Abstract
Among the pharmaceutical options available for treatment of ovarian cancer, much attention has been progressively focused on pegylated liposomal doxorubicin (PLD), whose unique formulation, which entraps conventional doxorubicin in a bilayer lipidic sphere surrounded by a polyethylene glycol layer, prolongs the persistence of the drug in the circulation and potentiates intratumor drug accumulation. These properties enable this drug to sustain its very favorable toxicity profile and to be used safely in combination with other drugs. PLD has been already approved for treatment of advanced ovarian cancer patients failing first-line platinum-based treatment. Moreover, phase III trials have been already completed, and results are eagerly awaited, which hopefully will expand the range of PLD clinical application in this neoplasia both in front-line treatment, and in the salvage setting in combination with other drugs. Moreover, attempts are continuing to enable this drug to be combined with novel cytotoxic drugs and target-based agents. This review aims at summarizing the available evidence and the new perspectives for the clinical role of PLD in the management of patients with epithelial ovarian cancer.
Keywords: pegylated liposomal doxorubicin, ovarian cancer, clinical trials
Introduction
Epithelial ovarian carcinoma (OvCa) is one of the most common gynecological malignancies, and the fifth most frequent cause of cancer death in women.1 Worldwide, more than 190,000 new cases of ovarian cancer are diagnosed each year accounting for around 5% of all cancers in women. In 2009, 21,550 new cases were estimated to have been diagnosed in the US.1
The standard of care for the management of OvCa patients includes surgery for staging and optimal cytoreduction (residual tumor < 1 cm) followed by adjuvant chemotherapy with a platinum/taxane combination.2,3 However, despite the advances in surgical efforts and the achievement of high response rates to front-line treatment, OvCa remains the most lethal gynecological malignancy, almost 50% to 75% of cases experiencing progression/recurrence of disease, and a 5-year overall survival OS of 25% to 30% in advanced stage disease.1,3
The major determinants of clinical outcome are represented by the extent of residual tumor at primary surgery and sensitivity to platinum-based therapy,4 the latter generally being defined according to the duration of the platinum-free interval (PFI). In particular, patients are considered platinum resistant if progression/recurrence of disease occurs during chemotherapy or within 6 months from its completion; in this clinical setting, second-line single-agent chemotherapy with non-platinum drugs results in short-lived response rates of approximately 10% to 25%, regardless of type of drugs used.5 On the other hand, patients defined as platinum sensitive, i.e. recurring/progressing after 6 months from the end of primary treatment, are usually treated with platinum-based combinations.4,5 Indeed, in the context of platinum sensitivity, relapse/progression within 6 to 12 months after the administration of primary chemotherapy represents a type of gray zone in terms of platinum resistance/responsiveness, and this is strongly supported by the clinical evidence in this subset of patients, the response rates range between 27% and 33% regardless of whether a platinum- based re-challenge or non-platinum drugs are used.6
In any case, besides the extent of response rates, other issues have to be taken into account in the choice of medical treatment, whether in front-line or second-line setting, including the rate and profile of side effects, especially for drug combinations and their impact on patients’ quality of life. In this context, among the pharmaceutical options currently available for medical treatment of OvCa, greater emphasis has been placed progressively on pegylated liposomal doxorubicin (PLD) (Doxil® in the US; Caelyx® in Canada and Europe), which was approved in 1999 by the FDA and in 2000 by the European Medicines Evaluation Agency (EMEA) as single agent for treatment of advanced OvCa patients failing first-line platinum-based treatment. Moreover, phase III trials have been already conducted, and results from other studies are eagerly awaited, exploring the efficacy of PLD in salvage setting and in front-line treatment in combination with other therapeutic drugs.
This review will focus on the clinical role of PLD in the management of patients with epithelial OvCa. A brief summary of the process of PLD development, as well as new perspectives on PLD use, will also be provided.
PLD: development, structure, and pharmacokinetic features
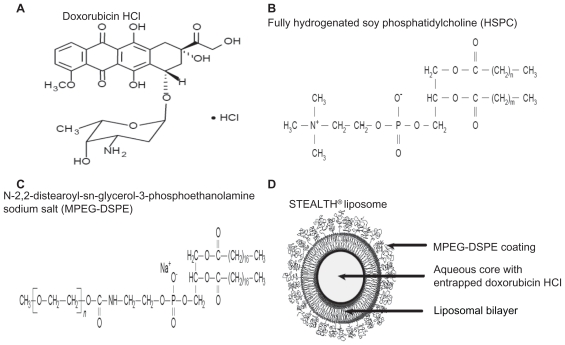
PLD is a unique formulation of conventional doxorubicin in which the drug is encapsulated in a bilayer lipidic sphere7 of approximately 100 nm (Figure 1): in contrast to other nanoparticles, the liposomal shell is surrounded by a polyethylene glycol (PEG) layer which represents a hydrophilic protective barrier between the liposome and the microenvironment, which prevents the interaction between circulating proteins and the lipidic bilayer: indeed, this phenomenon facilitates the activation of the reticulo endothelial system, thus leading to the destruction of the liposomal structure and release of the free drug. Therefore, the pegylation process plays a relevant role in prolonging the persistence of the drug in the circulation. It has also to be acknowledged that the size of PLD, while reducing or even preventing the extravasation of the drug in normal tissues, whose capillaries have a basal membrane and share very tight intercellular junctions, allows a facilitated uptake in tumor tissues which are characterized by loose capillary junctions. Finally, the absence of a structured lymphatic system in neoplastic tissues prevents PLD being cleared, and potentiates intratumor drug accumulation. These properties, which represent the rational basis for the exploitation of nanoparticle technology, sustain not only one of the major advantages of PLD, ie, lower cardiotoxicity and gastrointestinal toxicity compared to the free drug, but also its well-known pharmacokinetic features, such as long circulation time, minimal (<5%) drug leakage from circulating liposomes, as well as half-lives of approximately 60 to 80 hours for doses in the range of 35 to 70 mg/m2 in patients with solid tumors;8,9 this would, in turn, translate, as reported by Gabizon et al,9 to a PLD AUC approximately 250-fold higher than that of the free drug in humans. In particular, it has been shown that after PLD administration nearly 100% of the drug in the plasma is in the encapsulated form; moreover, compared to free doxorubicin, PLD plasma clearance is dramatically slower (0.1 L/h for PLD vs 45 L/h for free doxorubicin), and its volume of distribution is very small (4 L vs 254 L, respectively).9 The pharmacokinetics of PLD are still being investigated: there seems to be a complex interaction between pharmacokinetics and pharmacodynamics which could account for some patterns of toxicity; for instance, stomatitis/mucositis is documented more frequently at higher peak dose drug level, while cutaneous toxicity depends on dose interval or dose intensity, as shown by recent data showing that repeated PLD administrations result in cumulative inhibition of the clearance process.10
Figure 1.
Structural form of doxorubicin HCL (A), fully hydrogenated soy phosphatidylcholine (B), and N-2,2-distearoyl-sn-glycerol-3-phosphoethanolamine sodium salt (MPEG-DSPE) (C), comprising the STEALTH® liposome structure (D).
Advances in nanoparticle technology have fuelled great enthusiasm for the possibility of further enhancing the selective intratumor accumulation of PLD, and shifting the therapeutic index toward more tolerable toxicity profiles: in this context, the preliminary observations that recombinant serum albumine-conjugated PLD has longer blood circulating properties, smaller hepatic and splenic clearance, and more important, larger intratumor accumulation than PLD in pre-clinical models is very encouraging.11
Phase I studies with PLD as single agent or in combination
Table 1 summarizes the studies investigating the safety and assessing the maximum tolerated dose (MTD) of PLD used as a single agent:12–19 different dose escalation and schedules have been explored with PLD dose intensities, ranging from 10 to 15 mg/m2/week. Stomatitis was reported as the most frequent dose limiting toxicity (DLT) at PLD doses ≥ 60 mg/m2, while at lower doses with schedules < 21 days, the most common DLT was represented by hand–foot syndrome (HFS). Neutropenia was the DLT in two studies exploring dose-dense regimens,12,13 and in a series of 24 patients with pediatric solid tumors, treated with PLD doses of 40 to 50 mg/m2 every 28 days.19 The current PLD dosage as a single agent as indicated for ovarian cancer patients is 50 mg/m2 every 28 days.
Table 1.
Phase I studies with pegylated liposomal doxorubicin as a single agent
| Author | No. pts | Type of tumor | MTD | DI mg/m2/week | DLTs |
|---|---|---|---|---|---|
| Bogner12 | 40 | Kaposi sarcomas | 20 mg/m2, q15 | 10 | neutropenia |
| James13 | 15 | Kaposi sarcomas | 20 mg/m2, q15 | 10 | hematologic |
| Uziely14 | 56 | Solid tumors | 60 mg/m2, q28 | 15 | stomatitis (doses ≥ 60 mg/m2) for a single dose HFS (interval < 28 d) |
| Jahanzeb15 | 24 | Solid tumors | 40–50 mg/m2, q28 | 10–12.5 | neutropenia |
| Gabizon16 | 22 | MBC | 50 mg/m2, q28 | 12.5 | stomatitis (doses > 60 mg/m2) HFS (interval < 21 d) |
| Caponigro17 | 24 | Head/neck | 45 mg/m2, q21 | 15 | stomatitis |
| Hamilton18 | 20 | MBC | 60 mg/m2, q42 | 10 | mucositis |
| Marina19 | 22 | Pediatric solid tumors | 60 mg/m2, q28 | 15 | mucositis |
Abbreviations: DI, dose intensity; DLT, dose limiting toxicity; HFS, hand–foot syndrome; MBC, metastatic breast cancer; MTD, maximum tolerated dose; q, every; d, day.
Among the novel perspectives relative to PLD use, the investigation of escalating (15 to 100 mg/m2) doses of PLD plus hyperthermic intraperitoneal chemotherapy following optimal cytoreduction has been carried out in a phase I study including 21 advanced stage solid tumors including 3 patients with ovarian cancer.20 The most common grade 3/4 toxicities were superficial wound infections (n = 6), and prolonged ileus (n = 2). The most severe complication was represented by a post-operative anastomotic leakage requiring re-laparotomy.
Given the evidences of a different toxicity profile, PLD safety has been also investigated in combinations involving two or even three agents:21–64 in the dose-finding studies exploring combinations of PLD with cisplatin (CDDP) (Table 2), the DLTs were mostly represented by mucositis, and also neutropenia when CDDP was used at doses > 60 mg/m2 every 28 days.21,22 Similarly, when combining PLD with carboplatin (JM8), DLTs consisting in stomatitis/mucositis were documented at PLD doses > 50 mg/m2, while thrombocytopenia was the most frequently reported DLT for lower PLD doses, with the exception of the study by Hamilton et al.25
Table 2.
Phase I studies with pegylated liposomal doxorubicin in combination with platinum agents
| Author | No. pts | Type of tumor | MTD | DLTs |
|---|---|---|---|---|
| Klein21 | 25 | Solid tumors | CDDP 50 mg/m2, d8 PLD 50–60 mg/m2, d1, q28 |
mucositis skin toxicity |
| Lyass22 | 24 | Solid tumors | CDDP 60 mg/m2, PLD 50 mg/m2, q28 | mucositis neutropenia |
| Uys23 | 19 | Solid tumors | JM8 AUC 5 PLD 50 mg/m2, q28 |
stomatitis neutropenia thrombocytopenia |
| Goncalves24 | 22 | Solid tumors | JM8 AUC 5, q21,28 PLD 35 mg/m2 |
neutropenia thrombocytopenia |
| Hamilton25 | 20 | Solid tumors | JM8 AUC 6 PLD 30 mg/m2, q21 |
none |
| Gonzalez-Billalabeitia26 | 26 | Ovarian cancer | JM8 AUC 5 PLD 40 mg/m2, q28 |
mucositis thrombocytopenia |
| du Bois27 | 36a | Ovarian, peritoneal, tubal cancer | JM8 AUC 6 PLD 40 mg/m2, q28 |
neutropenia thrombocytopenia abdominal pain emesis, DVT |
| Recchia28 | 20 | Ovarian cancer | OXA 120 mg/m2 PLD 40 mg/m2, q21 |
neutropenia thrombocytopenia |
Abbreviations: CDDP, cisplatin; DLT, dose limiting toxicity; DVT, deep venous thromboembolism; JM8, carboplatin; MTD, maximum tolerated dose; OXA, oxaliplatin; q, every; d, day.
24 cases for phase I, 12 cases for confirmatory study.
The MTD of oxaliplatin (OXA) combined with fixed doses of PLD as salvage treatment of pre-treated advanced ovarian cancer was 130 mg/m2, as 2 out of 3 patients of this cohort showed dose-limiting thrombocytopenia and/or neutropenia during the first cycle of treatment.28
For the combination of PLD/taxanes29–46 (Table 3), the tolerability of PLD (at doses ranging from 30 to 40 mg/m2) combined with paclitaxel (PTX) (135 to 200 mg/m2 every 21 days) was acceptable; similarly, PLD at doses of 30 to 40 mg/m2, plus docetaxel (DTX) at doses of 67.5 to 80 mg/m2, was reported as the MTD for this combination. Based on the hypothesis that weekly administration could limit toxicity while keeping the dose intensity unchanged, weekly or bi-weekly administration of PLD (doses from 10 to 20 mg/m2) plus weekly PTX has been also explored.32,34–36
Table 3.
