrow head) represents the axonal terminals of one type of OFF cone BCs (Haeseleer et al., 2000; Haverkamp et al., 2003, Ghosh et al., 2004). The distributions of these BC axonal terminals in the laminar structured IPL of Spastic mutants are indistinguishable from that of wild type controls. In addition, antibody against calretinin was used to label ACs and their dendrites in OFF and ON layers of IPL (outer and inner bands) of both Spastic mutants (Fig. 1D, middle) and wild type controls (Fig. 1C, middle) (Ghosh et al., 2004; Wässle, 2004). The middle calretinin-positive band (Fig 1B, middle panels) represents the dendritic terminals of another group of ACs, which were previously shown to be nitric oxide synthase immunoreactive (Haverkamp and Wässle, 2000; Ghosh et al., 2004; Wässle, 2004). The distributions of these ACs dendritic arbors in the IPL of Spastic mutants are not different from that of wild type controls. In two Spastic mutants, however, the rods were totally lost, while the cones remained intact (data not shown). The retinas of these mice were significantly thinner than that of wild type controls.
Fig 1. The morphology and function of photoreceptors, BCs and ACs of most Spastic mutants are indistinguishable from wild type mice.
The morphology of photoreceptors, axonal/dendritic projections of BCs and ACs as well as the laminar structure of the IPL was evaluated by immuno-labeling of specific cell types and the function of photoreceptors and ON BCs was determined by ERG measurements. (A) Immuno-labeling of rods, M/L cones and S cones using anti-Rhodopsin, anti-M/L Opsin and anti-S Opsin antibodies in wild type mice. (B) Immuno-labeling of rods, M/L cones and S cones using anti-Rhodopsin, anti-M/L Opsin and anti-S Opsin antibodies in Spastic mice. (C) Immuno-labeling of retinal sections of a P33-aged wild type mouse with anti-CaBP5 and anti-calretinin antibodies. (D) Immuno-labeling of retinal sections of a P33-aged Spastic mouse with anti-CaBP5 and anti-calretinin antibodies. (E) Representative ERG waveforms from a P33-aged wild type mouse (WT) and a P33-aged Spastic (SPA) mouse. (F) Average amplitudes of ERG a-wave from P33-aged Spastic mice (7 mice, 14 eyes) and age-matched wild type controls (14 mice, 28 eyes). (F) Average amplitudes of ERG b-wave from the same two groups of mice as shown in panel F. Scale bars in panels A, B, C and D = 100 μm. * indicates 0.01 < p < 0.05. ** indicates 0.0001 < p < 0.01. *** indicates p < 0.0001 for this and following figures.
Physiologically, the light responses of photoreceptors, measured as ERG a-wave, were slightly decreased in Spastic mice for most of the tested light intensities (Fig. 1F). However, the responses of ON BCs measured as ERG b-wave of most (6 out of 7) mice were similar to that of wild type controls (Fig. 1G). One Spastic mouse (both eyes) showed significantly reduced a- and b-wave amplitudes (data not shown). These results support the notion that most Spastic mice have nearly normal functional rods, cones and BCs. In the following study of RGC light responses and dendritic morphology, we only used Spastic mice with normal morphology of rods and cones.
Mutation of glycine receptor β subunit significantly alters RGC synaptic inputs from ON and OFF synaptic pathways
GlyR-mediated synaptic activity is crucial to conduct OFF response from rod BCs/AII ACs to OFF RGCs (Strettoi et al., 1992, 1994; Deans et al., 2002). To determine the relative strength of synaptic inputs from ON and OFF pathways to RGCs mediated by rods and cones, we measured RGC responses to the onset and the offset of light stimulation under various light intensities. Light evoked action potentials were recorded from 696 RGCs of 8 wild type mice and 497 RGCs of 7 Spastic mice using a MEA system. The retinas were stimulated with full field steps of green light at six different light intensities. The light at the lowest intensity (−2.96 log, 173 Rh*/μm2/s) evoked RGC light responses driven by mixed rod-cone inputs, probably dominated by rod-driven inputs. The light at the highest intensity (0 log, 2.21 × 105 Rh*/μm2/s) saturated both rod and cone responses (Field and Rieke, 2002; Völgyi et al., 2004).
RGCs were simply classified into three groups based on their light responses as ON-OFF, ON and OFF cells to estimate the synaptic inputs from ON and OFF synaptic pathways. ON-OFF cells exhibited increased spike activity following both the onset and the termination of the stimulus (Fig. 2A). ON cells generated visually identifiable transient or sustained increases in spike frequency during the time the light was on (Fig. 2B). OFF cells produced an increase in spiking only following the termination of the stimulus (Fig. 2C). In mouse retina, even though the RGCs have center-excitation and surround-inhibition receptive fields, the response to a diffuse light is dominated by the central excitation region (Stone and Pinto, 1993). On this basis, our classification reflects the response properties of the excitatory synaptic inputs of the RGC receptive fields.
Fig 2. Mutation of GlyRs altered ON and OFF synaptic inputs to RGCs.
Light evoked action potentials were recorded from 696 RGCs of 8 wild type mice and 497 RGCs of 7 Spastic mice using a MEA system. RGCs were classified into ON-OFF, ON and OFF cells based on their light responses patterns. (A) A representative 2-second recording of light evoked action potentials (top), raster plot of 15 responses (middle) and frequency histogram (bottom) of an ON-OFF cell recorded from a wild type mouse. The bottom square waves indicate 1-second light stimuli. (B) A representative recording of light evoked action potentials, raster plots and frequency histogram an ON cell. (C) A representative recording of light evoked action potentials, raster plot and frequency histogram an OFF cell. (D) Average percentages of ON-OFF RGCs recorded from P33-aged wild type (WT) and Spastic (SPA) mice in responding to six different light intensities. (E) Average percentages of ON RGCs recorded from the same two groups of mice as shown in panel D. (F) Average percentages of OFF RGCs recorded from the same two groups of mice as shown in panel D. (G) Representative 2-second recordings of light evoked action potentials (upper) and frequency histograms (lower) of light responses of an ON-OFF RGC from a Spastic retina to five different light intensities. The light intensities are indicated as attenuation in log unit and the highest intensity is defined as 0 log unit. Note that the cell only responded to the onset of low intensity light stimulation but both the onset and the offset of higher intensity stimuli.
The Spastic mice have significantly reduced number of RGCs responding to the termination of light stimulation in the rod dominated light intensity range. At the lowest light intensity, only 14.2 ± 2.9% of the RGCs responded to both the onset and the offset of the light stimulation (Fig. 2D) and 3.1 ± 0.8% of the RGCs responded to the termination of light stimulation (Fig. 2F). These numbers are significantly lower than that of age-matched wild type controls (26.7 ± 2.1% for ON-OFF RGCs and 9.6 ± 0.9% for OFF RGCs, respectively; p = 0.00957 and 0.00211, respectively). The percentage of ON RGCs, on the other hand, is much higher in Spastic mice in comparison with that of wild type controls (Fig. 2E) (82.7 ± 3.1% versus 63.7 ± 1.8% for Spastic and control mice, respectively; p = 0.0005). With increase of light intensity, the percentage of ON-OFF responsive cells in Spastic mice sharply increased and reached 48.2 ± 1.4% of the total light responsive cells at the light intensity of −0.41 log unit, which is significantly higher than that of wild type controls (37.9 ± 1.7%, p = 0.0003). The increase of the population of ON-OFF RGCs with increasing of light intensity in Spastic mice was accompanied by a decrease of ON cells from 82.7 ± 3.1% to 36.7 ± 1.7%. Although the number of the OFF responsive cells increased with the increase of light intensity for both wild type mice and Spastic mutants, the Spastic mice had reduced numbers of the OFF responsive cells in all the light intensities tested. Carefully examining the light responses of each of the RGCs responded to both the onset and offset of high intensity light stimulations, a large number of them only responded to the onset of the low intensity light stimulations. Fig. 2G shows the light responses of such a RGC from a Spastic retina. This cell only responded to the onset of low intensity light stimulation but both the onset and the offset of high intensity lights. In wild type controls, much fewer RGCs showed the light intensity-dependent change in their response patterns.
