Summary
Des-γ-carboxy prothrombin (DCP), a protein induced by vitamin K absence or antagonist-II (PIVKA-II) was measured by an enzyme immunoassay (E-1023) using anti-DCP monoclonal antibody in 92 patients with various hepatobiliary diseases. Thirty-six of the 38 patients (94.7%) with hepatocellular carcinoma (HCC) had abnormal DCP levels greater than 0.1 arbitrary unit (AU)/ml, but only 18 of the 35 patients (51.4%) had AFP greater than 100 ng/ml (suspicious levels for HCC). There was no correlation between plasma or serum DCP and serum alpha-fetoprotein (AFP) levels. Serum alpha fetoprotein was elevated (above 20 ng/ml) in 23 of the 35 patients (65.7%), and DCP was elevated in all of the remaining 12 patients with normal AFP. DCP levels returned to normal levels following curative hepatic resection or orthotopic liver transplantation for HCC. DCP is a useful tumor marker in the diagnosis and postoperative monitoring of patients with HCC.
Keywords: Hepatocellular carcinoma, Abnormal prothrombin, Des-γ-carboxy prothrombin, PIVKA-II
Alpha-fetoprotein (AFP) has long been used as a tumor marker for hepatocellular carcinoma (HCC). However, AFP is significantly elevated in the sera of less than half of the patients with HCC (1, 2). Another tumor marker, desgamma-carboxy prothrombin (DCP), has been reported to be useful in the diagnosis of HCC (3-6). This marker is also known as a protein induced by vitamin K absence or antagonist-II (PIVKA-II) or abnormal prothrombin.
DCP was originally found in the blood of patients who were deficient in vitamin K or who were receiving a vitamin K antagonist (3). In 1984 Liebman et al. (4) reported for the first time that serum DCP was elevated in patients with HCC. The method of DCP measurement has improved from a competitive radioimmunoassay with conventional polyclonal antibody, used by Liebman et al., to an enzyme-linked immunosorbent assay (ELISA) using a monoclonal antibody prepared by a cell hybridization technique described by Motohara et al. (5), and more recently to a double antibody sandwich system developed by Naraki et al. (6).
In this report we examined the clinical significance of plasma and serum DCP measurement in the diagnosis of HCC and the follow-up after curative surgery including orthotopic liver transplantation. Also reported here are other clinical conditions in which DCP may be elevated in the absence of HCC.
Materials and methods
Plasma DCP levels were measured in a total of 92 patients. The histological diagnoses of 92 patients are listed in Table 1. There were 46 patients with primary hepatic malignancy, 10 patients with metastatic tumors of various origins, and 36 patients with non-cancerous liver diseases. The diagnosis was confirmed by pathological examination in all of the patients. The absence of HCC in the 27 patients with cirrhosis and 7 patients with hepatitis was confirmed by a careful examination of the diseased liver after transplantation.
Table 1.
The histological diagnosis of 92 patients
| Hepatocellular carcinoma (nonFL-HCC) . . . . . . . . . . . . . . . . . . . . .34 |
| Fibrolamellar HCC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4 |
| Hepatoblastoma . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1 |
| Cholangiocarcinoma . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 |
| Other primary hepatic malignancy . . . . . . . . . . . . . . . . . . . . . . . . . . .5 |
| EHE (2), angiosarcoma (1), neuroendocrine tumor (1), leiomyosarcoma (1) |
| Metastatic liver tumor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10 |
| Colon CA (5), pancreas CA (1), glucagonoma (1), somatostatinoma (1), duodenal papilla CA (1), esophagus CA (1) |
| Benign hepatic tumor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2 |
| Cirrhosis of the liver (decompensated) . . . . . . . . . . . . . . . . . . . . . .27 |
| Severe hepatitis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7 |
| Total . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .92 |
EHE: Epithelioid hemangioendothelioma
Venous blood was drawn into a vacuum tube containing a 3.8% sodium citrate solution (9 parts of blood to one part of anticoagulant). The plasma was separated and immediately stored at −20°C. In a few cases serum, rather than plasma, levels of DCP were measured.
