Abstract
Pro-angiogenic bone marrow (BM) cells include subsets of hematopoietic cells that provide vascular support and endothelial progenitor cells (EPCs), which under certain permissive conditions could differentiate into functional vascular cells. Recent evidence demonstrates that the chemokine stromal-cell derived factor-1 (SDF-1, also known as CXCL12) has a major role in the recruitment and retention of CXCR4+ BM cells to the neo-angiogenic niches supporting revascularization of ischemic tissue and tumor growth. However, the precise mechanism by which activation of CXCR4 modulates neo-angiogenesis is not clear. SDF-1 not only promotes revascularization by engaging with CXCR4 expressed on the vascular cells but also supports mobilization of pro-angiogenic CXCR4+VEGFR1+ hematopoietic cells, thereby accelerating revascularization of ischemic organs. Here, we attempt to define the multiple functions of the SDF-1–CXCR4 signaling pathway in the regulation of neo-vascularization during acute ischemia and tumor growth. In particular, we introduce the concept that, by modulating plasma SDF-1 levels, the CXCR4 antagonist AMD3100 acutely promotes, while chronic AMD3100 treatment inhibits, mobilization of pro-angiogenic cells. We will also discuss strategies to modulate the mobilization of essential subsets of BM cells that participate in neo-angiogenesis, setting up the stage for enhancing revascularization or targeting tumor vessels by exploiting CXCR4 agonists and antagonists, respectively.
Contribution of pro-angiogenic bone marrow cells to adult vascularization
The majority of the adult vasculature consists of mature, stable, non-leaky vessels invested by perivascular cells. Perivascular cells, which comprise pericytes, smooth muscle cells, or specialized hematopoietic cells, stabilize neo-vessels by releasing angiogenic factors or providing physical support. However, following injury or trauma, regeneration of blood vessels (neo-angiogenesis) is required to revascularize the wounded tissue. Injured tissues release pro-angiogenic factors that support assembly of the neo-vessels. However the precise identities of pro-angiogenic factors that recruit hematopoietic cells to stabilize the newly formed vessels are unknown.
Similarly, neo-angiogenesis is also essential for growth and progression of malignant tumors, which require nutrients and oxygen to overcome hypoxia and for tumor metastasis. Dysfunctional and leaky blood vessels contribute to the development of pathological conditions, such as retinopathies, atherosclerosis and rheumatoid arthritis. Generation of new blood vessels could be initiated from surrounding existing vessels (sprouting), and, under certain conditions, recruitment of endothelial progenitor cells (EPCs) could also contribute to vessel formation (adult vasculogenesis). Circulating EPCs can originate from the bone marrow (BM) [1], or also from other organs [2]. It is well established that EPCs are recruited in conjunction with pro-angiogenic hematopoietic cells and participate in driving neo-angiogenesis. The nature and exact functions of these mobilized BM-derived cells have been under intense scrutiny and are the subject of recent reviews [3,4]. Recruitment of EPCs and supporting cells contributes to the acceleration of ischemic revascularization and augments neo-angiogenesis in specific tumors, including lymphomas [5,6].
The chemokine stromal cell derived factor-1 (SDF-1, also known as CXCL12) is a constitutively expressed and inducible chemokine that regulates multiple physiological processes, including embryonic development and organ homeostasis [7]. This pleiotropic chemokine is expressed in several organs including lung, liver, skin and BM. The cognate receptor for SDF-1, CXCR4 (also known as CD184), is widely and constitutively expressed by numerous tissues, including hematopoietic and endothelial cells. CXCR4+ pro-angiogenic cells are composed of immature and mature hematopoietic cells, EPCs, and smooth muscle cell (SMC) progenitors, which all have direct or indirect pro-angiogenic properties. Accumulating evidence derived from mice deficient in SDF-1 and CXCR4, in addition to in vitro biochemical and migration studies, have set forth the concept that the SDF-1–CXCR4 pathway has a crucial role in modulating the trafficking and proper engraftment of hematopoietic stem cell (HSCs) and reconstitution of hematopoiesis [7]. The crucial roles of SDF-1 and CXCR4 in embryonic vasculogenesis are demonstrated by the blood vessel abnormalities manifested in SDF-1−/− and CXCR4−/− mice [8]. Indeed, the expression of SDF-1 in a large number of tumors and injured tissues strongly suggests that activation of CXCR4 participates in promoting neo-angiogenesis (Table 1). In the past few years, numerous studies have focused on studying the role ofSDF-1 in neo-vascularization models, suggesting a central role for this chemokine in triggering and supporting organ repair and tumor development. However, the precise mechanisms by which SDF-1 exerts its pro-angiogenic effects are not fully elucidated. In addition, it is not known how the CXCR4 antagonist, AMD3100, promotes or inhibits mobilization of pro-angiogenic hematopoietic cells.