To determine whether acutely block of glycinergic synaptic transmission in the wild type retina would alter RGC functional populations as that identified in Spastic mutants, we examined the effect of a GlyR antagonist, strychnine, on the functional population (ON, OFF and ON-OFF) of RGCs in wild type retinas. RGC light responses to full field light stimuli at a relatively strong light intensity (−0.4 log unit) were recorded before and during bath application of 1 μM strychnine. Unlike the Spastic mutants as shown in Fig. 2D–2F, the relative populations of ON, OFF and ON-OFF RGCs were not changed by acute block of glycinergic synaptic transmission in the wild type retina (Fig. 3B). In addition, we examined the frequencies of peak and sustained responses of each RGC before and during strychnine application. Pharmacologically block of GlyR had no significant effect on the peak frequencies of ON, OFF and ON-OFF RGCs (Fig. 3C, 3D and 3E, respectively) but increased the frequencies of sustained responses for all three groups of cells (Fig. 3F, 3G and 3H, respectively). Fig. 3A shows representative light responses of an ON-OFF RGC recorded before and during strychnine application. It is evident that both ON and OFF responses are significantly more sustained during strychnine application, while the peak responses to both the onset and offset of the light stimuli are not changed. Taken together, these results strongly suggest that the rod-driven OFF pathway in the Spastic mice is profoundly defective and the increase of ON-OFF RGCs and decrease of OFF RGCs in the Spastic retinas are more likely due to structural changes in RGC synaptic connectivity.
Fig 3. Pharmacologically block of GlyR function in wild type mice did not alter the number of RGCs receiving synaptic inputs from ON and OFF pathways.
Action potentials evoked by −0.4 log unit full field light stimulation were recorded from RGCs of wild type mice with and without bath application of 1 μM strychnine using a MEA system. The percentage of ON-OFF, ON and OFF RGCs as well as the peak and sustained ON and OFF responses of each RGC were measured before and during strychnine application. (A) Representative frequency histograms of the light responses of a RGC recorded before (Control) and during strychnine (Strychnine) application. The peak response was measured as the highest frequency within the first 200 ms after the light ON or OFF and the sustained response was measured as the average frequency of the second 500 ms after the light was ON or OFF. (B) Average percentages of ON-OFF, ON and OFF RGCs before and during strychnine application (n = 4). (C) Average peak response of ON RGCs before and during strychnine application from the same group of retinas (n = 168). (D) Average peak response of OFF RGCs before and during strychnine application of the same group of retinas (n = 50). (E) Average peak responses of ON-OFF RGCs before and during strychnine application of the same group of retinas (n = 101). (F) Average sustained response of ON RGCs before and during strychnine application. (G) Average sustained response of OFF RGCs before and during strychnine application. (H) Average sustained responses of ON-OFF RGCs before and during strychnine application.
Mutation of glycine receptors retards the developmental dendritic redistribution of RGCs after eye opening
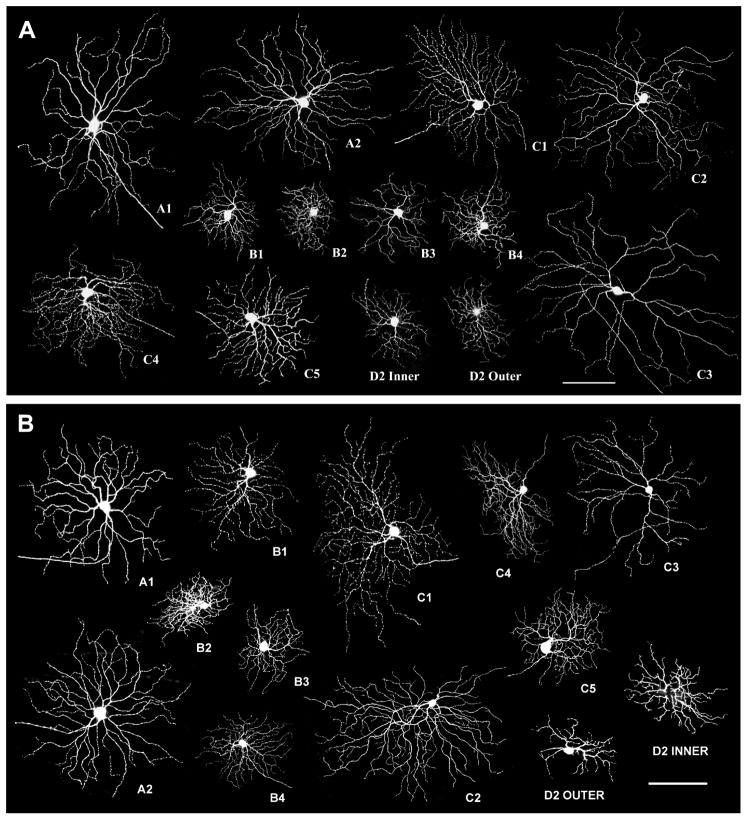
To further test this idea, we examined the RGC dendritic distribution of Spastic retinas in the sublaminae a and b of the IPL by generating Thy1-YFP/Spastic mouse colony. In the Thy1-YFP mice, several dozens of YFP labeled RGCs are randomly distributed throughout the retina. Confocal microscopy of these individual cells in whole mounted retinas allows the arborization patterns of the RGCs to be quantified in three-dimensional distribution (Tian and Copenhagen, 2003; Coombs et al., 2006, 2007; Xu and Tian, 2007). Every type of Thy1-YFP expression RGC found in wild type mice was also identified in Thy1-YFP/Spastic mutants, suggesting that GlyR mutation did not change the YFP expression pattern in the RGCs. Fig 4 shows examples of different morphological subtypes of RGCs obtained from P33-aged Thy1-YFP mice (Fig. 4A) and age-matched Thy1-YFP/Spastic mutants (Fig. 4B).
Fig 4. All YFP-expressing RGC types in wild type mice also express YFP in Spastic mutants.
(A) Stacked images representing twelve subtypes of morphologically identified RGCs from P33-aged Thy1-YFP mice. (B) Stacked images of same twelve subtypes of morphologically identified RGCs from P33-age Spastic/Thy1-YFP dual transgenic mice. The dendrites ramified in the ON and OFF sublaminae of the two D2 bistratified RGCs are shown in two different images in both (A) and (B), respectively. Scale bars, 100 μm.
The RGC dendritic distribution in different sublamina of the IPL was quantitatively determined by measuring the peak dendritic density and the dendritic width of each RGC based on the average pixel intensity of z-stacks of confocal images. As described in details previously (Xu and Tian, 2007), the ramification patterns of the dendrites of each RGC was quantitatively determined by measuring the peak dendritic density and the dendritic width of each RGC based on the average pixel intensity of a z-stack of confocal images (Fig. 5A). The average dendritic density of each frame of the images was calculated from the whole dendritic tree at that frame without the soma, axon and the initial portion of the primary dendrites. The calculated average dendritic density of each frame from the whole stack of the image was plotted as a function of IPL thickness and fitted with a single or double Gaussian distribution (Fig. 5D). The peak dendritic location was determined from the fitting and the dendritic width was defined as two times of the standard deviation (2 × SD) of the data distribution.
Fig 5. The age-dependent redistribution of RGC dendrites in the IPL was retarded in Spastic mutants.
The dendritic distribution of YFP-expressing RGCs in the IPL was quantified using confocal images from both Spastic and wild type controls at the ages of P12 and P33. (A) Five frames taken from a representative stack of confocal images of a mono-stratified ON RGC showing the soma and axon (first frame, green), the dendrites ramified in the sublamina a (second and third frame, green) of the RGC and the immuno-labeling of dopaminergic ACs dendritic processes (fourth frame, magenta) and the somas (Fifth frame, magenta). (B) A stacked image of the same RGC as shown in left panels and the dopaminergic ACs as shown in panel A. Scale bar: 50 μm. (C) The 90° rotation view of the same RGC and dopaminergic ACs as shown in panel A. The three dashed lines indicate the inner border of the IPL, the boundary of the sublaminae a and b and the outer border of the IPL, respectively. (D) Normalized pixel intensity of the dendrites of each frame (open circles) of the green channel was plotted as a function of IPL depth of the RGC. The data was fitted with a Gaussian distribution (green line). Double-arrows indicate dendritic widths. Single arrow indicates the location of the peak of dendritic density. (E) Average histogram of the peak dendritic location of mono-stratified (ON, OFF and mono-stratified ON-OFF) RGCs of P12-aged Spastic mutants (bar graph, SPA P12, 172 cells, 5 retinas). Age-matched wild type mice raised under cyclic light/dark conditions (dash line, WT P12, 145 cells, 4 retinas) were included for comparison. (F) Cumulative distributions of the dendritic width of the mono-stratified RGCs of P12-aged Spastic mutants and age-match wild type controls. (G) Average dendritic width of the same two groups of RGCs. (H) Average histograms of the peak dendritic location of mono-stratified (ON, OFF and mono-stratified ON-OFF) RGCs of P33-aged Spastic mutants (SPA P33, 214 cells, 8 retinas), age-matched wild type mice raised under either cyclic light/dark conditions (WT P33, 170 cells, 5 retinas) or constant darkness (D-WT P33, 194 cells, 5 retinas). (H) Cumulative distributions of the dendritic width of the mono-stratified RGCs of P33-aged Spastic mutants and age-match wild type controls. (H) Average dendritic width of the same two groups of RGCs.