Phase I studies with pegylated liposomal doxorubicin (PLD) in combination with taxanes
| Author | No. pts | Type of tumor | MTD | DLTs |
|---|---|---|---|---|
| Israel29 | – | Solid tumors | PTX 135 mg/m2, d1,8 PLD 30 mg/m2 d1, q28 |
mucositis, skin toxicity neutropenia |
| Muggia30 | 25 | Endometrial cancer | PTX 75 mg/m2, d1,8,15 PLD 24 mg/m2, d1, q28 |
HFS neutropenia |
| Modiano31 | 32 | Breast, gynecologic tumors | PTX 175 mg/m2 PLD 30 mg/m2, q21 |
HFS neutropenia |
| Schwonzen32 | 21 | MBC | PTX 80 mg/m2 weekly PLD 15 mg/m2, q15 |
mucositis, skin toxicity alopecia, neurotoxicity |
| Tolis33 | 21 | – | PTX 85 mg/m2, d1,8,15 PLD 30 mg/m2, d1, q28 or PTX 70 mg/m2, d1,8,15 PLD 35 mg/m2, d1, q28 |
neutropenia |
| Androulakis34 | 19 | Solid tumors | PTX 80 mg/m2, d1,8,15,21 PLD 10 mg/m2, d1,8,15,21, q42 |
neutropenia diarrhea |
| Mavroudis35 | 26 | Solid tumors | PTX 115 mg/m2, d2 PLD 15 mg/m2, d1, q15 |
neutropenia |
| Lortholary36 | 16 | MBC | PTX 80 mg/m2, weekly PLD 12.5–22.5 mg/m2, q15 |
– |
| Briasoulis37 | 44 | Solid tumors | PTX 80 mg/m2 d1,8,15 PLD 35 mg/m2 q28 or PTX 90 mg/m2 d1,8,15 PLD 30 mg/m2 q28 |
DVT HFS neutropenia |
| Hirsch38 | 17 | Solid tumors | DTX 67.5 mg/m2, d1 PLD 30 mg/m2, d1, q21 |
Stomatitis, neutropenia thrombocytopenia |
| Drinkard39 | 6 | Solid tumors | DTX 50 mg/m2, d5 PLD 30 mg/m2,d1, q21/28 |
esophagitis neutropenia |
| Pavlick40 | 26 | Solid tumors | DTX 80 mg/m2 PLD 30 mg/m2, q21 + GF |
HFS neutropenia |
| Tauer41 | 21 | Solid tumors | DTX 70 mg/m2, PLD 40 mg/m2, q28 | neutropenia |
| Sparano42 | 41 | Breast cancer | DTX 60 mg/m2, PLD 30 mg/m2, q21 or DTX 75 mg/m2, PLD 30 mg/m2, q28 + GF | neutropenia |
| Sikov43 | 22 | Solid tumors | DTX 25 mg/m2, d1,8,15 PLD 20 mg/m2, d1, q28 |
mucositis, diarrhea neutropenia |
| Gasparini44 | 15 | Breast cancer | DTX 35 mg/m2, d2,9 PLD 35 mg/m2, d1, q21 |
skin toxicity neutropenia |
| Fracasso45 | 22 | Solid tumors | DTX 40 mg/m2, PLD 20 mg/m2, bi-weekly | skin toxicity thrombocytopenia |
| Bischoff46 | 12 | MBC | DTX 30 mg/m2, d1,8,15 PLD 40 mg/m2, d1, q28 |
neutropenia |
Abbreviations: DLT, dose limiting toxicity; DTX, docetaxel; DVT, deep venous thromboembolism; GF, growth factor support; HFS, hand–foot syndrome; MBC, metastatic breast cancer; MTD, maximum tolerated dose; PTX, paclitaxel; q, every; d, day.
For weekly administration of PLD and DTX the MTDs corresponded to PLD 20 mg/m2 and DTX 25 mg/m2, every 28 days.43 The DLTs were mostly mucositis and neutropenia.
Given the peculiar not overlapping toxicity profile as well as the different mechanism of action of PLD compared to platinum agents and taxanes, it is not surprising that PLD tolerability has been also explored in combination with the most active regimen in ovarian cancer:47–49 the addition of PLD to JM8/PTX led to defining the MTD as corresponding to JM8 AUC 6, and PTX 175 mg/m2 every 21 days, plus PLD 30 mg/m2 administered, as recommended, every other cycle. With this 3-drug regimen the same pattern of DLTs documented with the doublets was reported. Given the strong biological rationale of combining PLD, an inhibitor of topoi-somerase II, with topoisomerase I inhibitors, as well as the non-overlapping toxicity profile of these two classes of drugs, the safety of PLD/topotecan (TPT) combination has been the subject of active investigation50–60 (Table 5). The MTD was reached at PLD doses of 30 and 40 mg/m2, every 21 or 28 days, and at TPT doses (0.5 to 1.0 mg/m2/day) administered with the classic shorter courses (days 1 to 3 or days 1 to 5). DLTs were represented in the vast majority of the studies by hematological toxicity. On the other hand, the use of longer courses (days 1 to 14, days 1 to 21), or longer intervals (up to 5 weeks) seemed to be associated with a higher tolerability profile.
Table 5.
Phase I studies with pegylated liposomal doxorubicin (PLD) in combination with topotecan (TPT)
| Author | No. pts | Type of tumor | MTD | DLTs |
|---|---|---|---|---|
| Ryan50 | 9 | Ovarian cancer | TPT 1.0 mg/m2, d1-5 PLD 20 mg/m2, q28 |
neutropenia thrombocytopenia |
| Yeung51 | 15 | Solid tumors | TPT 1.0 mg/m2, d1-5 PLD 30 mg/m2, q21 |
mucositis neutropenia |
| Hochster52 | 17 | Solid tumors | TPT 0.4 mg/m2, d1-21 PLD 30 mg/m2, q28 |
neutropenia |
| Hamilton53 | 27 | Solid tumors | TPT 0.4 mg/m2, d1-21 PLD 30 mg/m2, q28 |
neutropenia |
| Geertsen54 | 20 | Ovarian cancer | TPT 0.75 mg/m2, d1-5 PLD 30 mg/m2, d8, q35 |
neutropenia |
| Pautier55 | 16 | Solid tumors | TPT 0.5 mg/m2, d1-5 PLD 35 mg/m2, q28 |
neutropenia |
| Mirchandani56 | 21 | Ovarian cancer | TPT 0.4 mg/m2, d1-14 PLD 40 mg/m2, q28 |
neutropenia thrombocytopenia |
| Garcia57 | 32 | Solid tumors | TPT 1.0 mg/m2, d1-3 PLD 40 mg/m2, d4, q28 |
neutropenia 1 death |
| Ghesquieres58 | 23 | Solid tumors (84% ovarian cancer) | TPT 0.5 mg/m2, d1-5 PLD 35 mg/m2, d1, q28 |
neutropenia |
| Rose59 | 22 | Ovarian, tubal peritoneal cancer | TPT 1.5 mg/m2, d1-5 per os PLD 40 mg/m2, d1, q28 |
neutropenia |
| Penson60 | 18 | Müllerian tumors | TPT 0.5 mg/m2, d1-3 PLD 30 mg/m2, d1, q21 |
neutropenia |
Abbreviations: DLT, dose limiting toxicity; MTD, maximum tolerated dose; TPT, topotecan; q, every; d, day.
PLD safety has been investigated also in combination with other chemotherapeutics such as etoposide, vinorelbine, and gemcitabine;61–63 however, in spite of generally positive reports, only a few combinations have progressed to phase II evaluation (see below).
Phase II studies with PLD as single agent or in combination
A summary of phase II studies using PLD as single agent in ovarian cancer is presented in Table 6.65–80
Table 6.
Phase II studies with pegylated liposomal doxorubicin as single agent
| Author | Dose, schedule | Clinical setting | RR (%) | PFS (Median) months | Grade 3/4 toxicity/patient |
|||
|---|---|---|---|---|---|---|---|---|
| Hgb | Neu | PLT | HFS | |||||
| Muggia65 | 50 mg/m2, q21 | RES 35 | 25.7 | 5.7 | – | – | – | – |
| Gordon66 | 50 mg/m2, q21 | ALL 89 | 16.8 | 4.8 | 20.2 | 15.7 | 2.2 | 20.2 |
| RES 82 | 18.3 | 4.4 | ||||||
| Rose67 | 50 mg/m2, q28 | RES 37 | 13.5 | 4.0 | – | – | – | – |
| 40 mg/m2, q28 | RES 39 | 7.7 | 4.0 | |||||
| Arcuri68 | 50 mg/m2, q28 | ALL 30 | 26.6 | – | – | 23.3 | – | 10.0 |
| Katsumata69 | 50 mg/m2, q28 | RES 63 | 20.9 | 5.6 | 17.6 | 67.5 | 6.9 | 16.2 |
| SEN 11 | 27.3 | 5.3 | ||||||
| Gorumlu70 | 50 mg/m2, q28 | RES 17 | 17.0 | 3.7 | – | 12.0 | – | 6.0 |
| Steppan71 | 45 mg/m2, q28 | RES 29 | 30.5 | – | – | – | – | – |
| Chou72 | 45 mg/m2, q28 | RES 29 | 23.1 | 5.4 | – | – | – | – |
| Markman73 | 40 mg/m2, q28 | RES 44 | 9.1 | – | – | 2.0 | 0 | 0 |
| Campos74 | 40 mg/m2, q28 | ALL 72 | 27.0 | 5.3 | 11 | 2 | 1 | – |
| RES 29 | 29.0 | |||||||
| SEN 43 | 25.6 | |||||||
| Wilailak75 | 40 mg/m2, q21 | RES 14 | 23.0 | 6.0 | 0 | 14.3 | 0 | 2.0 |
| Lorusso76 | 35 mg/m2, q21 | ALL | 13.5 | 7.2 | 0 | 10.8 | 0 | 2.7 |
| RES 17a | 18.9 | – | ||||||
| SEN 20 | 10.0 | – | ||||||
| Strauss77 | 20 mg/m2, q15 | RES 50 | 40 | 4.1 | 0 | 4 | 0 | 2.0 |
| Oskay-Oezcelik78 | 20 mg/m2, q15 | RES 7 | 0 | 2.3 | 5 | 0 | 0 | 5.0 |
| SEN 13 | 7.7 | 4.1 | ||||||
| Sehouli79 | 20 mg/m2, q15 | ALL 64 | 10.9b | 4.3 | 4.7 | 0 | 1.6 | 4.7 |
| RES 21 | – | – | ||||||
| SEN 43 | – | – | ||||||
Abbreviations: ALL, ; HFS, hand–foot syndrome; Hgb, anemia; PFS, progression-free survival; RR, response rate; RES, platinum-resistant recurrent disease; Neu, neutropenia, PLT, platelet toxicity; SEN, platinum-sensitive recurrent disease; q, every; d, day.
Platinum sensitivity according to the cut-off of 12-month platinum free interval;
In assessed patients (n = 44).
When considering the studies including only or a very large proportion of platinum-resistant patients, the response rates ranged from 9.1% to 40.0%, and did not seem to present a schedule or dose dependence, although the relatively small sample series, and also patients’ heterogeneity in terms of the number of previously administered lines of chemotherapy, are a major limit to reliable comparisons.
In particular, several studies have shown that a more acceptable toxicity profile in terms of decreased rates of HFS and stomatitis/mucositis can be obtained with a PLD dose of 40 mg/m2 every 28 days, with comparable response rates and outcome.67,74,75
More recently, biweekly schedules have also been investigated in the same clinical setting in order to further improve the toxicity pattern.77–79 Although the direct comparison across non-randomized phase II studies is difficult, it seems that the biweekly regimen represents a good therapeutic option since it does not worsen clinical outcome while preventing severe hematological and non-hematological side effects.80 Results relative to platinum-sensitive recurrent ovarian cancer patients are not informative or refer to very low numbers of cases.69,76,78
The demonstrated efficacy of PLD in the poor prognosis subset of recurrent platinum-resistant ovarian cancer has supported the investigation of PLD/platinum doublets also in platinum-sensitive disease in which the currently administered partners of platinum agents are generally associated with elevated neurologic and hematological toxicity.81,82 As shown in Table 7, the association of PLD (50 mg/m2) with CDDP (60 mg/m2) was investigated in a 28-day schedule by Tas et al,83 who reported an overall response rate of 62.0% with 4 (19.0%) complete, and 9 (43.0%) partial responses. Although this schedule was well tolerated (only 10% grade 2 neurotoxicity, and grade 3/4 anemia, neutropenia, and HFS accounting for 18%, 41%, and 9%, respectively), PLD/JM8 combinations are considered more manageable due to the expected lower neurotoxicity.84–89 In 2007 Ferrero et al87 evaluated PLD (30 mg/m2) followed by JM8 (AUC 5), every 28 days, in 104 patients, of whom 53 were totally and 43 were partially platinum sensitive: overall, the objective response rate was 62.5%, with a 38% rate of complete response; median progression-free survival (PFS) and OS were 9.4 months, and 32 months, respectively. Comparable rates of response were achieved in the study by duBois et al87 who reported an objective response rate of 68% in 67 recurrent ovarian cancer patients.
Table 7.
Non-randomized studies with combinations of pegylated liposomal doxorubicin (PLD) and platinum agents
| Author | Drugs/dose | PFI mts | No. pts | RR (%) | PFS mts | % Grade 3/4 toxicity/patient |
|||
|---|---|---|---|---|---|---|---|---|---|
| Hgb | Neu | PLT | HFS | ||||||
| Tas83 | PLD (50 mg/m2) d1 | ≥6 | 22 | 62.0 | – | 18 | 41 | 0 | 9 |
| CDDP (60 mg/m2) d1, q28 | |||||||||
| Vorobiof84 | PLD (50 mg/m2) d1 | ≥6 | 29 | 76.2 | 9 | 52.3 | 38 | 9.5 | |
| JM8 (AUC 5) d1, q28 | |||||||||
| du Bois85 | PLD (40 mg/m2) d1 | ≥6 | 67 | 68 | 11.6 | 8 | 24 | 14 | 7 |
| JM8 (AUC 6) d1, q28 | |||||||||
| Rapoport86 | PLD (50 mg/m2) d1 | All | 40 | 67.5 | 11.9 | 10 | 55 | 42.5 | 7.5 |
| JM8 (AUC 5) d1, q28 | 7–12 | 19 | 52.6 | 9.7 | |||||
| >12 | 21 | 81.0 | 15.1 | ||||||
| Ferrero87 | PLD (30 mg/m2) d1 | All | 96 | 62.5 | 9.4 | 12 | 51 | 26 | 0 |
| JM8 (AUC 5) d1, q28 | 7–12 | 43 | – | 7.9 | |||||
| >12 | 53 | – | 11.4 | ||||||
| Power88 | PLD (30 mg/m2) d1 | 7–12 | 58 | 46 | 10 | 7 | 21 | 17 | 1.7 |
| JM8 (AUC 5) d1, q28 | |||||||||
| Weber89 | PLD (30 mg/m2) d1 | ≥6 | 81 | 65.4 | 13.6 | 13.0 | 55.0 | 29.0 | 1.0 |
| JM8 (AUC 5) d1, q28 | 6–12 | 32 | – | 9.8 | |||||
| ≥12 | 49 | – | 14.4 | ||||||
| Nicoletto90 | PLD (30 mg/m2) d1 | <6 | 14 | 28.6 | 5.9 | 0 | 9.3 | 0 | 0 |
| OXA (70 mg/m2) d1, q28 | ≥6 | 29 | 66.7 | 9.9 | |||||
| Recchia91 | PLD (20 mg/m2) d1,2 | <6 | 13 | 32.5 | 5.8 | 5 | 38 | 8 | 0 |
| OXA (60 mg/m2) d1,2, q21 | ≥6 | 27 | 67.5 | 12.1 | |||||
| Valerio92 | PLD (30 mg/m2) | <6 | 27 | 37.0 | 7 | 17 | 15 | 15 | 0 |
| OXA (85 mg/m2) | ≥6 | 12 | 66.3 | 8.5 | |||||
| cyclophosphamide (750 mg/m2) | |||||||||
Abbreviations: CDDP, cisplatin; HFS, hand–foot syndrome; Hgb, anemia; JM8, carboplatin; Neu, neutropenia; PFI, platinum-free interval; PFS, progression-free survival; PLT, platelet toxicity; RR, response rate; OXA, oxaliplatin; q, every; d, day.
