Abstract
Background
Tacrolimus (Tac) and mycophenolate mofetil (MMF) are newly approved immunosuppressive agents. However, the safety and efficacy of the combination of MMF and Tac in primary liver transplantation has not been determined.
Methods
An Institutional Review Board-approved, open-label prospective randomized protocol was initiated to study the efficacy and toxicity of Tac and steroids (double-drug therapy) versus Tac, steroids, and MMF (triple-drug therapy) in primary adult liver transplant recipients. Both groups of patients began on the same doses of Tac and steroids. Patients randomized to triple-drug therapy also received 1 g of MMF twice a day.
Results
Between August 1995 and January 1997,200 patients were enrolled, 99 in double-drug therapy and 101 in triple-drug therapy. All patients were followed until May 1997, with a mean follow-up of 12.7 months. During the study period, 28 of 99 patients in double-drug therapy received MMF to control ongoing acute rejection, nephrotoxicity, and/or neurotoxicity. On the other hand, 61 patients in triple-drug therapy discontinued MMF for infection, myelosuppression, and/or gastrointestinal disturbances. By an “intention-to-treat analysis,” the actuarial 1-year patient survival rate was 85.1% in double-drug therapy and 83.1% in triple-drug therapy (P=0.77). The actuarial 1-year graft survival rate was 80.2% for double-drug therapy and 79.2% for triple-drug therapy (P=0.77). Forty-one patients (41.4%) in double-drug therapy and 32 (31.7%) in triple-drug therapy had at least one episode of rejection, but this was not statistically significant (P=0.15). The mean maintenance dose of corticosteroids was slightly lower in triple-drug compared with double-drug therapy.
Conclusion
Patient and graft survival rates were similar in both groups. There was a trend to a lower incidence of rejection, reduced nephrotoxicity, and a lesser amount of maintenance corticosteroids in triple-drug therapy compared with double-drug therapy.
Within the past two years, the Food and Drug Administration has approved two new immunosuppressive agents, designed for chronic administration. Tacrolimus (Tac*) is a potent immunosuppressive agent, shown to significantly reduce episodes of acute rejection in primary liver transplant recipients when compared with cyclosporine (CsA)-based immunosuppression (1–3). It was determined in early clinical transplant trials that the principal limitations of Tac are nephrotoxicity, neurotoxicity, and diabetogenicity (reviewed in 4).
Prospective, randomized double-blinded clinical trials comparing CsA, steroids, and mycophenolate mofetil (MMF) to CsA, steroids, and placebo (or azathioprine) revealed significantly reduced episodes of rejection in kidney transplant patients who were randomized to MMF (5–6), The principal toxicities observed in these trials were bone marrow suppression and gastrointestinal toxicity.
Not only do Tac and MMF have different toxicity profiles but also different modes of action (7, 8). The aim of the present study was to evaluate the combination of Tac and steroids to Tac, steroids, and MMF in primary adult liver transplant recipients in terms of patient and graft survival, incidence of rejection, and drug toxicity profiles. This represents a scheduled interim analysis after enrollment of 50% of the projected number of patients (e.g., 400 total) at a single center.
A prospective randomized trial protocol for adult (age ≥18 years) primary orthotopic liver transplantation (OLT) patients comparing Tac and steroids versus Tac, steroids, and MMF was approved by the Institutional Review Board in August 1995. Randomization was based on a sequential draw of assignments using a variable block randomization procedure (9). All consenting adults with primary orthotopic liver transplants were enrolled in the study.
Patients in both arms received Tac at 0.03 to 0.05 mg/kg/day intravenously as a starting dose, commencing immediately after reperfusion of the liver allograft. Subsequent adjustments in the Tac dosage were made to achieve a whole blood Tac concentration of 20 ng/ml when on intravenous therapy and a trough level of 15 ng/ml when on oral Tac therapy during the first postoperative month. The target trough levels were 12–15 ng/ml in the second postoperative month and 10–12 ng/ml in the third postoperative month. All patients also received 1 g of methylprednisolone on reperfusion of the liver and a 6-day methylprednisolone taper, starting at 200 mg/day and ending at a baseline dose of 20 mg/day. Subsequent adjustment in baseline prednisone was made depending on the clinical course of the patient. Patients who experienced an acute rejection episode were initially treated with a single 1-g bolus of methylprednisolone and optimization of Tac levels. In the event that the liver function tests did not improve within 24 hr after the steroid bolus, a gradual steroid taper was introduced, starting at 200 mg of methylprednisolone and tapering to 20 mg of prednisone over the ensuing 5 days. Patients who failed augmented steroids were considered as having steroid-resistant rejection and were treated With 5 mg of OKT3 (Ortho Biotech, Raritan, NJ) for 7–10 days. Patients who were randomized to Tac, steroids, and MMF (triple-drug therapy; T) received 1 g of MMF twice a day from the day of transplant. Protocol allowed reduction or discontinuation of MMF if there were any side effects ascribed to MMF or if the clinical course of the patient deemed it necessary to do so. In addition, patients randomized to double-drug therapy could receive MMF to control acute rejection or Tac-related toxicity. Criteria used for the pathologic diagnosis of acute hepatic rejection were as described in an international consensus document (10).
