Abstract
Sixty-eight primary liver grafts were analyzed to see whether adenine nucleotides (AN: ATP, ADP, and AMP) or purine catabolites (PC: adenosine, inosine, hypoxanthine, and xanthine) of tissue or effluent can predict primary graft nonfunction. AN, PC, and nicotinamide adenine dinucleotide, oxidized form (NAD +) of the tissue before (pretransplant) and after graft reperfusion (post-transplant) and of the effluent were analyzed. The graft outcome was classified into two groups (group A: successful, n = 64; group B: primary nonfunctioning, n = 4). No significant differences were observed in pretransplant measurements between groups A and B, whereas ATP, ADP, total AN, total AN + total PC (T) and NAD +, in post-transplant tissues, were significantly higher in group A. Xanthine in the effluent was significantly higher in group B than in group A. ATP, ADP, total AN, T, and NAD + in post-transplant tissue were significantly associated with primary graft nonfunction by logistic regression analysis.
Keywords: Adenine nucleotides, viability, liver · Viability, liver, adenine nucleotides · Liver transplantation, nonfunction Nonfunction, liver transplantation
Introduction
With the widespread use of orthotopic liver transplantation and the acute organ shortage, livers at high risk of developing primary nonfunction should be discarded prior to implantation. Short of this, it is important to diagnose primary nonfunction as early as possible in order to increase the time interval for procuring a new liver for retransplantation.
Many animal studies have demonstrated the inverse correlation between liver viability and hepatocyte adenosine triphosphate (ATP) levels [6, 13]. These studies have shown that cellular ATP level or total adenine nucleotide [TAN: ATP + adenosine diphosphate (ADP) + adenosine monophate (AMP)] decreases during warm ischemia and correlates well with graft viability. In contrast to warm ischemia, tissue measurements of cellular ATP and TAN during periods of cold ischemia did not correlate with organ viability [6, 11, 17, 19].
Lanir et a1. [12] and González et al. [4] have shown that successful liver grafts preserved in Euro-Collins (EC) solution have higher concentrations of ATP, ADP, and energy charge [EC: (ATP + l/2ADP)/(ATP + ADP + adenosine monophosphate)] before implantation than do failed grafts. However, Kamiike et a1. have shown that only TAN of pretransplant (pre-tx) grafts preserved in EC solution was higher in successful grafts than in unsuccessful grafts. Higher concentrations of ATP and TAN were found in post-transplant (post-tx) liver biopsies obtained from viable grafts than from nonviable grafts [10]. However, “successful liver engraftment” in these studies was defined arbitrarily by the hepatic enzyme levels after transplantation, and the investigators did not report primary nonfunction. Thus, it is still controversial whether low adenine nucleotide (AN) levels of pre- or post-tx donor liver tissue can predict primary nonfunetion.
In this study, pre- and post-tx human liver grafts preserved in University of Wisconsin (UW) solution were examined to determine whether tissue AN and purine catabolite (PC) levels showed any correlation with primary nonfunction or could predict primary nonfunction. The effluent from the graft was also examined to determine whether PC levels in the effluent could predict primary nonfunction.
Materials and methods
Case material
From 6 October 1990 to 31 December 1990, 134 orthotopic liver transplantations were performed at Presbyterian University Hospital, University of Pittsburgh. Of the 134 patients, tissue biopsies of 97 grafts in 88 patients (56 male and 32 female, mean age 48.1 ± 13.3 years, range 13–74 years) were successfully collected. Of these 97 grafts, 81 grafts were primary, 13 were secondary, and 3 were tertiary. Thirteen grafts were excluded from the study due to either technical problems (n = 8: 5 vascular problems, 2 severe biliary complications, and 1 technical difficulty) or positive cytotoxic crossmatches (n = 5). These primary grafts were then classified into two groups according to graft outcome during the first 7 postoperative days: group A, successful graft; group B, primary nonfunctioning (PNF) graft. While there is no uniform definition of PNF, a diagnosis of PNF was made if the graft never demonstrated evidence of initial function following transplantation and if retransplantation had to be performed urgently to prevent the patient’s death within 1 week of the initial transplant. A diagnosis of PNF could not be made when these were technical complications during the transplant procedure. Clinical findings strongly associated with PNF included stage 4 coma, sluggish or no bile flow, progressive jaundice, uncorrectable coagulopathy. metabolic acidosis, and renal failure. The pathology of nonfunctioning grafts mainly showed ischemia/preservation injury, which was represented by small infarcts und/or zonal hepatocellular coagulative necrosis (either centrilobular or periportal) or severe cholestasis without evidence of rejection (Table 1).