Table 1. Increased expression of SDF-1 in tumors and pathological conditionsa.
| Cancers | Pathological conditions | ||
|---|---|---|---|
| Tumor cells | Tumor stromal cells | Vasculature | Injured tissues |
| Ovarian cancer | Breast cancer fibroblasts | MS blood vessels | Myocardium infarct |
| Neuroblastoma | ICC fibroblasts | Systemic sclerosis endothelial cells | Diabetic retinopathy |
| Breast cancer | RA endothelium | Injured liver | |
| Prostate cancer | Diabetic retinopathy | Irradiated BM | |
| Rhabdomyosarcoma | Asthmatic endothelium | Ischemic brain | |
| Melanoma | Vascular intima | Ischemic kidney | |
| Lung cancer | Atherosclerotic plaque | RA synovium | |
| AML | Systemic sclerosis | ||
| See Ref. [24] | |||
Abbreviations: AML, acute myelogenous leukemia; ICC, intrahepatic cholangiocarcinoma; MS, multiple sclerosis; RA: rheumatoid arthritis.
Here, we describe the potential mechanisms by which activation of the SDF-1–CXCR4 pathway supports recruitment of the pro-angiogenic cells from the BM and other organs, thereby facilitating the formation of stable vasculature during ischemic revascularization and tumor growth. Moreover, we set forth a novel concept whereby AMD3100, through modulation of plasma SDF-1 levels, acutely promotes, whereas chronic treatment blocks, mobilization of CXCR4+VEGFR1+ cells. Taken together, accumulating evidence suggests that the magnitude of SDF-1 plasma level is the key regulator of recruitment of pro-angiogenic cells and extent of revascularization.
Expression of SDF-1 in hypoxic tissues
Chemokine production at sites in demand of rapid vascularization is crucial for recruiting circulating cells that are essential for neo-angiogenesis. SDF-1 is upregulated in damaged tissues, such as the liver [9], arteries [10] or irradiated BM [11] (Table 1). Indeed, hypoxic and/or apoptotic conditions are the trigger to induce expression of cytokines and chemokines. Ceradini et al. have demonstrated that SDF-1 expression in ischemic sites is directly correlated with the amplitude of hypoxia [12]. Signaling activated by hypoxia leading to SDF-1 upregulation involves the recruitment of integrin-linked kinase and hypoxia-inducible factor-1 (HIF-1) [12,13]. Increased expression of SDF-1 in human and rodent models of infarcted myocardium has been confirmed by several studies [14–17]. SDF-1 expression was increased as early as 1h after induction of hypoxia in the myocardium or hindlimbs, suggesting a role in the initiation of tissue repair and revascularization [13,18,19]. Taken together, these data suggest that induction of SDF-1 by hypoxia provides an effective means to recruit circulating cells into neo-angiogenic niches.
A myriad of other cytokines are also upregulated in damaged ischemic tissues, including vascular endothelial growth factor-A (VEGF-A), or hematopoietic cytokines, such as erythropoietin (EPO), Kit-ligand (KitL, also known as stem cell factor), thrombopoietin (TPO), granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) [20]. Notably, enforced VEGF-A elevation in the liver induces SDF-1 expression [21]. Similarly, hematopoietic cytokines increase SDF-1 secretion, thereby augmenting neo-angiogenic processes [20]. Therefore, induction of SDF-1 expression seems to have a major role in initiating revascularization of the ischemic injured tissues. In support of these findings, SDF-1 is expressed on endothelial cells and pericytes of hypoxic, injured or pathological tissues, including injured carotid arteries and atherosclerotic plaques (Table 1) [22]. Finally, EPCs themselves express and secrete SDF-1 together with VEGF-A [23]. Mimicking injured normal tissues, both main components of the tumor microenvironment, namely tumor cells and tumor stroma cells, also secrete SDF-1. A large variety of tumor cells express SDF-1 (recently reviewed in Ref. [24]) (Table 1). Moreover, tumor stromal fibroblasts, but not normal fibroblasts, elaborate SDF-1, which in turn promotes tumor growth and angiogenesis [25,26] (Table 1). Finally, SDF-1 expression by tumor vasculature has been reported in brain tumors [27,28].