Since platinum sensitivity is more likely to be a continuum instead of being defined by operational time-based cut-off points, it is not surprising that much interest has been focused on that subset of partially platinum = sensitive patients which show a substantially similar rate of response to platinum, as well as to non-platinum agents,6 thus questioning if they should really be considered as partially platinum sensitive or partially platinum resistant. The studies by Ferrero et al87 and by Weber et al89 do not report the specific response rates in partially vs totally platinum-sensitive disease, but median PFS was longer in the latter group in both studies. The same trend has been reported by Rapoport et al,86 who documented in the whole population an overall response rate of 67.5%, but clearly distinguished totally vs partially sensitive patients (response rate = 81.0% vs 52.6%; median PFS = 15.1 vs 9.7 months, respectively).
The largest phase II study (n = 58) specifically focusing on partially sensitive recurrent ovarian cancer reported an overall response rate of 46% (4% complete and 42% partial responses), with an additional 33% of cases experiencing disease stabilization longer than 6 months.88 For those patients with measurable Ca125 levels, the response rate was 66% (28% complete and 38% partial responses), with an additional 18% of cases experiencing disease stabilization longer than 6 months. Median PFS was 10 months, and median OS 19.1 months. On the basis of the present literature, it seems that phase III randomized studies comparing platinum-based vs non-platinum agents in this clinical subset are urgently needed in order to correctly allocate patients to salvage treatment. In this context, a multicentric phase III study (MITO-8, NCT00657878) has been recently launched comparing PLD (40 mg/m2 every 28 days) vs JM8/PTX (AUC 5, 175 mg/m2) with cross-over on progression in OvCa patients recurring within 6 to 12 months from the completion of primary treatment.
Overall, the toxicity rate/severity related to combinations of PLD and JM8 was consistent across the studies, with grade 3/4 anemia ranging from 7% to 13%,84–89 and grade 3/4 neutropenia occurring in around 50% of cases with the exception of two studies.86,88 The rate of severe thrombocytopenia seems closely related to PLD dosage: indeed, in studies using PLD at 30 to 40 mg/m2, grade 3/4 thrombocytopenia remained within 14% and 26%, while at PLD doses of 50 mg/m2 it increased to approximately 40%. As expected, severe HFS was negligible in studies using PLD at a dosage of 30 mg/m2.87–89
Combinations of PLD with OXA seem very promising since the reported rates of response appear in the range of those reported with PLD/JM8 combinations.90–92 Moreover, with the limits of the sample size, a very acceptable rate of stomatitis/mucositis and HFS has been shown, likely due to the use of the PLD at the dosage of 30 mg/m2, every 21/28 days.
For neurotoxicity, grade 2 sensory neuropathy was reported in 7% of cases in the study by Nicoletto et al90 which seems quite an acceptable figure considering that 37% of patients had already received >1 previous lines of chemotherapy; 77% of patients had also been administered prior platinum/taxanes. However, a high rate of grade 2 neurotoxicity was documented by Recchia et al91 whose study, nevertheless, used a cumulative OXA dosage of 120 mg/m2.
Attempts to add PLD to combinations of two drugs have been also reported: in particular, Valerio et al92 explored the combination PLD (30 mg/m2), OXA (85 mg/m2), and cyclophosphamide (750 mg/m2) on a 3-week schedule in a series of 39 recurrent ovarian cancer patients (12 platinum sensitive, 27 platinum resistant). The response rate was 66.3% in platinum-sensitive (median PFS 8.5 months), and 37% in platinum-resistant (median PFS = 7 months). Overall, the regimen was well tolerated with grade 2 neurotoxicity observed in 20% of patients, and grade 3/4 anemia, neutropenia, and thrombocytopenia documented in 17%, 15%, and 15% of cases, respectively. Severe HFS was not reported.
Finally, a phase II front-line study (S9912) has been recently conducted by the Southwest Oncology Group, which investigated the addition of intravenous (iv) PLD (30 to 40 mg/m2, day 8, × 2 cycles) to intraperitoneal (ip) CDDP (75 mg/m2, day 2, every 21 days), and both iv and ip paclitaxel (135 mg/m2, day 1, every 21 days, and 60 mg/m2, day 8, every 21 days, respectively).93 This regimen gave clinical outcome measures similar to those reported in comparable patient populations treated with classical CDDP-containing combinations; however, an unacceptable rate of severe toxicity (5 treatment related deaths, and 32 patients with at least on grade 3–5 toxicity) was documented, thus discouraging any further development.
One of the most frequently studied partners in PLD-based combination is represented by gemcitabine (GEM), on the basis of the demonstrations of the synergistic antiproliferative activity of the drugs, and of their non overlapping toxicity profiles. As summarized in Table 8, in the subset of platinum resistant disease the response rate range from 22 to 33%, with median PFS from 2.7 to 6.0 months regardless of the schedule administered.94–100
Table 8.
Non-randomized studies with combinations of pegylated liposomal doxorubicin (PLD) and non-platinum agents
| Author | Drugs/dose | No. | RR (%) | PFS mts | % Grade 3/4 toxicity/patient |
|||
|---|---|---|---|---|---|---|---|---|
| Hgb | Neu | PLT | HFS | |||||
| Tas94 | PLD (20 mg/m2) d1,15 | RES 18 | 28.0 | – | 16.7 | 0 | 0 | 5.5 |
| GEM (2000 mg/m2) d1,15 q28 | ||||||||
| Skarlos95 | PLD (25 mg/m2) d1 | RES 37 | 22 | 2.7 | – | – | – | 2.7 |
| GEM (650 mg/m2) d1,8 q28 | ||||||||
| Holloway96 | PLD (25 mg/m2) d1 | ALL 25 | 64.0 | – | – | 24.0 | 4.0 | – |
| GEM (650 mg/m2) d1,8 | ||||||||
| Karaoglu97 | PLD (25 mg/m2) d1 | RES 35 | 28.6 | 6 | 2.9 | 8.6 | – | 0 |
| GEM (1000 mg/m2) d1,8 q28 | ||||||||
| Petru98 | PLD (30 mg/m2) d1 | RES 31 | 33.0 | 3.8 | 3.0 | 26.0 | 10 | 3.0 |
| GEM (650 mg/m2) d 1,8 q28 | ||||||||
| D’Agostino99 | PLD (30 mg/m2), d1 | RES 36 | 25.0 | – | 7.0 | 32.6 | 8.5 | 10 |
| GEM (1000 mg/m2), d1,8 q21 | SEN 31 | 45.2 | ||||||
| Ferrandina100 | PLD (30 mg/m2), d1 | RES 66 | 21.6 | 5 | 9.0 | 28.8 | 10.8 | 14.4 |
| GEM (1000 mg/m2), d1,8 q21 | SEN 45 | 53.7 | 8.7 | |||||
| Verhaar-Langereis102 | PLD (30 mg/m2), d1 | RES 27 | 28.0 | 7.5 | – | 70.4a | 48.1 | 44.4 |
| TPT (1.0 mg/m2), d1-5 q21 and PLD (40 mg/m2), d1 | ||||||||
| TPT (0.75 mg/m2), d1-5 q21 | ||||||||
| Campos103 | PLD (30 mg/m2), d1, q21 | ALL 37 | 29.0 | – | 2.5 | 40.0 | 0 | 52.5 |
| PTX (70 mg/m2), weekly | RES 24 | 17.0 | ||||||
| for 18 weeks | SEN 13 | 54.0 | ||||||
| Katsaros104 | PLD (30 mg/m2), d1 | ALL 30 | 37.0 | 5.5 | 0 | 4.0 | 0 | 2.0 |
| vinorelbine (30 mg/m2), d1, q21 | ||||||||
| Joly105 | PLD (40 mg/m2), d1 | ALL 98 | 28.0 | – | 7.0 | 48.0 | 3.0 | 2.0 |
| ifosfamide (1700 mg/m2), d1-3 q28 | RES 57 | 19.0 | ||||||
| SEN 41b | 41.0 | |||||||
Abbreviations: GEM, gemcitabine; HFS, hand–foot syndrome; Hgb, anemia; Neu, neutropenia; PFS, progression free survival; RR, response rate; RES, platinum resistant recurrent disease; SEN, platinum sensitive recurrent disease; PLT, platelet toxicity; PTX, paclitaxel; TPT, topotecan; q, every; d, day.
leukopenia;
platinum-sensitive patients are defined as having a 6–12-month platinum free interval.
Considering that the percentage of patients who had already received >1 lines of chemotherapy was high in some studies,99,100 the regimen was relatively well tolerated: indeed, grade 3/4 anemia was documented at 3% to 9% in studies using the classical 3-week and 4-week schedules, increasing up to 17% in the only study using the bi-weekly schedule.94 Grade 3/4 neutropenia was negligible in bi-weekly regimens but increased up to 30% in the 3-week schedule.99,100 The rate of grade 3/4 thrombocytopenia was consistent across all studies, at around 9% to 10%, while a wide heterogeneity in the rate of severe HFS was noted. However, it has to be taken into account that the two studies reporting >10% grade 3/4 HFS99,100 included heavily pretreated patients in >50% of the sample series: indeed, evidence has been reported that the incidence of HFS is correlated with the presence of neuropathy and also with the number of previous chemotherapy regimens, regardless of type of chemotherapeutic agent used.101
Some phase II studies explored the efficacy of PLD associated with topotecan,102 as well as PTX,103 vinolrebine,104 and ifosphamide.105 Overall, the rate of response ranged from 28% to 37% with a median PFS of 5.5 to 7.5 months, figures which are quite comparable to those reported with other non-platinum combinations. The combination PLD/weekly PTX was well tolerated, as was the PLD/vinorelbine combination. 104 In contrast, PLD/TPT, even if tested at different doses of the two drugs, was characterized by an unacceptable rate of severe anemia (48%), leukopenia (70%), and thrombocytopenia (44%).102
PLD: phase III studies
Table 9 summarizes the results from randomized trials using PLD alone or in combination in the salvage setting:106–110,112,113,117 in the study by O’Byrne et al,106 214 recurrent ovarian cancer patients (not defined according to platinum sensitivity) were randomized to either PLD (50 mg/m2 every 28 days) or PTX (175 mg/m2 every 21 days). Preliminary analysis of the data revealed that there were no significant differences in response rates, PFS, OS, or rate of adverse events. However, since the study was suspended because of poor accrual as paclitaxel became incorporated into first-line chemotherapy, no definitive analysis was carried out.
Table 9.
Randomized studies with pegylated liposomal doxorubicin (PLD) alone or in combinations in salvage setting
| Author | Pts (No.) | Drugs/dose | No. | RR (%) | PFS mts | OS mts | % Grade 3/4 toxicity/patient |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Hgb | Neu | PLT | HFS | |||||||
| O’Byrne106 | REC (214) | PLD (50 mg/m2) q28 vs | 107 | 17.8 | 5.4 | 11.4 | – | – | – | – |
| PTX (175 mg/m2) q21 | 107 | 22.4 | 6.0 | 14.0 | ||||||
| Gordon107,108 | RES (255) | PLD (50 mg/m2) d1, q28 vs | 130 | 12.3 | 2.3 | 8.9 | 5 | 12 | 1 | 23 |
| TPT (1.5 mg/m2) d1–5 q21 | 125 | 6.5 | 3.4 | 10.3 | 28 | 77 | 34 | 0 | ||
| SEN (219) | PLD (50 mg/m2) d1, q28 vs | 109 | 28.4 | 7.2 | 27 | |||||
| TPT (1.5 mg/m2) d1–5, q21 | 110 | 28.8 | 5.8* | 17.5* | ||||||
| Mutch109 | RESa (195) | PLD (50 mg/m2) d1, q28 vs | 96 | 8.3 | 3.6 | 12.7 | 2.1 | 18.7 | 5.2 | 10.4 |
| GEM (1,000 mg/m2) d1,8, q21 | 99 | 6.1 | 3.1 | 13.5 | 3.0 | 38.4 | 6.1 | 0 | ||
| Ferrandina110 | RESb (153) | PLD (40 mg/m2) q28 vs | 76 | 16 | 4.0 | 14 | 5 | 6 | 0 | 5 |
| GEM (1,000 mg/m2) d1,8,15 q28 | 77 | 29 | 5.0 | 12.7* | 7 | 23 | 5 | 0 | ||
| Vergote112 ASSIST-1 | RESc (461) | PLD (50 mg/m2) d1, q28 or TPT 1.5 mg/m2 d1–5 q21 vs |
229 | 10.9 | 4.3* | 13.5* | – | – | – | – |
| CAN (1,000 mg/m2) q21 | 232 | 4.3 | 2.3 | 8.5 | – | – | – | – | ||
| Vergote113 ASSIST-5 | RESc (125) | PLD (50 mg/m2) d1 CAN (1000 mg/m2) q28 vs |
65 | 12.3d | 5.6d | – | – | – | – | – |
| PLD (50 mg/m2) d1, q28 | 60 | 8.3 | 3.7 | – | – | – | – | – | ||
| Monk117 OVA-301 | ALL (672) | PLD (30 mg/m2) d1 TRAB (1.1 mg/m2) d1,q21 vs |
335 | 28.0* | 7.3* | 20.5e | 14.0 | 63.0 | 18.0 | 4.0 |
| PLD (50 mg/m2) q28 | 337 | 19.0 | 5.9 | 19.4 | 6.0 | 22.0 | 2.0 | 20.0 | ||
| SEN (430) | PLD (30 mg/m2) d1 TRAB (1.1 mg/m2) d1,q21 vs |
– | 35* | 9.2* | – | – | – | – | – | |
| PLD (50 mg/m2) q28 | – | 23 | 7.5 | – | – | – | – | – | ||
Abbreviations: CAN, canfosfamide; GEM, gemcitabine; HFS, hand–foot syndrome; Hgb, anemia; Neu, neutropenia; OS, overall survival; PFS, progression-free survival; PLT, platelet toxicity; PTX, paclitaxel; REC, not otherwise specified recurrent disease; RES, platinum-resistant recurrent disease; RR, response rate; SEN, platinum-sensitive recurrent disease; TRAB, trabectedin; q, every; d, day.
statistically significant.
up to 2 prior regimens allowed;
platinum resistance = recurrence/progression within 12 months from primary chemotherapy;
patients progressed on 2nd line treatment;
in the subgroup of platinum refractory/resistant (n = 75), the combination achieved a high response rate (15.0% vs 5.7%) and a longer PFS (median = 5.6 months vs 2.9 months) (HR = 0.55; P = 0.042);
results from interim analysis.