Table 1.
Summary of failed grafts with respect to histopathology and liver function
| Graft no.a |
Age | Sex | Graft survival (day) |
Highest ASTb (U/l) |
Highest PTc (sec) |
Highest T-Bilc (mg/dl) |
Histopathology |
|---|---|---|---|---|---|---|---|
| 22 | 55 | F | 1 | 5028 | 20.0 | 13.5 | Widespread hemorrhagic necrosis |
| 26 | 66 | M | 4 | 4844 | 27.5 | 14.6 | Centrilobular congestion and necrosis |
| 60 | 58 | F | 4 | 3669 | 14.5 | 8.2 | Diffuse microvesicular steatosis and some areas of hemorrhagic necrosis |
| 78 | 56 | M | 1 | 7505 | 18.5 | 7.6 | Distortion of sinusoidal architecture with extracellular fat globules and centrilobu- lar congestion |
Graft no. is a serial number of the graft in this study in the order of sampling
AST increase is the difference in AST from preoperative value and highest value until retransplantation
Highest postoperative values until retransplantation
Operative procedures
Donor operation
All liver procurements were performed by in situ infusion with UW solution [24] as part of multiple organ procurements [20, 21). All of the liver grafts in this study were preserved with UW solution at 4 °C.
Transplant procedure
Recipient hepatectomy and hepatic replacement were performed using standard techniques [3]. Before revascularizing the new liver, the organ was perfused with 300–500 ml of cold, lactated Ringer’s solution to remove air and excess potassium. The anhepatic phase was defined as the interval between the interruption of the recipient’s hepatic circulation and the restoration of portal or arterial circulation to the graft.
Immunosuppression
An intravenous dose of 0.1 mg/kg FK 506 was infused over 24 h, starting in the operating room, and repeated every 24 h until oral intake began. One gram of intravenous methylprednisolone was given to all patients after reperfusion of the liver allograft. A daily dose of 20 mg methylprednisolone was given thereafter.
Sampling
Liver tissue
Pretransplant wedge biopsies (about 50 mg) were performed just before starting the suprahepatic vena cava anastomosis (n = 68). The biopsies were cut and promptly stored in liquid nitrogen within 10 s.
Post-transplantation wedge biopsies (approximately 50 mg) were taken 1.685 ± 0.655 h after graft revascularization and cut and stored in the same way as in the pre-tx biopsies (n = 58).
Normal liver tissue from six patients with cellular carcinoma (without liver cirrhosis or metastatic liver carcinoma) was taken in the same way as the olher biopsies. These served as controls.
Effluent
After flushing the liver with cold, lactated Ringer’s solution but before finishing the anastomosis of the infrahcpatic vcna cava, the first 10 ml of the effluent was collected and centrifuged for 15 min at 1500 g at 4 °C. The supernatant was frozen at −70°C. (n = 40).
Histopathology
Histopathology of the failed grafts was studied to determine the extent and nature of liver damage. Tissues were fixed with buffered formalin and stained with hematoxylin-eosin.
Analytical methods
All of the specimens were processed at 4 °C, unless otherwise indicated.
Tissue extraction
The tissue biopsies were taken out of the liquid nitrogen, weighed, and homogenized in 1 ml of cold 6 % perchloric acid containing 0.8 mM EDTA with Polytron homogenizer (Brinkmann, Westbury, N. Y., USA). The homogenates were centrifuged for 10 min at 10000 g. The supernatants were adjusted to a pH of 4–6 with 69 % K2CO3 solution, centrifuged again for 15 min at 10,000 g, and the supernatants stored at −70 °C.
Effluent extraction
The effluent was mixed with the same volume of 6% perchloric acid solution containing 0.8 mM EDTA and vortexed and centrifuged for 10 min at 10000 g. The supernatant was collected, its pH adjusted with 69 % K2CO3 solution to 4–6, and centrifuged again for 15 min at 1500 g. The supernatant was then frozen at −70° C.