Several studies have demonstrated that SDF-1 expression is sufficient to induce cell mobilization, enhancing angiogenesis and tissue recovery. Enforced release of SDF-1 locally in ischemic tissue leads to increased EPC mobilization and induction of neo-angiogenesis. Of note, those effects are abrogated in the absence of injury, suggesting that other signals emitted by damaged tissue are required for the incorporation of EPCs in the vascular niche within tissues [29,30]. Moreover, implantation of SDF-1-overexpressing cardiac fibroblasts leads to significant revascularization and cardiac functions [14]. Altogether, SDF-1 seems to be a key molecule released by ischemic injured tissues into the circulation that regulates the recruitment of pro-angiogenic cells to the injured and tumoral tissues.
SDF-1 is the primary chemokine that supports mobilization of pro-angiogenic cells
The main SDF-1 receptor, CXCR4, is widely expressed on BM cells, which endows SDF-1 with the capacity to affect a broad range of effector cells. VEGFR2+, CXCR4+ and c-Kit+ cells represent specific subsets of pro-angiogenic and vascular cells with overlapping phenotypes that are mobilized by their respective ligands: VEGF-A, SDF-1 and KitL. The precise roles of each of these ligands in BM cell mobilization are still under investigation. Mobilization of specific subpopulations is likely to be regulated by tight modulation in terms of timing and concentration of those chemotactic factors. Overexpression of VEGF-A induces BM cell recruitment and retention, in a SDF-1 dependent manner [20,21]. SDF-1 and VEGF-A could converge chemotactic signals to attract VEGFR2+ and CXCR4+ cells, two cell populations that are not totally overlapping in their phenotype and angiogenic properties (Box 1). Increased expression of SDF-1 in the tissue is rapidly followed by its release into the circulation [18,29]. In vitro, SDF-1 is a chemoattractant for EPCs in addition to hematopoietic CXCR4+ cells.
Direct evidence for the role of SDF-1 in regulating mobilization of pro-angiogenic BM cells in vivo has been demonstrated by plasma elevation of SDF-1, which stimulates mobilization of CXCR4+ BM cells, including HSCs and EPCs [31,32]. Increased elevation of SDF-1 concentration in the blood has been observed in ischemic models, while its concentration in the BM was decreased, thereby resulting in the mobilization of the pro-angiogenic cells [14,18,33].
In addition to SDF-1 released by injured tissues, recent evidence has demonstrated that platelets express and release SDF-1 following cytokine-induced activation in vitro and in vivo during vessel injury [20,34,35]. In thrombocytopenic mice, such as TPO-deficient mice, SDF-1 plasma elevation, in addition to mobilization of pro-angiogenic CXCR4+VEGFR1+ cells (hemangiocytes) and tissue revascularization are severely impaired. However, restoration of platelet production or enforced SDF-1 expression restores neo-angiogenesis, providing evidence for the essential role of platelet-derived SDF-1 in regulating neo-vascularization [20].
These data suggest that the magnitude of plasma elevation of SDF-1 is a tightly regulated process and is the primary molecular thermostat regulating the mobilization of pro-angiogenic hematopoietic cells and the extent of revascularization.
Box 1. SDF-1 and VEGF-A - a powerful angiogenic couple.
Similarities
Production by tumors and injured organs
Upregulated by HIF-1a
Chemotactic properties
Overlapping targeted cells: HSCs, EPCs and mature endothelial cells
Angiogenic properties on endothelial cells
Differences
Targeted cells: VEGFR1+ and/or VEGFR2+ cells versus CXCR4+ cells (overlapping populations)
Signaling: CXCR4 and CXCR7 are G protein-coupled receptors, VEGFR1 and VEGFR2 are tyrosine kinases
Regulation
SDF-1 upregulates VEGF-A
VEGF-A upregulates SDF-1 and CXCR4
Synergy
Matrigel plug assay
Chemotaxis
Recruitment and incorporation of EPCs
How does activation of the SDF-1-CXCR4 pathway support mobilization of the pro-angiogenic cells?