To determine whether GlyR mutation alters the development of RGC dendritic distributions before and after eye opening, we first examined the peak dendritic distribution of YFP expressing RGC, which arborized their dendrites in only one layer in the IPL (mono-stratified RGCs), in Thy1-YFP/Spastic mice at the age of P12. In a total of 172 RGCs recorded from 5 mutant retinas, 162 (94.2%) of them were mono-stratified cells. This number is very close to that of age-matched wild type controls (91.5%). Both the peak location (Fig. 5E) and the dendritic width (Fig. 5F and 5G) of the mono-stratified RGCs from mutants are similar to that of age-matched wild type controls. K-S tests showed that neither the peak location nor the dendritic width of Spastic RGCs are statistically different from that of controls (p = 0.1236 and 0.556 for peak location and dendritic width, respectively). These results demonstrated that RGC dendritic structures develop normally in Spastic mutants before eye opening.
As we reported previously, RGCs redistribute their dendrites from the center of the IPL to both the inner and outer portion of the IPL after eye opening and light deprivation retards the developmental redistribution of RGC dendrites from the center to sublamina a of the IPL (Xu and Tian, 2007). To illustrate whether glycine receptor-mediated synaptic transmission plays a role in the developmental redistribution of RGC dendrites after eye opening, we examined the dendritic distribution of RGCs in P33-aged Spastic mutants and compared with age-matched wild type controls. In Spastic mice, the developmental redistribution of RGC dendrites from the center to sublamina a of the IPL is substantially retarded (Fig. 5H). The dendritic distribution of mono-stratified RGCs of P33-aged Spastic mice raised under cyclic light/dark conditions was significantly different from that of age-matched controls raised under the same conditions (K-S test, p = 0.0029) but not different from that of the wild type mice raised in constant darkness (KS test, p = 0.4671). In addition, the dendritic width of RGCs of P33-aged Spastic mice had a wider distribution range in comparison with age-matched controls (K-S test, p = 0.0025, Fig. 5I and 5J), suggesting that glycine receptor-mediated synaptic transmission selectively affects the developmental redistribution of RGC dendrites after eye opening.
To estimate the relative populations of RGCs receiving synaptic inputs from ON and OFF pathways, YFP-expressing RGCs were classified into four groups based on their peak dendritic locations and dendritic width. RGCs with dendrites exclusively ramified in either sublamina a or b were classified as OFF or ON cells, respectively. RGCs that arborized their dendrites in only one layer but ramified in both sublaminae a and b might receive synaptic inputs from both ON and OFF BCs and, therefore, were classified as mono-stratified ON-OFF RGCs. RGCs with dendrites clearly separated into two layers and are ramified in sublaminae a and b of the IPL, respectively, were classified as bistratified ON-OFF RGCs. The inner and outer boundaries of the IPL were identified by the peak location of the somas of RGCs in the ganglion cell layer and dopaminergic ACs in the INL, respectively. The boundary between sublaminae a and b of the IPL was determined by the middle calretinin band. The representative images and the dendritic pixel distribution curves of an ON, OFF, mono-stratified ON-OFF RGCs and bistratified ON-OFF RGC from wild type and Spastic mice are show in Fig 6A and 6B, respectively.
Fig 6. The retardation of the age-dependent redistribution of RGC dendrites in the IPL altered the synaptic inputs from ON and OFF synaptic pathways in Spastic mutants.
RGCs were classified into ON, OFF, mono-stratified ON-OFF and bistratified ON-OFF RGCs based on their dendritic distribution patterns in the IPL. (A) Upper panels: stacked images of an ON RGC, an OFF RGC, a mono-stratified ON-OFF RGC and a bistratified ON-OFF RGC from wild type mice. The dendrites ramified in the sublamina a of the bistratifed ON-OFF RGC were color-coded in green and the dendrites ramified in the sublamina b were color-coded in magenta. Middle panels: 90° rotation views of the same four cells. Lower panels: normalized dendritic distribution of each cell (open circles) and the Gaussian fitting of the data (green/magenta lines). The shadowed area indicates the sublamina a. (B) Stacked images (upper panels), 90° rotation views (middle panels) and normalized dendritic distribution and the Gaussian fitting (lower panels) of an ON RGC, an OFF RGC, a mono-stratified ON-OFF RGC and a bistratified ON-OFF RGC from Spastic mice. Scale bars in both panels A and B: 50 μm. (C) Average percentages of ON, OFF, bistratified ON-OFF and mono-stratified ON-OFF RGCs of P12-aged Spastic mutants (SPA P12) and age-match wild type controls (WT P12). (D) Average percentages of ON, OFF, bistratified ON-OFF and mono-stratified ON-OFF RGCs of P33-aged Spastic mutants (SPA33) and age-match wild type mice raised under either cyclic light/dark conditions (WT P33) or constant darkness (D-WT P33).
Since the dendritic distribution pattern of the mutants was similar to that of wild type controls at the age of P12 (Fig. 5E), the relative populations of ON, OFF, mono-stratified ON-OFF and bistratified ON-OFF RGCs were not different between these two groups of mice (Fig. 6C). On the other hand, the relative populations of RGCs were significantly altered in Spastic retinas at the age of P33 (Fig. 6D). The numbers of morphologically identified OFF and bistratified ON-OFF RGCs of Spastic mice were significantly lower than that of the age-matched wild type controls raised under cyclic light/dark conditions but not different from that of dark reared wild type mice. The population of mono-stratified ON-OFF RGCs of Spastic mice was, however, 2.8-fold higher than that of age-matched controls under cyclic lighting conditions but not different from that of dark reared wild type mice. In addition, mutation of GlyR β subunit had no effect on the relative population of ON cells.
To further determine whether GlyR β subunit mutation affects specific morphological subtypes of RGCs, we analyzed the dendritic distribution patterns of each subtype of RGCs. RGCs were grouped into four categories, A, B, C and D and 12 subtypes (Figs. 4, 7A and 7B) based on their morphological properties using the criteria of morphological classification of mouse RGCs developed by Sun et al., (2002). Before eye opening, similar dendritic distribution patterns were found between wild type and mutants for each group of RGCs (Fig. 7C, 7E and 7G). There was 5.8% RGCs were group D (bistratified) RGCs in the mutants. All of these cells showed two clearly separated dendritic layers. Since the dendritic distribution pattern of the mutants was similar to that of wild type controls at the age of P12, the relative populations of ON, OFF, mono-stratified ON-OFF and bistratified ON-OFF RGCs were not different between these two groups of mice (Fig. 7D, 7F and 7H). At the age of P33, it was evident that GlyR β subunit mutation blocked the developmental redistribution of RGC dendrites from the center to sublamina a of the IPL for all three groups of mono-stratified RGCs (Fig. 7I, 7K and 7M) and, therefore, reduced the relative populations of OFF RGCs and increased the relative populations of mono-stratified ON-OFF RGCs (Fig. 7J, 7L and 7N). These are very similar to the effects induced by light deprivation on wild type mice (Xu and Tian, 2007). More detailed information about the cell percentage, dendritic stratification depth and width of each morphological subtype of RGC of Spastic mice are listed in the Table 2. These results clearly demonstrated that GlyR β subunit mutation affects the dendritic maturation of all morphological subtypes of RGCs in an OFF-pathway specific manner.
Fig 7. Spastic mutation retarded the developmental dendritic redistribution of all morphological subtypes of RGCs after eye opening.
RGCs were classified into four groups and 12 subtypes based on their dendritic distribution patterns. The peak dendritic distribution in the depth of the IPL of each group of mono-stratified RGCs was analyzed. (A) Stacked images and 90° rotation views of a type A (left), type B (middle) and type C (right) RGC from Spastic mice. (B) Stacked images and 90° rotation views of a type A (left), type B (middle) and type C (right) RGC from wild type mice. Scale bars for both panel A and B: 50 μm. (C) Distributions of the peak dendritic location as a function of the IPL depth of ON, OFF and mono-stratified ON-OFF RGCs of group A RGCs from P12-aged Spastic mutants and age-matched wild type mice. (D) Average percentages of ON, OFF and mono-stratified ON-OFF RGCs of group A RGCs from P12-aged spastic mutants and age-matched wild type mice. (E) Distributions of the peak dendritic location as a function of the IPL depth of ON, OFF and mono-stratified ON-OFF RGCs of group B RGCs from P12-aged Spastic mutants and age-matched wild type mice. (F) Average percentages of ON, OFF and mono-stratified ON-OFF RGCs of group B RGCs from P12-aged spastic mutants and age-matched wild type mice. (G) Distributions of the peak dendritic location as a function of the IPL depth of ON, OFF and mono-stratified ON-OFF RGCs of group C RGCs from P12-aged Spastic mutants and age-matched wild type mice. (H) Average percentages of ON, OFF and mono-stratified ON-OFF RGCs of group C RGCs from P12-aged spastic mutants and age-matched wild type mice. (I) Distributions of the peak dendritic location as a function of the IPL depth of ON, OFF and mono-stratified ON-OFF RGCs of group A RGCs from P33-aged Spastic mutants and age-matched wild type mice. (J) Average percentages of ON, OFF and mono-stratified ON-OFF RGCs of group A RGCs from P33-aged spastic mutants and age-matched wild type mice. (K) Distributions of the peak dendritic location as a function of the IPL depth of ON, OFF and mono-stratified ON-OFF RGCs of group B RGCs from P33-aged Spastic mutants and age-matched wild type mice. (L) Average percentages of ON, OFF and mono-stratified ON-OFF RGCs of group B RGCs from P33-aged spastic mutants and age-matched wild type mice. (M) Distributions of the peak dendritic location as a function of the IPL depth of ON, OFF and mono-stratified ON-OFF RGCs of group C RGCs from P33-aged Spastic mutants and age-matched wild type mice. (N) Average percentages of ON, OFF and mono-stratified ON-OFF RGCs of group C RGCs from P33-aged spastic mutants and age-matched wild type mice.
