The allocation of organs and the optimal form of postoperative management are two of the most important issues in renal transplantation. On 1 January 1986, a system for equitable deployment of cadaveric organs was put into place at the University of Pittsburgh Transplantation Center [1]. The system provided merit points for time waiting, quality of antigen match, degree of presensitization as reflected by a preformed antibody analysis (PRA), medical urgency, and logistical factors which would add to the risk by increasing preservation time. The point system was a step toward avoiding ad hoc decisions about who would receive a given kidney and a movement toward computerization in selection.
Ultimately, the foregoing point system was adopted essentially without change for national use by the United Network for Organ Sharing (UNOS), a private and previously voluntary organization which had been given by law the responsibility for developing a distribution scheme. However, the influence of such a system which systemically assures equitable access for all patients, including those at high medical and immunologic risk, has never been assessed.
It is our intention to analyze here our experience with the point system, with particular reference to the effect of immunosuppressive regimens.
Methods
Four hundred and sixty-three renal transplantations were performed at the University of Pittsburgh hospitals (Presbyterian-University Hospital and Children’s Hospital of Pittsburgh) between 1 January 1986 and 31 December 1987. Two of the kidneys were from living-related donors and were excluded from analysis. Similarly excluded were 11 cases of cadaver kidney transplantation in conjunction with a liver or heart transplant. Otherwise, there were no exclusions whatsoever in the 450 consecutive cadaveric kidney transplantations.
Case Material
Three hundred and eighty-seven adults, whose mean age was 39.6 ± 15.4 (SD) years, received 407 transplants. The most common disease of the native kidneys was glomerulonephritis. Eighty-five of the adult recipients (22%) were diabetics, almost all type I. Thirty-eight children received 43 transplants; 25 were 10–18 years old and 13 were 6 months to 9 years old.
Tissue Typing
The HLA typing for all donors and all recipients was carried out in an accredited laboratory in which all known class 1 and class 2 antigens can be measured.
Antidonor antibodies were systematically looked for, and crossmatches with current recipient sera were performed in every case. A negative crossmatch was required in most cases, but in 39 instances, mostly involving highly sensitized recipients who had been waiting for long periods of time, transplantation was performed in spite of a weakly positive or doubtfully positive cytotoxic crossmatch. This experience has been reported separately [2] and will not be further discussed in this paper, except to mention that the results were not materially degraded from those in the overall group of highly sensitized recipients who had unequivocally negative crossmatches.
Point Allocation System
The Pittsburgh allocation system ranks potential recipients of a given kidney on the basis of several factors [1]. Waiting time, defined as beginning with the date of referral, can account for up to 10 points. Each class 1 or class 2 antigen match between donor and recipient accounts for two points, for a potential total of 12 if there is complete HLA identity. Every 10% of preformed antibody analysis (PRA; also called panel reactive antibody) accounts for one point, for a potential total of 10 points if there are antibodies against all of the lymphocyte test panel (100% PRA). Finally, medical urgency or logistic factors can add points, although these are rarely used. Thus, the system gives priority to those who have waited the longest, those with the best antigen match, and those with the greatest degree of presensitization who have a negative crossmatch.
Operative Procedures
Renal transplantation was with the standard operation [3], but using many variations when indicated. Because of the large number of older recipients, hypogastric to renal arterial anastomoses were not commonly performed, and in the vast majority, a Carrell patch of aorta containing the renal arterial orifice(s) was anastomosed to the external iliac artery. Ureteral reconstruction was with a nipple-tunnel technique [3] or with modifications of the extra-vesical operation of Lich et al. [4].
In the 1986–1987 period, the organs from all local donors, and from the majority of donors in distant centers, were removed with the technique developed for multiple organ harvest [5, 6]. In our center, the presence of a long cold ischemia time has not had an adverse affect on ultimate outcome [7], although the need for early postoperative dialysis increases with time.
