Abstract
The distribution of estrogen receptor between the cytosolic and nuclear compartments were evaluated in liver of male rats to determine whether a circadian rhythm exists. Cytosolic receptor reached a maximum level at 400 hours and a minimum at 2000 and 2400 hr. Nuclear receptor reached a maximum level at 800 hr and was lowest at 1600 and 2000 hr. Serum estradiol levels were also highest at 800 hr and lowest at 1600 hr. The variations in cytosolic and nuclear receptors are not reciprocal; in fact, the overall content of receptor in the liver is not constant and also displays a circadian rhythm.
Keywords: Circadian rhythm, estrogen receptors, serum estradiol, liver
Introduction
Mammalian liver of both sexes is responsive to sex steroid hormones. Estrogen administration increases the synthesis of many serum transport proteins as well as others such as the cell surface low density lipoprotein receptor (reviewed in 1). These estrogenic effects are presumably mediated through the estrogen receptor (ER) which has been characterized in the liver of both male and female rats (2-8), green monkeys (9) and human beings (10). The level of hepatic ER is controlled in a positive manner by estrogen (2), negatively by testosterone (2), and is maintained by a pituitary factor thought to be growth hormone (6, 11). Partial hepatectomy results in a rapid translocation of cytosolic ER (cER) to the nucleus (nER); this cellular redistribution of ER correlates with increased DNA synthesis and mitotic indices (12). Because serum estradiol levels demonstrate circadian rhythmicity, it was of interest to determine whether circadian variations could be observed in both cER and nER, and whether these variations have any relationship to serum estradiol levels.
Materials and Methods
Animals
Thirty male Sprague–Dawley rats (220–240 g) were used in these experiments. Animals were caged two per cage and maintained on a normal 12 hr light–dark cycle (light 0630 to 1830 hr) with food and water available ad libitum. Six groups of five animals each were killed by exsanguination under ether anesthesia at 4-hr intervals: 0400, 0800, 1200, 1600, 2000 and 000/2400 hr. Blood was removed via the abdominal aorta, and cold isotonic saline was perfused through the aorta to wash most of the residual blood from the liver. The livers were removed quickly and placed immediately in ice cold TEM (0.01 M Tris–HCl, 1.5 mM EDTA, 20 mM sodium molybdate, pH 7.4) buffer. Livers were homogenized immediately after all animals were killed.
Materials
Radioactive [2, 4, 6, 7 3H]-estradiol, 97 Ci/mmol ([3H]-E2), was obtained from New England Nuclear, Boston, MA. Purity of radio-labeled compounds was assessed periodically by thin layer chromatography (2). Sources of other substances were previously described (2, 10, 12). Buffers used in preparation of nuclei and cytosol contained leupeptin (0.15 mM) and benzamidine (1.0 mM), protease inhibitors which stabilize the activity of the estrogen receptor.
Preparation of subcellular fractions
The fresh livers were homogenized in 3 volumes of TEM/g tissue using a Brinkman polytron. The homogenate was centrifuged at 800 g for 15 min at 4°C. The crude nuclear pellet was resuspended and washed five times in SMgHM buffer (0.25 M sucrose, 3 mM MgCl2, 10 mM HEPES, 20 mM sodium molybdate, pH 7.4) with a recentrifugation using the same conditions as above after each wash. The nuclear pellet was then re-suspended in SMgHM buffer in a volume equal to that of the original homogenate. Nuclei so prepared appeared rounded under light microscopy and stained blue with hematoxylin–eosin; no cytosolic staining was observed. The final nuclear preparation was free of cytosolic contamination as judged by the fact that the preparation contained no detectable cytosolic alcohol dehydrogenase activity (13). Cytosol was prepared from the same liver by centrifugation of the supernatant fluid from the crude nuclear pellet at 49,500 g for 20 min, and the resulting supernate at 150,000 g for 45 min. The supernatant fluid was removed carefully in both steps to avoid lipid contamination.