Table 2.
Dendritic stratification of mophologically identified RGCs in Spastic mice
| Monostratified RGCs |
Bistratified RGCs |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RGCA |
RGCB |
RGCC |
RGCD |
|||||||||||
| A1 | A2a | B1 | B2 | B3a | B4 | C1a | C2a | C3 | C4 | C5a | D2 | |||
| Cell no. % (mean±SE) | P12 | 32 18.5±5 |
7 3.8±1.3 |
7 3.4±1.9 |
11 7.5±4 |
6 2.3±1.3 |
21 12.4±2.1 |
10 6.1±1.6 |
16 8.3±2.8 |
2 1.1±0.6 |
16 8.4±3.1 |
34 21.4±4.2 |
10 5.9±0.8 |
|
| P33 | 29 13.2±2.9 |
19 9.8±2.1 |
17 9.1±2.2 |
11 6.4±2.4 |
12 5.5±1.8 |
11 7.1±3.1 |
19 9.8±2.4 |
23 11.8±1.5 |
2 1±0.6 |
19 8.9±1.7 |
27 15±2.9 |
25 13.6±2.4 |
||
| P33D | 29 15.7±2.1 |
19 9.9±3 |
15 8.7±2.3 |
4 2.3±1.1 |
11 6.6±1.9 |
11 4.9±2.3 |
17 9.3±3.3 |
18 8.4±3.3 |
2 1.3±0.8 |
13 5.6±2 |
28 14±2.5 |
26 13.3±2.5 |
||
| Stratification depth (% of IPL) (mean±SE) | P12 | 66.2±1.2 | 40.6±4.6 (28.6%)b |
65.2±2.5 | 48.3±5.6 |
- (0%)b |
55.7±2.4 | 25.8±7 (27.3%)b |
29.2±2.6 (43.8%)b |
58.5±3.7 | 47.2±4.4 | 38.9±2 (17.6%)b |
64.7±2.2c | 42.1±1.8d |
| 62.9±1.2 (71.4%)b |
56.4±3.2 (100%)b |
64.1±1.7 (72.7%)b |
62.5±2 (56.2%)b |
62±1.2 (82.4%)b |
||||||||||
| P33 | 69.7±1.3 | 33.4±2.5 (27.8%)b |
50±3.6 | 50.4±2.3 | 41.4 (8.3%)b |
50.1±4.7 | 36.5±5.1 (10.5%)b |
38.5±2.7 (21.7%)b |
71.8±1.5 | 52.6±1.9 | 35.9±3.2 (15.4%)b |
62±1.6c | 38.5±1.1d | |
| 64.6±3.3 (83.3%)b |
66.7±2.2 (91.7%)b |
62.7±1.7 (89.5%)b |
65.8±2.4 (78.3%)b |
61.2±2.1 (84.6%)b |
||||||||||
| P33D | 73.5±1.6 | 33.9±3.2 (42.1%)b |
58.2±4.6 | 50.9±10.7 |
- (0%)b |
50.7±2.4 | 34.9±4.8 (23.5%)b |
33.4±4.3 (22.2%)b |
83.4±2.4 | 48.1±1.7 | 31.9±3.4 (21.4%)b |
55.2±0.8c | 38.5±1d | |
| 66.3±2.2 (57.9%)b |
76.9±2.1 (100%)b |
67.5±2.3 (96.5%b |
67.2±2.5 (77.8%)b |
61.5±2.6 (78.6%)b |
||||||||||
| Stratification width (% of IPL) (mean±SE) | P12 | 20.3±1.6 | 18.8±2.6 | 18.7±1.4 | 21.1±3 | 24.8±5.7 | 18.9±1.4 | 17.6±1.3 | 23.4±2.7 | 10.2±1.1 | 29.1±3.5 | 21.4±1.4 | 19.5±2.1c | 18.2±2.8d |
| P33 | 23.3±1.2 | 25.8±1.7 | 26±1.7 | 33.4±3.7 | 22.7±2.3 | 28.8±2.2 | 26.8±2 | 24.9±1.8 | 26.9±0.2 | 32.1±2 | 25.4±2.5 | 20±0.9c | 16.5±1.3d | |
| P33D | 25.5±1.3 | 26.2±1.1 | 23.5±1.5 | 28.6±0.6 | 23±2.1 | 21.7±2.4 | 21±1.3 | 24.7±2.1 | 24.7±5.8 | 25.7±2 | 27.5±1.3 | 12.7±0.8c | 10.7±0.7d | |
Monostratified cell type with both on and off population.
percentage of on and off population whithin the type.
and d, on and off dendrites of bistratified RGCs, respectively.
Light deprivation had no additional effect on the RGC dendritic distribution of Spastic mice
If visual stimulation regulated the developmental dendritic redistribution of RGCs through GlyR-mediated synaptic activity, dark rearing of Spastic mice would have no additional effects on the RGC dendritic redistribution. To test this idea, we examined the dendritic distribution and light responsiveness of RGCs of Spastic mice raised in constant darkness. Fig 8A shows that the relative populations of all four morphologically identified groups of RGCs from Spastic mice raised in constant darkness are not different from that of Spastic mice raised under cyclic lighting conditions. Functionally, the percentages of ON, OFF and ON-OFF responsive RGCs of dark reared Spastic mice were similar to those of Spastic mice reared under cyclic light/dark conditions (Fig. 8B), suggesting that light deprivation had no additional impact on the functional maturation of RGC synaptic activity in Spastic retina. Further analysis of dendritic distribution of each morphological group of mono-stratified RGCs also showed that neither the peak dendritic distributions nor the relative populations of morphologically identified ON, OFF and mono-stratified ON-OFF RGCs of dark reared Spastic mice are different from that of Spastic mice raised under cyclic lighting conditions (Fig. 8C–H; Table 2). These results strongly support the notion that the visual activity-dependent developmental redistribution of RGC dendrites from the center of the IPL to the sublamina a after eye opening is regulated by GlyR-mediated synaptic activity.
Fig 8. Light deprivation had no additional effect on the RGC dendritic distribution of Spastic mice.
Spastic mice were raised in constant darkness from birth to P33 and the confocal images and light evoked action potentials were recorded from RGCs of these mice. (A) Average percentages of morphologically identified ON, OFF, bistratified ON-OFF and mono-stratified ON-OFF RGCs of P33-aged Spastic mutants raised under either cyclic light/dark conditions (SPA P33) or constant darkness (D-SPA P33). (B) Average percentages of functional ON, OFF and ON-OFF responsive RGCs of P33-aged Spastic mice raised in constant darkness or under cyclic light/dark conditions. (C) Distributions of the peak dendritic location as a function of the IPL depth of group A RGCs from the same two groups of Spastic mice as shown in panel A. (D) Average percentages of ON, OFF and mono-stratified ON-OFF group A RGCs. (E) Distributions of the peak dendritic location of group B RGCs from the same two groups of Spastic mice. (F) Average percentages of ON, OFF and mono-stratified ON-OFF group B RGCs. (G) Distributions of the peak dendritic location of group C RGCs from the same two groups of Spastic mice. (H) Average percentages of ON, OFF and mono-stratified ON-OFF RGCs in group C.
Discussion
Our results revealed that GlyR β subunit mutation had minimum effect on the structure and function of the synaptic circuitry of outer retina and no detectable effect on RGC dendritic distribution before eye opening. However, the Spastic mutants had altered RGC light evoked synaptic inputs from ON and OFF pathways, which could not be mimicked by pharmacologically blocking of glycinergic synaptic transmission on wild type animals, and retarded developmental redistribution of RGC dendrites from the center to the sublamina a of the IPL after eye opening. These results are similar to that induced by light deprivation in wild type animals. In addition, light deprivation had no additional effect on the RGC dendritic distribution in Spastic mice. We interpret these results as that visual stimulation regulates the maturation of RGC synaptic activity and connectivity primarily through GlyR-mediated synaptic transmission.