Immunosuppression
During 1986, all patients were managed initially with ciclosporin and prednisone. In 1987, just under half of the recipients were started on ciclosporin, azathioprine and prednisone. Variations of this triple-drug regimen were described in 1984 at the International Transplantation Society [8-10] or shortly after [11]. Earlier, the combination of ciclosporin and azathioprine had been tested in primates by Reitz et al. [12] and synergism was demonstrated in rats and dogs by Squifflet et al. [13]. By the time of the 1986 meeting in Helsinki of the International Transplantation Society, more than a dozen papers describing the advantages of three-drug or four-drug therapy were presented.
OKT3 was used for steroid-resistant rejection episodes [14].
Statistical Methods
Actuarial patient and graft survivals were calculated for the 2-year period. Statistical analysis was performed using BMPD Software; significance was assessed by the Mantel-Cox test.
Results
Patient Survival and Causes of Death
Four hundred and twenty-five patients received 450 kidneys. Overall actuarial patient survival at 1 and 2 years was 92 and 89% (fig. 1). Thirty-seven (8.7%) of the 425 recipients have died.
Fig. 1.
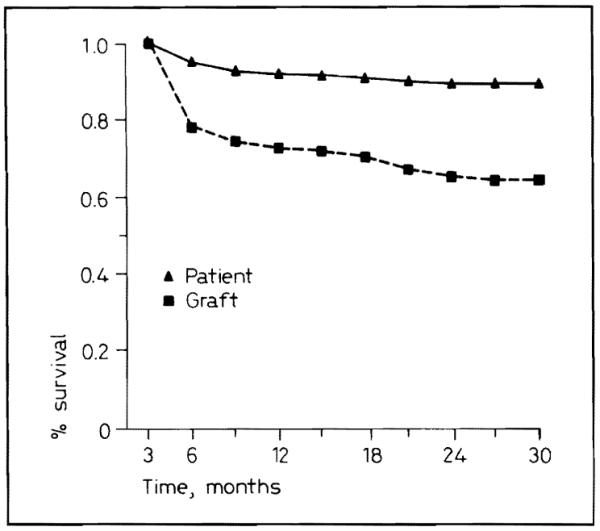
Patient and graft survival for 1986–1987.
An effort was made to assign a single cause of failure (table I), realizing that before the time of death, multiple diagnoses almost invariably were applicable. However, an initial complication usually triggered a series of adverse consequences, often including infection as well as deterioration of the renal graft if this had not already occurred. The combinations of deadly complications after renal transplantation and how these interrelate have been described in detail previously, long before the advent of ciclosporin [15].
Table I.
Causes of death after renal transplantation
| Cause of death | Graft functioning |
Graft removed or nonfunctioning |
Total |
|---|---|---|---|
| Infection | 1 | 12 | 13 |
| Cardiovascular | 4 | 5 | 9 |
| Gastrointestinal | 1 | 4 | 5 |
| Respiratory | 0 | 2 | 2 |
| Malignancy | 0 | 1 | 1 |
| Technical | 0 | 1 | 1 |
| Miscellaneous (DIC, multiple organ, failure, hyperkalemia, bleed after biopsy) |
1 | 3 | 4 |
| Unknown | 0 | 2 | 2 |
|
| |||
| 7 (19%) | 30 (81%) | 37 | |
In over 80% of the cases, there had been difficulty in maintaining good renal graft function (table I), either early because of acute rejection, or later because of chronic rejection or other factors. Apart from this factor, the most common principal cause of the events leading to death was infection, usually caused by opportunistic organisms or viruses. For example, 2 young men were given kidneys from a 23-year-old donor killed in a motorcycle accident. Later, the donor was proved to be a herpes simplex carrier. The two renal recipients died of herpes hepatitis 17 and 19 days postoperatively. The liver from this donor was given to a recipient who was being treated with acyclovir because of a ‘fever blister’ at the time of his operation; he escaped harm.
The second principal cause of death (9 patients) was cardiovascular disease. Gastrointestinal disease was also an important cause of death, with 2 lethal colonic perforations, 2 cases of severe upper gastrointestinal hemorrhage requiring emergency operations, and 1 case of liver failure (table I). A lymphoma caused the death of 1 patient. If diagnosed in time, these lymphomas usually involute with discontinuance or lightening of immunosuppression [16]. No deaths were caused by epithelial malignancies in the 1986–1987 recipients.