High-performance liquid chromatography (HPLC)
The separation of AN, PC, and NAD + was performed by single-run-HPLC using the modified method of Wynants et al. [27]. Extracted samples were analyzed with Waters’ HPLC system [Model 501 pumps, Model 484 absorbance module (path length 10 mm), and Model 700 WISP system; Waters Chromatography Division/Millipore, Milford, Mass., USA] equipped with a Maxima 820 chromatography workstation (Waters). Reverse phase column [LiChrospher 100 RP-18 (5 μm), 4 mm × 250 mm; E.Merk, Darmstadt, Germany] was used with a precolumn (RCSS Guard-PAK; Waters) at 26 °C. The mobile phases consisted of solution A, a 0.15 mol/l ammonium dihydrogen phosphate buffer, pH 5.7, and solution B, acetonitrile and methanol (50:50) containing 1 % triethanolamine. The elution of the first 4 min was isocratic of solvent A followed by linear gradient to 82 % of solution A and 18 % of solution B for 16 min, and then returned to isocratic separation with 100 % of solvent A for 20 min at a now rate of 0.8 ml/min. Thc eluted PCs were monitored at 254 nm.
Statistical analysis
The Mann-Whitney U-test, a nonparametrie equivalent of the standard. two-sample t-test, was used to compare median liver function values between grafts in groups A and B. AN and PC in pre-tx biopsies were compared between grafts in groups A and B and those in a control group using the Kruskal-Wallis test, a nonparametric equivalent to the one-way analysis of variance. The Kruskal-Wallis test was also used to compare AN and PC post-tx biopsies between grafts in groups A and B and a control. If there was evidence to support a significant difference among the groups, then the Mann-Whitney U-test was used as a post hoc technique. Comparison of values between pre-tx biopsies and post-tx biopsies was carried out using a Wilcoxon signed ranks test. Logistic regression analysis was used to predict the probability of PNF based on AN, PC, and NAD + values [26].
Results
Measurement of AN, PC, and NAD + with HPLC
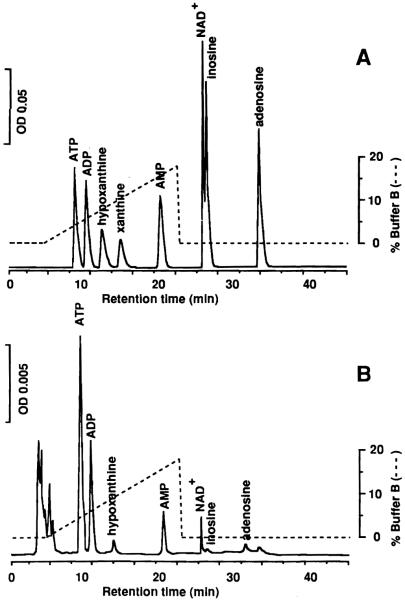
As shown in Fig. 1, all components of AN, PC, and NAD + were well separated, except for the poor separation of inosine (INO) and NAD +. Even with less than 50 mg of tissue, each peak was quantitated satisfactorily.
Fig.1A, B.
Elution profiles of AN, PC and NAD + with HPLC. Column: reverse-phase column 4 mm × 250 mm. Injection volume: 10 μl. Flow rate: 0.8 ml/min. Column temperature: 26°C. AN, PC and NAD + were detected bv absorbance at 254 nm: A standard mixturc: 1.0 μM of AN, PC, and NAD +; B normal human liver tissue. Tissue weight was 63 mg
Failed grafts
Table 1 shows liver function results and histopathological findings of group B grafts (n = 4). These grafts failed as a result of PNF and the patients required retransplantation within 4 days. Two patients died after retransplantation of multiple organ failure. Aspartate aminotransferate (AST) values in the failed grafts were higher than corresponding values in successful group A grafts (n = 64; Table 2).
Table 2.
Comparison of the two groups with respect to liver function, renal function, and gas analysis. CIT, Cold ischemia time; WIT, warm ischemia time; LSP, lowest systolic pressure
| Group A (n = 64) |
Group B (n = 4) |
|
|---|---|---|
| Donor | ||
| AST (U/l) | 64.67 ± 5.10 | 80.25 ± 21.04 |
| ALT(U/l) | 47.52 ± 5.80 | 60.00 ± 6.25 |
| PT | 13.88 ± 0.29 | 14.70 ± 1.02 |
| PTT | 31.07 ± 1.99 | 29.67 ± 6.92 |
| T-Bil (mg/dl) | 0.919 ± 0.095 | 0.975 ± 0.246 |
| LDH (U/l) | 494.4 ± 67.78 | 1089 ± 607 |
| pO2 (mm Hg) | 228.2 ± 20.25 | 128.8 ± 35.32a |
| BUN (mg/dl) | 14.28 ± 1.046 | 19.33 ± 2.848 |
| Cr (mg/dl) | 1.16 ± 0.061 | 1.25 ± 0.38 |
| LSP (mm Hg) | 82.26 ± 3.534 | 78.25 ± 7.261 |
| Recipient | ||
| Age (years) | 47.25 ± 1.65 | 58.75 ± 2.50 |
| CIT (h) | 13. 94 ± 0.415 | 14.13 ± 0.446 |
| WIT (min) | 63.7 ± 15.20 | 56.75 ± 7.05 |
| AST (U/l) | 1444 ± 213.3 | 5262 ± 1612a |
| ALT (U/l) | 1526 ± 247.8 | 2499 ± 990.9 |
| PT | 20.37 ± 2.83 | 20.13 ± 5.44 |
| PTT | 33.68 ± 1.49 | 42.73 ± 2.47 |
P < 0.05 versus group A by Mann-Whitney U-test
Comparison of two groups
The condition of the donor and of the recipient in both groups with respect to hepatic, renal cardiac, and pulmonary functions is summarized in Table 2. There were no significant differences between the two groups except for donor pO2 and recipient AST.