GlyR β subunit mutation in Spastic mice affects the developmental refinement of RGC synaptic connectivity in OFF pathway after eye opening
The major finding of this study is that Spastic mutation retarded the activity-dependent developmental redistribution of RGC dendrites from the center to the sublamina a of the IPL. During normal postnatal development, mouse RGCs redistribute their dendrites in the IPL significantly. Before eye opening, a large portion of RGCs distributed their dendrites near the center of the IPL and receive synaptic inputs from both ON and OFF pathways. After eye opening, RGCs redistribute their dendrites toward the inner and outer borders of the IPL and receive synaptic inputs only from ON or OFF pathway (Tian and Copenhagen, 2003; Xu and Tian, 2007). This developmental refinement of RGC synaptic connectivity and dendritic distribution are thought to be regulated by light evoked glutamate release from presynaptic BCs (Bodnarenko and Chalupa, 1993; Bodnarenko et al., 1995; 1999; Tian and Copenhagen, 2003; Liu et al., 2007). In Spastic mutants, the RGC dendritic distribution at the age of P12 is not different from that of wild type controls, whereas the RGC synaptic inputs from ON and OFF pathways and RGC dendritic distribution of P33-aged Spastic mice are significantly different from that of age-matched wild type mice raised under cyclic light/dark conditions but indistinguishable from that of age-matched wild type mice raised under constant darkness. These results provide the evidence at the first time showing that glycinergic synaptic transmission plays a key role in the activity-dependent developmental refinement of RGC synaptic connectivity and dendritic distribution.
Interestingly, our recent study showed that dark rearing of mice, under which condition photoreceptors continuously release glutamate, preferentially blocked RGC dendritic redistribution from the center to sublamina a (OFF sublamina) of the IPL (Xu and Tian, 2007). Similarly, intraocular injection of APB of cats, which mimics the dark rearing by constantly activating mGluR6 receptors on the rod and cone ON BC dendritic terminals reduced the number of α RGCs ramified in the sublamina a and increased the number of α cells ramified in both sublaminae a and b of the IPL (Deplano et al., 2004). These asymmetric effects induced by light deprivation and intraocular APB injection on the RGC dendritic distribution support a notion that the cellular and synaptic mechanisms, with which glutamates released from BCs regulate the RGC dendritic distribution in the ON and OFF layers of the IPL, are different. The difference in these cellular/synaptic mechanisms ought to be reflected in the cellular/synaptic structures of the ON and OFF synaptic pathways and these cellular/synaptic structures should be located between BCs and RGCs. If this was the case, the most likely structures for this differential modulation would be the synaptic connections between AII ACs and cone BCs, where AII ACs depolarize ON cone BCs through gap junctions and hyperpolarize OFF cone BCs through glycinergic synaptic transmission.
Consistent to this idea, our results demonstrated that reduction of GlyR-mediated synaptic transmission in Spastic mice increased the ON-OFF responsive RGCs, which ramify their dendrites in both sublaminae a and b of the IPL, and reduced OFF RGCs, which ramify their dendrites only in the sublamina a of the IPL. These results closely resemble the effects induced by light deprivation on the RGC light responses and dendritic distribution in wild type mice (Tian and Copenhagen, 2003; Xu and Tian, 2007) and intraocular injection on cats (Deplano et al., 2004). Given that both light deprivation and Spastic mutation blocked the developmental refinement of RGC dendrites in a similar manner and light deprivation of Spastic mice had no additional effect on the RGC dendritic distribution, it is highly likely that light deprivation and Spastic mutation affected the same group of RGCs. Since GlyR-mediated synaptic transmission for rod driven OFF signals is “downstream” of rod BC synaptic outputs but “upstream” of RGC synaptic inputs in the visual signal processing, we interpret these results as that visual stimulation regulates the maturation of RGC dendrites through GlyR-mediated synaptic transmission.
What are the possible cellular and synaptic mechanisms with which glycinergic synaptic transmission regulates the RGC developmental dendritic distribution?
Mechanistically, it is commonly assumed that RGCs in mammalian retinas achieve their mature stratified patterns from early diffuse ramification patterns by removing “misplaced” dendrites, namely dendritic pruning (Wong and Ghosh, 2002). It is well documented that the dendrites of RGCs are diffusely ramified in the IPL in early development and then gradually stratified into narrow stratum during postnatal development in many mammals, including cats (Dann et al., 1988; Maslim and Stone, 1988; Bodnarenko et al., 1995), ferrets (Bodnarenko et al., 1999), rabbits (Wong, 1990), rats (Yamasaki and Ramoa, 1993), and mice (Bansal et al., 2000; Diao et al., 2004). However, recent studies of mouse retina showed that, similar to that of RGCs in adult animals, the dendrites of nearly all RGCs are narrowly stratified in the IPL by the time of eye opening (Bansal et al., 2000; Diao et al., 2004; Xu and Tian, 2007), suggesting dendritic pruning is largely completed at the time of eye opening (Mumm, et al., 2005). Our recent study showed that RGCs redistribute their dendrites in the IPL significantly after eye opening without decrease of the width of their dendritic distribution (Xu and Tian 2007), suggesting the maturational redistribution of RGC dendrites after eye opening could not be achieved by simply removing some “misplaced” dendrites from already narrowly stratified dendritic plexus. However, it is unclear how a narrowly stratified RGC dendritic plexus could be relocated from one stratum of the IPL to another in developing mammalian retina. A recent study of zebrafish retina using in vivo imaging demonstrated that lamina-restricted dendritic plexus of RGCs could be redistributed from the inner border of the IPL to the outer border of the IPL in 2–3 days without diffusely elaborating their dendrites throughout the IPL (Mumm et al., 2006). This redistribution of a stratified dendritic plexus involves simultaneous adding and eliminating of dendrites in different strata of the IPL. Given that the dendritic widths of Spastic mice are not different from that of wild type mice at the age of P12 but significantly wider than the age-matched controls at the age of P33, these results further support the idea that glycinergic synaptic transmission regulates the activity-dependent redistribution of RGC dendrites after eye opening but not the dendritic pruning before eye opening.
It needs to be further determined whether glycine regulates the OFF RGC dendritic redistribution by directly activating the GlyRs on the OFF RGC dendritic terminals or through influencing the excitatory (glutamatergic) synaptic outputs of OFF cone BCs. GlyRs are expressed in several different types of mammalian retinal neurons, including rod BCs, OFF cone BCs, ACs and RGCs (Koulen et al., 1996; Lin et al., 2000; Ivanova, et al., 2006; Frazao et al., 2007; Majumdar et al., 2007; Eggers et al., 2007; Veruki et al., 2007; Heinze et al., 2007). The activation of GlyRs on the OFF RGCs might regulate the RGC dendritic redistribution by interacting with excitatory synaptic activity. Such interaction has been observed in an auditory nucleus, the lateral superior olive, in which block of glycinergic synaptic inputs prevented a developmental decrease of NMDA receptor-mediated synaptic currents (Kotak and Sanes, 1996) and retarded an activity-dependent dendritic maturation of postsynaptic neurons (Sanes et al., 1992). Consistent with this idea, we recently found that the kinetics of light evoked synaptic currents of RGCs undergo an age-dependent change after eye opening, which can be readily interpreted as a developmental down-regulation of NMDA receptor-mediated synaptic currents. Light deprivation suppressed this age-dependent change of RGC synaptic currents (Xu and Tian, 2004). However, it is unclear whether Spastic mutation alters the NMDA receptor-mediated synaptic currents in RGCs as that in the lateral superior olive.
GlyRs might also regulate the RGC dendritic redistribution through influencing the excitatory glutamatergic synaptic outputs of OFF cone BCs. In mouse retina, OFF cone BCs receive rod OFF inputs from AII ACs mediated by glycinergic synaptic transmission (Bloomfield and Dacheux, 2001; Tsukamoto et al. 2001; Protti et al., 2005; Ivanova, et al., 2006). If the strength of excitatory synaptic outputs of OFF cone BCs regulated the dendritic redistribution of RGCs to sublamina a of the IPL, Spastic mutation would reduce the rod OFF inputs to the OFF cone BCs and weaken their excitatory synaptic outputs and, therefore, retard the developmental distribution of RGC dendrites to sublamina a of the IPL.