The exact mechanism by which stress-induced release of SDF-1 mediates mobilization of BM-derived pro-angiogenic cells has just recently been elucidated. SDF-1 released within the intravascular compartment is translocated from the plasma to the BM compartment through a complex vesicular transport system, a process referred to as transcytosis [36]. When SDF-1 enters the BM microenvironment, it induces the activation of matrix metalloproteinase-9 (MMP-9) and the release of soluble kit-ligand (sKitL). Subsequently, sKitL induces the release of more SDF-1, enhancing mobilization of the CXCR4+ and c-Kit+ cells to the circulation [32] (Figure 1).
Figure 1.
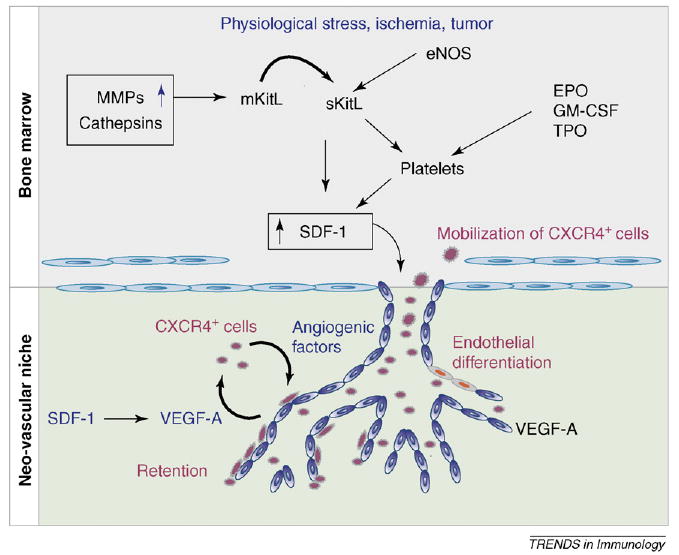
Regulation of neo-vascularization by SDF-1. The chemokine SDF-1 is produced by hypoxic conditions, vascular injury or tumors, and is released in the circulation. SDF-1 signaling induces a complex remodeling of the BM microenvironment involving proteases, Kit-ligand (KitL) and NO production, leading to mobilization of CXCR4+ angiogenic cells. Ultimately, SDF-1 expression in the neo-angiogenic niche recruits CXCR4+ cells and mediates their proper retention, differentiation and pro-angiogenic activities in coordination with other angiogenic factors such as VEGF-A.
Another pathway participating in amplifying the SDF-1 response is the nitric oxide (NO) pathway. SDF-1 induces the release of NO by endothelial cells, resulting in the upregulation of MMP-9. Remarkably, activation of MMP-9 and subsequent release of sKitL and mobilization of pro-angiogenic cells are impaired in eNOS−/− mice, delineating a new role for the NO signaling pathway in SDF-1-induced BM cell mobilization [37]. In addition, NO-induced vasodilatation of BM endothelium facilitates cell egress. Notably, caveolin expression on BM cells is upregulated after ischemia, resulting in a transient CXCR4 sequestration and SDF-1-induced BM cell mobilization [38]. Activation of osteoclasts and cathepsins has also been implicated in the mobilization of the hematopoietic and endothelial progenitor cells [39,40]. Finally, additional molecules such as extracellular matrix (ECM) compounds, matrix degradation products and activated complement proteins have crucial roles in priming CXCR4+ cells and modulating the amplitude of SDF-1 signals [41–43]. Of note, during G-CSF-induced BM cell mobilization, plasma is enriched for soluble molecules that synergize with SDF-1 to attract CXCR4+ cells [43]. Collectively, these data suggest that SDF-1-mediated mobilization of CXCR4+ hemangiogenic cells is a multi-pronged process and is driven by activation of a cascade of molecular pathways, including protease activation, release of cytokines and matrix remodeling (Figure 1).
