Abstract
The immunosuppressive qualities and other features of a new cyclosporine (CsA) analogue, Nva2-cyclosporine (Nva2-CsA) were examined using canine orthotopic liver allografts. The mean survival time was 11.8±9.6 (SD) days in dogs without treatment, 60.8±34.4 days with Nva2-CsA and 65.1±33.0 days with CsA. Functional abnormalities indicating toxic side effects were not noted either with Nva2-CsA or with CsA. Using the same oral dose, the rate of blood level rise and the amount of the rise were greater with Nva2-CsA. Histopathologically, Nva2-CsA treatment was associated with the same degree of hydropic vocuolation in the pars recta of the proximal tubules as CsA treatment. Thus, in the dog, Nva2-CsA had identical immunosuppressive properties as CsA, with no functionally detectable toxicity affecting the liver and kidney.
The most limiting side effects of the new immunosuppressive agent, cyclosporine (CSA),3 have been from nephrotoxicity (1-3). Consequently, the announcement in 1984 of a cyclosporine analogue, hereafter called Nva2-cyclosporine (Nva2-CsA), which was not nephrotoxic (4), attracted more than casual interest. Nva2-CsA was shown by Hiestand et al. (4) to have potent immunosuppressive qualities in rats.
In this report, we will describe the immunosuppressive qualities and other features of Nva2-CsA in comparison to those of CsA in canine recipients of orthotopic liver homografts. In addition, particular attention was paid to the histopathologic changes or lack thereof denoting nephrotoxicity in the kidneys of dogs treated with one or the other of these agents for 90 days, or for lesser periods in the event of earlier death.
The results have shown that Nva2-CsA has immunosuppressive properties equal to those of CsA. Neither drug caused detectable changes in renal function but the use of both agents was associated with an equal amount of histologically demonstrable hydropic change in the renal tubules.
MATERIALS AND METHODS
Female beagle and mongrel dogs, weighing 10–15 kg, were obtained from the University animal farm. Beagle-to-beagle (B/B)4 or mongrel-to-beagle (M/B) combinations were used. On the morning of operation, the dogs were not fed and were anesthetized with sodium pentobarbital and ketamine. The donor was exsanguinated during removal of the liver and the blood was stored for transfusions. Usually, no other blood was given. The principles of the transplantation were as described before (5) but with several important modifications. Low flow venovenous bypasses (6) were used to decompress the portal and systemic venous systems during the anhepatic period while the liver was orthotopically transplanted. Drainage of the splanchnic bed during bypass was through the splenic vein after splenectomy. Drainage of the inferior vena caval bed was through the femoral vein, and pump driven delivery of the blood was to the external jugular vein. The suprahepatic vena caval, infrahepatic vena caval, and portal venous reconstructions were with end-to-end anastomoses. Hepatic arterial flow was restored with an end-to-side anastomosis of the donor to recipient abdominal aorta (5, 6). The biliary tract was reconstructed with cholecystoduodenostomy after ligation of the common duct. Antibiotics were given for 3 days. Oral intake, including CsA or Nva2-CsA was started on the morning after operation.
Both CsA and Nva2-CsA were supplied4 as a powder. They were dissolved in ethanol and in olive oil to a concentration of 100 mg/ml of solution. One or the other drug was randomly selected for a given dog on the morning after operation and given orally in doses of 20 mg/kg body weight on that morning and daily for the first 30 days. Then the doses were reduced to 15 mg/kg. The drug was discontinued after the 90th postoperative day or earlier in 2 dogs for which there was not sufficient Nva2-CsA.
A total of 46 liver transplantations were performed. Animals that did not survive for 6 days or more were excluded from analyses in order to eliminate the artifact introduced by lethal technical and surgical complications. To obtain 19 dogs which survived 6 days or more in the immunosuppressed groups, 27 transplantations were required for an inclusion rate of 70%. In contrast, 19 transplantations were required in untreated dogs to obtain 8 6-day survivors for an inclusion rate of only 42%. The culled final groups were as follows:
Group 1—no treatment, 8 dogs; 6 were B/B and 2 were M/B.
Group 2—Nva2-CsA, 10 dogs; 5 were B/B and 5 were M/B.