In the Gordon et al study,107 whose updated findings were presented in 2004,108 ovarian cancer patients recurring/progressing after front-line chemotherapy were randomized to receive PLD (50 mg/m2 every 28 days) vs TPT (1.5 mg/m2 days 1 to 5, every 21 days): in platinum resistant disease (n = 255) no significant difference was seen in response rate, PFS, or OS between the two treatment arms, while in platinum-sensitive patients (n = 219), median PFS and OS were significantly prolonged in PLD- vs TPT-treated patients (P value = 0.037, and P value = 0.008, respectively). More mature survival analysis confirmed the long-term advantage for platinum-sensitive patients receiving PLD vs TPT (median OS = 27 months vs 17.5 months, hazard ratio [HR] = 1.432, P value = 0.017).108 Moreover, for partially platinum-sensitive disease (n = 122), the HR favored PLD vs TPT (HR = 1.58, P value = 0.021).
The toxicity profiles of the two drugs were completely different, grade 3/4 hematological toxicity occurring more frequently and more severely in TPT vs PLD: in particular, severe neutropenia was documented in 77% of TPT- vs 12% of PLD-treated patients (P < 0001), and thrombocytopenia was found in 34% of TPT vs 1% of PLD cases (P < 0.001). No case of severe HFS was documented in the TPT arm while it was registered in 23% of PLD-treated patients (P < 0.001). Although compliance to fulfil the EORTC-QLQ-C30 questionnaire was poor, thus leaving at 12 weeks of treatment only 200 patients available for comparison, there were no differences in terms of functional and symptom scale QoL scores between the two arms.
Two more recent phase III trials commpared PLD with GEM in recurrent platinum-resistant ovarian cancer patients: Mutch et al109 studied 195 cases experiencing progressive disease within 6 months of completing first-line platinum-based therapy: patients were randomly assigned to either PLD (50 mg/m2 every 28 days) or GEM (1000 mg/m2, days 1, 8, every 21 days) until progression or undue toxicity. Almost 36% of cases in the overall series had already received 2 prior regimens; moreover, response was assessed on the basis of CA15 levels only, in approximately 36% of cases. There was no difference in the response rate between the two treatment arms; median PFS was 3.6 and 3.1 months in PLD vs GEM-treated arms, respectively, while median OS was 12.7 vs 13.5 months: none of the survival end-points showed a statistically significant difference between the two treatment groups.
In contrast to the study of Mutch et al,109 the MITO-3 trial on behalf of the Multicenter Italian Trials in Ovarian cancer (MITO) Group was conducted on patients who recurred/progressed within 12 months from the completion of primary treatment and had received only one platinum/paclitaxel regimen;110 the study included 153 cases (86 patients with a PFI < 6 months and 67 patients with a PFI within 7 to 12 months) who were randomized to PLD (40 mg/m2, every 28 days) vs GEM (1,000 mg/m2, d1,8,15, every 28 days). In the whole series the response rate was 16% in PLD vs 29% in GEM treated patients (P value = 0.066). No statistically significant difference was documented between the two treatment arms in terms of PFS; however, a more favorable OS was registered in the PLD- vs the GEM-treated arm (median OS = 14 months vs 12.7 months, respectively; P value = 0.048). With the limits inherent in the small sample series, the survival advantage reported with PLD over GEM was maintained in the subgroup of partially sensitive patients (P value = 0.016).
Higher global QoL scores especially for physical and emotional findings and fatigue were found in PLD-treated patients at the first and second post-baseline assessments, and this is likely to reflect the profile of toxicity: indeed, hematological toxicity was negligible, with only 6% of grade 3/4 neutropenia compared to 23% in the GEM arm. Moreover, severe HFS was documented in only 5% of cases, in line with the results of previously reported phase II studies (see Table 6).
Very recently, the activity of canfosfamide (CAN) ( Telcyta®), a glutathione analog prodrug which, upon activation by glutathione S-transferase P1-1, is able to induce cellular apoptosis,111 has been tested with a control arm represented by PLD or TPT in platinum-resistant recurrent ovarian cancer patients who had already progressed on second-line treatment with PLD or TPT112 (ASSIST-1, NCT00057720). Patients (n = 461) were randomized to receive PLD (50 mg/m2 every 28 days) or TPT (1.5 mg/m2, days 1 to 5, every 21 days) vs CAN (1000 mg/m2 every 21 days). The overall response rate was higher in the control arm vs CAN (10.9% vs 4.3%, respectively), as was also median PFS (4.3 months vs 2.3 months, P value = 0.01). An overall survival advantage favoring the control arm vs CAN was also documented; in particular, median OS was 13.5 months in the control (14.2 months in PLD, 10.8 months in TPT) vs 8.5 months in the CAN arm (P < 0.01). Based on these results, which basically represent the first evidence from a randomized study of prolongation of OS with third-line treatment, a phase III trial (ASSIST-5, NCT00350948) comparing the combination PLD/CAN vs PLD alone had been planned based on the demonstration that PLD might favor glutathione S-transferase expression, thus potentially increasing cancer cell susceptibility to CAN: patients were randomized to receive PLD (50 mg/m2) plus CAN (1000 mg/m2) every 28 days, vs PLD (50 mg/m2 every 28 days). The primary end-point of the study was originally represented by OS and the planned sample size corresponded to 244 platinum resistant recurrent OvCa patients who had already been administered at least 2 previous lines of chemotherapy. However, the study was temporarily suspended while waiting for the data from the ASSIST-1 study, and, at the time enrolment was re-started, the primary end-point had been replaced by PFS, while 35 out of 125 patients enrolled had not received the planned drug.113 In the intention to treat analysis, no difference emerged between the two treatment arms in terms of response rate and PFS; however, when considering the subgroup of platinum-refractory and primary platinum-resistant patients, a statistically significant advantage in favor of the combination was observed in terms of response rate (15.0% vs 5.7%) and PFS (median 5.6 months vs 2.9 months, HR = 0.55, P value = 0.042). A trend for a longer OS was also observed in the combination vs single agent arm (median OS = 11.8 months vs 7.8 months), although statistical significance was not reached. While the hematological toxicity was generally higher in the combination arm vs PLD alone, the rate of grade 2/3 HFS was lower in PLD/CAN than PLD alone (9% vs 21%, respectively), although the reasons for the protection conferred by the combination remain unclear. These data are the first to report a potential advantage related to the use of a doublet in the poor-prognosis subset of platinum-resistant OvCa patients, and need to be confirmed in the final analysis.
At this time, available data support the phase II-derived suggestions that in platinum-resistant disease none of the currently most frequently used drugs, such as PLD, GEM, or TPT, shows superiority over the others in terms of response rate and survival; in this context the 3-week schedule of administration of PLD at 40 mg/m2 seems to offer the most favorable toxicity profile, which is likely to sustain the achievement of better QoL scores, at least in comparison to GEM.110
Among the most intriguing novel drugs, trabectedin (TRAB) (ET743; Yondelis®), the marine compound derived from Ecteinascidia turbinata, has become relevant for treatment of sarcomas and other solid tumors.114 TRAB has a unique mechanism of action, in that, unlike most other agents, it binds to the minor groove of DNA thus affecting a variety of transcription factors, cell proliferation, and the nucleotide excision repair system. In addition, TRAB inhibits the MDR-1 gene coding for the protein responsible for chemoresistance.114 Based on safety and efficacy results from phase I/II studies in several advanced malignancies, including resistant and particularly platinum-sensitive recurrent OvCa patients,115,116 a phase III trial (OVA-301, NCT00113607) has been planned to compare PLD 50 mg/m2 every 28 days with the combination PLD 30 mg/m2 and TRAB 1.1 mg/m2 every 21 days, in ovarian, peritoneal, and tubal cancer recurring/progressing after first-line chemotherapy,117 with the exclusion of refractory cases; patients were stratified according to ECOG PS (0–1 vs 2), and platinum sensitivity (PFI < 6 months vs PFI ≥ 6 months). Originally, the primary end-point was the OS but it was later amended to PFS at the end of 2006. Secondary end-points included OS, response rates, safety, and pharmacokinetics. Overall, 672 patients were enrolled (337 allocated to PLD/TRAB vs 335 allocated to PLD). In the whole series the response rate as assessed by independent radiology review by Response Evaluation Criteria In Solid Tumors (RECIST) was significantly higher in PLD/TRAB than PLD alone group, as was also median PFS (HR = 0.79, P value = 0.019). However, in platinum-resistant cases (n = 242), no difference was observed in the combination vs PLD alone in terms of response rate (13.4% vs 12.2%, respectively), and PFS, while a clear advantage favoring the combination compared to single-agent PLD was evident in platinum-sensitive disease (n = 430) (response rate 35.3% vs 22.6%, P = 0.0042; median PFS 9.2 months vs 7.5 months; HR = 0.73, P = 0.017). In the context of platinum-sensitive disease, these findings were also evident in the subset of partially platinum-sensitive disease with median PFS of 7.4 vs 5.5 months in PLD/TRAB vs PLD arm (HR = 0.65, P = 0.0152).
Grade 3/4 anemia, neutropenia and thrombocytopenia were documented in 14%, 63%, and 18% of PLD/TRAB cases, and were significantly more frequent compared to PLD alone. Among non-hematological toxicities, grade 3/4 elevation of sGOT, and sGPT was reported in 38% of cases: they were described as of short duration, and of decreased magnitude with succeeding cycles. On the other hand, HFS was documented in 4% of the PLD/TRAB arm compared to 20% in the PLD alone arm. In spite of the increased hematological toxicity in the PLD/TRAB group there was no deterioration of QoL/patient reported outcome (PRO), QLQ-C30 and OV28 and EQ-5D scales.118 Based on these results, which support the PLD/TRAB combination as the most effective non-platinum based combination in platinum-sensitive disease, the combination PLD (30 mg/m2) and TRAB (1.1 mg/m2), every 3 weeks, has been recently approved by the EMEA (September 2009), and is currently submitted for FDA approval for treatment of patients with relapsed platinum-sensitive OvCa.
Among platinum combinations, which are well established to be superior to platinum agents alone in the recurrent setting, PLD/JM8 regimens seem to offer the most effective therapeutic index compared to combinations with taxanes and gemcitabine,81,82 as also suggested by phase II studies. Indeed, the SWOG SO200 trial119 compared PLD/JM8 combination to JM8 alone, but was prematurely stopped because of slow accrual probably related to the introduction of PTX as the JM8 partner in the salvage setting; however, final re-analysis of survival analysis has been very recently published,120 showing that with longer follow up and additional events, a statistically significant improvement of PFS with the combination has been registered, although the previously reported more favorable OS could no longer be detected. Interestingly, for unknown reasons, the association of PLD with JM8 drastically reduced the rate of hypersensitivity reactions compared to JM8 alone (9% vs 0%, P = 0.0008).
The other randomized trials tested PLD/JM8 regimen against PTX/JM8 doublet: in particular, the phase II randomized study by Linardou et al121 documented no difference between the two arms in terms of response rate and PFS, probably because of the small sample size, while confirming the very favorable toxicity profile for the combination, which resulted in a lower rate of severe thrombocytopenia and, more importantly, in no case of severe neurotoxicity.
At the 2009 ASCO meeting the Gynecologic Cancer Intergroup presented the preliminary results of CALYPSO trial (EORTC 55051),122 a randomized phase III study which accrued 976 OvCa patients recurring after 6 months after their first- or second-line platinum based therapy. Patients were randomized: the control group received JM8 AUC 5 and PTX 175 mg/m2 every 21 days and the experimental group received JM8 AUC 5 and PLD 30 mg/m2 every 28 days. The trial showed a statistically significant superiority of PLD/JM8 over PTX/JM8 combination in terms of PFS. Moreover, the superiority of PLD/JM8 was also confirmed in the subset of partially platinum-sensitive disease.123 With a median follow up of 21 months and 308 events, data on OS were be reported early. While grade ≥ 2 HFS was documented in 13% of the PLD/JM8 vs 2% of the PTX/JM8 arm, lower rates of grade 2/3 neurotoxicity were reported in the experimental than in the standard arm (5% vs 28%, respectively). Interestingly, severe hypersensitivity reactions occurred less frequently in PLD/JM8 than in PTX/JM8 group (2% vs 9% ).
While waiting for the mature OS data from the CALYPSO trial, it can be reasonably stated that the PLD/JM8 combination represents a valid alternative to other platinum-based regimens in recurrent platinum-sensitive OvCa especially for patients whose QoL is recognized to be heavily compromised by alopecia, or who had experienced or had not yet been rescued from taxane-induced neurotoxicity.
Attempts to include PLD in front-line treatment have also been attempted: in particular, with the aim of improving PTX/JM8 efficacy, sequential doublets or triplet combinations including PLD have been investigated based also on the very favorable and not overlapping toxicity profile. The use of 4 cycles of standard PTX/JM8 (175 mg/m2, AUC 6, every 21 days) followed by 4 cycles of PLD/JM8 (40 mg/m2, AUC 6, every 21 days) has been first investigated by Potamianou et al124 in a phase II study including 41 patients. At the end of the 8 courses the response rate was 66%, and median PFS was 20 months. Toxicity mainly consisted of neutropenia, which occurred in 48.7% of patients at the end of JM8/PTX and 63.8% at the end of PLD/JM8 treatment. There was no undue or unexpected non-hematological toxicity, but grade 2 and 3 neurotoxicity after PTX/JM8 was registered in 9% and 34.1% of patients, respectively. The potential efficacy of triplets and sequential doublets has been also investigated in the GOG182/ICON5 randomized trial,125 which represents the largest cooperative effort attempted worldwide by the Gynecologic Oncology Group in the US, and the Medical Research Council in the UK on behalf of the International Collaborative Ovarian Neoplasm (ICON) Group. The GOG182/ICON5 trial enrolled 4312 stage III/IV patients who were randomized to 5 arms including the standard treatment 1) JM8 AUC 6, PTX 175 mg/m2 every 21 days, 8 cycles, vs 2 triplets; 2) JM8 AUC 5, PTX 175 mg/m2, GEM 800 mg/m2 days 1, 8, every 21 days, 8 cycles; 3) JM8 AUC 5, PTX 175 mg/m2, every 21 days plus PLD 30 mg/m2, every other cycle, 8 cycles, or 2 sequential doublets: 4) JM8 AUC 6, TPT 1.25 mg/m2 days 1 to 3, 4 cycles, followed by JM8 AUC 6, PTX 175 mg/m2, 4 cycles; 5) JM8 AUC 6, GEM 800 mg/m2, days 1, 8 every 21 days, 4 cycles followed by JM8 AUC 6, PTX 175 mg/m2, 4 cycles.