Between August 1995 and January 1997, 200 patients were enrolled in the study. There were no significant differences in recipient age, sex, or United Network of Organ Sharing status at the time of transplant. The mean age was 53.1 ± 12 years in D and 52.8 ± 12 years in T. The proportion of male/female was 58/41 in D versus 53/48 in T. Fourteen donors were above the age of 65 years in T compared with 11 in D. For the purposes of this report, all patient statuses were updated to May 5, 1997. The mean follow-up was 12.7 ± 0.4 SE months (median 12.9; range 3.5 to 20.9). Patient and graft survival rates were calculated using the Kaplan-Meier method and compared by the log-rank test. Differences between means were tested by the standard two-sample t test, whereas differences in proportions were tested by the Pearson chi-square test. Analyses were performed by “intention-to-treat” analysis. A P value less than 0.04, therefore, was considered statistically significant. Continuous data are presented as mean ± SD, and categorical data are presented as proportions.
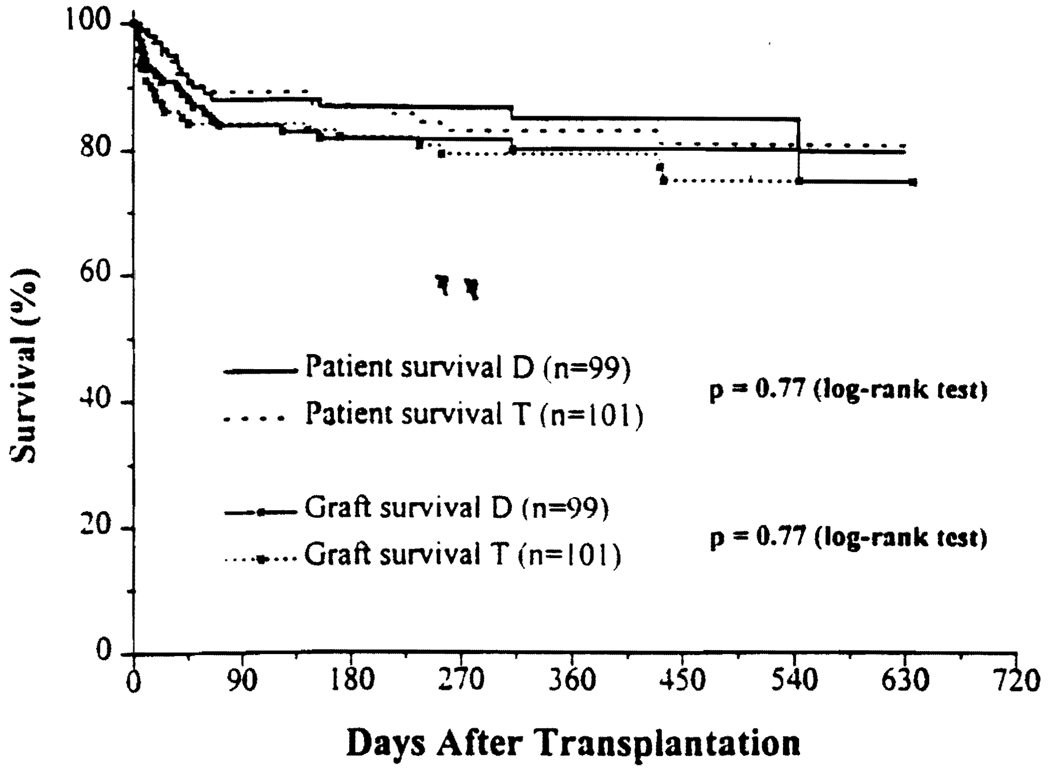
The Kaplan-Meier actuarial patient and graft survival rates are shown in Figure 1. The 1-year actuarial patient survival rate was 85.1% for D and 83.1% for T (P=0.77). Patients who died with a functioning graft were considered as graft loss. Fifteen (15.2%) patients in D and seventeen (16.8%) patients in T died during the follow-up period. The main causes of deaths were sepsis and multisystem organ failure (9 in D, 8 in T). Other causes of deaths included intracranial bleeding (1 in D, 3 in T), primary graft nonfunction (1 in D, 2 in T), cardiopulmonary (1 in D, 2 in T), cryptococcus (1 in D, 1 in T), hemorrhagic gastritis (1 in D), recurrent hepatitis C (1 in D), and status epilepticus (1 in T).