Miscellaneous causes of death included hemorrhage after a renal biopsy, a technical error in performing ureteroureteroneocystotomy, respiratory arrest during changing of a tracheostomy, and a respiratory arrest which may have been caused by an OKT3 infusion 12 h earlier.
Graft Survival
Overall Graft Survival
One- and 2-year actuarial graft survival was 72 and 64% (fig. 1). Although a sophisticated examination of tissue typing was not part of this study, there was no obvious affect of tissue matching (table II). The incidence of current success was about the same with all levels of compatibility.
Table II.
Matching in 418 cases, in which there was adequate donor and recipient typing: kidney function has been from 4 to 28 months
| Number of antigens matched | Functioning/total |
|
|---|---|---|
| n | % | |
| 6 | 1/2 | 50 |
| 5 | 2/3 | 67 |
| 4 | 8/13 | 62 |
| 3 | 33/55 | 60 |
| 2 | 82/113 | 72 |
| 1 | 103/142 | 73 |
| 0 | 53/90 | 61 |
|
| ||
| 282/418 | 67 | |
Because some of the patients received more than one graft during the 1986–1987 study period, the actual number of patients represented by the 450 cadaveric transplantations was 425. Of these 425 patients, 388 (91%) are alive, and 311 (73%) are off dialysis (table III). Thus, the gap between patient survival and graft survival shown in figure 1 underestimates the effectiveness of renal transplantation, in terms of liberating patients from dialysis (table III).
Table III.
Fate of 425 recipients of 450 grafts
| n | % | |
|---|---|---|
| Alive | 388 | 91.3 |
| Off dialysis | 311 | 73.2 |
| On dialysis | 77 | 18.1 |
| Dead | 37 | 8.7 |
Adults versus Children
Adults and children did not differ significantly in overall graft survival (fig. 2). Of the 38 pediatric recipients of 43 grafts 4 (10.5%) died, for a mortality that was similar to that in adults.
Fig. 2.
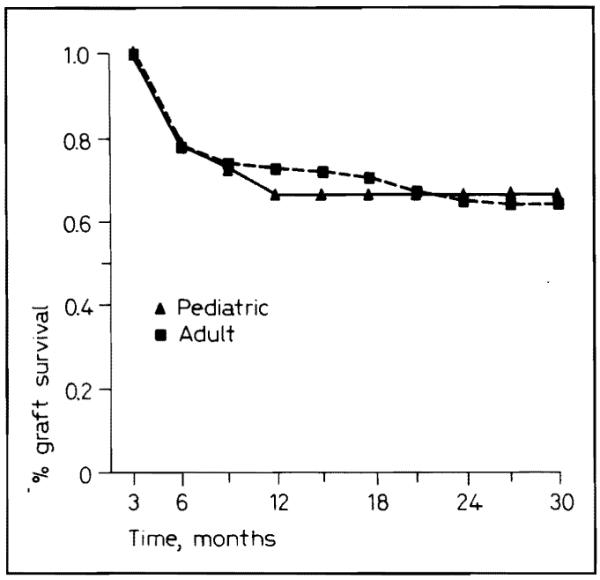
Pediatric and adult graft survival for 1986–1987.
Primary Transplantation versus Retransplantation
The results in transplanting patients for the first time were slightly but not significantly better than the results of retransplantation (fig. 3).
Fig. 3.