Steroid binding assays
Cytosol was diluted to a protein concentration of 5 mg/ml; protamine sulfate precipitates were prepared as indicated previously (12) and incubated at 0°C overnight with 0.2–5.0 nM [3H]-E2 with and without a 100-fold excess of unlabeled diethylstilbestrol (DES). The precipitates were washed as described (12) and the radioactivity content determined. The nuclear receptor content was determined by an exchange assay described previously (12), which involves incubation of aliquots (200 μl) of the nuclear suspension with 10 nM of [3H]-E2 in the presence and absence of a 250-fold excess of unlabeled DES for 60 min at 30°C. The unbound steroid was removed by washing the nuclear pellet 5 times with SMgHM buffer. The washed pellet was then extracted with 2 ml of ethanol for 2 hr at 30°C and the entire extract and pellet were transferred to a 20 ml scintillation vial, and 8 ml of ACS scintillation fluid (Amersham) were added. Additional details of subcellular fractionation and assay conditions may be found in Reference (12).
Methods
Binding data were analyzed according to the method of Scatchard (14). Linearity and values from standard curves and Scatchard plots were determined using the unweighted linear regression program of the TI55-II calculator (Texas Instruments). Protein content was determined by the method of Lowry et al. (15). DNA content of homogenates and nuclear preparations was determined by the method of Burton (16). Serum estradiol concentrations were determined by radioimmunoassay as described previously (3). Statistical analysis was performed using Student's t-test and verified by analysis of variance, using programs available on the Hewlett Packard 9815S, and Tukey's multiple comparison procedure for analysis of variance. Radioactivity content of samples was determined using a Packard TriCarb 4530 with automatic dpm conversion.
Results and Discussion
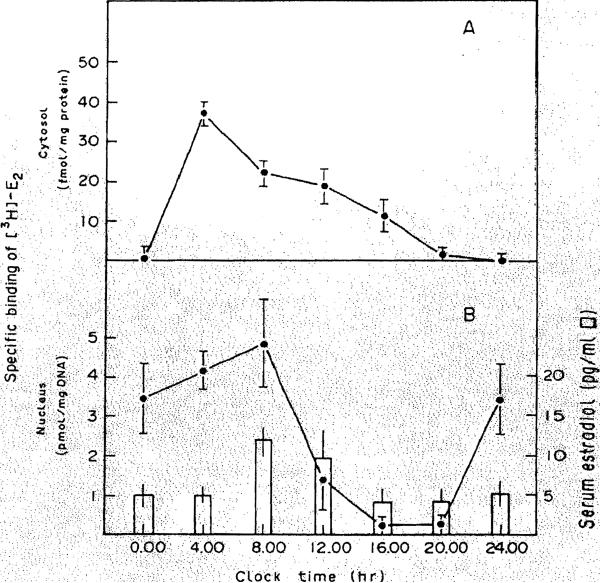
In this study, we have determined that the localization of estrogen receptors in cytosolic and nuclear fractions of liver shows considerable variation as a function of time of day. As shown in Figure 1, cytosolic ER is virtually undetectable (0.48 ± 0.48 fmol/mg protein) at midnight, and rapidly increases to a peak of 37.2 ± 3.71 fmol/mg protein at 400 hr. This latter value is significantly different from those of all other groups (P < 0.001). After this time, the level of cER falls until at 2400 hr, it is almost undetectable. In fact, in two animals at 2000 hr and three animals at 2400 hr, no specific binding of [3H]-E2 was detectable. The affinity of the cER for E2 did not vary as a function of these changes in receptor levels; the Kd values for cER at all time points were similar (range, 0.8–1.5 nM).
Figure 1. Variations in cystosolic and nuclear content of estrogen receptor as a function of circadian phase.