Despite the documentation of increased hematological and non-hematological toxicity in the triplet regimens, there was no PFS or OS advantage with sequential doublets or with triplets compared with the control arm.
In the front-line setting, mature results from the MITO-2 (NCT00326456)126,127 trial are eagerly awaited; this study, which first investigated the combination PLD/JM8 (30 mg/m2, AUC = 5, every 21 days) vs the standard treatment, has randomized 820 stage IC/IV ovarian cancer patients since January 2003 to November 2007. The primary objective was PFS, while secondary objectives were OS, response rate, toxicity and QoL. Data presented at the 2009 ASCO meeting documented the equivalence of the two treatment arms in terms of response rate (59.0% in the standard vs 57.0% in the experimental arm, P = 0.70).128 As of March 2009, with a median follow up of 35 months, 530 events for PFS and 269 deaths were documented; therefore survival data can-not be considered mature enough for final analysis, which will be hopefully available in 2010. As expected, the pattern of toxicity differed between the two groups: severe anemia and thrombocytopenia were more frequently detected in PLD/JM8 vs the standard arm (10% vs 4%, P < 0.001, for anemia) (16% vs 2%, P < 0.001, for thrombocytopenia). On the other hand, grade ≥ 3 neurotoxicity was registered in 3% of standard vs 0.3% in the experimental treatment (P = 0.004). Finally, alopecia (any grade) occurred in 63% of cases allocated to the standard vs 14% allocated the experimental arm (P < 0.001).
PLD and target-based agents
Given the relevance of PLD alone or in combination with platinum as well as non-platinum agents in almost all clinical settings of ovarian cancer, it is not surprising that attempts are ongoing to study combinations of this drug with target-based agents: the rationale of this approach is represented by the enormous potential inherent in classes of drugs with a different mechanism of action. Moreover, the availability of target-based agents at an advanced stage of clinical development, and therefore with a well known spectrum of activity and toxicity, has fuelled great enthusiasm for exploring their association with PLD. Among the most appealing classes of biological drugs, the angiogenesis inhibitors seem to be the most promising: in particular, several phase II trials have shown the activity of bevacizumab (BEV) (Avastin®), the monoclonal antibody against vascular endothelial growth factor (VEGF), in platinum-resistant and -sensitive disease. 129 Preliminary results from an ongoing phase II study have been recently presented at the 2009 ASCO meeting, on the PLD/BEV combination in second-line treatment of OvCa patients with a PFI ≤ 6 months and with < 3 previous regimens.130 The study was started in 2007 and aimed at recruiting 48 patients. PLD (30 mg/m2 every 21 days) was administered alone at the first cycle, and then with BEV (15 mg/kg every 21 days) for the following 6 cycles or until progression. In the 21 patients available for analysis, response was documented in 14.3% of cases according to the RECIST criteria, and in 8 out of 14 cases (57.1%) who had elevated Ca125 levels at enrolment. In 62% of cases PFS duration exceeded 18 weeks. Interestingly, the pharmacokinetics measures obtained after 1 hour, and at day 7 and day 21 of the first 2 cycles did not show any BEV-induced modification of PLD pharmacokinetics. Grade 3 HFS was registered in 5% of cases, and PLD dose reduction was required in 33% of cases, while grade 3 hypertension and BEV reduction > 10% was reported in 15% of cases. With the aim of reducing the rate/severity of side effects, a weekly regimen of PLD/BEV (PLD 10 mg/m2 and BEV 2 mg/kg days 1, 8, 15, every 28 days, for at least 3 cycles) was investigated in 30 recurrent OvCa patients who had been heavily pre-treated.131 According to the Gynecologic Cancer Intergroup criteria, an overall response rate of 45% was achieved in 26 evaluable patients with a clinical benefit in 75% of cases. On the basis of the RECIST criteria, response was achieved in 38.4% of cases with disease stabilization in 34.6%. No severe hematological toxicity was observed, and the only cases experiencing gastrointestinal perforation were treated conservatively. HFS was documented in 13.6% and required treatment in only one case. Overall, the weekly regimen seems well tolerated although the potential cumulative cardiovascular side effects of the two drugs need to be explored in a larger series. A large phase II randomized study (AURELIA, NCT00976911) is ongoing, which recruits patients with recurrent platinum-resistant ovarian, fallopian tube and peritoneal cancer, who are randomized to standard treatment (PTX, or TPT, or PLD 40 mg/m2 every 28 days) vs the experimental arm in which BEV 10 mg/m2 bi-weekly or 15 mg/m2 every 21 days is added to the same drugs. Among novel VEGFR kinase inhibitors, vandetanib is currently being investigated in combination with PLD in recurrent OvCa (NCT00862836).
The interest in other angiogenesis inhibitors is shown by the efforts to study molecules acting on different targets, such as volociximab (VOL). This is a chimeric monoclonal antibody able to directly target α5/β1 integrin, a protein characterizing the activated endothelial cells, thus preventing its interaction with the extracellular fibronectin and disrupting tumor neoangiogenesis.132 A phase II study by Vergote et al133 has been carried out in recurrent OvCa patients who had already been administered up to 2 lines of chemotherapy; patients received PLD 40 mg/m2 every 28 days (n = 15), or PLD 40 mg/m2, every 28 days and VOL 15 mg/m2 biweekly (n = 15) or PLD 40 mg/m2 every 28 days and VOL 15 mg/m2 weekly (n = 15). According to the PFS duration documented in each arm, an increasing number of cases would be enrolled in more favorable arms. 66 patients were enrolled in the first arm while 34 and 27 patients were allocated to the remaining groups. Median PFS was 27.5 weeks in the first arm, 18 weeks in the second and 31.6 weeks in the third, thus suggesting no superiority of the combination vs PLD alone. Severe side effects have been documented in <5% of cases in each treatment arm and, interestingly, in this study also, the addition of the target-based agent did not seem to alter PLD pharmacokinetics.
A summary of the ongoing trials investigating the combination of PLD with other growth factor receptor inhibitors such as IMC-3G3, an inhibitor of PDGF-R (NCT00913835), panitumumab (an EGF-R blocker) (NCT00861120), and pazopanib (which interferes with VEGF-R1,2,3 kinase, PDGF-R and c-kit oncogene product) (NCT01035658) can be found at the www.clinicaltrials.gov.
Besides the attempts to study the association of PLD with drugs interfering with the angiogenic and growth factor driven mitogenic processes, other novel biological targets crucial for cancer cell biology have been considered for designing PLD/target based therapy: for instance farletuzumab (MORAB-003), an inhibitor of folate receptor-α, is under investigation combined with PLD and JM8 in recurrent platinum-sensitive recurrent OvCa (NCT01004380). Moreover, based on preclinical studies and encouraging phase I data showing the absence of any interference of bortezomib (BOR; Velcade®), a proteasome inhibitor, with PLD pharmacokinetics,134 preliminary results of a phase II study combining the two drugs were presented at the 2008 ASCO meeting;135 recurrent platinum-resistant (n = 15), and -sensitive (n = 15) OvCa patients were administered PLD 30 mg/m2 and BOR 1.3 mg/m2 days 1, 4, 8, 11 every 21 days. Responses were seen only in the platinum-sensitive disease group which proceeded to the second step of enrolment.
Although all these data are very preliminary, it seems that quite tolerable combinations of PLD with target-based agents can be used without interfering with PLD pharmacokinetics.
PLD: toxicity issues
The very favorable PLD toxicity profile is widely recognized as the advantage of this drug, which does not accumulate in normal tissues and especially in cardiac muscle, thus eliminating the cardiotoxicity commonly associated with conventional doxorubicin administration.136 However, some adverse side effects have to be taken into account: acute hypersensitivity reaction, characterized by flushing, headache, facial edema, back pain, rigors, dyspnea, hypotension and chest/throat tightness can occur during drug infusion (and differently from hypersensitivity reactions with other drugs which generally are documented after a previous exposure), they can be registered even during the first administration. PLD-related reactions are seen in about 6.8% of patients; however, if they are not documented initially, they rarely occur in subsequent cycles.14 Moreover, hypersensitivity reactions seem to depend on the infusion rate, and can therefore be prevented, in principle, by administering the drug at an initial rate of 1 mg/min. Muco-cutaneous toxicity is the most frequent PLD-related side effect, and represents the most important dose-limiting toxicity.19 In particular, palmar-plantar erythrodysesthesia, also known as HFS and originally described as associated with 5-fluorouracil infusion, represents a distinctive toxic reaction to PLD administration. Pathogenesis remains unclear, although it is generally accepted that the prolonged accumulation of PLD in areas where subclinical trauma occurs (due to friction, tight-fitting clothing or shoes, repeated skin pressure or chemical insults), leading to inflammation and subsequent altered vascular per-meability, might play a relevant role.137 It has been suggested that PLD transport by sweat could lead to an easy localization of the drug into the stratum corneum where free radicals are produced and HFS can be induced.138
HFS is characterized by paresthesia of the outer extremities occurring 2 to 12 days after chemotherapy administration, and is followed 3 to 4 days later by patch erythema, edema, and desquamation of hands and soles. The natural history of HFS is often self-limiting with resolution within 1 to 5 weeks from stopping treatment. However, some cases develop blistering and ulceration which can limit daily functions and reduce patient quality of life. Recently, the investigation of factors favoring the occurrence of HFS during PLD treatment has been carried out in a very large series of recurrent ovarian, peritoneal and fallopian tube cancer patients: the number of PLD cycles and doses ≥ 50 mg/m2 as well as the concomitant occurrence of neutropenia, and peripheral neuropathy were predisposing factors for HFS.101 Moreover, the incidence of HFS was higher in patients receiving >3 lines of previous chemotherapy lines regardless of chemotherapeutic agents used. Surprisingly, the proportion of cases who suffered PPE was higher in cases of cooling mechanism adoption,101 while patient age and mean body mass index did not affect HFS development, confirming previously published results.139 Besides the cumulative dose, the schedule of administration also seems to be an important risk factor: in particular, it has been suggested that the 3-week schedule may coincide with the interval of epidermal turnover, a phenomenon that would thus emphasize the potential PLD-induced keratinocyte damage.140 Patient education to avoid risk factors by preventing mechanical, physical, or chemical skin insults, and to recognize early the initial signs/symptoms of skin toxicity is relevant. More specifically, the use of ice pack cooling of hands and feet associated with consumption of iced liquids during chemotherapy administration has been empirically explored,141 as well as the administration of corticosteroids, pyridoxine supplement, topical application of dimethylsulfoxide, and emollient or moisturizing lotions which are often used in clinical practice.142 However, with the exception of pyridoxine supplement which has been recently tested in a phase III study and shown not to confer any advantage compared to placebo in terms of HFS prevention,143 the true efficacy of the other approaches has not been proven in prospective trials. Apart from the studies suggesting that the bi-weekly schedule, or the 4-weekly administration of PLD at doses of 40 mg/m2 are associated with negligible if any severe HFS (see Table 6), a randomized phase II trial in metastatic breast cancer has also shown that by reducing PLD dose intensity to 10 mg/m2/week, HFS tends to be mild or modest in the vast majority of cases.144 Recently, an international panel of experts was convened to develop recommendations for management of PLD-associated HFS according to the grade of symptoms and clinical findings;145 however, phase III trials are urgently needed to support the rigorous adoption of any of these previously cited interventions.
Conclusions
The pegylated liposomal formulation of doxorubicin, because of its unique and favorable toxicity profile, has greatly expanded the clinical applications of the parent compound: indeed, whether used alone or in combination with non-platinum and platinum agents, PLD has been introduced in the management of almost all clinical settings in OvCa patients.
In particular, results from phase II and phase III randomized trials have led to FDA approval of PLD in the salvage treatment of recurrent disease; moreover, the upcoming mature results of the CALYPSO trial suggest that PLD/carboplatin combination is a very valid option in recurrent platinum-sensitive disease especially in patients who had experienced or had not yet been rescued from taxane-induced neurotoxicity or just refuse to tolerate alopecia. The MITO-2 final results might lead to the replacement of paclitaxel as the carboplatin partner in front-line treatment, or at least provide a useful alternative to carboplatin/paclitaxel depending on patient performance status and preference. Meanwhile efforts will continue in combining PLD with target-based agents which have already shown preliminary promising activity in ovarian malignancies, and do not seem to alter the unique and advantageous pharmacokinetics of PLD.
Table 4.
Phase I studies with pegylated liposomal doxorubicin (PLD) in combination with platinum/taxanes
| Author | No. pts | Type of tumor | MTD | DLTs |
|---|---|---|---|---|
| Eng47 | 23 | Solid tumors | CDDP 60 mg/m2 PTX 90 mg/m2 (1 cycle) then 130 mg/m2 PLD 30 mg/m2, q21 |
neutropenia |
| Rose48 | 12 | Ovarian, tubal peritoneal cancer | JM8 AUC 5 PTX 175 mg/m2, q21 PLD 30 mg/m2, every other cycle |
neutropenia |
| Gibbs49 | 31 | Ovarian carcinomas and MMMT | JM8 AUC 6 PTX 175 mg/m2 PLD 30 mg/m2, q28 or JM8 AUC 5 PTX 175 mg/m2 PLD 20 mg/m2, q21 |
neutropenia stomatitis HFS |
Abbreviations: CDDP, cisplatin; DLT, dose limiting toxicity; HFS, hand–foot syndrome; JM8, carboplatin; MMMT, mixed malignant Müllerian tumors; MTD, maximum tolerated dose; PTX, paclitaxel; q, every; d, day.
Table 10.
Randomized studies with pegylated liposomal doxorubicin (PLD) in combination with platinum in salvage setting
| Author | Pts | Drugs/dose | No. pts | RR (%) | PFS mts | OS mts | % Grade 3/4 toxicity/patient |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Hgb | Neu | PLT | HFS | |||||||
| Alberts119,a SWOG SO200 | SEN 6–24 mts | PLD (30 mg/m2) d1 JM8 (AUC 5) d1, q28 vs |
31 | 52 | 12* | 26* | 16 | 48.0 | 39.0 | 3.0 |
| JM8 (AUC 5) d1, q28 | 30 | 29 | 8 | 18 | 0 | 3.0 | 0 | 0 | ||
| Markman120 SWOG SO200 | SEN 6–24 mts | PLD (30 mg/m2) d1 JM8 (AUC 5) d1, q28 vs |
31 | 59* | 12* | 31 | – | – | – | – |
| JM8 (AUC 5) d1, q28 | 30 | 28 | 8 | 18 | – | – | – | – | ||
| Linardou121,b | SEN >6 mts | PLD (45 mg/m2) d1 JM8 (AUC 5) d1, q28 vs |
93 | 51 | 11.7 | 24.4 | – | 35.0 | 12.0 | 0 |
| PTX (175 mg/m2) d1 JM8 (AUC 5) d1, q21 |
96 | 58 | 10.8 | 30.4 | – | 30.0 | 12.0* | – | ||
| Pujade-Lauraine122 CALYPSO (EORTC 55051) | SEN >6 mts | PLD (30 mg/m2) d1 JM8 (AUC 5) d1, q28 vs |
467 | – | 11.3* | –c | – | 35.0 | 16.0 | 1.0 |
| PTX (175 mg/m2) d1 JM8 (AUC 5) d1, q21 |
509 | – | 9.4 | – | – | 46.0 | 6.0 | 1.0 | ||
statistically significant.
prematurely closed for slow accrual;
randomized phase II study;
too early to be reported.