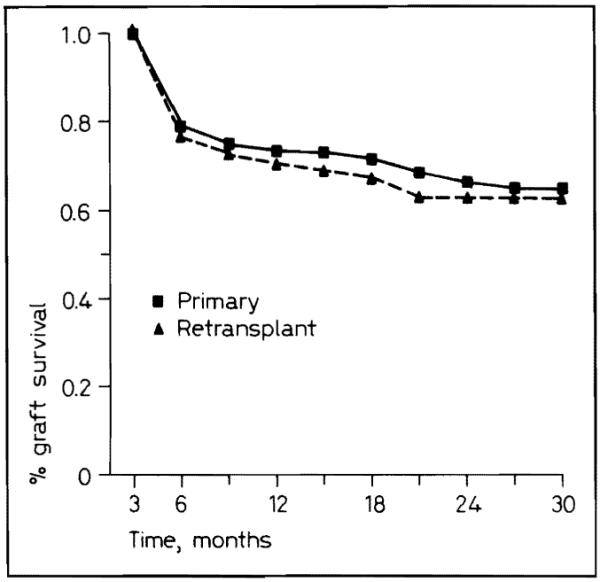
Primary and retransplant graft survival for 1986–1987.
Transplantation to ‘Clean’ versus Sensitized Patients
Grafts in patients with a PRA less than 40% had a significantly (p < 0.025) better survival than in patients with a PRA greater than 40% (fig. 4). In most reports, a great preponderance of highly sensitized patients have had previous transplantations and because of this, retransplantations have been less successful. The discordance with this expectation was due to the fact that many highly sensitized patients were undergoing primary transplantation in this series.
Fig. 4.
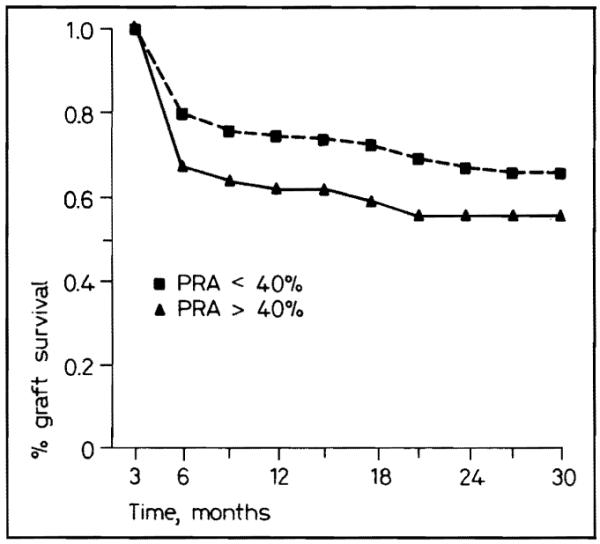
Graft survival for PRA less than and greater than 40%.
Effect of Triple- versus Double-Drug Immunosuppression
Overall Results
In the subgroup of patients treated beginning in January 1987 with ciclosporin, azathioprine, and prednisone, graft survival has been significantly better (p < 0.02) than with the cohort of patients receiving ciciosporin and steroids alone (fig. 5). The actuarial projections at 1 year predict 86% survival with triple therapy versus 69% with double therapy.
Fig. 5.
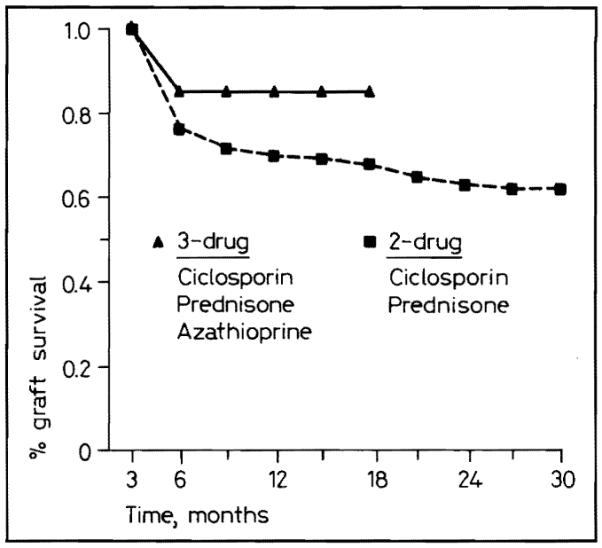
Graft survival for two- and three-drug immunosuppression.
Adults versus Children
The advantage with triple-drug therapy was approximately the same whether the recipients were in the adult or pediatric population (fig. 6), although the numbers in the pediatric group were too small to permit statistical significance.
Fig. 6.