Graft outcome versus AN, PC, and NAD +
Biopsies
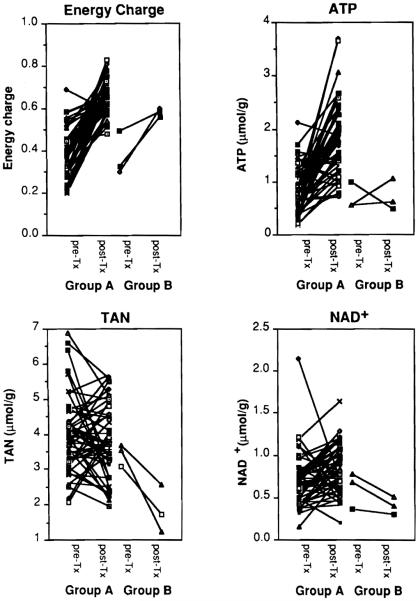
AN and PC in pre- and post-tx biopsies are summarized in Table 3 and Fig.2. Despite varying periods of cold ischemia (9.8–21.2 h), ATP for both groups decreased to less than half that of the control group. After reperfusion. ATP in group A recovered to the level of the control group; however, ATP levels remained statistically lower in group B (P = 0.0099 for group A vs group B). Also, ADP in group B decreased significantly as compared to ADP in group A after reperfusion. For both groups AMP increased more than three times that of the control group during cold ischemia and decreased to normal levels after reperfusion. There was no evidence to support the hypothesis that TAN levels changed during cold ischemia. After reperfusion, TAN decreased significantly in group B (P = 0.0109 for group A vs B). During cold ischemia there was a tenfold increase in PC and total PC (TPC). Both PC and TPC decreased significantly following reperfusion. After reperfusion, however, PC values remained higher in group A than in group B. Total AN + total PC (T) increased during cold preservation. The increase was largely due to the increase in PC. After reperfusion, the T of group A grafts returned to the control level, whereas the T of group B grafts was significantly lower than that of group A (P= 0.0072 for group A vs B). NAD + remained unchanged during cold ischemia in both groups, whereas it increased in group A and decreased in group B after reperfusion (P= 0.012 for group A vs group B post-Tx).
Table 3.