Abbreviations: HFS, hand–foot syndrome; Hgb, anemia; JM8, carboplatin; Neu, neutropenia, PFS, progression-free survival; PLT, platelet toxicity; PTX, paclitaxel; RR, response rate; RES, platinum-resistant recurrent disease; SEN, platinum-sensitive recurrent disease; q, every; d, day.
Footnotes
Disclosures
The authors declare no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Eisenkop SM, Spirtos NM, Friedman RL, Lin WC, Pisani AL, Perticucci S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecol Oncol. 2003;90:390–396. doi: 10.1016/s0090-8258(03)00278-6. [DOI] [PubMed] [Google Scholar]
- 3.Ozols RF. Systemic therapy for ovarian cancer: current status and new treatments. Semin Oncol. 2006;33(2 Suppl 6):S3–S11. doi: 10.1053/j.seminoncol.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Salzberg M, Thurlimann B, Bonnefois H, et al. Current concepts of treatment strategies in advanced or recurrent ovarian cancer. Oncology. 2005;68:293–298. doi: 10.1159/000086967. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong DK. Relapsed ovarian cancer: challenges and management strategies for a chronic disease. Oncologist. 2002;7(Suppl 5):20–28. doi: 10.1634/theoncologist.7-suppl_5-20. [DOI] [PubMed] [Google Scholar]
- 6.Colombo N, Gore M. Treatment of recurrent ovarian cancer relapsing 6–12 months post platinum-based chemotherapy. Crit Rev Oncol Haematol. 2007;64:129–138. doi: 10.1016/j.critrevonc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration, Center for Drug Evaluation and Research. [Accessed Dec 17, 2009]. [updated 2008 Oct 06]. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050718s033lbl.pdf.
- 8.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 9.Gabizon A, Catane R, Uziely B, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54:987–992. [PubMed] [Google Scholar]
- 10.Gabizon A, Isacson R, Rosengarten O, Tzemach D, Shmeeda H, Sapir R. An open-label study to evaluate dose and cycle dependence of the pharmacokinetics of pegylated liposomal doxorubicin. Cancer Chemother Pharmacol. 2008;4:695–702. doi: 10.1007/s00280-007-0525-5. [DOI] [PubMed] [Google Scholar]
- 11.Yokoe J, Sakuragi S, Yamamoto K, et al. Albumin-conjugated PEG liposome enhances tumor distribution of liposomal doxorubicin in rats. Int J Pharm. 2008;353:28–34. doi: 10.1016/j.ijpharm.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Bogner JR, Kronawitter U, Rolinski B, Truebenbach K, Goebel FD. Liposomal doxorubicin in the treatment of advanced AIDS-related Kaposi sarcoma. J Acquir Immune Defic Syndr. 1994;7:463–468. [PubMed] [Google Scholar]
- 13.James ND, Coker RJ, Tomlinson D, et al. Liposomal doxorubicin (Doxil): an effective new treatment for Kaposi’s sarcoma in AIDS. Clin Oncol (R Coll Radiol) 1994;6:294–296. doi: 10.1016/s0936-6555(05)80269-9. [DOI] [PubMed] [Google Scholar]
- 14.Uziely B, Jeffers S, Isacson R, et al. Liposomal doxorubicin: antitumor activity and unique toxicities during two complementary phase I studies. J Clin Oncol. 1995;13:1777–1785. doi: 10.1200/JCO.1995.13.7.1777. [DOI] [PubMed] [Google Scholar]
- 15.Jahanzeb M, Vogel C, Elkrish M, et al. Stealth liposomal doxorubicin (Doxil) causes a mild and delayed leukopenia compared to doxorubicin in patients with solid tumors. Proc Am Soc Clin Oncol. 1997;16:239. Abstr 843. [Google Scholar]
- 16.Gabizon A, Uziely B, Lotem M, et al. Doxil in patients with pretreated metastatic breast cancer (MBC): a dose-schedule finding study with pharmacokinetics. Proc Am Soc Clin Oncol. 1997;16:147. Abstr 516. [Google Scholar]
- 17.Caponigro F, Comella P, Budillon A, et al. Phase I study of Caelyx (doxorubicin HCL, pegylated liposomal) in recurrent or metastatic head and neck cancer. Ann Oncol. 2000;11:339–342. doi: 10.1023/a:1008319618638. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton A, Biganzoli L, Coleman R, et al. EORTC 10968: a phase I clinical and pharmacokinetic study of polyethylene glycol liposomal doxorubicin (Caelyx, Doxil) at a 6-week interval in patients with meta-static breast cancer. European Organization for Research and Treatment of Cancer. Ann Oncol. 2002;13:910–918. doi: 10.1093/annonc/mdf157. [DOI] [PubMed] [Google Scholar]
- 19.Marina NM, Cochrane D, Harney E, et al. Dose escalation and pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in children with solid tumors: a pediatric oncology group study. Clin Cancer Res. 2002;8:413–418. [PubMed] [Google Scholar]
- 20.Harrison LE, Bryan M, Pliner L, Saunders T. Phase I trial of pegylated liposomal doxorubicin with hyperthermic intraperitoneal chemotherapy in patients undergoing cytoreduction for advanced intra-abdominal malignancy. Ann Surg Oncol. 2008;15:1407–1413. doi: 10.1245/s10434-007-9718-8. [DOI] [PubMed] [Google Scholar]
- 21.Klein P, Wasserheit C, Hochster H, et al. Apparent protection of doxil skin and oral toxicities when combined with cisplatin (CDDP): results of a Phase I study. Proc Am Soc Clin Oncol. 1999;18:217. Abstr 835. [Google Scholar]
- 22.Lyass O, Hubert A, Gabizon AA. Phase I study of doxil-cisplatin combination chemotherapy in patients with advanced malignancies. Clin Cancer Res. 2001;7:3040–3046. [PubMed] [Google Scholar]
- 23.Uys A, Rapoport BL, Mahomed R. Phase I trial of pegylated-liposomal doxorubicin (Caelyx, Doxil) and carboplatin in patients with advanced malignancy. Proc Am Soc Clin Oncol. 2002;21 Abstr 2138. [Google Scholar]
- 24.Goncalves A, Braud AC, Viret F, et al. Phase I study of pegylated liposomal doxorubicin (Caelyx) in combination with carboplatin in patients with advanced solid tumors. Anticancer Res. 2003;23:3543–3548. [PubMed] [Google Scholar]
- 25.Hamilton AL, Pavlick AC, Volm M, et al. Pegylated liposomal doxorubicin (PLD) and carboplatin: a Phase I study of combination therapy with maintenance PLD. Proc Am Soc Clin Oncol. 2003;22 Abstr 1986. [Google Scholar]
- 26.Gonzalez-Billalabeitia E, Mendiola C, Mellado B, et al. for the Grupo PSAMOMA. A phase I/II clinical study of pegylated liposomal doxorubicin plus carboplatin in advanced ovarian cancer. Proc Am Soc Clin Oncol. 2003;22 Abstr 1919. [Google Scholar]
- 27.du Bois A, Burges A, Meier W, et al. Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom. Pegylated liposomal doxorubicin and carboplatin in advanced gynecologic tumors: a pro-spective phase I/II study of the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) Ann Oncol. 2006;17:93–96. doi: 10.1093/annonc/mdj032. [DOI] [PubMed] [Google Scholar]
- 28.Recchia F, Saggio G, Amiconi G, et al. A multicenter phase II study of pegylated liposomal doxorubicin and oxaliplatin in recurrent ovarian cancer. Gynecol Oncol. 2007;106:164–169. doi: 10.1016/j.ygyno.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Israel VK, Jeffers S, Bernal G, et al. Phase I study of doxil (liposomal doxorubicin) in combination with paclitaxel. Proc Am Soc Clin Oncol. 1998 Abstr 938. [Google Scholar]
- 30.Muggia FM, Hornreich G, Wadler S, et al. Overview of toxicities from a combination of Doxil-paclitaxel (PachiDox) in a New York Gynecologic Oncology Group (NYGOG) study on endometrial carcinomas and sarcomas. Proc Am Soc Clin Oncol. 2000 Abstr 1616. [Google Scholar]
- 31.Modiano M, Taylor C, Sharpington T, Ng M, Martinez A. Phase I study of Doxil (pegylated liposomal doxorubicin) plus escalating doses of Taxol in the treatment of patients with advanced breast or gynecologic malignancies. Proc Am Soc Clin Oncol. 1999 Abstr 848. [Google Scholar]
- 32.Schwonzen M, Kurbacher CM, Mallmann P. Liposomal doxorubicin and weekly paclitaxel in the treatment of metastatic breast cancer. Anticancer Drugs. 2000;11:681–685. doi: 10.1097/00001813-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Tolis C, Briasoulis E, Tzamakou E, et al. Phase I trial and interaction pharmacokinetics of pegylated-liposomal doxorubicin (Caelyx; Doxil) and weekly Taxol. Proc Am Soc Clin Oncol. 2000;19 Abstr 803. [Google Scholar]
- 34.Androulakis N, Kouroussis C, Mavroudis D, et al. Phase I study of weekly paclitaxel and liposomal doxorubicin in patients with advanced solid tumours. Eur J Cancer. 2002;38:1992–1997. doi: 10.1016/s0959-8049(02)00168-5. [DOI] [PubMed] [Google Scholar]
- 35.Mavroudis D, Kouroussis C, Kakolyris S, et al. Phase I study of paclitaxel (taxol) and pegylated liposomal doxorubicin (caelyx) administered every 2 weeks in patients with advanced solid tumors. Oncology. 2002;62:216–222. doi: 10.1159/000059568. [DOI] [PubMed] [Google Scholar]
- 36.Lortholary A, Delozier T, Bourgeois H, et al. A phase I study of paclitaxel in combination with pegylated liposomal doxorubicin (PLD) as second-line chemotherapy in metastatic breast cancer (MBC) Proc Am Soc Clin Oncol. 2003;22 Abstr 206. [Google Scholar]
- 37.Briasoulis E, Pentheroudakis G, Karavasilis V, et al. Weekly paclitaxel combined with pegylated liposomal doxorubicin (CaelyxTM) given every 4 weeks: dose-finding and pharmacokinetic study in patients with advanced solid tumors. Ann Oncol. 2004;15:1566–1573. doi: 10.1093/annonc/mdh404. [DOI] [PubMed] [Google Scholar]
- 38.Hirsch R, Jahanzeb M. Phase I Study of Doxil in Combination with Escalating Doses of Taxotere in the Treatment of Patients with Advanced Malignancies (Meeting abstract) Proc Am Soc Clin Oncol. 1999 Abstr 820. [Google Scholar]
- 39.Drinkard L, Blumenschein G, DiStefano A, Adams J. A Phase I trial of liposomal doxorubicin (Doxil) given on day one followed by docetaxel (Taxotere) given on day five in patients with advanced solid tumors. (Meeting abstracts) Proc Am Soc Clin Oncol. 1999;18 Abstr 801. [Google Scholar]
- 40.Pavlick AC, Chodkiewicz C, Liebes L, et al. A phase I and pharmacokinetic study of docetaxel combined with Doxil (pegylated liposomal doxorubicin) without and with granulocyte colony stimulating factor. Anticancer Drugs. 2004;15:119–125. doi: 10.1097/00001813-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Tauer K, Hussein A, Birch R, et al. A phase i study of Taxotere (T) and Doxil (D) in refractory cancer. Proc Am Soc Clin Oncol. 2000;19 Abstr 901. [Google Scholar]
- 42.Sparano JA, Malik U, Rajdev L, et al. Phase I trial of pegylated iposomal doxorubicin and docetaxel in advanced breast cancer. J Clin Oncol. 2001;15;19:3117–3125. doi: 10.1200/JCO.2001.19.12.3117. [DOI] [PubMed] [Google Scholar]
- 43.Sikov WM, Hsieh M, Lopez F. Phase I weekly docetaxel with doxorubicin or Doxil: a BrUOG study. Proc Am Soc Clin Oncol. 2001;20 Abstr 2088. [Google Scholar]
- 44.Gasparini G, Morabito A, Fanelli M, et al. Phase I–II study of liposomal doxorubicin and docetaxel as first-line treatment in patients with advanced breast cancer. Proc Am Soc Clin Oncol. 2002;21 Abstr 2029. [Google Scholar]
- 45.Fracasso PM, Rodriguez LC, Herzog TJ, et al. Phase I dose and sequencing study of pegylated liposomal doxorubicin and docetaxel in patients with advanced malignancies. Cancer. 2003;98:610–617. doi: 10.1002/cncr.11547. [DOI] [PubMed] [Google Scholar]
- 46.Bischoff J, Hesse S, Stemmler H, Zauner S, Gutschow K. A phase I study of PEG liposomal doxorubicin (PLD) plus docetaxel (D) in metastatic breast cancer (MBC) Proc Am Soc Clin Oncol. 2003:22. Abstr 282. [Google Scholar]
- 47.Eng C, Mauer AM, Fleming GF, et al. MJ. Phase I study of pegylated liposomal doxorubicin, paclitaxel, and cisplatin in patients with advanced solid tumors. Ann Oncol. 2001;12:1743–1747. doi: 10.1023/a:1013574328938. [DOI] [PubMed] [Google Scholar]
- 48.Rose PG, Greer BE, Horowitz IR, Markman M, Fusco N. Paclitaxel, carboplatin, and pegylated liposomal doxorubicin in ovarian, and peritoneal carcinoma: A phase I study of the Gynecologic Oncology Group. Gynecol Oncol. 2007;104:114–119. doi: 10.1016/j.ygyno.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 49.Gibbs DD, Pyle L, Allen M, et al. A phase I dose-finding study of a combination of Pegylated liposomal doxorubicin (Doxil), carboplatin and paclitaxel in ovarian cancer. Br J Cancer. 2002;86:1379–1384. doi: 10.1038/sj.bjc.6600250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ryan CW, Fleming GF, Janisch L, et al. A phase I study of liposomal doxorubicin (Doxil) with topotecan. Am J Clin Oncol. 2000;23:297–300. doi: 10.1097/00000421-200006000-00019. [DOI] [PubMed] [Google Scholar]
- 51.Yeung AW, Wong A, Ali M. Phase I trial of doxil and topotecan in solid tumors. Proc Am Soc Clin Oncol. 1998 Abstr 979. [Google Scholar]
- 52.Hochster H, Rosenthal M, Chachoua M, Sorich J, Muggia F. Prolonged topotecan infusion combined with doxil. a well tolerated regimen. Proc Am Soc Clin Oncol. 1999;18:214. Abstr 822. [Google Scholar]
- 53.Hamilton A, Hoechster H, Rosenthal M, et al. Continuous infusion topotecan (TP-CI) with Doxil (DX): a phase I study of dual topoisomerase inhibition. Proc Am Soc Clin Oncol. 2000;19 Abstr 777. [Google Scholar]