Group 3—CsA, 9 dogs; 4 were B/B and 5 were M/B.
Morning blood samples were taken every 3 days postoperatively and analyzed for serum glutamic oxaloacetic transaminase (SGOT), total bilirubin, and direct bilirubin (T- and D-bili), blood urea nitrogen (BUN) and creatinine (Cr). The blood concentrations of CsA and Nva2-CsA were measured with heparinized blood by high-performance liquid chromatography using cyclosporine D (CsD) as a standard for CsA determination and CsA as an internal standard for Nva2-CsA measurements. In addition, tissue distributions and concentrations of Nva2-CsA and CsA were measured in specimens frozen at −20°C after sampling at biopsy or autopsy. These studies are reported elsewhere.
At the end of 90 days, all surviving dogs underwent unilateral nephrectomy and liver biopsy. Specimens were fixed in 4% phosphate buffered formalin and in 2.5% cacodylate-buffered glutaraldehyde for light microscopic and electron microscopic examinations, respectively. Animals that died before 90 days were autopsied as soon as possible and the tissues were fixed in formalin. Structural changes were graded according to severity on a subjective 0 to +++ scale.
Statistics were performed using Students t test, Chi square test, or Fisher’s exact test. Group means were considered significantly different if probability of error was less than 0.05.
RESULTS
The results with the two donor-to-recipient strain combinations, B/B and M/B, were not statistically significantly different. Therefore, these were pooled in all 3 of the groups.
Survival
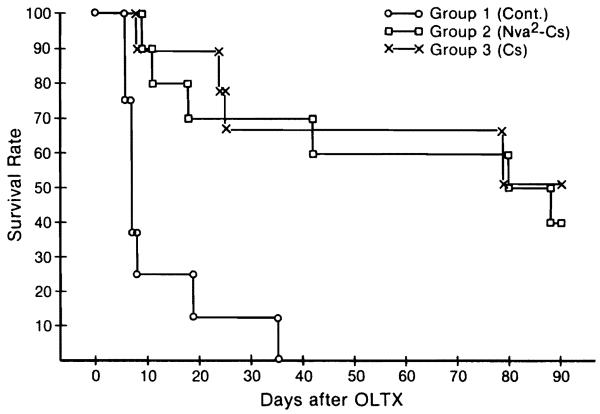
Of the 8 control animals, 7 died within 19 days. The eighth dog survived for 35 days; this was a B/B strain combination. Mean survival was 11.8±9.6 (SD) days (Fig. 1).
Figure 1.
Survival of group 1 (control), group 2 (Nva2-CsA), and group 3 (CsA) animals after orthotopic liver transplantation. Differences between group 2 and group 3 are not significant.
In the treated dogs, an arbitrary observation limit of 90 days was set in calculating survival. Nva2-CsA administration had to be discontinued in two dogs of group 2 after 82 days and 73 days because of a shortage of the drug. The former animal survived for more than 90 days and the latter dog died at 80 days, 3 days after unilateral nephrectomy. Credit to the time points of 90 and 80 days for these 2 dogs were used in the calculation of survival.
Using a ceiling of 90 days for calculation, dogs in group 2 (Nva2-CsA) lived for a mean of 60.8±34.4 (SD) days, and those in group 3 (CsA) lived for 65.1±33 (SD) days (Fig. 1). At 30, 60, and 90 days, the survival rates in the group 2 dogs (Nva2-CsA) and the group 3 animals (CsA) were 70 versus 67, 60 versus 67, and 40 versus 56. The differences were not significant.
At the end of 90 days, 9 dogs were still alive, 4 from group 2 (Nva2-CsA) and 5 from group 3 (CsA). Four were B/B and 5 were M/B stain combinations. Therapy was stopped. Five dogs died subsequently after a total survival of 94 (CsA), 118 (Nva2-CsA), 122 (CsA), 123 (Nva2-CsA), and 138 (CsA) days. All 5 animals had progressive hepatic failure. Histopathologic examination of their livers at autopsy compared with the livers at the 90-day biopsy showed a striking reduction in the number of small bile ducts, an increase in the amount of collapse of the reticulin fibrils at the center of the lobular framework, formation of septa linking portal tracts and cutting across lobules, and the appearance of cholestasis.