One might argue that glycinergic synaptic activities provide a push/pull action between ON and OFF synaptic pathways (McGuire et al., 1986; Belgum et al., 1987). Such glycinergic synaptic inhibition is involved in the contrast enhancement and shaping the light responses of RGCs (Belgum et al., 1984; O’Brien et al., 2003). This glycinergic push/pull action might also regulate the developmental redistribution of RGC dendrites in the IPL in a competing manner and a bias of the glycinergic push/pull action would imbalance the synaptic “driven force” from ON and OFF synaptic pathways and cause uneven developmental distribution of RGC dendrites in the ON and OFF sublaminae of the IPL. If this were the case, one would expect to have reduced number of RGCs ramified in the OFF sublamina and increased number of the RGCs ramified in the ON sublamina of the IPL in the Spastic mutants. However, our results showed that, although the number of RGCs ramified in the OFF sublamina of the IPL was significantly reduced, the number of the RGCs ramified in the ON sublamina was not different from that of age-matched wild type controls. Instead, more RGCs in adult Spastic mutants have their dendrites remaining near the center of the IPL as that of immature wild type mice. These results indicate that the developmental redistribution of RGC dendrites in the ON and OFF sublaminae of the IPL is unlikely regulated by a competing mechanism between ON and OFF synaptic pathways.
Recent studies showed that a neurotrophic factor, brain-derived neurotrophic factor (BDNF), modulates the visual experience-dependent developmental refinement of RGC dendrites (Landi et al., 2007; Liu et al., 2007). Over-expression of BDNF or increase BDNF expression by enriching the physical environment of mice accelerated the laminar refinement of RGC dendrites, whereas decrease TrkB, the receptor for BDNF, expression in the retina or intraocular injections of BDNF antisense oligonucleotides retarded the developmental refinement of RGC dendrites (Liu et al., 2007; Landi et al., 2007). Since visual deprivation reduces the levels of retinal BDNF expression (Seki et al., 2003; Mandolesi et al., 2005; Landi et al., 2007) and BDNF over-expression overrode the requirement for visual inputs to stimulate laminar refinement of RGCs (Liu et al., 2007), it is likely that visual stimulation per se might not, but the activity-dependent expression of BDNF/TrkB, be required for the developmental refinement of RGC dendrites. Consistent with this idea, we recently found that deletion of mGluR6, which blocks the light evoked responses of the rod and ON cone BCs, profoundly increased the excitatory spontaneous synaptic inputs of RGCs and overrode the retardation effects induced by light deprivation on RGC dendritic refinement in developing retina (our unpublished data). To further determine whether glycinergic synaptic transmission regulates the RGC dendritic distribution in the IPL through BDNF/TrkB pathway, it would be necessary to determine whether Spastic mutation specifically alters the activity-dependent expression of BDNF/TrkB in sublamina a of the IPL or the dendrites of OFF RGCs.
Could Spastic mutation affect RGC dendritic maturation through mechanisms other than rod OFF synaptic pathway?
A previous report showed that taurine signaling pathway acted through GlyR plays a crucial role for rod production in mouse retina. Reduction of GlyR α2 level decreased the number of rod photoreceptors in vivo (Young and Cepko, 2004). The rod-promoting effect is thought to be induced through activation of neonatal isoform of GlyRs, which are non-synaptic α2 subunit homopentamers (Young and Cepko, 2004). The Spastic mutation reduces the expression of β subunits and selectively interferes with the postnatal accumulation of the adult isoform of GlyRs, which are heteropentamers of 3α1:2β subunits (Langosch et al., 1988) and bind with gephyrin at the synaptic sites in more mature animals (Sassoé-Pognetto et al., 1994). Therefore, Spastic mutation does not affect the perinatal expression of the neonatal GlyR isoform and the formation of functional GlyRs by α subunits (Becker et al., 1992; Lynch, 2004). However, lack of β subunits prevents clustering of adult isoform of GlyRs at the postsynaptic membrane (Lynch, 2004). In Spastic retinas, the GlyR labeling of the IPL was reduced markedly but the distribution and intensity of both a presynaptic marker (synaptophysin) and a marker for the rod BCs (protein kinase C) were indistinguishable from those in normal retinas (Pinto et al, 1994). Our results also showed that Spastic mutation has minimum effect on the photoreceptor and BC morphology and function as indicated by the immuno-labeling of photoreceptors and BCs as well as the ERG measurements, suggesting the overall structure and function of the Spastic retina was not disrupted. In addition, the RGC dendritic distribution of Spastic mutants before eye opening is indistinguishable from that of age-matched wild type controls. Therefore, the changes we reported here on the RGC dendritic distribution in Spastic retinas are unlikely the consequences of alterations of GlyR-mediated rod-promoting effect during early development.
One might argue that OFF signals could be conducted to RGCs through alternative rod OFF synaptic pathways and cone OFF pathway, which are independent of glycinergic synaptic transmission. Although alternative rod pathways have been identified for the transmission of rod OFF signals in mammals (DeVries and Baylor, 1995; Soucy et al., 1998; Tsukamoto et al., 2001; Hack et al., 1999; Li et al., 2004), almost all of the rod OFF signals are conducted to RGCs through glycinergic synaptic transmission between AII ACs and OFF cone BCs in mouse retina (Ivanova, et al., 2006). This was evident that almost all of the scotopic OFF responses of RGCs were blocked by the GlyR antagonist, strychnine, or the mGluR6 agonist, APB, in both mice and rats (Protti et al., 2005). Therefore, blockade of the primary rod OFF pathway would block most of the rod OFF signal in mouse retina.
Since both GABA and glycine mediate inhibitory synaptic transmission in the retina, could GABAergic synaptic transmission compensate the reduction of glycinergic synaptic transmission in Spastic mutants to influence the maturation of RGC dendrites? Such GABAergic compensation has been observed in the neurons of spinal cord of Spastic mutants, on which GABARs and GlyRs co-exist (Graham et al., 2003). Recent immunocytochemistry study showed that GlyRs do not colocalize with either GABA(A)Rs or GABA(C)Rs in mouse retina (Frazao et al., 2007). In addition, Spastic mutants had reduced, but not enhanced, GABA and GABA(A)Rs staining in the retina (Yazulla et al., 1997). Further more, Spastic mutation affects RGC light responses by reducing the OFF surround responses of ON-center RGCs and the OFF responses of ON-OFF RGCs (Stone and Pinto, 1992), which are very similar to effects induced by pharmacologically blockade of GlyRs on wild type animals (Müller et al., 1988; Saito, 1981, 1983; Stone and Pinto, 1992; Protti et al., 2005). Therefore, it is unlikely that GABAR-mediated synaptic transmission would compensate the loss of glycinergic synaptic transmission in Spastic mutants.
Could the dendritic maturation of RGCs be controlled by more than one synaptic mechanism?
Interestingly, neither light deprivation nor GlyR mutation had significant effect on the developmental change of the number of RGCs ramifying in the sublamina b of the IPL (Xu and Tian, 2007; Fig 6 of this study). Similarly, long-term APB treatment had no effect on the number of α RGCs ramifying in the sublamina b of cat retina (Deplano et al., 2004). However, suppression of BDNF/TrkB expression in the retina significantly altered the number of ON RGCs but not OFF or bilaminated ON-OFF RGCs (Liu et al., 2007). The most plausible interpretation of these results would be that different aspects of the dendritic maturation of RGCs, such as the peak dendritic location in different stratum of the IPL and the size and density of dendritic arbors, are regulated by different mechanisms.
Acknowledgments
We wish to thank Dr. Ling Diao for her assistance on the morphological analysis of RGC subtypes and Dr. Francoise Haeseleer for her kindly proving antibody against CaBP5. This work was supported by NIH grant R01 EY 012345, Research to Prevent Blindness (RPB), Funds from Connecticut Lion, Eye Research Foundation and a James Hudson Brown-Alexander B. Coxe fellowship (for HX) from Yale University.