FIGURE 1.
Kaplan Meier actuarial survival curve for primary liver transplant patient and graft survival rates for tacrolimus and steroids (double-drug; D) therapy, v/s tacrolimus, steroids, and MMF (triple-drug; T) therapy.
Seven patients in D and 11 patients in T required retransplantation. The causes of retransplantation included: primary nonfunction (4 in D and 8 in T; not significant); hepatic artery thrombosis (3 in D); and recurrence of hepatitis C viral infection resulting in liver failure (2 in T). One patient in T was retransplanted because of graft failure due to posttransplant lymphoproliferative disease and graft-versus-host disease. The 1-year actuarial graft survival rate was 80.2% for D and 79.2% for T (Fig. 1).
In the follow-up period of 3.5 to 12 months, 41 (41.4%) patients in D and 32 (31.7%) patients in T had at least one episode of rejection. Eleven patients in D and 8 patients in T had more than one episode of rejection (Table 1). The total episodes of rejections were 57 in D and 44 in T. The median time to first episode of rejection was 16 days in D and 18 days in T. Freedom from rejection was observed in 58 patients (58.5%) in D and 69 (68.3%) in T (P=0.15). In D, 26 of 59 (44%) episodes of rejection responded to 1 g of methylprednisolone alone, whereas 27 of 59 (46%) required an additional steroid taper over the next 5 days. In four patients, OKT3 was used to control steroid-resistant rejection. In T, 17 (43%) episodes of rejection responded to 1 g of methylprednisolone, and 22 (50%) episodes required additional steroid taper over the next 5 days. Three of these episodes of rejection were treated with OKT3. No grafts in either group were lost due to rejection.
TABLE 1.
| Rejection | Random group |
Episodes of rejection | Freedom from rejection, n (%) |
||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Total | |||
| Early (n) | Double | 36 | 6 | 2 | 0 | 44 | |
| <3 Mo | Triple | 24 | 2 | 1 | 0 | 27 | |
| Late (n) | Double | 5 | 5 | 2 | 1 | 13 | |
| 3–12 Mo | Triple | 8 | 6 | 2 | 1 | 17 | |
| Total (n) | Double | 41 | 11 | 4 | 1 | 57 | 58 (58.5%) |
| Early – Late | Triple | 32 | 8 | 3 | 1 | 44 | 69 (68.3%) |
| Treatment of episodes of rejection |
|||||||
| SMa | SM– RC |
RC | OKT3 | ||||
| Double | 26 | 28 | 0 | 4 | 57 | ||
| Triple | 17 | 22 | 2 | 3 | 44 | ||
SM, 1 g of methylprednisolone: RC, 600 mg of methyprednisolone (or oral prednisone) over 5 days.
The maintenance dose of Tac and the whole blood trough concentration of Tac in both groups of patients was similar. However, the mean maintenance baseline dose of prednisone per patient was slightly lower in the triple-drug therapy group compared with the double-drug therapy group. This was not statistically significant (Table 2).
TABLE 2.