Four dogs are still living after discontinuance of therapy with Nva2-CsA (2 dogs) or CsA (2 dogs). All four were M/B. The longest survival (Nva2-CsA) is now 241 days.
Hepatic and renal function
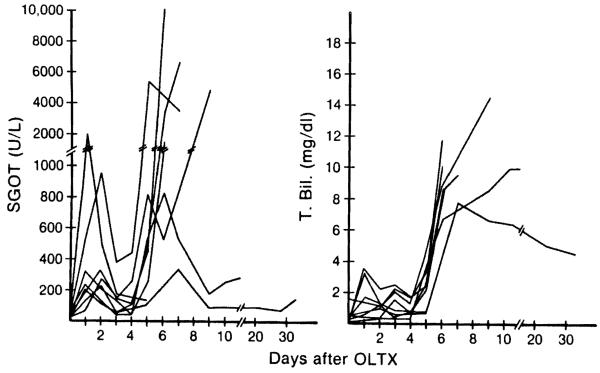
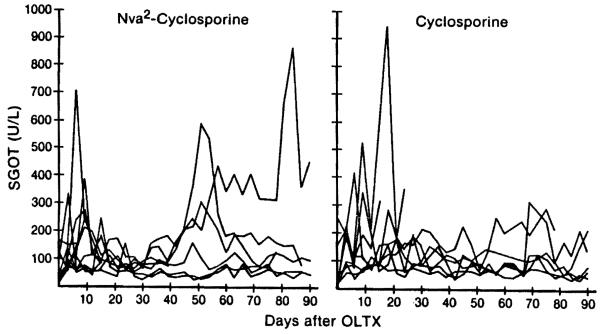
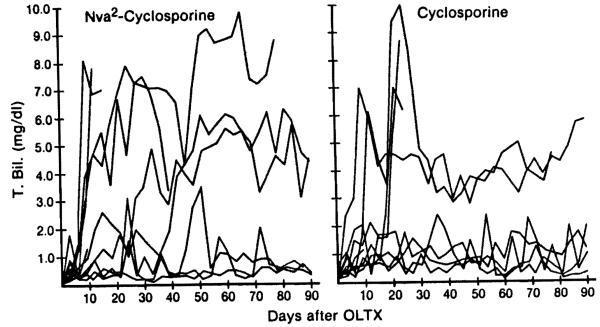
In the untreated controls, abrupt elevations of SGOT and T-bili and D-bili were observed between the fourth and the sixth postoperative days (Fig. 2). This acute deterioration of liver function caused by rejection led eventually to the death of all animals. In contrast, the SGOT of animals treated either with Nva2-CsA or CsA showed only moderate abnormalities that tended to stabilize (Fig. 3). There were 3 animals in group 2 (Nva2-CsA) and 4 in group 3 (CsA) that survived for 90 days with only slight or no elevations of bilirubin throughout this time (Fig. 4). The other long-surviving dogs had variable jaundice in spite of continuous therapy. No significant changes in Cr and BUN were noted in the control, Nva2-CsA or CsA animals.
Figure 2.
Changes in SGOT and T-bili of untreated control animals after liver transplantation.
Figure 3.
Changes in SGOT of group 2 (Nva2-CsA) and group 3 (CsA) animals after liver transplantation.
Figure 4.
Changes in T-bili of group 2 (Nva2-CsA) and group 3 (CsA) animals after liver transplantation.