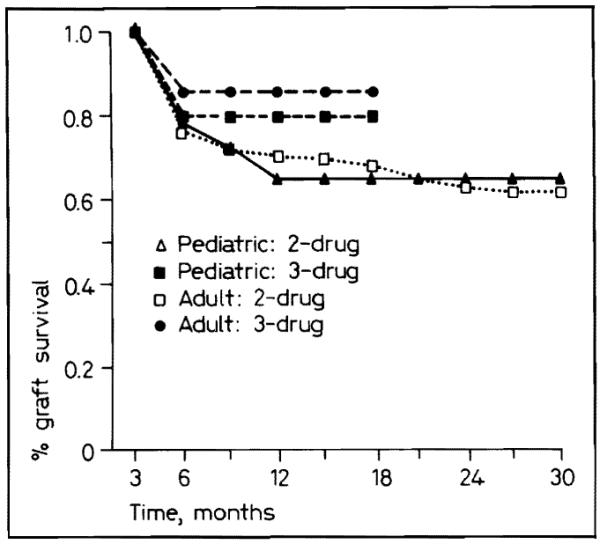
Pediatric and adult graft survival with two- and three-drug immunosuppression.
Primary Transplantation versus Retransplantation
The advantage of triple-drug therapy was evident in recipients of primary grafts as well as in those undergoing retransplantation, and in the larger group of primary transplantations, the advantage was statistically significant (p < 0.025) (fig. 7).
Fig. 7.
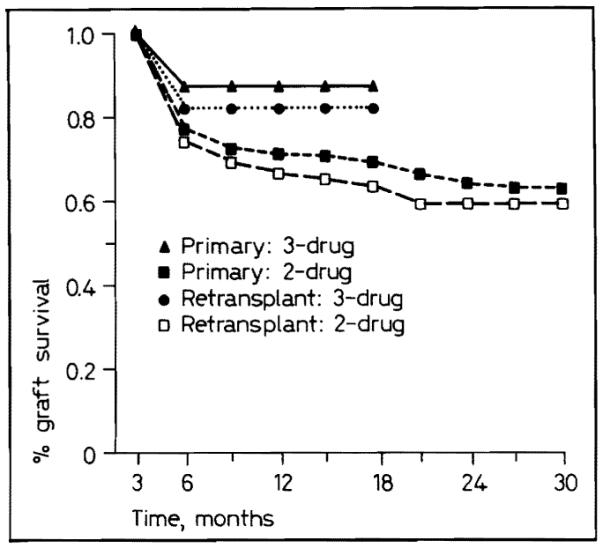
Primary and retransplant graft survival with two- and three-drug immunosuppression.
Low versus High PRA
Triple-drug therapy was advantageous for highly sensitized patients who have a predicted 1-year survival of 76%, even though they did not do as well as patients with low PRAs who have a projected 1-year survival of 88% (fig. 8). In contrast, patients in our 2-year sample of double-drug therapy including contemporaneous 1987 controls, have a 1-year actuarial survival of 57% when highly sensitized versus 71% with a low PRA (fig. 8). Thus, triple-drug therapy has upgraded survival in both the favorable and immunologically unfavorable patient categories.
Fig. 8.
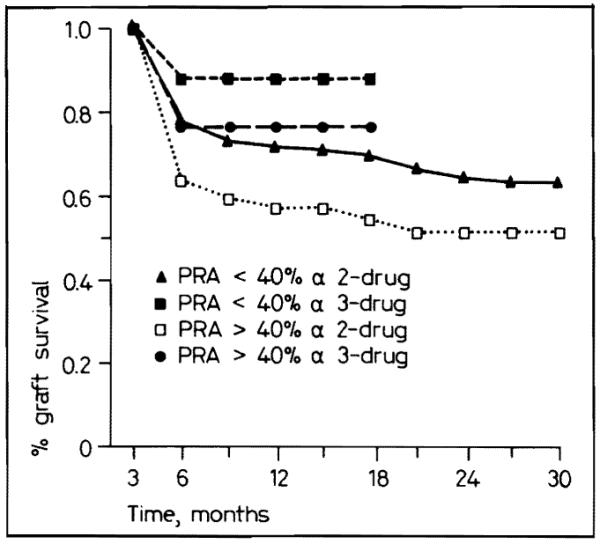
Graft survival for PRA less than and greater than 40% with two- and three-drug immunosuppression.