Adenine nucleotides and purine catabolites of pre- and post-transplantation biopsies. Values indicate mean ± SEM. EC, Energy charge; ATP, adenosine triphosphate; ADP, adenosine diphosphate; AMP, adenosine monophosphate; ADE, adenosine; lNO, inosine; HX, hypoxanthine; X, xanthine; TAN, total adenine nucleotide; TPC, total purine catabolite; T, TAN + TPC; NAD +, nicotinamide adenine dinucleotide, oxidized form
| Control (μmol/g) | Pre-tx (μmol/g) |
Post-tx (μmol/g) |
|||
|---|---|---|---|---|---|
| Group A (n = 68) |
Group B (n = 4) |
Group A (n = 58) |
Group B (n = 3) |
||
| EC | 0.713 ± 0.0465 | 0.384 ± 0.0133* | 0.362 ± 0.0445* | 0.655 ± 0.00963 | 0.583 ± 0.0108 |
| ATP | 2.080 ± 0.298 | 0.853 ± 0.0517* | 0.640 ± 0.123* | 1.77 ± 0.0929 | 0.712 ± 0.172** |
| ADP | 1.17 ± 0.0628 | 1.39 ± 0.0814 | 1.12 ± 0.125 | 1.45 ± 0.0524 | 0.726 ± 0.127*, ** |
| AMP | 0.445 ± 0.115 | 1.93 ± 0.190* | 1.53 ± 0.204* | 0.549 ± 0.0302 | 0.398 ± 0.0829 |
| ADE | 0.0358 ± 0.00899 | 0.423 ± 0.0420* | 0.348 ± 0.0146* | 0.0916 ± 0.00667* | 0.0593 ± 0.00145 |
| INO | 0.121 ± 0.0198 | 1.18 ± 0.0708* | 1.20 ± 0.0774* | 0.314 ± 0.0273* | 0.161 ± 0.0350 |
| HX | 0.118 ± 0.0244 | 1.14 ± 0.135* | 1.21 ± 0.210* | 0.473 ± 0.0668* | 0.161 ± 0.0913 |
| X | 0.0087 ± 0.0052 | 0.216 ± 0.0296* | 0.285 ± 0.0511* | 0.0538 ± 0.01 09 | 0.0713 ± 0.0270 |
| TAN | 3.69 ± 0.223 | 4.17 ± 0.285 | 3.30 ± 0.174 | 3.77 ± 0.138 | 1.84 ± 0.379*, ** |
| TPC | 0.284 ± 0.0336 | 2.95 ± 0.232* | 3.04 ± 0.271* | 0.932 ± 0.0905* | 0.452 ± 0.114 |
| T | 3.97 ± 0.233 | 7.12 ± 0.49* | 6.34 ± 0.309* | 4.704 ± 0.167 | 2.29 ± 0.426*, ** |
| NAD + | 0.632 ± 0.0440 | 0.671 ± 0.364 | 0.582 ± 0.0900 | 0.821 ± 0.0382 | 0.406 ± 0.0609* |
P < 0.05 vs control
P < 0.05 vs group A of the same biopsy
Fig.2.
Tissue energy charge, ATP, total adenine nucleotide (TAN), and NAD + levels in pre-transplant (pre-tx) and post-transplant (post-tx) biopsies
Effluent
Scattergrams of hypoxanthine (HX) and xanthine (X) in the effluent are shown in Fig.3. HX, INO, and X were abundant in this order. Adenosine (ADE) was hardly detected even though UW solution contains 5 mm of ADE. All of these PCs were higher in group B, although a significant difference was obtained only for X (P= 0.045).
Fig.3.
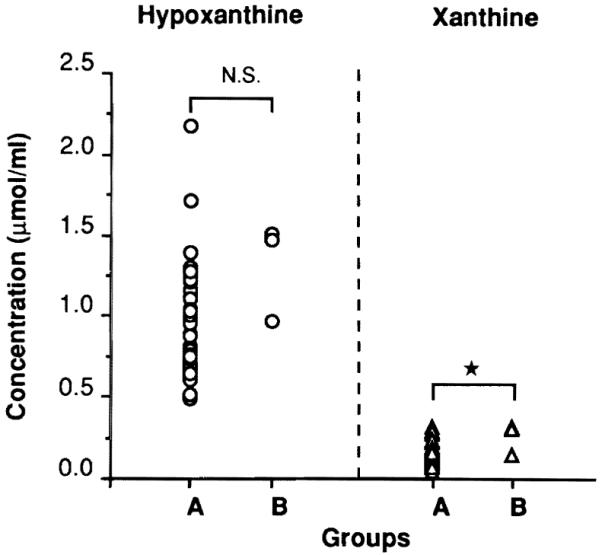
Scattergrams of hypoxanthine (HX) and xanthine (X) in the effluent. * P = 0.045
Predictability of primary nonfunction (PNF)
Logistic regression analysis was used to test whether PNF was associated with post-tx AN and PC values. There were five factors significantly associated with PNF and the results are summarized in Table 4. All factors have odds ratios significantly less than 1. This implies that a decrease in any one of these values is associated with an increased likelihood of PNF. Figure 4 shows logistic regression curves of these parameters. From these analyses it was determined that when the graft has post-tx ATp, ADP, NAD, or TAN less than 0.518, 0.529, 0.137, or 1.444 μmol/g, respectively, the likelihood of PNF within 1 week is more than 80 %.
Table 4.