- 54.Geertsen PF, Str⊘yer I, Herrstedt J, Werner Hansen S. Phase I study of topotecan (T) and pegylated liposomal doxorubicin (Caelyx) in patients (pts) with progressive ovarian cancer within 12 months after first-line platinum-paclitaxel containing chemotherapy. Proc Am Soc Clin Oncol. 2001;20 Abstr 2522. [Google Scholar]
- 55.Pautier P, Germann N, Faivre S, et al. Phase I study of Caelyx (KLX – stealth stabilized liposomal doxorubicin – Doxil) in combination with topotecan (TPT) Proc Am Soc Clin Oncol. 2000;19 Abstr 910. [Google Scholar]
- 56.Mirchandani D, Hochster H, Hamilton A, et al. Phase I study of combined pegylated liposomal doxorubicin with protracted daily topotecan for ovarian cancer. Clin Cancer Res. 2005;11:5912–5919. doi: 10.1158/1078-0432.CCR-04-1240. [DOI] [PubMed] [Google Scholar]
- 57.Garcia AA, Roman L, Muderspach L, et al. Phase I clinical trial of topotecan and pegylated liposomal doxorubicin. Cancer Invest. 2005;23:665–670. doi: 10.1080/07357900500359877. [DOI] [PubMed] [Google Scholar]
- 58.Ghesquieres H, Faivre S, Djafari L, et al. Phase I dose escalation study of pegylated liposomal doxorubicin (Caelyx) in combination with topotecan in patients with advanced malignancies. Invest New Drugs. 2006;24:413–421. doi: 10.1007/s10637-006-7520-2. [DOI] [PubMed] [Google Scholar]
- 59.Rose PG, Smrekar M, Haba P, Fusco N, Rodriguez M. A phase I study of oral topotecan and pegylated liposomal doxorubicin (doxil) in platinum-resistant ovarian and peritoneal cancer. Am J Clin Oncol. 2008;31:476–480. doi: 10.1097/COC.0b013e31816a6221. [DOI] [PubMed] [Google Scholar]
- 60.Penson RT, Seiden MV, Goodman A, et al. Gynecologic Oncology Research Program at Dana Farber/Partners CancerCare. Phase I trial of escalating doses of topotecan in combination with a fixed dose of pegylated liposomal doxorubicin in women with müllerian malignancies. Gynecol Oncol. 2004;93(3):702–707. doi: 10.1016/j.ygyno.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 61.Laufman LR, Spiridonidis CH, Jones JJ, Rhodes V, Rossi K, Wallace K. Phase I study of Doxil and vinorelbine in patients with advanced malignancies. Cancer Invest. 2004;22:344–352. doi: 10.1081/cnv-200034893. [DOI] [PubMed] [Google Scholar]
- 62.D’Agostino G, Ferrandina G, Garganese G, et al. Phase I study of gemcitabine and liposomal doxorubicin in relapsed ovarian cancer. Oncology. 2002;62:110–114. doi: 10.1159/000048255. [DOI] [PubMed] [Google Scholar]
- 63.Fracasso PM, Blum KA, Tan BR, et al. Phase I study of pegylated liposomal doxorubicin and gemcitabine in patients with advanced malignancies. Cancer. 2002;95:2223–2229. doi: 10.1002/cncr.10937. [DOI] [PubMed] [Google Scholar]
- 64.Bozionelou V, Vamvakas L, Pappas P, et al. A dose escalation and pharmacokinetic study of biweekly pegylated liposomal doxorubicin, paclitaxel and gemcitabine in patients with advanced solid tumours. Br J Cancer. 2007;97:43–49. doi: 10.1038/sj.bjc.6603832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muggia FM, Hainsworth JD, Jeffers S, et al. Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. J Clin Oncol. 1997;15:987–993. doi: 10.1200/JCO.1997.15.3.987. [DOI] [PubMed] [Google Scholar]
- 66.Gordon AN, Granai CO, Rose PG, et al. Phase II study of liposomal doxorubicin in platinum- and paclitaxel-refractory epithelial ovarian cancer. J Clin Oncol. 2000;18:3093–3100. doi: 10.1200/JCO.2000.18.17.3093. [DOI] [PubMed] [Google Scholar]
- 67.Rose PG, Maxson JH, Fusco N, et al. Liposomal doxorubicin in ovarian, peritoneal, and tubal carcinoma: a retrospective comparative study of single-agent dosages. Gynecol Oncol. 2001;82:323–328. doi: 10.1006/gyno.2001.6272. [DOI] [PubMed] [Google Scholar]
- 68.Arcuri C, Sorio R, Tognon G, et al. A phase II study of liposomal doxorubicin in recurrent epithelial ovarian carcinoma. Tumori. 2006;90:556–561. doi: 10.1177/030089160409000604. [DOI] [PubMed] [Google Scholar]
- 69.Katsumata N, Fujiwara Y, Kamura T, et al. Phase II clinical trial of pegylated liposomal doxorubicin (JNS002) in Japanese patients with mullerian carcinoma (epithelial ovarian carcinoma, primary carcinoma of fallopian tube, peritoneal carcinoma) having a therapeutic history of platinum-based chemotherapy: a Phase II Study of the Japanese Gynecologic Oncology Group. Jpn J Clin Oncol. 2008;38:777–785. doi: 10.1093/jjco/hyn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gorumlu G, Kucukzeybek Y, Kemal-Gul M, et al. Pegylated liposomal doxorubicin in heavily pretreated epithelial ovarian cancer patients. J BUON. 2008;13(3):349–352. [PubMed] [Google Scholar]
- 71.Steppan I, Reimer D, Sevelda U, Ulmer H, Marth C, Zeimet AG. Treatment of recurrent platinum-resistant ovarian cancer with pegylated liposomal doxorubicin-an evaluation of the therapeutic index with special emphasis on cardiac toxicity. Chemotherapy. 2009;55:391–398. doi: 10.1159/000262452. [DOI] [PubMed] [Google Scholar]
- 72.Chou HH, Wang KL, Chen CA, et al. 2005. Pegylated liposomal doxorubicin (Lipo-Dox(R)) for platinum-resistant or refractory epithelial ovarian carcinoma: A Taiwanese gynecologic oncology group study with long term follow-up. Gynecol Oncol. 2006;101:423–428. doi: 10.1016/j.ygyno.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 73.Markman M, Kennedy A, Webster K, et al. Phase 2 trial of liposomal doxorubicin (40 mg/m2) in platinum/paclitaxel-refractory ovarian and fallopian tube cancers and primary carcinoma of the peritoneum. Gynecol Oncol. 2000;78:369–72. doi: 10.1006/gyno.2000.5921. [DOI] [PubMed] [Google Scholar]
- 74.Campos SM, Penson RT, Mays AR, et al. The clinical utility of liposomal doxorubicin in recurrent ovarian cancer. Gynecol Oncol. 2001;81:206–212. doi: 10.1006/gyno.2000.5980. [DOI] [PubMed] [Google Scholar]
- 75.Wilailak S, Linasmita V. A study of pegylated liposomal Doxorubicin in platinum-refractory epithelial ovarian cancer. Oncology. 2004;67:183–186. doi: 10.1159/000081315. [DOI] [PubMed] [Google Scholar]
- 76.Lorusso D, Naldini A, Testa A, et al. Phase II study of pegylated liposomal doxorubicin in heavily pretreated epithelial ovarian cancer patients. May a new treatment schedule improve toxicity profile? Oncology. 2004;67:243–239. doi: 10.1159/000081324. [DOI] [PubMed] [Google Scholar]
- 77.Strauss HG, Hemsen A, Karbe I, Lautenschläger C, Persing M, Thomssen C. Phase II trial of biweekly pegylated liposomal doxorubicin in recurrent platinum-refractory ovarian and peritoneal cancer. Anticancer Drugs. 2008;19:541–545. doi: 10.1097/CAD.0b013e3282fcbbf7. [DOI] [PubMed] [Google Scholar]
- 78.Oskay-Oezcelik G, Koensgen D, Hindenburg HJ, et al. Biweekly pegylated liposomal doxorubicin as second-line treatment in patients with relapsed ovarian cancer after failure of platinum and paclitaxel: results from a multi-center phase II study of the NOGGO. Anticancer Res. 2008;28:1329–1334. [PubMed] [Google Scholar]
- 79.Sehouli J, Oskay-Ozcelik G, Kühne J, et al. Biweekly pegylated liposomal doxorubicin in patients with relapsed ovarian cancer: results of a multicenter phase-II trial. Ann Oncol. 2006;17:957–961. doi: 10.1093/annonc/mdl079. [DOI] [PubMed] [Google Scholar]
- 80.Sehouli J, Camara O, Schmidt M, et al. North-Eastern German Society of Gynecological Oncology. Pegylated liposomal doxorubicin (CAELYX) in patients with advanced ovarian cancer: results of a German multicenter observational study. Cancer Chemother Pharmacol. 2009;64:585–591. doi: 10.1007/s00280-008-0909-1. [DOI] [PubMed] [Google Scholar]
- 81.Parmar MK, Ledermann JA, Colombo N, et al. ICON and AGO Collaborators. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer:the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–2106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 82.Pfisterer J, Plante M, Vergote I, et al. AGO-OVAR; NCIC CTG; EORTC GCG. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–4707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 83.Tas F, Derin D, Guney N, Aydiner A, Topuz E. Chemotherapy with pegylated liposomal doxorubicin and cisplatin in recurrent platinumsensitive epithelial ovarian cancer. Int J Clin Oncol. 2008;13:330–334. doi: 10.1007/s10147-007-0757-8. [DOI] [PubMed] [Google Scholar]
- 84.Vorobiof DA, Rapoport BL, Slabber CF, et al. Phase 2 study of combination therapy with liposomal doxorubicin and carboplatin in patients with relapsed, platinum sensitive ovarian cancer. Proc Am Soc Clin Oncol. 2004;23:471s. [Google Scholar]
- 85.du Bois A, Pfisterer J, Burchardi N, et al. Arbeitsgemeinschaft Gynäekologische Onkologie Studiengruppe Ovarialkarzinom; Kommission Uterus. Combination therapy with pegylated liposomal doxorubicin and carboplatin in gynecologic malignancies: a prospective phase II study of the Arbeitsgemeinschaft Gynäekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and Kommission Uterus (AGO-K-Ut) Gynecol Oncol. 2007;107:518–525. doi: 10.1016/j.ygyno.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 86.Rapoport BL, Vorobiof DA, Slabber C, et al. Phase II study of pegylated liposomal doxorubicin and carboplatin in patients with platinum-sensitive and partially platinum-sensitive metastatic ovarian cancer. Int J Gynecol Cancer. 2009;19:1137–1141. doi: 10.1111/IGC.0b013e3181a8b938. [DOI] [PubMed] [Google Scholar]
- 87.Ferrero JM, Weber B, Geay JF, et al. Second-line chemotherapy with pegylated liposomal doxorubicin and carboplatin is highly effective in patients with advanced ovarian cancer in late relapse: a GINECO phase II trial. Ann Oncol. 2007;18:263–268. doi: 10.1093/annonc/mdl376. [DOI] [PubMed] [Google Scholar]
- 88.Power P, Stuart G, Oza A, et al. Efficacy of pegylated liposomal doxorubicin (PLD) plus carboplatino in ovarian cancer patients who recur within six to twelve months: a phase II study. Gynecol Oncol. 2009;114:410–414. doi: 10.1016/j.ygyno.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 89.Weber B, Lortholary A, Mayer F, et al. Pegylated liposomal doxorubicin and carboplatin in late-relapsing ovarian cancer: a GINECO group phase II trial. Anticancer Res. 2009;29:4195–4200. [PubMed] [Google Scholar]
- 90.Nicoletto MO, Falci C, Pianalto D, et al. Phase II study of pegylated liposomal doxorubicin and oxaliplatin in relapsed advanced ovarian cancer. Gynecol Oncol. 2006;100:318–323. doi: 10.1016/j.ygyno.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 91.Recchia F, De Filippis S, Saggio G, et al. Phase I study of liposomal doxorubicin and oxaliplatin as salvage chemotherapy in advanced ovarian cancer. Anticancer Drugs. 2003;14:633–638. doi: 10.1097/00001813-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 92.Valerio MR, Tagliaferri P, Raspagliesi F, et al. A phase II study of pegylated liposomal doxorubicin oxaliplatin and cyclophosphamide as second-line treatment in relapsed ovarian carcinoma. Int J Gynecol Cancer. 2006;16(Suppl 1):79–85. doi: 10.1111/j.1525-1438.2006.00324.x. [DOI] [PubMed] [Google Scholar]
- 93.Smith HO, Moon J, Wilczynski SP, et al. Southwest Oncology Group Trial S9912: intraperitoneal cisplatin and paclitaxel plus intravenous paclitaxel and pegylated liposomal doxorubicin as primary chemotherapy of small-volume residual stage III ovarian cancer. Gynecol Oncol. 2009;114:206–209. doi: 10.1016/j.ygyno.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tas F, Guney N, Derin D, Aydiner A, Topuz E. A pilot study evaluating the efficacy and toxicity of biweekly gemcitabine and pegylated liposomal doxorubicin in recurrent platinum-resistant epithelial ovarian cancer. Int J Clin Oncol. 2008;13:156–160. doi: 10.1007/s10147-007-0740-4. [DOI] [PubMed] [Google Scholar]
- 95.Skarlos DV, Kalofonos HP, Fountzilas G, et al. Gemcitabine plus pegylated liposomal doxorubicin in patients with advanced epithelial ovarian cancer resistant/refractory to platinum and/or taxanes. A HeCOG phase II study. Anticancer Res. 2005;25:3103–3108. [PubMed] [Google Scholar]
- 96.Holloway RW, Finkler NJ, Nye LP, et al. Doxil and gemcitabine combination therapy for recurrent ovarian cancer: results of a phase II trial. Proc Am Soc Clin Oncol. 2004 Abstr 5090. [Google Scholar]
- 97.Karaoglu A, Arslan UY, Ozkan M, et al. Efficacy and toxicity of gemcitabine and pegylated liposomal Doxorubicin in recurrent platinum-resistant/refractory epithelial ovarian cancer. Asian Pac J Cancer Prev. 2009;10:63–66. [PubMed] [Google Scholar]