Discussion
Equal access to organs for patients in need is a matter of intense concern to the public as well as to health care providers. The point system [1] was designed to simplify recipient selection and to remove from the process the kind of bias against certain classes of potential recipients that could easily creep into an ad hoc system of patient selection. For example, there are no advantages or disadvantages for being old, afflicted by diseases of other organ systems, belonging to specific ethnic groups or religious persuasions, or being foreign-born.
One of the major arguments against the point system [17] comes from a school-of-thought that seeks to maximize graft survival by transplanting to the ‘best’ recipients, namely those who are young, healthy, and with low PRAs. The point system assures that highly sensitized patients will come to transplantation and considers irrelevant the question of obtaining good graft survival curves. In our series, 1 of every 7 patients had a PRA greater than 40%, connoting a poorer prognosis in all multicenter collections. The average age of adults in this sample was 39.6 ± 15.4 years.
The possibility that the point system could degrade results could be argued from our own experience during this bellwether period of 1986–1987. Even in our first trials with ciclosporin-steroid therapy in 1979–1980, the 1-year graft survival was with primary cadaveric transplantation 80% [18], and, in 1981, this expectation at 1-year rose to nearly 90% [19]. In these patients with whom ciclosporin-steroid therapy was first tried and standardized, the results in 1979–1981 were superior to those in 1986–1987, even though all of the earlier work was done without any means of pharmacologic monitoring with ciclosporin blood or plasma assays. Although the recipients were not highly selected, they were younger than in the 1986–1987 period, had a lower incidence of diabetes mellitus, and had less disease of other organ systems.
Nevertheless, acceptable results were obtained in the first 2 years of the point system. Of equal importance, the triple-drug trials of 1987 demonstrated the possibility of achieving even better levels of success. The additive and possibly synergistic combination of ciclosporin, azathioprine, and steroids has been recognized worldwide since its introduction in 1984 [8-10] and 1985 [11]. The superiority of and probable safety of the triple-drug regimen has been established in several recent randomized trials and our observations with shorter follow-up are in accord with these claims.
In the United States, and for the first time, the establishment of the United Network of Organ Sharing Kidney Transplant Registry will allow assessment of results after all of the cadaveric renal transplantations nationwide. From these data, analyses should begin to show if any of the factors used to compute points for the recipient scores will affect, either favorably or adversely, graft or patient life survival curves. Since our own experience with the point system precedes by almost 2 years that of all of the other centers which eventually were asked to adopt the system, some inkling of the implications of details of the point system will be watched for with interest in our patients. For example, a spectrum of donor-recipient matching is ensured by the point system, but so far, no major affect on the outcome has been identifiable as the result of extremely good or extremely poor compatibility or any permutations in between. The effect of age itself may prove to be important. In our own series, a high number of complications which would be expected in older patients were seen including colonic perforations, and many lethal cardiovascular complications.
Probably, it will be a number of years before enough data can be collected to justify concluding that easy entry into candidacy for high-risk patients and equitable access to organs thereafter will lead to truly inefficient use of organs. Even if this proves to be a valid conclusion, the loss of organs is not apt to be so great as to encourage an idolatry of survival numbers or worship at the altar of statistical morality.
Acknowledgements
We wish to thank the following people: Lisa Streb, RN, Loraine Kaminski, RN, Joan Murray, RN, Regina Fenton, RN and Deborah Good, RN for their invaluable contributions to the care of the patients and the data collection; Toni Pratt, for her untiring work in the preparation of the manuscript; and Robert W. Karausky, Verdere C. Philpot and Terry N. Trees, PhD for their help in fashioning the slides, figures and tables.
Footnotes
Supported by Research Grants from the Veterans Administration and Project Grant No. AM-29961 from the National Institutes of Health, Bethesda, Md.