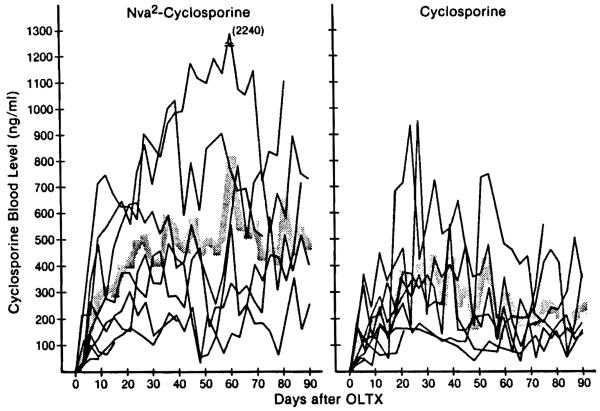
Cyclosporine blood levels
The whole-blood levels of cyclosporine measured with high-performance liquid chromatography varied considerably in individual dogs. However, the mean blood level of cyclosporine in the Nva2-CsA-treated animals of group 2 was higher than in the CsA-treated dogs of group 3 at all postoperative intervals (Fig. 5). The differences were significant at 36, 57, 63, 66, 69, 81, 84, and 87 days. A reduction of dose from 20 mg/kg to 15 mg/kg caused no decline of the blood level in either group, but 2 Nva2-CsA dogs (Fig. 6) and 1 CsA dog died of rejection after this reduction (Fig. 6).
Figure 5.
Changes in the blood level of Nva2-CsA (group 2) and CsA (group 3) after liver transplantation. Shaded zone indicates mean values.
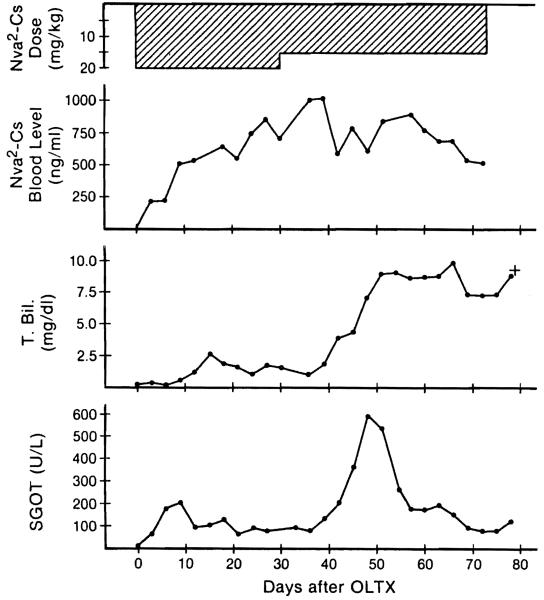
Figure 6.
Changes in Nva2-CsA dose, blood level, T-bili, and SGOT of No. 30 dog (group 2). Despite no appreciable change of blood Nva2-CsA level after dose reduction, the animal died of rejection.
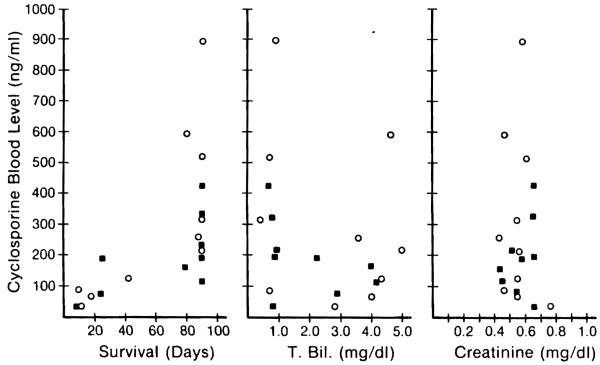
The average 24-hr trough level of cyclosporine throughout treatment with Nva2-CsA was 409.4 ng/ml (range 25–2240) compared with 235.8 ng/ml (range 25–935) during treatment with CsA, a difference that was highly significant. The number of dogs that had an average value more than 500 ng/ml was 3 in the Nva2-CsA group and none in the CsA group (Fig. 7). The interval for the mean level to rise to 200, 300, and 400 ng/ml after transplantation was 9, 12, and 21 days in group 2 (Nva2-CsA) compared with 12, 21, and 27 days in group 3 (CsA) (Fig. 5). Thus, the rate of postoperative blood level rise as well as the amount of the rise was greater in group 2.
Figure 7.
Correlations of trough blood level of Nva2-CsA (open circle) and CsA (black box) with the survival, average T-bili, and average creatinine of each animal.
The animals in both group 2 and group 3 that had blood levels averaging more than 200 ng/ml survived for significantly longer periods than those with lower levels, and they had lower concentrations of serum bilirubin and SGOT but did not show significant correlations with Cr (Fig. 7). Animals with low blood cyclosporine levels commonly had significant body weight loss, probably reflecting poor control of rejection.