Adenine nucleotide and purine catabolite values associated with acute graft failure. ATP, Adenosine triphosphate; ADP, adenosine diphosphate; TAN, total adenine nucleotides; T, TAN + total purine catabolite; NAD +, nicotine-amide adenine dinucleotide
| Measurement | Group A (μmol/g) |
Group B (μmol/g) |
Odds ratio | P value |
|---|---|---|---|---|
| post-ATP | 1.77 ± 0.093 | 0.712 ± 0.172 | 0.0012 | 0.0007 |
| post-ADP | 1.45 ± 0.052 | 0.726 ± 0.127 | 0.0008 | 0.0010 |
| post-TAN | 3.77 ± 0.138 | 1.84 ± 0.379 | 0.0447 | 0.0007 |
| post-T | 4.70 ± 0.167 | 2.29 ± 0.426 | 0.0156 | 0.0002 |
| post-NAD + | 0.821 ± 0.038 | 0.406 ± 0.061 | 0.0004 | 0.0064 |
Fig.4.
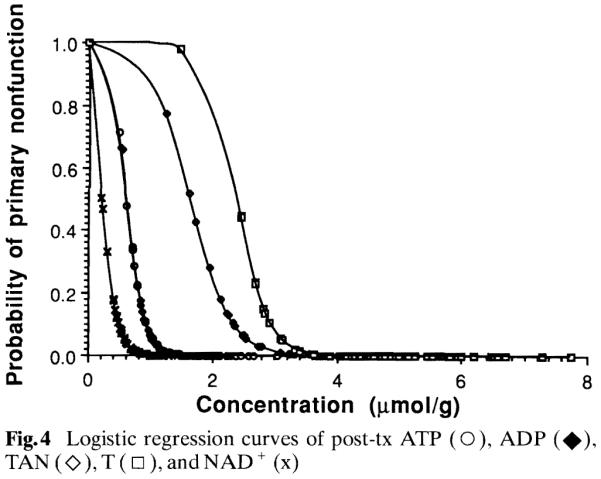
Logistic regression curves of post-tx ATP (○), ADP (◆), TAN (◇), T (□), and NAD + (x)
Discussion
Prolonged warm ischemia causes irreversible injury and necrosis of the liver, which is well documented by the decrease in cellular ATP and TAN levels [6, 19] and the retardation of the recovery of cellular ATP after reperfusion [6, 13, 17]. This irreversibility is partially due to the loss of mitochondrial oxidative phosphorylation capacity [14, 25]. In addition to a decrease in AN, PC accumulates during warm ischemia, and this plays an important role in superoxide generation after reperfusion. The superoxide then causes derangement of sinusoidal lining cells, disturbance of microcirculation [1], and subsequent retardation of ATP recovery upon reperfusion. In this context, it has been pointed out that cellular ATP and TAN levels and their recovery rates are good indices of the viability of the ischemic liver [6, 9, 13].
Many studies have attempted to apply the index of liver viability used in warm ischemia to cold ischemic injury [6, 10, 12, 18, 19, 23]. However, animal studies in the rat and dog have demonstrated that cold-preserved, viable livers have extremely low ATP values when measured by HPLC [6, 18]. Thus, neither ATP nor TAN levels during cold preservation in UW solution could predict graft outcome. On the other hand, tissue ATP detected by nuclear magnetic resonance spectroscopy (MRS), which can detect cytosolic ATP, seems to be predictive [5]. There are two main differences between these two measurements. The HPLC method detects all of the ATP in the tissue, whereas the MRS method only detects cytosolic-free ATP. Moreover, since tissue biopsies are very small for the HPLC, the error in the measurements could be larger than that of the MRS method, which can detect the signal of β-ATP from the wider part of the liver. Although it is not clear what is responsible for the different conclusions, the usefulness of tissue ATP level during cold ischemia for the viahility estimation is still controversial.
As the degradation of AN is halted at the level of AMP in cold ischemia, as shown by Harvey et aI., HX and X levels and their increase with perfusion were much lower than those of warm ischemic livers [6]. This study suggests that the change in TAN expressed by cold ischemic injury is not the same as the change in TAN expressed by warm ischemia. As shown in the present study, ATP levels were decreased, AMP levels were reciprocally increased, and TAN levels were kept almost constant. This indicates that degradation of ATP was halted at the AMP level in clinical liver transplantation mainly due to cold ischemic injury. However, a considerable number of PCs were detected in the preserved graft compared with the number of PCs in the control grafts. This is probably due to the 5 mm of ADE contained in UW solution, which may be metabolized to INO, HX, and X during cold ischemia.