Panel A, Protamine sulfate precipitates of rat liver cytosol were prepared and incubated with varying concentrations of [3H]E2 in the absence (total binding) and presence (nonspecific binding) of a 100-fold excess of unlabeled DES for 16 hr at 4°C. Specific binqing is calculated as the difference of these two values. Each point is the mean ± S.E.M. of cER in five animals, using duplicate determinations for each animal. Scatchard plots of the specific binding values were used to determine the number of binding sites and affinity of binding. Panel B, Nuclear suspensions were incubated with 10 nM [3H]-E2 in the absebce and presence of a 250-fold excess of unlabeled DES for 1 hr at 30°C, as detailed in Methods. Also shown (open bars) are the serum E2 concentrations as determined by radioimmunoassay.
The level of the nuclear receptor reached a maximal value at 800 hr (5.0 ± 1.4 pmol/mg DNA), although this value was not significantly different from that observed at 400 hr and 2400 hr. It was, however, significantly different than the values at either 1200 hr (P < 0.005), or at 1600 hr or 2000 hr (P < 0.001). The lowest value for the nuclear ER was observed at 1600 hr (0.2 ± 0.2 pmol/mg DNA). The level of nuclear ER varied in the same fashion as serum estradlol levels (Figure 1) in that the latter was relatively high at 800 hr (12 pg/ml) as compared to that at 1600 hr (4 pg/ml).
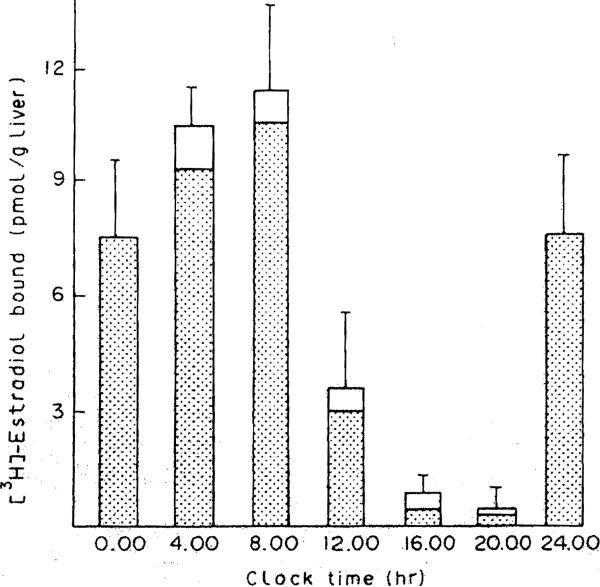
Hepatic content of both forms of the receptor is illustrated in Figure 2. At all time points the nuclear form of the receptor predominates. Recent studies (17-19) have indicated that steroid receptors may in fact reside only in the nucleus of the cell, and that cytosolic receptors represent artifacts of cell breakage. However, the surprising finding in our study is that the overall level of hepatic receptor does not remain constant over a 24-hr span. At 1600 and 2000 hr, very little receptor is apparent in either the nuclear or the cytosolic fractions. At this point, we do not know the reason for this apparent loss of receptor. Perhaps the receptor is degraded becomes refractory during this time period. Another possibility is that the ER is unavailable for assay, possibly because of shunting to another subcellular compartment or by complexing with other cellular substances which prevent binding of radiolabeled ligand. It is unlikely that our methods do oot detect the receptor if present in the cytosolic and nuclear fractions, since we used an exchange assay for measurement of nuclear receptor, and the cER assay should be quantitative, since essentially all hepatic cER in an untreated animal is ligand-free (20). These various possibilities remain under investigation.
Figure 2. Variations in total hepatic content of estrogen receptor at different times of day.
The height of each bar represents the total receptor content (cytosolic and nuclear) corrected for and expressed as binding per gram of liver. The shaded area of each bar represents the contribution of the nuclear receptor to the total amount.