DCP was measured by the enzyme immunoassay (EIA) using a monoclonal antibody against DCP (E-1023: Eitest® MONO P-II, Eisai Company, Ltd. Tokyo) (6). One arbitrary unit (1 AU) is equivalent to 1 mg of purified prothrombin (7). Levels of DCP greater than 0.1 AU/ml were considered to be abnormally elevated (8).
Serum alpha-fetoprotein (AFP) was measured by AFP-EIA kit (Hybritech Inc. San Diego) and vitamin K-dependent blood coagulation factors (Factor II, VII, IX and X) were tested by Normotest (Hepaplastintest®, Eisai Company, Ltd, Tokyo). Serum total bilirubin was also measured at the same time.
Results
Plasma DCP levels were essentially equal (r = 0.97) to those in the serum, when they were measured simultaneously in 21 cases with liver tumor.
The DCP levels of patients with hepatocellular carcinoma (HCC) and those with other diseases are summarized in Figure 1. DCP was elevated (greater than 0.1 AU/ml) in 32 of the 34 patients with HCC (94.1%). Elevated DCP levels were also observed in all of the 4 patients with fibrlamellar HCC and in one patient with hepatoblastoma. None of the 2 patients with peripheral cholangiocarcinoma or the 5 patients with other primary hepatic malignancy had elevated DCP levels; nor did the 10 patients with metastatic liver tumor. The DCP was slightly elevated in one patient who had a large liver cell adenoma and two lesions of focal nodular hyperplasia.
Fig. 1.
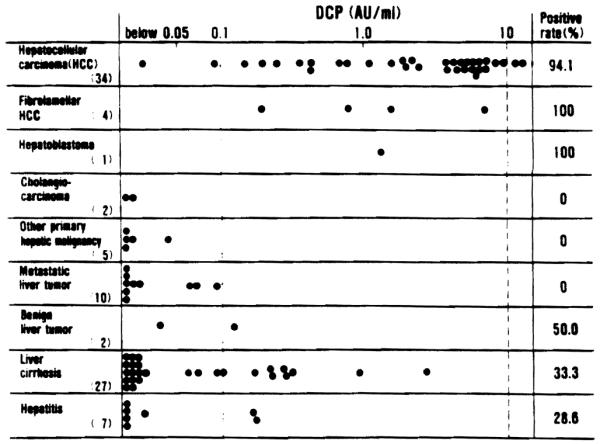
DCP levels in 92 patients with various diseases
The DCP was elevated in 9 of the 27 patients with liver cirrhosis (33.3%), and in 2 of the 7 patients with hepatitis (28.6%) who did not have any malignancy. All these patients with liver cirrhosis and hepatitis had very poor hepatic function, and were waiting for orthotopic liver transplantation.
Both AFP and DCP were measured at the same time in the 31 patients with non-fibrolamellar hepatocellular carcinoma (non-FL-HCC) and in the 4 patients with fibrolamellar HCC (FL-HCC), and the results are summarized in Figure 2. The AFP levels were higher than 20 ng/ml (normal AFP < ng/ml) in 23 (65.7%) of the 35 patients with hepatocellular carcinoma (HCC), and higher than 100 ng/ml (suspicious for HCC) in 18 (51.4%) of them. On the other hand, the DCP levels were above 0.1 AU/ml in 33 (94.3%) of the 35 patients with HCC.
Fig. 2.
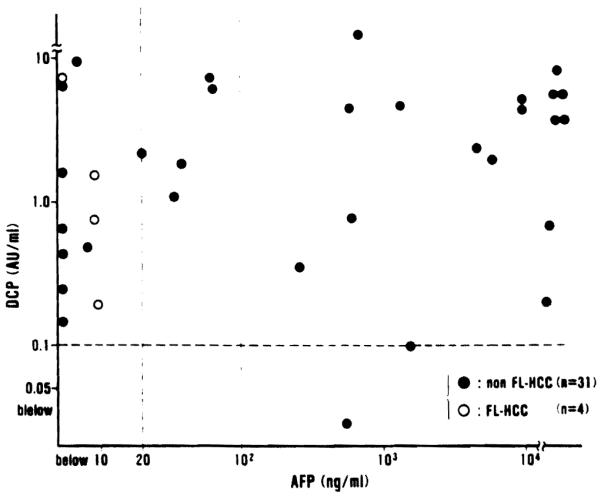
Relationship between DCP and AFP levels in 31 patients with non-fibrolamellar hepatocellular carcinoma (nonFL-HCC) and 4 patients with fibrolamellar hepatocellular carcinoma (FL-HCC)
It is worth noting that all 12 patients whose AFP levels were within normal range (below 20 ng/ml) and all 17 patients with the AFP levels of less than 100 ng/ml had elevated DCP levels. Thus, in all of the 35 patients with HCC, either AFP or DCP was abnormally elevated. All of the 4 patients with fibrolamellar HCC had normal levels (less than 20 ng/ml) of AFP, but their DCP levels were markedly elevated.