References
- Amthor FR, Oyster CW, Takahashi ES. Morphology of on-off direction-selective ganglion cells in the rabbit retina. Brain Res. 1984;298:187–190. doi: 10.1016/0006-8993(84)91167-3. [DOI] [PubMed] [Google Scholar]
- Bansal A, Singer JH, Hwang BH, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. doi: 10.1523/JNEUROSCI.20-20-07672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable CJ. Monoclonal antibodies which recognize different cell types in the rat retina. Nature. 1980;286:231–235. doi: 10.1038/286231a0. [DOI] [PubMed] [Google Scholar]
- Becker CM, Schmieden V, Tarroni P, Strasser U, Betz H. Isoform-selective deficit of glycine receptors in the mouse mutant spastic. Neuron. 1992;8:283–289. doi: 10.1016/0896-6273(92)90295-o. [DOI] [PubMed] [Google Scholar]
- Belgum JH, Dvorak DR, McReynold JS. J Physiol. Vol. 354. Lond: 1984. Strychnine blocks transient but not sustained inhibition in mudpuppy retinal ganglion cells; pp. 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgum JH, Dvorak DR, McReynold JS, Miyachi E. Push-pull effect of surround illumination on excitatory and inhibitory inputs to mudpuppy retinal ganglion cells. J Physiol. 1987;388:233–243. doi: 10.1113/jphysiol.1987.sp016612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res. 2001;20:351–384. doi: 10.1016/s1350-9462(00)00031-8. [DOI] [PubMed] [Google Scholar]
- Bodnarenko SR, Chalupa LM. Stratification of ON and OFF ganglion cell dendrites depends on glutamate-mediated afferent activity in the developing retina. Nature. 1993;364:144–146. doi: 10.1038/364144a0. [DOI] [PubMed] [Google Scholar]
- Bodnarenko SR, Jeyarasasingam G, Chalupa LM. Development and regulation of dendritic stratification in retinal ganglion cells by glutamate mediated afferent activity. J Neurosci. 1995;15:7037–7045. doi: 10.1523/JNEUROSCI.15-11-07037.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnarenko SR, Yeung G, Thomas L, McCarthy M. The development of retinal ganglion cell dendritic stratification in ferrets. Neuroreport. 1999;10:2955–2959. doi: 10.1097/00001756-199909290-00015. [DOI] [PubMed] [Google Scholar]
- Coombs J, Van Der List D, Wang GY, Chalupa LM. Morphological properties of mouse retinal ganglion cells. Neuroscience. 2006;140:123–136. doi: 10.1016/j.neuroscience.2006.02.079. [DOI] [PubMed] [Google Scholar]
- Coombs J, Van Der List D, Chalupa LM. Morphological properties of mouse retinal ganglion cells during postnatal development. J Comp Neurol. 2007;503:803–814. doi: 10.1002/cne.21429. [DOI] [PubMed] [Google Scholar]
- Dann JF, Buhl EH, Peichl L. Postnatal dendritic maturation of alpha and beta ganglion cells in cat retina. J Neurosci. 1988;8:1485–1499. doi: 10.1523/JNEUROSCI.08-05-01485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–712. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplano S, Gargini C, Maccarone R, Chalupa LM, Bisti S. Long-term treatment of the developing retina with the metabotropic glutamate agonist APB induces long-term changes in the stratification of retinal ganglion cell dendrites. Dev Neurosci. 2004;26:396–405. doi: 10.1159/000082282. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Baylor DA. An alternative pathway for signal flow from rod photoreceptors to ganglion cells in mammalian retina. Proc Natl Acad Sci USA. 1995;92:10658–10662. doi: 10.1073/pnas.92.23.10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao L, Sun W, Deng Q, He S. Development of the mouse retina: emerging morphological diversity of the ganglion cells. J Neurobiol. 2004;61:236–249. doi: 10.1002/neu.20041. [DOI] [PubMed] [Google Scholar]
- Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol. 2007;582(Pt 2):569–582. doi: 10.1113/jphysiol.2007.131763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV, Kolb H., Jr Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science. 1976;194:193–195. doi: 10.1126/science.959847. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes J. Imaging neural subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Frazao R, Nogueira MI, Wässle H. Colocalization of synaptic GABA(C)-receptors with GABA (A)-receptors and glycine-receptors in the rodent central nervous system. Cell tissue Res. 2007 doi: 10.1007/s00441-007-0446-y. in press. [DOI] [PubMed] [Google Scholar]
- Field GD, Rieke F. Mechanisms regulating variability of the single photon responses of mammalian rod photoreceptors. Neuron. 2002;35:733–747. doi: 10.1016/s0896-6273(02)00822-x. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004;469:70–82. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- Graham BA, Schofield PR, Sah P, Callister RJ. Altered inhibitory synaptic transmission in superficial dorsal horn neurones in spastic and oscillator mice. J Physiol. 2003;551(Pt 3):905–16. doi: 10.1113/jphysiol.2003.049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack I, Peichl L, Brandstatter JH. An alternative pathway for rod signals in the rodent retina: rod photoreceptors, cone bipolar cells, and the localization of glutamate receptors. Proc Natl Acad Sci USA. 1999;96:14130–14135. doi: 10.1073/pnas.96.24.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Sokal I, Verlinde CLMJ, Erdjument-Bromage H, Tempst P, Pronin AN, Beovic JL, Fariss RN, Palczewski K. Five members of a lovel Ca2+-binding protein (CABP) subfamily with similarity to calmodulin. J Biol Chem. 2000;275:1247–1260. doi: 10.1074/jbc.275.2.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline HK. The response of single optic nerve fibers of the vertebrate eye to illumination of the retina. Amer J Physiol. 1938;121:400–415. [Google Scholar]
- Haverkamp S, Ghosh KK, Hirano AA, Wässle H. Immunocytochemical description of five bipolar cell types of the mouse retina. J Comp Neurol. 2003;455:463–476. doi: 10.1002/cne.10491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424:1–23. [PubMed] [Google Scholar]
- Haycock JW, Waymire JC. Activating antibodies to tyrosine hydroxylase. J Biol Chem. 1982;257:9416–9423. [PubMed] [Google Scholar]
- Heinze L, Harvey RJ, Haverkamp S, Wässle H. Diversity of glycine receptors in the mouse retina: localization of the alpha4 subunit. J Comp Neurol. 2007;500:693–707. doi: 10.1002/cne.21201. [DOI] [PubMed] [Google Scholar]
- Hicks D, Barnstable CJ. Different Rhodopsin monoclonal antibodies reveal different binding patterns on developing and adult rat retina. J Histochem and Cytochem. 1987;35:1317–1328. doi: 10.1177/35.11.3655327. [DOI] [PubMed] [Google Scholar]
- Huang Z, Zang K, Reichardt LF. The origin recognition core complex regulates dendrite and spine development in postmitotic neurons. J Cell Biol. 2005;170:527–535. doi: 10.1083/jcb.200505075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova E, Müller U, Wässle H. Characterization of the glycine input to bipolar cells of the mouse retina. Eur J Neurosci. 2006;23:350–364. doi: 10.1111/j.1460-9568.2005.04557.x. [DOI] [PubMed] [Google Scholar]
- Kingsmore SF, Giros B, Suh D, Bieniarz M, Caron MC, Seldin MF. Glycine receptor β-subunit gene mutation in spastic mouse associated with LINE-1 element insertion. Nature Genetics. 1994;7:136–142. doi: 10.1038/ng0694-136. [DOI] [PubMed] [Google Scholar]
- Koulen P, Sassoè-Pognetto M, Grünert U, Wässle H. Selective clustering of GABAA and glycine receptor in the mammalian retina. J Neurosci. 1996;16:2127–2140. doi: 10.1523/JNEUROSCI.16-06-02127.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH. Developmental influence of glycinergic transmission: regulation of NMDA receptor-mediated EPSEs. J Neurosci. 1996;16:1836–1843. doi: 10.1523/JNEUROSCI.16-05-01836.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langosch D, Thomas L, Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc Natl Acad Sci USA. 1998;85:7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi S, Cenni MC, Maffei L, Berardi N. Environmental enrichment effects on development of retinal ganglion cell dendritic stratification require retinal BDNF. PLoS ONE. 2007;2:e346. doi: 10.1371/journal.pone.0000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lèvai O, Strotmann J. Projection pattern of nerve fibers from the septal organ: DiI-tracing studies with transgenic OMP mice. Histochem Cell Biol. 2003;120:483–492. doi: 10.1007/s00418-003-0594-4. [DOI] [PubMed] [Google Scholar]
- Li W, Keung JW, Massey SC. Direct synaptic connections between rods and off cone bipolar cells in the rabbit retina. J Comp Neurol. 2004;474:1–12. doi: 10.1002/cne.20075. [DOI] [PubMed] [Google Scholar]
- Li W, DeVries SH. Separate blue and green cone networks in the mammalian retina. Nat Neurosci. 2004;7:751–756. doi: 10.1038/nn1275. [DOI] [PubMed] [Google Scholar]
- Lin B, Martin PR, Solomon SG, Grunert U. Distribution of glycine receptor subunits on primate retinal ganglion cells: a quantitative analysis. Eur J Neurosci. 2000;12:4155–4170. [PubMed] [Google Scholar]