| Mean | Treatment group |
Pre-LTxa | PostLTx days | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 14 | 27 | 28 | 60 | 90 | 180 | 270 | 360 | |||
| Renal function | |||||||||||
| BUN (mg/dl) | Double | 21 | 52 | 56 | 51 | 45 | 29 | 28 | 29 | 26 | 23 |
| Triple | 22 | 45 | 45 | 49 | 44 | 28 | 24 | 27 | 26 | 23 | |
| Creatinine (mg/dl) | Double | 1.26 | 1.77 | 2 | 2.1 | 2.1 | 1.41 | 1.37 | 1.37 | 1.47 | 1.39 |
| Triple | 1.32 | 1.55 | 1.6 | 1.8 | 1.6 | 1.44 | 1.31 | 1.38 | 1.46 | 1.42 | |
| Hematology | |||||||||||
| Anemia % (Hct<25%) | Double | 12 | 14 | 25 | 19 | 22 | 9 | 4 | 4 | 0 | 3 |
| Triple | 13 | 12 | 15 | 20 | 22 | 7 | 6 | 2 | 2 | 0 | |
| Leukopenia % (WBC<4.000/mm3 | Double | 24 | 0 | 6 | 0 | 4 | 17 | 15 | 22 | 14 | 22 |
| Triple | 31 | 4 | 3 | 9 | 9 | 20 | 27 | 20 | 22 | 20 | |
| Thrombocytopenia % (platelets<50.000/mn3 | Double | 24 | 20 | 12 | 3 | 2 | 2 | 4 | 2 | 0 | 3 |
| Triple | 25 | 25 | 5 | 4 | 6 | 3 | 3 | 2 | 2 | 8 | |
| Baseline immunosuppression | |||||||||||
| Tacrolimus dose (mg/day) | Double | 8.4 | 9.5 | 10.5 | 9.9 | 8.6 | 8.2 | 7.2 | 5.7 | 5.3 | |
| Triple | 9.2 | 10.8 | 9.6 | 9.2 | 8 | 8 | 6.9 | 6.5 | 5.3 | ||
| Tacrolimus level (ng/ml) | Double | 15.8 | 14.5 | 15.3 | 14.5 | 11.9 | 12.2 | 11.1 | 10.1 | 10.6 | |
| Triple | 15.1 | 14.2 | 13.9 | 14.2 | 11.8 | 11.6 | 11.5 | 10.6 | 10.7 | ||
| Prednisone (mg/day) | Double | 20 | 18.6 | 16.1 | 14.2 | 10 | 18.7 | 5.7 | 4.6 | 3.3 | |
| Triple | 20 | 18.3 | 15.6 | 13.2 | 9.1 | 7.2 | 5.3 | 3.6 | 2 | ||
Abbreviation used in table: LTx, liver transplantation.
During the study period, 61 of the 101 patients who were randomized to T discontinued MMF for infection (n = 23: sepsis 12; cytomegalovirus 7; recurrent hepatitis C virus 3; and cryptococcus 1), myelosuppression (n = 20: leukopenia 12; anemia 7; and thrombocytopenia 1), gastrointestinal disturbances (n = 14: diarrhea 10; nausea/vomiting 2; anorexialprolonged ileus 1), and other miscellaneous reasons (n = 4). Mean time to discontinue MMF was 64 ± 82 days. During the same time, 28 of 99 patients who were randomized to D required the addition of MMF for ongoing acute rejection (16), nephrotoxicity (6), nephrotoxicity with rejection (3), neurotoxicity (2), neurotoxicity, and nephrotoxicity and rejection (1). The mean interval from OLT to the addition of MMF was 53 ± 80 days.
The rate of positive blood cultures was 40 (40.4%) in D versus 41 (40.6%) in T. Cytomegalovirus infection was 16.2% in D versus 17.8% in T. The rate of fungal infection was 10.1% in D versus 5% in T.
Before OLT, five patients who were subsequently randomized to T were on dialysis as compared with two in D. After OLT, 21 (all new onset) patients required dialysis in D, and 19 (14 new onset) required dialysis in T (P=0.188). The majority of patients with renal failure (either pre- or postOLT) improved; only one patient in D and two patients in T remain on dialysis (one of the patients in T was transplanted for hyperoxalosis and is awaiting kidney transplantation). The pre- and postOLT mean serum creatinine levels and blood urea nitrogen levels are shown in Table 2. Although the serum creatinine level was lower in the triple-drug therapy group during the first month compared with the double-drug therapy group (Table 2), this difference did not reach statistical significance (P=0.07).
A considerable number of patients were anemic (hematocrit <25%; 12% in D and 13% in T), leukopenic (leukocyte count <4,000/mm3; 24% in D and 31% in T), and/or thrombocytopenic (platelet count <50,000/mm3; 24% in D and 25% in T) before OLT. Most often these hematologic parameters were ascribed to hypersplenism. The percentage of patients with anemia, leukopenia, and/or thrombocytopenia before OLT and at various times after OLT is shown in Table 2 for both groups of patients.
Diarrhea was the most common gastrointestinal symptom observed in both groups of patients, surprisingly with equal proportions in both groups. Twenty-five patients in T and 24 patients in D experienced diarrhea in the postoperative period; only four of these patients in D experienced diarrhea after the addition of MMF for rejection. In T, 19 patients had diarrhea when they were on MMF, and the remaining 6 patients were not on MMF. In T, two patients were reported to have nausea and vomiting, one patient had anorexia, and one other patient experienced prolonged paralytic ileus.