Pathologic studies
All but one of the control untreated animals showed acute rejection of the liver allograft. In the expanded portal tracts, there was dense mononuclear cell infiltration, particularly around the smaller bile ducts and portal vein branches. Blast cells and mitoses were frequent. Small bile duct epithelium was damaged, and hepatocytes in the central parts of the lobules were atrophic. In the two animals that survived for 19 days and 35 days, respectively, many of the smaller bile ducts had been destroyed and there was collapse of the reticulin fibril framework around central veins. The one exception was an animal that died at six days from acute renal tubular necrosis. In this dog, mild mononuclear cell infiltration of the portal tracts was the only abnormality.
The CsA and Nva2-CsA-treated liver allografts also showed mononuclear cell infiltration of portal tracts, but this was less severe and there were fewer blast cells. In the nine dogs that survived for 90 days, there was also proliferation of bile ductules and portal tract fibrosis; these changes were slight in four of the animals (two from each group). Disappearance of small bile ducts, collapse of the centrilobular recticulin fibrils, septa linking portal tracts and cutting across lobules, destruction of the limiting plates, and atrophy of centrilobular hepatocytes and cholestasis were striking additional features of the five liver allografts that survived for 18–88 days after discontinuance of therapy. All of these animals died as a result of this chronic rejection. The death of a CsA-treated dog at 8 days was due to hepatic artery thrombosis. The Nva2-CsA dog that died at 9 days had destroyed its graft by a combination of acute rejection and suppurative cholangitis; an animal that survived for 11 days perished from peritonitis with minor changes in the graft. In the treated animals, there was no structural evidence to suggest additional damage to the liver allograft by either CsA or Nva2-CsA.
Hydropic vacuoles were found in the cytoplasm of the pars recta of the proximal convoluted tubules in kidneys from dogs in all three groups. Inclusion bodies and microcalcification were not seen. These changes were more common in the two treated groups, affecting 75% of the kidneys from the animals given CsA and 80% of the kidneys from the Nva2-CsA group, compared with 38% of the kidneys from the control dogs. The juxtaglomerular apparatus, which was normal in the controls, was enlarged with increased numbers of granular cells in 75% of the kidneys from the dogs treated with Nva2-CsA and in 40% of those given CsA. Small electron-dense deposits were present in the glomerular mesangium of 80% of the dogs treated for 90 days with Nva2-CsA, and 60% of those treated for a similar period with CsA. Mesangial deposits were also present in the two controls that survived for 19 and 35 days, respectively. There were no arteriolar changes.
DISCUSSION
Both Nva2-CsA and CsA are produced by the fungus Tolypocladium inflatum GAMS. The Nva2-CsA differs in structure from CsA only in that nonvaline is in position 2 of the cyclic endecapeptide instead of an alpha-amino butyric acid residue (4). Good immunosuppression of Nva2-CsA in rats was documented by Hiestand et al. (4), an effort that has not yet been made in larger animals. The provision of such information was the main purpose of the present investigation in dogs.
The strain combinations used in these studies provided some advantages as well as disadvantages. The principal advantage was a closer genetic control with a presumed smaller variance in immunologic response. The principal disadvantage could have been that the B/B combination may be “too easy.” In actual fact, the results with the B/B or M/B strain combinations were not significantly different. It was noteworthy that all 4 of the dogs with long survival after discontinuance of drug therapy were from the presumably less-favorable M/B combination. The results with either Nva2-CsA or CsA were remarkably good, with survival of more than 60 days even when credit for a maximum of 90 days was the limit for any individual dog. Thus, there were neither any subtle or obvious differences between Nva2-CsA and CsA in terms of immunosuppression.
The evidence that Nva2-CsA is nonnephrotoxic so far has come from studies in strains of rats that develop renal disease or spontaneous hypertension (7). The backdrop of incipient kidney damage apparently has been important in unmasking the additional nephrotoxicity that is imposed with CsA. In such test systems, Nva2-CsA has not caused or amplified kidney damage (7).