There was no statistically signficant correlation between the AFP levels and the DCP levels (r = 0.08, Fig. 2).
In the 12 patients with DCP-positive hepatocellular carcinoma (HCC), the plasma levels of DCP were measured after 4 hepatic resections and 8 orthotopic liver transplantations. As shown in Figure 3, the DCP levels decreased sharply after curative operation in all cases, and the levels returned to below 0.1 AU/ml within 3 days after transplantation and 3 weeks after hepatic resection.
Fig. 3.
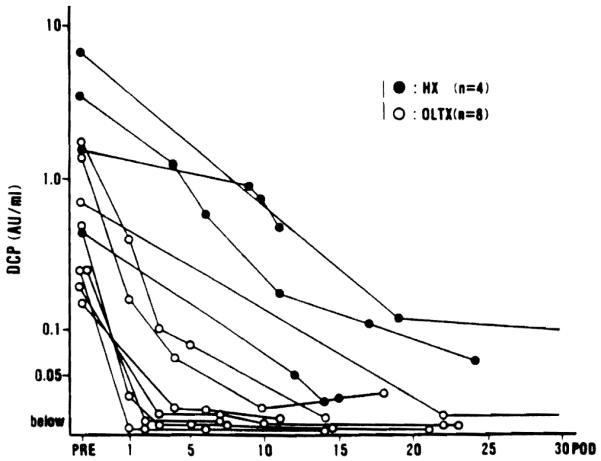
Postoperative changes in DCP level in 4 patients after hepatic resection (HX) and in 8 patients after orthotopic liver transplantation (OLTx) for hepatocellular carcinoma
In order to clarify the influence of vitamin K-deficiency upon DCP levels the relationship between DCP and serum bilirubin was examined in Fig. 4, and that between DCP and Normotest in Fig. 5. High levels of serum total bilirubin were observed in many of the patients with advanced cirrhosis and severe hepatitis waiting for liver transplantation, and these patients had abnormal DCP levels in the absence of hepatic malignancy. In patients with hepatocellular carcinoma (HCC) extreme hyperbilirubinemia was rare (Fig. 4). Low levels of Normotest (less than 50%) were observed in 21 out of 34 patients (61.8%) with advanced cirrhosis and severe hepatitis, but only in 7 of the 26 patients (26.9%) with hepatocellular carcinoma (HCC) (Fig. 5).
Fig. 4.
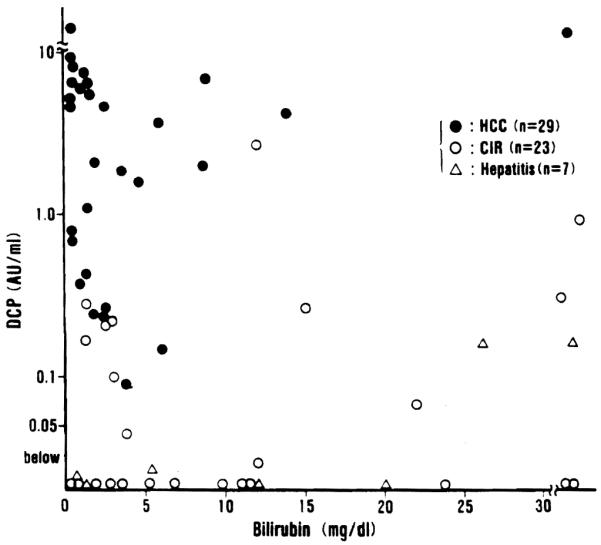
Relationship between DCP and serum total bilirubin in 29 patients with hepatocellular carcinoma (HCC), 23 patients with liver cirrhosis (CIR) and 7 patients with hepatitis
Fig. 5.