A number of trials using MMF have been conducted in renal transplantation using CsA-based immunosuppression (6–8). This is the first report on a large randomized clinical trial of MMF with Tac in OLT patients. Although the principle of immunosuppressive therapies in liver and kidney transplantation is similar, the nature of liver transplant patients and the complexity of their postoperative management often dictates practical changes. For example, a significant number of patients with end-stage liver disease have portal hypertension and splenomegaly at the time of OLT, resulting in hypersplenism leading to thrombocytopenia, leukopenia, and anemia. The use of myelosuppressive agents, such as azathioprine, has historically been associated with further bone marrow suppression, leading to greater risk of postOLT infection (11). Similar concerns are apparent when using MMF, which can potentially depress bone marrow. Reduction in immunosuppression is a mainstay in addressing life-threatening infections (12). Sixty percent of the patients randomized to T discontinued the MMF for adverse events related to postOLT infection, suppressed formed blood elements, and gastrointestinal disturbances. MMF could have been continued in the presence of infection, however, in the absence of rejection and normal liver function it was felt that three antirejection medications are not necessary. The interim analysis has highlighted that many of these side effects were also seen in the D group.
Despite the concerns with the addition of a new immunosuppressive agent (MMF) in combination with another potent immunosuppressive agent (Tac), there was a trend to a lower rejection rate in T. In addition, the maintenance and cumulative doses of steroids were lower in T. Despite similar doses of Tac and trough concentrations of Tac, renal function seemed to be better preserved in T. Patient survival and graft survival were similar in both groups of patients, although there were more retransplants in T, mainly because of more marginal donors rather than because of the effect of MMF.
This interim report, comparing Tac and steroids versus Tac, steroids, and MMF has shown similar patient and graft survival rates in both groups. The rates of postoperative infection, pre- and postoperative hematological side effects, and gastrointestinal side effects were similar in both groups of patients. However, their presence posed an additional clinical management problem and led to discontinuation of MMF in nearly 60% of the patients in the triple-drug therapy group. There was a trend of a lower rate of rejection, improved renal function, and lower use of steroids in triple-drug therapy as compared with double-drug therapy. However, none of these parameters reached a statistical significance.
Acknowledgments
We thank William Irish for his help with the statistical analysis and generating the randomization codes.
Footnotes
This paper was presented at the 16 Annual Meeting of the American Society of Transplant Physicians, Chicago, IL, May 11–14, 1997.
Abbreviations: CsA, cyclosporine: D, tacrolimus and steroids: double-drug therapy; MMF, mycophenolate mofetil; OLT, orthotopic liver transplantation; T, tacrolimus. steroids. and mycophenolate mofetil: triple-drug therapy; Tac, tacrolimus.
REFERENCES
- 1.Fung JJ, Eliasziw M, Todo S, et al. The Pittsburgh randomized trial of tacrolimus vs. cyclosporine for liver transplantation. J Am Coll Surg. 1996;183:117. [PMC free article] [PubMed] [Google Scholar]
- 2.European FK506 Multicentre Liver Study Group. Randomized trial comparing tacrolimus (FK506) and cyclosporine in prevention of liver allograft rejection. Lancet. 1994;344:423. [PubMed] [Google Scholar]
- 3.The U.S. Multicenter FK506 Liver Study Group. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med. 1994;331:1110. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 4.Jain AB, Fung JJ. Cyclosporine and tacrolimus in clinical transplant: a comparative review. Clin Immunother. 1996;5:351–373. [Google Scholar]
- 5.The European Multicenter Renal Transplant Mycophenolate Mofetil Study Group. Placebo-controlled study of mycophenolate mofetil combined with cyclosporine and corticosteroids for prevention of acute rejection. Lancet. 1995;345:1321. [PubMed] [Google Scholar]
- 6.HW Sollinger for the US Renal Transplant Mycophenolate Mofetil Study Group. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. Transplantation. 1995;60(3):225. doi: 10.1097/00007890-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Schreiiber SL, Crabtree GR. The mechanisms of action of cyclosporine A and FK 506. Immunol Today. 1992;13:136. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 8.Allison AC, Almquist SJ, Muller CD, Eugui EM. In vitro immunosuppressive effects of mycophenalic acid and an ester prodrug, RS-61443. Transplant Proc. 1991;23:3143. [PubMed] [Google Scholar]
- 9.Friedman LM, Furberg CD, Demeb A. Fundamentals of clinical trials. St. Louis, MO: Mosby-Year Book; 1985. [Google Scholar]
- 10.Demetris AJ, Batts AP, Ferrell L, et al. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 11.Drummer JS, Hardy A, Poorsattar A, Ho M. Early infections in kidney, heart, and liver transplant recipients on cyclosporine. Transplantation. 1983;36:259. doi: 10.1097/00007890-198309000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Manez R, Kusne S, Linden P, et al. Temporary withdrawal of immunosuppression for life-threatening infections after liver transplantation. Transplantation. 1994;57:149. doi: 10.1097/00007890-199401000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]