Serum E2 levels’ also display circadian rhythms (21). Many points of control of these E2 levels are possible, such as variations in testicular and adrenal synthesis and secretion of estradiol; in addition, the metabolism of some hormones by the liver may vary as well (22, 23). Our data show a correspondence between the peak of serum E2 and the maximal measurement of nuclear receptor concentration, both occurring at 800 hr. However, nuclear receptor levels demonstrate a decline at the next interval (1200 hr), whereas the serum estradiol remains elevated. It is difficult to tell why this occurs, but similar observations of apparent disappearance of nuclear receptor after hormone administration have been made. This disappearance has been termed ‘processing’ (24, 25) and is as yet unexplained, but may involve conformational changes in or proteolytic cleavage of the receptor to a form which is resistant to radioligand exchange. Since these previous studies involved administration of exogenous hormone, and our study involves only endogenous hormone, it is difficult to determine whether the effect of low receptor content we observed at 1200 hr, or even at other time intervals, involved processing. More studies and perhaps alternate technology will be needed to determine the causes of fluctuation in receptor content in the liver and other tissues. HOwever, to our knowledge, this present study represents the first demonstration that hepatic estrogen receptors undergo circadian rhythmicity.
Acknowledgements
This work was supported in part by the Veterans Administration, and grants AM30001, AM31577 and AM29961 from the National Institutes of Health, and Grant 84/0058644 from Consiglio Nazionale Delle Ricerche.
Abbreviations used
- cER and nER
cytosolic and nuclear estrogen receptor, respectively
- E2
estradiol
- DES
diethylstilbestrol (4,4′-[1,2-diethyl-1,2-ethenediyl] bisphenol)
References
- 1.Eagon PK, Porter LE, Francavilla A, DiLeo A, Van Thiel D. Estrogen and androgen receptors in liver: their role in liver disease and regeneration. Semin Liver Dis. 1985;5:59–69. doi: 10.1055/s-2008-1041758. [DOI] [PubMed] [Google Scholar]
- 2.Eagon PK, Fisher SE, Imhoff AF, Porter LE, Stewart RR, Van Thiel DH, Lester R. Estrogen binding proteins of male rat liver: influence of hormonal changes. Arch Biochem Biophys. 1980;201:486–499. doi: 10.1016/0003-9861(80)90537-8. [DOI] [PubMed] [Google Scholar]
- 3.Eagon PK, Zdunek JR, Van Thiel DH, Singletary BK, Egler KM, Gavaler JS, Porter LE. Alcohol induced changes in hepatic estrogen binding proteins. Arch Biochem Biophys. 1981;211:48–55. doi: 10.1016/0003-9861(81)90428-8. [DOI] [PubMed] [Google Scholar]
- 4.Aten RF, Dickson RB, Eisenfe1d AJ. Estrogen receptors in adult male rat liver. Endocrinology. 1978;103:1629–1635. doi: 10.1210/endo-103-5-1629. [DOI] [PubMed] [Google Scholar]
- 5.Powell-Jones W, Thompson C, Nayfeh SN, Lucier GW. Sex differences in estrogen binding by cytosolic and nuclear components of rat liver. J Steroid Biochem. 1980;13:219–229. doi: 10.1016/0022-4731(80)90195-8. [DOI] [PubMed] [Google Scholar]
- 6.Norsted G, Wrange O, Gustafsson JA. Multihormonal regulation of the estrogen receptor in rat liver. Endocrinology. 1981;108:1190–1196. doi: 10.1210/endo-108-4-1190. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson HA. Estrogen binding sites of mammalian liver: endocrine regulation of estrogen receptor synthesis in the regenerating rat liver. J Steroid Biochem. 1982;17:471–478. doi: 10.1016/0022-4731(82)90004-8. [DOI] [PubMed] [Google Scholar]