Paradox of the SDF-1-CXCR4 signaling pathway in the regulation of stem cell trafficking
Currently, published evidence has indicated that both activation, through plasma elevation of SDF-1, and inhibition of CXCR4 result in the mobilization of pro-angiogenic hematopoietic cells. This apparent paradox emanates from the finding that a single injection of the CXCR4 antagonist, AMD3100, efficiently induces rapid mobilization of HSCs, pro-angiogenic cells and EPCs into the circulation [44,45] (Figure 2c). However, plasma elevation of SDF-1 also supports mobilization of BM cells over a period of several days, entertaining the notion that the mechanism by which CXCR4 inhibition or activation promotes mobilization is driven by two different molecular or cellular pathways.
Figure 2.
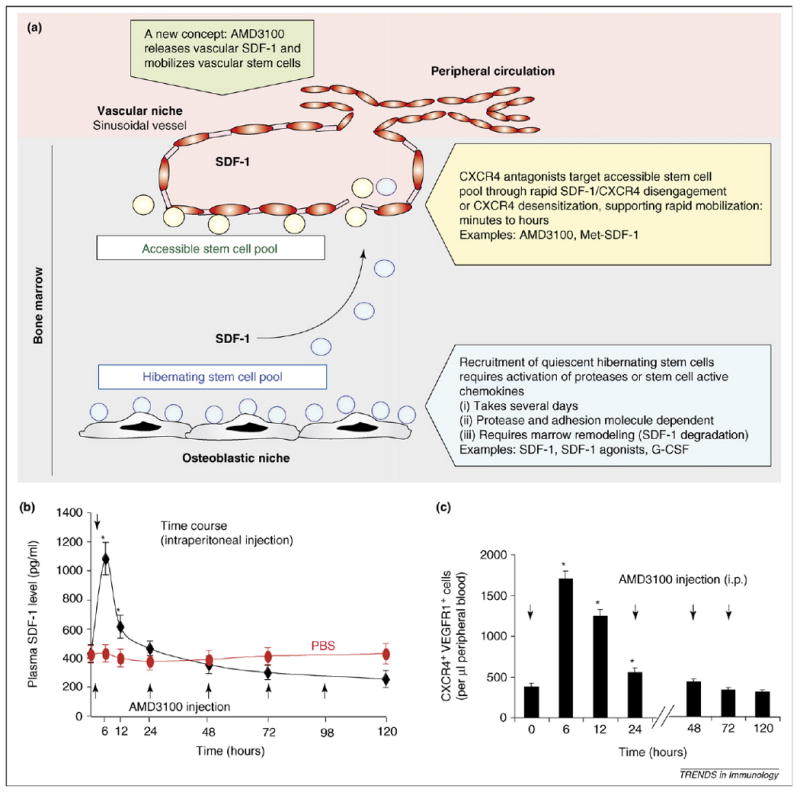
Reconciling the SDF-1-mediated mobilization paradox: a matter of stem cell niches? (a) Localization of HSCs to the osteoblastic and vascular niches might provide an explanation as to how both SDF-1 signaling and CXCR4 inhibition lead to stem cell mobilization. We propose that CXCR4 inhibition by antagonists, such as AMD3100, rapidly mobilizes readily accessible progenitor cells located within the vicinity of the vascular niche. Alternatively, AMD3100 induces a rapid surge in intravascular release of SDF-1 that recruits readily available perivascular stem cells. SDF-1-dependent mobilization requires remodeling of the constituents within the BM, where HSCs are tightly bound to the osteoblastic niche, and as such are not readily available to enter the peripheral circulation. (b) AMD3100 induces rapid elevation of plasma SDF-1. C57bl/6J mice were injected with either phosphate buffered saline or AMD3100 at 1.25 mg/kg [intraperitoneally (i.p.)] twice daily and levels of SDF-1 in the plasma were measured at different time points by ELISA. Plasma levels of SDF-1 peaked at 6 h with AMD3100 (2.8-fold increase as compared with the PBS-treated controls, n = 6, *P < 0.05, where * represents statistical significance compared with PBS-treated controls) and then rapidly declined within the first 24 h of treatment. Plasma levels of SDF-1 were below baseline after 72 h of treatment with AMD3100 as compared with the PBS-treated controls. Arrows indicate time points of AMD3100 administration. (c) Number of circulating CXCR4+VEGFR1+ cells in the peripheral blood was measured and analyzed by two-color flow cytometry at the indicated time points after treatment with AMD3100 (1.25 mg/kg i.p. twice daily) in C57Bl/6J mice (n = 5, *P < 0.05, where * represents statistical significance compared with time 0). Consistent with the trend observed in SDF-1 plasma levels, the number of mobilized CXCR4+VEGFR1+ cells peaked at 6 h after AMD3100 treatment, then progressively declined in the ensuing 5-day period. Arrows indicate time points of AMD3100 administration.