- 98.Petru E, Angleitner-Boubenizek L, Reinthaller A, et al. Combined PEG liposomal doxorubicin and gemcitabine are active and have acceptable toxicity in patients with platinum-refractory and -resistant ovarian cancer after previous platinum-taxane therapy: A phase II Austrian AGO study. Gynecol Oncol. 2006;101:18–23. doi: 10.1016/j.ygyno.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 99.D’Agostino G, Ferrandina G, Ludovisi M, et al. Phase II study of liposomal doxorubicin and gemcitabine in the salvage treatment of ovarian cancer. Br J Cancer. 2003;89:1180–1184. doi: 10.1038/sj.bjc.6601284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ferrandina G, Paris I, Ludovisi M, et al. Gemcitabine and liposomal doxorubicin in the salvage treatment of ovarian cancer: updated results and long-term survival. Gynecol Oncol. 2005;98:267–273. doi: 10.1016/j.ygyno.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 101.Tanyi JL, Smith JA, Ramos L, Parker CL, Munsell MF, Wolf JK. Predisposing risk factors for palmar-plantar erythrodysesthesia when using liposomal doxorubicin to treat recurrent ovarian cancer. Gynecol Oncol. 2009;114:219–224. doi: 10.1016/j.ygyno.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 102.Verhaar-Langereis M, Karakus A, van Eijkeren M, et al. Phase II study of the combination of pegylated liposomal doxorubicin and topotecan in platinum-resistant ovarian cancer. Int J Gynecol Cancer. 2006;16:65–70. doi: 10.1111/j.1525-1438.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- 103.Campos SM, Matulonis UA, Penson RT, et al. 2003. Phase II study of liposomal doxorubicin and weekly paclitaxel for recurrent Mullerian tumors. Gynecol Oncol. 2003;90:610–618. doi: 10.1016/s0090-8258(03)00373-1. [DOI] [PubMed] [Google Scholar]
- 104.Katsaros D, Oletti MV, Rigault de la Longrais IA, et al. Clinical and pharmacokinetic phase II study of pegylated liposomal doxorubicin and vinorelbine in heavily pretreated recurrent ovarian carcinoma. Ann Oncol. 2005;16:300–306. doi: 10.1093/annonc/mdi055. [DOI] [PubMed] [Google Scholar]
- 105.Joly F, Sevin E, Lortholary A, et al. Association of pegylated liposomal doxorubicin and ifosfamide in early recurrent ovarian cancer patients: A multicenter phase II trial. Gynecol Oncol. 2009 Nov 2; doi: 10.1016/j.ygyno.2009.09.036. [Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 106.O’Byrne KJ, Bliss P, Graham JD, et al. A Phase III study of Doxil/Caylex versus paclitaxel in platinum treated taxane naive relapsed ovarian cancer. Proc Am Soc Clin Oncol. 2002;21 Abstr 808) [Google Scholar]
- 107.Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–3322. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 108.Gordon AN, Tonda M, Sun S, Rackoff W. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol. 2004;95:1–8. doi: 10.1016/j.ygyno.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 109.Mutch DG, Orlando M, Goss T, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2007;25:2811–2818. doi: 10.1200/JCO.2006.09.6735. [DOI] [PubMed] [Google Scholar]
- 110.Ferrandina G, Ludovisi M, Lorusso D, et al. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol. 2008;26:890–896. doi: 10.1200/JCO.2007.13.6606. [DOI] [PubMed] [Google Scholar]
- 111.Satyam A, Hocker MD, Kane Maguire KA, Morgan AS, Villar HO, Lyttle MH. Design, synthesis and evalution of latent alkylating agents activated by glutathione S-transferase. J Med Chem. 1996;39:1736–1747. doi: 10.1021/jm950005k. [DOI] [PubMed] [Google Scholar]
- 112.Vergote I, Finkler N, del Campo J, et al. Phase 3 randomised study of canfosfamide (Telcyta, TLK286) versus pegylated liposomal doxorubicin or topotecan as third-line therapy in patients with platinum-refractory or -resistant ovarian cancer. Eur J Cancer. 2009;45:2324–2332. doi: 10.1016/j.ejca.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 113.Vergote I, Finkler J, Hall JB, et al. Randomized phase III study of canfosfamide (C, TLK286) plus pegylated liposomal doxorubicin (PLD) versus PLD as second-line therapy in platinum (P) refractory or resistant ovarian cancer (OC) Proc Am Soc Clin Oncol. 2009 Abstr 5552. [Google Scholar]
- 114.Ganjoo KN, Patel SR. Trabectedin: an anticancer drug from the sea. Exp Opin Pharmacother. 2009;10:2735–2743. doi: 10.1517/14656560903277236. [DOI] [PubMed] [Google Scholar]
- 115.von Mehren M, Schilder RJ, Cheng JD, et al. A phase I study of the safety and pharmacokinetics of trabectedin in combination with pegylated liposomal doxorubicin in patients with advanced malignancies. Ann Oncol. 2008;19:1802–1809. doi: 10.1093/annonc/mdn363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McMeekin S, Del Campo JM, Colombo N, et al. Trabectedin (T) in relapsed advanced ovarian cancer (ROC): a pooled analysis of three phase II studies. Proc Am Soc Clin Oncol. 2007;25 18sAbstr 5579. [Google Scholar]
- 117.Monk BJ, Herzog T, Kaye S, et al. A randomized Phase III study of trabectedin with pegylated liposomal doxorubicin (PLD) versus PLD in relapsed ovarian cancer (OC) Proceedings of ESMO; Ann Oncol. 2008;19 doi: 10.1016/j.ygyno.2012.06.034. 9Abstr LBA4. [DOI] [PubMed] [Google Scholar]
- 118.Krasner CN, Poveda A, Herzog T, et al. Health-related quality of life/patient-reported outcomes in relapsed ovarian cancer: results from a randomized phase III study of trabectedin with pegylated liposomal doxorubicin (PLD) versus PLD alone. Proc Am Soc Clin Oncol. 2009;27 doi: 10.1016/j.ygyno.2012.06.034. 15sAbstr 5526. [DOI] [PubMed] [Google Scholar]
- 119.Alberts DS, Liu PY, Wilczynski SP, et al. Randomized trial of pegylated liposomal doxorubicin (PLD) plus carboplatin in platinum sensitive (PS) patients with recurrent epithelial ovarian or peritoenal carcinoma after failure of initial platinum-based chemotherapy (South-west Oncology Group Protocol S0200) Gynecol Oncol. 2008;108:90–94. doi: 10.1016/j.ygyno.2007.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Markman M, Moon J, Wilczynski S, et al. Single agent carboplatin versus carboplatin plus pegylated liposomal doxorubicin in recurrent ovarian cancer: Final survival results of a SWOG (S0200) phase 3 randomized trial. Gynecol Oncol. 2009 Dec 29; doi: 10.1016/j.ygyno.2009.11.026. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Linardou H, Bafaloukos D, Bamlas A, et al. A randomized, Phase II study of carboplatin plus liposomal doxorubicin (CLD) vs carboplatin plus paclitaxel (CP) in potentially platinum sensitive ovarian cancer patients. A Hellenic Cooperative Oncology Group. Eur J Cancer. 2007;5(Suppl) doi: 10.1186/1741-7015-8-3. 3122Abstr 5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pujade-Lauraine E, Mahner S, Kaern J, et al. A randomized, phase III study of carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in relapsed platinum-sensitive ovarian cancer (OC): CALYPSO study of the Gynecologic Cancer Intergroup (GCIG) J Clin Oncol. 2009;27(18s suppl) Abstr LBA5509. [Google Scholar]
- 123.Vasey P, Largillier R, Gropp M, et al. A GCIC randomized phase III study of carboplatin © and pegylated liposomal doxorubicin (PLD) (C-D) vs carboplatin and paclitaxel (P) (C-P): CALYPSO results in partially platinum-sensitive ovarian cancer (OC) Proc ESMO. 2009 Abstr LBA18. [Google Scholar]
- 124.Potamianou A, Androulakis N, Papakotoulas P, et al. Sequential combination of paclitaxel-carboplatin and paclitaxel-liposomal doxorubicin as a first-line treatment in patients with ovarian cancer. A multicenter phase II trial. Oncology. 2005;69:348–353. doi: 10.1159/000089767. [DOI] [PubMed] [Google Scholar]
- 125.Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;20;27:1419–1425. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pignata S, Scambia G, Savarese A, et al. MITO Investigators. Safety of a 3-weekly schedule of carboplatin plus pegylated liposomal doxorubicin as first line chemotherapy in patients with ovarian cancer: preliminary results of the MITO-2 randomized trial. BMC Cancer. 2006;6:202. doi: 10.1186/1471-2407-6-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pignata S, Scambia G, Savarese A, et al. Carboplatin and pegylated liposomal doxorubicin for advanced ovarian cancer: preliminary activity results of the MITO-2 phase III trial. Oncology. 2009;76:49–54. doi: 10.1159/000178760. [DOI] [PubMed] [Google Scholar]
- 128.Pignata S, Scambia G, Savarese A, et al. Carboplatin plus paclitaxel (CP) versus carboplatin plus stealth liposomal doxorubicin (CLD) in patients with advanced ovarian cancer: activity and safety results of the MITO-2 randomised multicenter trials. Proc Am Soc Clin Oncol. 2009;27 LBA 5508. [Google Scholar]
- 129.Sánchez-Muñoz A, Pérez-Ruiz E, Mendiola Fernández C, Alba Conejo E, González-Martín A. Current status of anti-angiogenic agents in the treatment of ovarian carcinoma. Clin Transl Oncol. 2009;11:589–595. doi: 10.1007/s12094-009-0409-8. [DOI] [PubMed] [Google Scholar]
- 130.Muggia FM, Boyd L, Liebes L, et al. Pegylated liposomal doxorubicin (PLD) with bevacizumab (B) in second-line treatment of ovarian cancer (OC): pharmacokinetics (PK), safety, and preliminary outcome results. Proc Am Soc Clin Oncol. 2009 Abstr 5548. [Google Scholar]
- 131.Kikuchi Y, Kouta H, Kikuchi, et al. Effects of weekly bevacizumab and pegylated liposomal doxorubicin in heavily pretreated patients with recurrent or progressed ovarian cancer. Proc Am Soc Clin Oncol. 2009 Abstr 5547. [Google Scholar]
- 132.Ramakrishnan V, Bhaskar V, Law DA, et al. Preclinical evaluation of an anti alpha5beta1 integrin antibody as a novel antingiogenic agent. J Exp Ther Oncol. 2006;5:273–286. [PubMed] [Google Scholar]
- 133.Vergote I, Colombo N, Kutarska E, et al. Phase II study comparing volociximab (an antiangiogenic antibody) and pegylated liposomal doxorubicin (PLD) with PLD alone in recurrent ovarian or primary peritoneal cancer. Proc Am Soc Clin Oncol. 2009 Abstr 5560. [Google Scholar]
- 134.Dees EC, O’Neil BH, Lindley CM, et al. Phase I and pharmacologic study of the combination of bortezomib and pegylated liposomal doxorubicin in patients with refractory solid tumors. Cancer Chemother Pharmacol. 2008;63:99–107. doi: 10.1007/s00280-008-0716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Scambia G, Parma G, Del Conte D, et al. A phase II combination study of bortezomib with pegylated-liposomal doxorubicin in patients with ovarian cancer failing platinum containing regimens. Proc Am Soc Clin Oncol. 2008 doi: 10.1097/IGC.0b013e318251051a. Abstr 5581. [DOI] [PubMed] [Google Scholar]
- 136.O’Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 137.Martschick A, Sehouli J, Patzelt A, et al. Pathogenetic mechanism of anthracycline-induced palmar-plantar erythrodysesthesia. Anticancer Res. 2009;29:2307–2313. [PubMed] [Google Scholar]
- 138.Jacobi U, Waibler E, Schulze P, et al. Release of doxorubicin in sweat: first step to induce the palmar-plantar erythrodysesthesia syndrome? Ann Oncol. 2005;16:1210–1211. doi: 10.1093/annonc/mdi204. [DOI] [PubMed] [Google Scholar]
- 139.Gordinier ME, Dizon DS, Fleming EL, et al. Elevated body mass index does not increase the risk of palmar-plantar erythrodysesthesia in patients receiving pegylated liposomal doxorubicin. Gynecol Oncol. 2006;103:72–74. doi: 10.1016/j.ygyno.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 140.Lyass O, Uziely B, Ben-Yosef R, et al. Correlation of toxicity with pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in metastatic breast carcinoma. Cancer. 2000;89:1037–1047. doi: 10.1002/1097-0142(20000901)89:5<1037::aid-cncr13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 141.Molpus KL, Anderson LB, Craig CL, Puleo JG. The effect of regional cooling on toxicity associated with intravenous infusion of pegylated liposomal doxorubicin in recurrent ovarian carcinoma. Gynecol Oncol. 2004;93:513–516. doi: 10.1016/j.ygyno.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 142.Lorusso D, Di Stefano A, Carone V, Fagotti A, Pisconti S, Scambia G. Pegylated liposomal doxorubicin-related palmar-plantar erythrodysesthesia (‘hand-foot’ syndrome) Ann Oncol. 2007;18:1159–1164. doi: 10.1093/annonc/mdl477. [DOI] [PubMed] [Google Scholar]
- 143.Von Gruenigen VE, Frasure H, Fusco N, et al. A double-blind ranodmized trial of pyridoxine versus placebo fort he prevention of pegylated liposomal doxorubicin hydrochloride-related palamr-plantar erythrodysesthesia. Proc Am Soc Clin Oncol. 2009 Abstr 5594. [Google Scholar]
- 144.Coleman RE, Biganzoli L, Canney P, et al. A randomised phase II study of two different schedules of pegylated liposomal doxorubicin in metastatic breast cancer (EORTC-10993) Eur J Cancer. 2006;42:882–887. doi: 10.1016/j.ejca.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 145.von Moos R, Thuerlimann BJ, Aapro M, et al. Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts. Eur J Cancer. 2008;44:781–790. doi: 10.1016/j.ejca.2008.01.028. [DOI] [PubMed] [Google Scholar]