- 8.Kneifel R, Katzenellenbogen BS. Comparative effects of estrogen and antiestrogen on plasma reninsubstrate levels and hepatic estrogen receptors in rats. Endocrinology. 1981;108:545–552. doi: 10.1210/endo-108-2-545. [DOI] [PubMed] [Google Scholar]
- 9.Aten RF, Dickson RB, Eisenfeld AJ. Female and male green monkey liver estrogen receptor. Biochem Pharmacol. 1979;28:2445–2449. doi: 10.1016/0006-2952(79)90006-6. [DOI] [PubMed] [Google Scholar]
- 10.Porter LE, Elm MS, Van Thiel DH, Dugas MC, Eagon PK. Characterization and quantitation of human hepatic estrogen receptor. Gastroenterology. 1983;84:704–712. [PubMed] [Google Scholar]
- 11.Mode A, Norstedt G, Eneroth P, Gustafsson JA. Purification of liver feminizing factor from rat pituitaries and demonstration of its identity with growth hormone. Endocrinology. 1983;113:1250–1260. doi: 10.1210/endo-113-4-1250. [DOI] [PubMed] [Google Scholar]
- 12.Francavilla A, DiLeo A, Eagon PK, Wu SQ, Ove P, Van Thiel DH, Starzl TE. Regenerating rat liver: correlation between estrogen receptor localization and DNA synthesis. Gastroenterology. 1984;86:552–567. [PMC free article] [PubMed] [Google Scholar]
- 13.Vallee BL, Hoch FL. Zinc, a component of yeast alcohol dehydrogenase. Proc Natl Acad Sci USA. 1958;41:327–332. doi: 10.1073/pnas.41.6.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scatchard G. The attraction of proteins for small molecules and ions. Ann NY Acad Sci. 1949;46:660–672. [Google Scholar]
- 15.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 16.Burton K. Determinations of DNA concentration with diphenylamine. Methods Enzymol. 1968;12:163–168. [Google Scholar]
- 17.King WJ, Greene GL. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984;307:745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- 18.Welshons WV, Lieberman ME, Gorski J. Nuclear localization of unoccupied oestrogen receptors. Nature. 1984;307:747–749. doi: 10.1038/307747a0. [DOI] [PubMed] [Google Scholar]
- 19.Perrot-Applanat M, Logeat F, Groyer-Picard MT, Milgrom E. Immunocytochemical study of mammalian progesterone receptor using monoclonal antibodies. Endocrinology. 1985;116:1473–1478. doi: 10.1210/endo-116-4-1473. [DOI] [PubMed] [Google Scholar]
- 20.Dickson RB, Eisenfeld AJ. Estrogen receptor in rat liver: translocation to the nucleus in isolated parenchymal cells. Endocrinology. 1979;105:627–632. doi: 10.1210/endo-105-3-627. [DOI] [PubMed] [Google Scholar]
- 21.Kalra PS, Kalra SP. Regulation of gonadal steroid rhythms in rats. J Steroid Biochem. 1979;11:981–987. doi: 10.1016/0022-4731(79)90041-4. [DOI] [PubMed] [Google Scholar]
- 22.Graef V, Golf SW. Circadian rhythm of hepatic steroid rhythms in rats. J Steroid Biochem. 1979;11:1299–1305. doi: 10.1016/0022-4731(79)90199-7. [DOI] [PubMed] [Google Scholar]
- 23.Stenberg A. Developmental, diurnal and oestrous cycle dependent changes in the activity of liver enzymes. J Endocrinol. 1976;68:265–277. doi: 10.1677/joe.0.0680265. [DOI] [PubMed] [Google Scholar]
- 24.McGuire WL, Horwitz KB. Action of estrogen and antiestrogen in human breast cancer cells. Cold Spring Harbor Conf Cell Proliferation. 1979;6:937–947. [Google Scholar]
- 25.Strobl JS, Kasid A, Huff KK, Lippman ME. Kinetic alterations in estrogen receptor processing in human breast cancer cells. Endocrinology. 1984;115:1116–1124. doi: 10.1210/endo-115-3-1116. [DOI] [PubMed] [Google Scholar]