It was recognized in advance that the dog was an inappropriate experimental animal in which to compare the nephrotoxicity of Nva2-CsA with CsA. Neither drug ever caused an increase in BUN or Cr. The majority of the kidneys from animals treated with either CsA or Nva2-CsA showed hydropic vacuolation of the epithelium lining the pars recta or straight part of the proximal tubules. Comparable lesions were far less frequent in the controls. This part of the canine tubule is the equivalent of the S2 and S3 segment of the rat tubule where vacuolation is characteristically found after high doses of CsA (8). The mesangial electron dense deposits possible resulted from gut-derived antigens or complexes reaching the circulation in excessive quantities because of either defective reticuloendothelial activity or spontaneously developing portal-systemic shunts in the liver recipients.
Thus, the question of nephrotoxicity will have to be clarified in other experimental situations and presumably in animals other than dogs. For the moment, it may be said that Nva2-CsA has immunosuppressive qualities as impressive as those of CsA. From the results, further clarification of the pharmacokinetics of Nva2-CsA versus CsA will be important. It is suggested that Nva2-CsA is more effectively absorbed, since the average blood levels were somewhat greater than with the administration of CsA. The implication of this latter finding is not yet clear—although it may be important, since in both the Nva2-CsA and CsA-treated animals there was a definite, although imperfect, positive correlation between the blood levels of the drug, the quality of graft function, survival, and the ability to maintain weight and good health.
Note added in proof
After the manuscript for this article was submitted, a study of Nva2-CsA in dogs with kidney transplantation was reported by Calne et. al. in a Letter to the Editor of Lancet (1985; 2: 1342–1343). The conclusion of the English group—that Nva2-CsA is more immunosuppressive and less nephrotoxic than CsA—could not be supported from our experiments with a different kind of graft.
Footnotes
This work was supported by Research Grants from the Veterans Administration, and by Project Grant AM-29961 from the National Institutes of Health, Bethesda, MD.
- B/B
- beagle-to-beagle
- BUN
- blood urea nitrogen
- Cr
- creatinine
- CsA
- cyclosporine
- CsD
- cyclosporine D
- D-bili
- direct bilirubin
- M/B
- mongrel-to-beagle
- SGOT
- serum glutamic oxaloacetic transaminase
- T-bili
- total bilirubin
Sandoz Corporation, Basel, Switzerland.
REFERENCES
- 1.Calne RY, Rolles K, White DJG, et al. Cyclosporin A initially as the only immunosuppressant in 34 patients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2:1033. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 2.Starzl TE, Weil R, III, Iwatsuki S, et al. The use of cyclosporin-A and prednisone in cadaver kidney transplantation. Surg Gynecol Obstet. 1980;151:17. [PMC free article] [PubMed] [Google Scholar]
- 3.Klintmalm GBG, Iwatsuki S, Starzl TE. Nephrotoxicity of cyclosporin-A in liver and kidney transplant patients. Lancet. 1981;1:470. doi: 10.1016/s0140-6736(81)91851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiestand PC, Gunn H, Gale J, et al. The immunosuppressive profile of a new natural cyclosporine analogue: Nva2-cyclosporine. Transplant Proc. 1985;17:362. [Google Scholar]
- 5.Starzl TE, Kaupp HA, Brock DR, Lazarus RE, Johnson RJ. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet. 1960;111:733. [PMC free article] [PubMed] [Google Scholar]
- 6.Kam I, Lynch S, Todo S, et al. Low flow veno-venous bypasses in small animals and pediatric patients. Surg Gynecol Obstet. in press. [PMC free article] [PubMed] [Google Scholar]
- 7.Hiestand PC, Gunn HC, Gale JM, Ryffel B, Borel JF. Comparison of the pharmacological profiles of cyclosporine (Nva2)-cyclosporine and (Val2) dihydro-cyclosporine. Immunology. 1985;55:249. [PMC free article] [PubMed] [Google Scholar]
- 8.Ryffel B, Siegl H, Mueller A-M, Hauser R, Mihatsch MJ. Nephrotoxicity of cyclosporine in spontaneously hypertensive rats. Transplant Proc. 1985;17:1430. [PubMed] [Google Scholar]