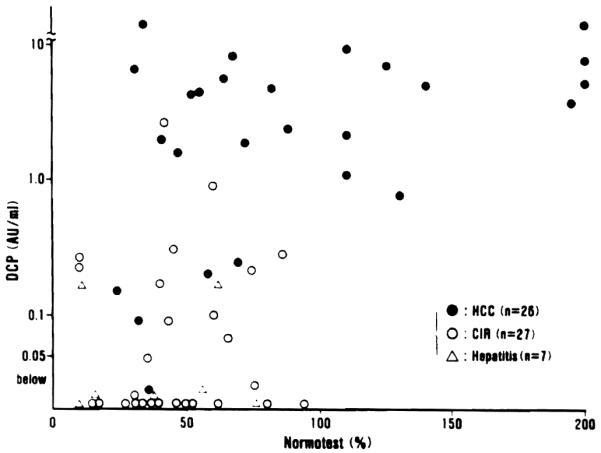
Relationship between plasma DCP and Normotest levels in 26 patients with hepatocellular carcinoma (HCC), 27 patients with advanced liver cirrhosis (CIR) and 7 patients with hepatitis
Discussion
Vitamin K, a fat soluble vitamin, is derived from food and intestinal microflora. It is absorbed through the intestinal wall in the presence of bile and pancreatic juice. In the liver, vitamin K is required to convert the precursor of prothrombin to active prothrombin by carboxylation of specific glutamic acid (Glu) residues in the precursor protein (3). The precursor (DCP) of prothrombin contains 10 Glu residues in the NH2-terminal domain. When these residues undergo carboxylation in the presence of vitamin K, they are converted to gamma carboxyglutamic acid (Gla) residues. The prothrombin in which all Glu residues are converted to Gla residues is designated as normal prothrombin, and that with incomplete conversion as abnormal prothrombin (DCP or PIVKA-II).
The exact mechanism by which abnormal prothrombin (DCP) is produced by hepatocellular carcinoma (HCC), has not been determined. However, Okuda et al. (9) detected abnormal prothrombin in the culture medium of hepatoma cells, and Motohara et al. (10) stained abnormal prothrombin in the hepatoblastoma cells. The normalization of abnormal prothrombin (DCP) after curative operation, as shown in Fig. 3, is further evidence that the HCC is a source of its production.
The half-life of abnormal prothrombin (DCP) was reported to be approximately 30 hours in the patients of vitamin K deficiency (10). However, the half-life was 3 to 4 days after curative hepatic resection in our four patients (Fig. 3). Rapid normalization of DCP in our 8 patients after orthotopic liver transplantation (Fig. 3) may reflect the dilution or washout effect of a large amount of transfusion during the transplant procedure. The long half-life of DCP in cancer patients is possibly due either to the impaired hepatic function after major hepatic resection, or to the molecular structural difference (11) between DCP in vitamin K deficiency and that produced by cancer. Further investigation is needed to establish the mechanism of DCP production by cancer cells and to examine the possible difference in the molecular structure of DCP.
Ninety-five percent of our 38 patients with hepatocellular carcinoma (HCC) had DCP levels greater than 0.1 AU/ml. Fujiyama et al. (8) reported that 63% of their 120 patients with HCC had DCP levels greater than 0.1 AU/ml, and Hattori et al. (12) reported an incidence of 53% in their 558 patients with HCC. The difference in the positive rates between the present report and reports from Japan (8, 9, 12) may suggest that HCCs in the United States and those in Japan differ in terms of cell function or etiology, or else this may be due simply to the difference in tumor size or stage. And, indeed, small HCCs are diagnosed more frequently in Japan than in the United States.
Our experience with DCP measurements clearly indicates that DCP is a better tumor marker for HCC than AFP (94.3%, versus 51.4%). Japanese investigators (8, 9, 12) reported that AFP was elevated in 36.5% to 53.6% of their patients with HCC, and DCP in 48% to 57.7%, and that either AFP or DCP was elevated in 65.4% to 71.6%. They therefore concluded that both AFP and DCP should be measured in patients with HCC. The present authors agree with this conclusion.