- Liu X, Grishanin RN, Tolwani RJ, Renteria RC, Xu B, Reichardt LF, Copenhagen DR. Brain-derived neurotrophic factor and TrkB modulate visual experience-dependent refinement of neuronal pathways in retina. J Neurosci. 2007;27:7256–7267. doi: 10.1523/JNEUROSCI.0779-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- McGuire BA, Stevens JK, Sterling P. Microcircuitry of beta ganglion cells in cat retina. J Neurosci. 1986;6:907–918. doi: 10.1523/JNEUROSCI.06-04-00907.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslim J, Stone J. Time course of stratification of the dendritic fields of ganglion cells in the retina of the cats. Brain Res Dev Brain Res. 1988;44:87–93. doi: 10.1016/0165-3806(88)90120-4. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Heinze L, Haverkamp S, Ivanova E, Wässle H. Glycine receptors of A-type ganglion cells of the mouse retina. Vis Neurosci. 2007;29:1–17. doi: 10.1017/S0952523807070174. [DOI] [PubMed] [Google Scholar]
- Mandolesi G, Menna E, Harauzov A, von Bartheld CS, Caleo M, Maffei L. A role for retinal brain-derived neurotrophic factor in ocular dominance plasticity. Curr Biol. 2005;15:2119–2124. doi: 10.1016/j.cub.2005.10.045. [DOI] [PubMed] [Google Scholar]
- Merbs SL, Nathans J. Absorption spectra of human cone pigments. Nature. 1992;356:433–435. doi: 10.1038/356433a0. [DOI] [PubMed] [Google Scholar]
- Meister M, Pine J, Baylor DA. Multi-neuronal signals from the retina: acquisition and analysis. J Neurosci Methods. 1994;51:95–106. doi: 10.1016/0165-0270(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Müller F, Wässle H, Voigt T. Pharmacological modulation of the rod pathway in the cat retina. J Neurophysiol. 1988;59:1657–1672. doi: 10.1152/jn.1988.59.6.1657. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Godinho L, Morgan JL, Oakley DM, Schroeter EH, Wong ROL. Laminar circuit formation in the vertebrate retina. Prog in Brain Res. 2005;147:155–169. doi: 10.1016/S0079-6123(04)47012-5. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Willams PR, Godinho L, Koerber A, Pittman AJ, Roeser T, Chien CB, Bailer H, Wong ROL. In vivo imaging reveals dendritic targeting of laminated afferents by zebrafish retinal ganglion cells. Neuron. 2006;52:609–621. doi: 10.1016/j.neuron.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R, Famiglietti EV, Jr, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978;41:472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- O’Brien BI, Richardson RC, Berson DM. Inhibitory network properties shaping the light evoked responses of cat alpha retinal ganglion cells. Vis Neurosci. 2003;20:351–361. doi: 10.1017/s0952523803204016. [DOI] [PubMed] [Google Scholar]
- Otani A, Dorrell MI, Kinder K, Moreno SK, Nusinowitz S, Banin E, Heckenlively J, Friedlander M. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Invest. 2004;114:765–774. doi: 10.1172/JCI21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL. Seizures accelerate functional integration of adult-generated granule cells. J Neurosci. 2006;26:4095–4103. doi: 10.1523/JNEUROSCI.5508-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto LH, Grünert U, Studholme K, Yazulla S, Kirsch J, Becker CM. Glycine receptors in the retinas of normal and spastic mutant mice. Inv Ophthal Vis Sci. 1994;35:3633–3639. [PubMed] [Google Scholar]
- Protti DA, Flores-Herr N, Li W, Massey SC, Wassle H. Light signaling in scotopic conditions in the rabbit, mouse and rat retina: a physiological and anatomical study. J Neurophysiol. 2005;93:3479–3488. doi: 10.1152/jn.00839.2004. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Hendrickson A, McGuire CR, Reh TA. Retinoid X receptor γ is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Invest Ophthalmol Vis Sci. 2005;46:2897–2904. doi: 10.1167/iovs.05-0093. [DOI] [PubMed] [Google Scholar]
- Sassoé-Pognetto M, Wässle H, Grunert U. Glycinergic synapses in the rod pathway of the rat retina: cone bipolar cells express the alpha1 subunit of the glycine receptor. J Neurosci. 1994;14:5131–5146. doi: 10.1523/JNEUROSCI.14-08-05131.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H. The effects of strychnine and bicuculline on the responses of X- and Y-cells of the isolated eye-cup preparation of the cat. Brain Res. 1981;212:243–248. doi: 10.1016/0006-8993(81)90061-5. [DOI] [PubMed] [Google Scholar]
- Saito H. Pharmacological and morphological differences between X- and Y-type ganglion cells in the cat’s retina. Vision Res. 1983;23:1299–1308. doi: 10.1016/0042-6989(83)90105-0. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Markowitz S, Bernstein J, Wardlow J. The influence of inhibitory afferents on the development of postsynaptic dendritic arbors. J Comp Neurol. 1992;321:637–644. doi: 10.1002/cne.903210410. [DOI] [PubMed] [Google Scholar]
- Seki M, Nawa H, Fukuchi T, Abe H, Takei N. BDNF is upregulated by postnatal development and visual experience: quantitative and immunohistochemical analyses of BDNF in the rat retina. Invest Ophthalmol Vis Sci. 2003;44:3211–3218. doi: 10.1167/iovs.02-1089. [DOI] [PubMed] [Google Scholar]
- Soucy E, Wang Y, Nirenberg S, Nathans J, Meister M. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998;21:481–493. doi: 10.1016/s0896-6273(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Stone C, Pinto LH. Receptive field organization of retinal ganglion cells in the spastic mutant mouse. J Physiol. 1992;456:125–142. doi: 10.1113/jphysiol.1992.sp019330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone C, Pinto LH. Response properties of ganglion cells in the isolated mouse retina. Vis Neurosci. 1993;10:31–39. doi: 10.1017/s0952523800003205. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Dacheux RF, Raviola E. Cone bipolar cells as interneurons in the rod pathway in the rabbit retina. J Comp Neurol. 1994;347:139–149. doi: 10.1002/cne.903470111. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol. 1992;325:152–168. doi: 10.1002/cne.903250203. [DOI] [PubMed] [Google Scholar]
- Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol. 2002;451:115–126. doi: 10.1002/cne.10323. [DOI] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual Stimulation is required for refinement of ON and OFF pathway in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci. 2001;21:8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Gill SB, Hartveit E. Spontaneous IPSCs and glycine receptors with slow kinetics in wide-field amacrine cells in the mature rat retina. J Physiol. 2007;581(Pt 1):203–219. doi: 10.1113/jphysiol.2006.127316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vistamehr S, Tian N. Light deprivation suppresses the light response of inner retina in both young and adult mouse. Vis Neurosci. 2004;21:23–37. doi: 10.1017/s0952523804041033. [DOI] [PubMed] [Google Scholar]
- Völgyi B, Deans MR, Paul DL, Bloomfield SA. Convergence and segregation of the multiple rod pathways in mammalian retina. J Neurosci. 2004;24:11182–11192. doi: 10.1523/JNEUROSCI.3096-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Macke JP, Merbs SL, Zack DJ, Klaunberg B, Bennett J, Gearhart J, Nathans J. A locus control region adjacent to the human red and green visual pigment genes. Neuron. 1992;9:429–440. doi: 10.1016/0896-6273(92)90181-c. [DOI] [PubMed] [Google Scholar]
- Warnecke M, Oster H, Revelli J, Alvarez-Bolado G, Eichele G. Abnormal development of the locus coeruleus in Ear2(Nr2f6)-deficient mice impairs the functionality of the forebrain clock and affects nociception. Genes and Dev. 2005;19:614–625. doi: 10.1101/gad.317905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H. Parallel processing in the mammalian retina. Nature Neurosci. 2004;5:1–11. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- White WF, Heller AH. Glycine receptor alteration in the mutant mouse spastic. Nature. 1982;298:655–657. doi: 10.1038/298655a0. [DOI] [PubMed] [Google Scholar]
- Winsky L, Isaacs KR, Jacobowitz DW. Calretinin mRNA and immunoreactivity in the medullay reticular formation of the rat: colocalization with glutamate receptors. Brain Research. 1996;741:123–133. doi: 10.1016/s0006-8993(96)00908-0. [DOI] [PubMed] [Google Scholar]
- Wong ROL. Differential growth and remodelling of ganglion cell dendrites in the postnatal rabbit retina. J Comp Neurol. 1990;294:109–132. doi: 10.1002/cne.902940109. [DOI] [PubMed] [Google Scholar]
- Wong ROL, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nature Rev Neurosci. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- Xu HP, Tian N. Pathway-specific maturation, visual deprivation, and development of retinal pathways. The Neuroscientist. 2004;10:337–346. doi: 10.1177/1073858404265254. [DOI] [PubMed] [Google Scholar]
- Xu HP, Tian N. Retinal ganglion cell dendrites undergo a visual activity-dependent redistribution after eye opening. J Comp Neurol. 2007;503:244–259. doi: 10.1002/cne.21379. [DOI] [PubMed] [Google Scholar]
- Yamasaki EN, Ramoa AS. Dendritic remodelling of retinal ganglion cells during development of the rat. J Comp Neurol. 1993;329:277–289. doi: 10.1002/cne.903290209. [DOI] [PubMed] [Google Scholar]
- Young TL, Cepko CL. A role for ligand-gated ion channels in rod photoreceptor development. Neuron. 2004;41:867–879. doi: 10.1016/s0896-6273(04)00141-2. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Studholme KM, Pinto LH. Differences in the retinal GABA system among control, spastic mutant and retinal degeneration mutant mice. Vision Res. 1997;37:3471–3482. doi: 10.1016/S0042-6989(96)00223-4. [DOI] [PubMed] [Google Scholar]