In 1988, two reports appeared, one showing that pre-tx values of ATP, ADP, and TAN were associated with post-tx success [12], and the other reporting that only pre-tx TAN was predictive of graft outcome. The latter reported that post-tx ATP and TAN were also associated with a positive graft outcome [10]. In another recent study, grafts that had high ATP, ADP, or EC before implantation functioned well after transplantation [4]. In addition, effluent PC related closely to the degree of hepatic cold ischemic damage [17]. In our study, there was no evidence to suggest that pre-tx measurements were significantly different between successful and PNF grafts. However, PNF grafts were well characterized as having a poorer recovery of ATP, TAN, T, and NAD + after reperfusion, as well as a higher concentration of X in the effluent. To test the predictabilit y of these values for PNF, logistic regression analysis was performed. Post-tx values of ATP, ADP, TAN, T, and NAD + were significantly associated with PNF, even though there were only three PNF cases, an obvious limitation in the power of our statistical analysis.
It is concluded from the study that ATP values from biopsies taken immediately after recirculation of the liver graft may have predictive value for PNF. In contrast, biopsies taken shortly before implantation may not be predictive of PNF. A possible explanation for a nonviable graft after cold preservation may not only be decreased AN metabolism but, more importantly, nonparenchymal cell injury. Extensive injury to nonparenchymal cells, such as endothelial cells, rather than damage to parenchymal cells during cold preservation, was shown ultrastructurally with rat livers [8, 15]. In addition, nutritional status of donors [2, 16, 22] and warm ischemia during procurement or liver replacement [7, 10] may influence initial graft function in human livers.
Acknowledgements
This work was supported by research grants from the Veterans Administration and project grant no. DK 29961 from the National Institute of Health, Bethesda, Maryland.
References
- 1.Adkison D, Höllwarth ME, Benoit JN, Parks DA, McCord JM, Granger DN. Role of free radicals in ischemia-reperfusion injury to the liver. Acta Physiol Scand. 1986;548:101–107. [PubMed] [Google Scholar]
- 2.Boudjema K, Lindell SL, Southard JH, Belzer FO. The effects of fasting on the quality of liver preservation by simple cold storage. Transplantation. 1990;50:943–948. doi: 10.1097/00007890-199012000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Furukawa H, Todo S, lmventarza O, Casavilla A, Wo YM, Foglieni CS, Broznick B, Bryant J, Day R, Starzl TE. Effect of cold ischemia time on the early outcome of human hepatic allografts preserved with UW solution. Transplantation. 1991;51:1000–1004. doi: 10.1097/00007890-199105000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.González FX, Rimola GA, Antolín M, Valdecasas JCG, Fuster J, de Lacy AM, Cugat E, Robusté J, Visa J. Adenine nucleotides in liver tissue and organ viability in human liver transplantation. Transplant Proc. 1992;24:133–134. [PubMed] [Google Scholar]
- 5.Gulian JM, Dalmasso C, Desmoulin F, Scheiner C, Cozzone PJ. Twenty-four-hour hypothermic preservation of the rat liver with Euro-Collins and UW solutions. Transplantation. 1992;54:599–603. doi: 10.1097/00007890-199210000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Harvey PRC, Iu S, McKeown CMB, Petrunka CN, Ilson RG, Strasberg SM. Adenine nucleotide tissue concentrations and liver allograft viability after cold preservation and warm ischemia. Transplantation. 1988;45:1016–1020. doi: 10.1097/00007890-198806000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Higashi H, Takenaka K, Fukuzawa K, Yoshida Y, Sugimachi K. Restoration of ATP contents in the transplanted liver closely relates to graft viability in dogs. Eur Surg Res. 1989;21:76–82. doi: 10.1159/000129006. [DOI] [PubMed] [Google Scholar]
- 8.Holloway CMB, Harvey PRC, Mullen JBM, Strasberg SM. Evidence that cold preservation-induced micro-circulatory injury in liver allografts is not mediated by oxygen-free radicals or cell swelling in the rat. Transplantation. 1989;48:179–188. doi: 10.1097/00007890-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Kamiike W, Watanabe F, Hashimoto T, Tagawa K, Ikeda Y, Nakao K, Kawashima Y. Changes in cellular levels of ATP and its catabolites in ischemic rat liver. J Biochem. 1982;91:1349–1356. doi: 10.1093/oxfordjournals.jbchem.a133822. [DOI] [PubMed] [Google Scholar]