Although DCP is an excellent tumor marker for HCC; it is not a tumor-specific antigen. There are other conditions in which DCP is abnormally high. Patients with advanced cirrhosis, severe hepatitis or obstructive jaundice also have elevated levels of DCP in the absence of HCC, as described in the present report and in others (4, 8, 9, 12). Under these conditions, marked hyperbilirubinemia, a low Normotest (indication of vitamin K-dependent coagulation factors II, VII, IX, and X deficiency) and/or very poor liver function tests, are also present. Therefore, the abnormal DCP levels must be carefully interpreted after detailed liver function tests and blood coagulation factors.
Nevertheless, abnormal prothromin (DCP, PIVKA-II) is a useful tumor marker for hepatocellular carcinoma.
Footnotes
Supported by Research Grants from the Veterans Administration and Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, Maryland
References
- 1.Foli AK, Sherlock S, Adinolfi M. α1-fetoprotein in patients with liver disease. Lancet. 1961;ii:1267–1269. [Google Scholar]
- 2.Okuda K, Kotoda K, Obata H, et al. Clinical observations during a relatively early stage of hepatocellular carcinoma, with special reference to serum alpha-fetoprotein levels. Gastroenterology. 1975;69:226–234. [PubMed] [Google Scholar]
- 3.Stenflo J, Suttie JW. Vitamin K-dependent formation of carboxyglutamic acid. Ann. Rev. Biochem. 1977;46:157–172. doi: 10.1146/annurev.bi.46.070177.001105. [DOI] [PubMed] [Google Scholar]
- 4.Liebman HA, Furie BC, Tong MJ, et al. Des-γ-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. New Engl. J. Med. 1984;310:1427–1431. doi: 10.1056/NEJM198405313102204. [DOI] [PubMed] [Google Scholar]
- 5.Motohara K, Kuroki Y, Kan H, Endo F, Matsuda I. Detection of vitamin K deficiency by use of an enzyme-linked immunosorbent assay for circulating abnormal prothrombin. Pediat. Res. 1985;19:354–357. doi: 10.1203/00006450-198519040-00008. [DOI] [PubMed] [Google Scholar]
- 6.Naraki T, Watanabe K, Shimosuru Y, et al. Development and evaluation of EIA Kit for the detection of PIVKA-II using a double antibody sandwich system; monoclonal antibody to PIVKA-II and polyclonal antibody to prothrombin. Clin. Immunol. (Japanese) 1986;18:479–492. [Google Scholar]
- 7.Laurell CB. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal. Biochem. 1966;15:45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- 8.Fujiyama S, Morishita T, Hashiguchi O, Sato T. Plasma abnormal prothrombin (des-γ-carboxy prothrombin) as a marker of hepatocellular carcinoma. Cancer. 1988;61:1621–1628. doi: 10.1002/1097-0142(19880415)61:8<1621::aid-cncr2820610820>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 9.Okuda H, Obata H, Nakanish T, Furukawa R, Hashimoto E. Production of abnormal prothrombin (des-γ-carboxy prothrombin) by hepatocellular carcinoma. J. Hepatol. 1987;4:357–363. doi: 10.1016/s0168-8278(87)80546-9. [DOI] [PubMed] [Google Scholar]
- 10.Motohara K, Endo F, Matsuda I, Iwamasa T. A carboxy prothrombin (PIVKA-II) as a marker of hepatoblastoma in infants. J. Pediat. Gastroenterol. Nutr. 1987;6:42–45. doi: 10.1097/00005176-198701000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Liebman HA. Isolation and characterization of a hepatoma associated abnormal (des-γ-carboxy) prothrombin. Cancer Res. 1989;49:6493–6497. [PubMed] [Google Scholar]
- 12.Hattori N, Ohmizo R, Unoura M, Tanaka N, Kobayashi K, PIVKA-II collaborative working group Abnormal prothrombin measurements in hepatocellular carcinoma. J. Tumor Marker Oncol. 1988;3:207–216. [Google Scholar]