References
- 1.Starzl TE, Hakala T, Tzakis A, et al. A multifactorial system for equitable selection of cadaveric kidney recipients. JAMA. 1987;257:3073–3075. [PMC free article] [PubMed] [Google Scholar]
- 2.Tzakis A, Hakala T, Makowka L, et al. Renal transplantation in the presence of a positive cytotoxic antibody. Transplant Proc. 1988;20:92–94. [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE. Experience in Renal Transplantation. Saunders; Philadelphia: 1964. [Google Scholar]
- 4.Lich R, Jr, Howerton, Connie W, et al. Recurrent urosepsis in children. J Urol. 1961;86:554–558. [Google Scholar]
- 5.Starzl TE, Hakala TR, Shaw BW, Jr, et al. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223–230. [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Miller C, Broznick B, et al. An improved technique for multiple organ harvesting. Surg Gynecol Obstet. 1987;165:343–348. [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor RJ, Landreneau MD, Makowka L, et al. Cyclosporine immunosuppression and delayed graft function in 455 cadaveric renal transplants. Transplant Proc. 1987;19:2100–2103. [PMC free article] [PubMed] [Google Scholar]
- 8.Illner W-D, Land W, Habersetzer R, et al. Cyclosporine in combination with azathioprine and steroids in cadaveric renal transplantation. Transplant Proc. 1985;17:1181–1184. [Google Scholar]
- 9.Slapak M, Goeghegan T, Digard N, et al. The use of low-dose cyclosporine in combination with azathioprine and steroids in renal transplantation. Transplant Proc. 1985;17:1222–1226. [PubMed] [Google Scholar]
- 10.Fries D, Kechrid C, Charpentier B, et al. A prospective study of a triple association: Cyclosporine, corticosteroids, and azathioprine in immunologically high-risk renal transplantation. Transplant Proc. 1985;17:1231–1234. [Google Scholar]
- 11.Simmons RL, Canafax DM, Strand M, et al. Management and prevention of cyclosporine nephrotoxicity after renal transplantation. Use of low doses of cyclosporine, azathioprine and prednisone. Transplant Proc. 1985;17:266–275. [PubMed] [Google Scholar]
- 12.Reitz BA, Bieber CP, Raney AA, et al. Orthotopic heart and combined heart and lung transplantation with cyclosporin A immune suppression. Transplant Proc. 1981;13:393–396. [PubMed] [Google Scholar]
- 13.Squifflet J-P, Sutherland DER, Rynasiewicz JJ, et al. Combined immunosuppressive therapy with cyclosporin-A and azathioprine. Transplantation. 1982;34:315–318. doi: 10.1097/00007890-198212000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Fung JJ, Demetris AJ, Porter KA, et al. Use of OKT3 with cyclosporine and steroids for reversal of acute kidney and liver allograft rejection. Nephron. 1987;96:19–33. doi: 10.1159/000184431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starzl TE, Porter KA, Andres G, et al. Long-term survival after renal transplantation in humans: (with special reference to histocompatibility matching, thymectomy, homograft, glomerulonephritis, heterologous ALG, and recipient malignancy) Ann Surg. 1970;172:437–472. doi: 10.1097/00000658-197009000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starzl TE, Nalesnik MA, Porter KA, et al. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporine-steroid therapy. Lancet. 1984;i:583–587. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fryd DS. The selection of cadaver kidney recipients. JAMA. 1988;259:840. doi: 10.1001/jama.1988.03720060012012. [DOI] [PubMed] [Google Scholar]
- 18.Starzl TE, Hakala TR, Iwatsuki S, et al. Cyclosporin A and steroid treatment in 104 cadaveric renal transplantations. In: White DJG, editor. Cyclosporin A. Elsevier, Biomedical Press; Amsterdam: 1982. pp. 365–378. [Google Scholar]
- 19.Rosenthal JT, Hakala TR, Iwatsuki S, et al. Cadaveric renal transplantation under cyclosporine-steroid therapy. Surg Gynecol Obstet. 1983;157:309–315. [PMC free article] [PubMed] [Google Scholar]