- 10.Kamiike W, Burdelski M, Steinhoff G, Ringe B, Lauchart W, Pichlmayr R. Adenine nucleotide metabolism and its relation to organ viability in human liver transplantation. Transplantation. 1988;45:138–143. doi: 10.1097/00007890-198801000-00030. [DOI] [PubMed] [Google Scholar]
- 11.Lambotte L, Pontegnie-Istace S, Otte JB, Kestens PJ. The effect of isoproterenol and Collin’s solution on the preservation of canine livers with simple cooling. Transplant Proc. 1974;6:301–303. [PubMed] [Google Scholar]
- 12.Lanir A, Jenkins RL, Caldwell C, Lee RGL, Khettry U, Clouse ME. Hepatic transplantation survival: correlation with adenine nucleotide level in donor liver. Hepatology. 1988;8:471–475. doi: 10.1002/hep.1840080306. [DOI] [PubMed] [Google Scholar]
- 13.Marubayashi S, Takenaka M, Dohi K, Ezaki H, Kawasaki T. Adenine nucleotide metabolism during hepatic ischemia and subsequent blood reflow periods and its relation to organ viability. Transplantation. 1980;30:294–296. doi: 10.1097/00007890-198010000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Mittnacht S, Sherman SC, Farber JL. Reversal of ischemic mitochondrial dysfunction. J Bioi Chem. 1979;254:9871–9878. [PubMed] [Google Scholar]
- 15.Momii S, Koga A. Time-related morphological changes in cold-stored rat livers. A comparison of Euro-Collins solution with UW solution. Transplantation. 1990;50:745–750. doi: 10.1097/00007890-199011000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Morgan GR, Sanabria JR, Clavicn P-A, Phillips MJ, Edwards PC, Harvey PRC, Strasberg SM. Correlation of donor nutritional status with sinusoidal lining cell viability and liver function in the rat. Transplantation. 1991;51:1176–1183. doi: 10.1097/00007890-199106000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Nishida T, Koseki M, Kamiike W, Nakahara M, Nakao K, Kawashima Y, Hashimoto T, Tagawa K. Levels of purine compounds in a perfusate as a biochemical marker of ischemic injury of cold-preserved liver. Transplantation. 1987;44:16–21. doi: 10.1097/00007890-198707000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Palombo JD, Pomposelli JJ, Hirschberg Y, Blackburn GL, Bistrian BR. Glycolytic support of adenine nucleotides in rat liver flush-preserved with UW or Collin’s. II. Importance of donor nutritional status. Transplantation. 1989;48:901–905. doi: 10.1097/00007890-198912000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Pontegnie-Istace S, Lambotte L. Liver adenine nucleotidc metabolism during hypothermic anoxia and a recovery period in perfusion. J Surg Res. 1977;23:339–347. doi: 10.1016/0022-4804(77)90071-3. [DOI] [PubMed] [Google Scholar]
- 20.Starzl TE, Hakala TR, Shaw BW, Jr, Hardesty RL, Rosenthal TJ, Griffith BP, Iwatsuki S, Bahnson HT. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223–230. [PMC free article] [PubMed] [Google Scholar]
- 21.Starzl TE, Miller C, Broznick B, Makowka L. An improved technique for multiple organ harvesting. Surg Gynecol Obstet. 1987;165:343. [PMC free article] [PubMed] [Google Scholar]
- 22.Sumimoto R, Southard JH, Belzer FO. Livers from fasted rats acquire resistance to warm or cold ischemic injury (abstract) Transplantation. 1993;55:728–732. doi: 10.1097/00007890-199304000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Teperman L, Rao PN, Venkataramanan R, Gordon R, Todo S, Caldwell C, Makowka L, Starzl TE. The relationship of purine catabolism to human liver preservation using the University of Wisconsin (UW) solution. Hepatology. 1988;8:1289. [Google Scholar]
- 24.Wahlberg JA, Love R, Landegaard L, Southard JA, Belzer FO. 72-Hour preservation of the canine pancreas. Transplantation. 1987;43:5–8. doi: 10.1097/00007890-198701000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe F, Kamiike W, Nishimura T, Hashimoto T, Tagawa K. Decrease in mitochondrial levels of adenine nucleotides and concomitant mitochondrial dysfunction in ischemic rat liver. J Biochem. 1983;94:493–499. doi: 10.1093/oxfordjournals.jbchem.a134380. [DOI] [PubMed] [Google Scholar]
- 26.Hosmer DW, Lemeshow S. Applied logistic regression. John Wiley and Sons; New York: 1989. pp. 25–133. [Google Scholar]
- 27.Wynants J, Belle H. Single-run high-performance liquid chromatography of nucleotides, nucleosides, and major purine bases and its application to different tissue extracts. Anal Biochem. 1985;144:258–266. doi: 10.1016/0003-2697(85)90114-9. van. [DOI] [PubMed] [Google Scholar]