One potential explanation for this paradox might be owing to localization of the stem cells within the BM, where it has been demonstrated that HSCs could reside in two different geographical microenvironments, namely the osteoblastic or vascular niches. Based on this emerging paradigm, we propose a novel ‘niche-dependent mobilization model’, which could reconcile the SDF-1-mediated mobilization paradox. Based on these findings, we introduce the concept that the SDF-1–CXCR4 signaling pathway could be involved in a biphasic mobilization pattern, in which CXCR4 inhibition allows for recruitment of the readily available HSCs from a perivascular niche, while activation of CXCR4 results in the mobilization of cells from another protected niche where the HSCs are tethered to the stromal or osteoblastic niches (Figure 2a). Notably, G-CSF-induced mobilization relies on a severe, but relatively slow, decrease in BM SDF-1 protein owing to transcriptional regulation and/or protease-mediated degradation, leading to the progressive disruption of SDF-1–CXCR4-dependent anchorage of HSCs within their niches [46–48].
This biphasic effect of the CXCR4-mediated mobilization fits with the observed early and late phases of the recruitment of stem cells to the circulation. The rapid mobilization of the HSCs induced by the CXCR4 antagonist AMD3100 suggests that cells in close apposition of vascular cells were forced to enter the circulation, appearing in the peripheral blood within an hour of interfering with SDF-1 binding to CXCR4. By contrast, SDF-1 or its agonists [49], or G-CSF, which require activation of a cascade of events and, possibly, cycling of hematopoietic cells, need several days to allow for the recruitment of quiescent tethered HSCs from the BM, delaying the entry of the cells to the peripheral circulation (Figure 2a).
Alternatively, CXCR4 antagonists might not mobilize CXCR4+ cells by inhibiting CXCR4 signaling as suggested. Indeed, acute treatment with AMD3100 induces a rapid displacement and release of SDF-1, which peaks at 6 h, into the plasma (Figure 2b), resulting in the mobilization of CXCR4+ hematopoietic stem or progenitor cells (Figure 2c). SDF-1 is possibly deployed rapidly into the plasma from intravascular sources, including platelets and endothelial cells. Additional experimentation is necessary to define the precise mechanisms by which CXCR4 agonists or antagonists support mobilization of hemangiogenic cells.
Arrest, extravasation and retention at the neo-angiogenic site
Once mobilized into the circulation, CXCR4+ cells must extravasate through the blood vessel wall into the ischemic site or tumor microenvironment. EPCs preferably incorporate into the tumoral and ischemic microenvironments, indicating that specific adhesion molecules expressed on the vessel wall could direct this specific homing [50]. SDF-1 expressed and presented by endothelial cells at the site of injury probably has an important role in triggering cell arrest and emigration into the neo-angiogenic niches [51] (Table 1). Recognition and tethering of CXCR4+ circulating cells leads to SDF-1-induced integrin activation, which triggers cell arrest and extravasation. SDF-1 induces integrin expression on EPCs [18] in addition to vascular cell adhesion molecule-1 on the endothelium [52]. Other crucial integrins mediating the incorporation of EPCs include β2 integrins, lymphocyte function-associated antigen-1 and intercellular adhesion molecule-1 [53–55]. In addition, Massberg et al. have demonstrated that SDF-1 released from platelets supports the recruitment of EPCs and facilitates their adhesion to the injured vascular wall [34]. Finally, membrane-bound KitL (mKitL) in atherosclerotic lesions induces the adhesion and recruitment of EPCs, which could be indirectly driven by the activation of the SDF-1–CXCR4 pathway [54].
After their extravasation, recruited cells take up a position in perivascular niches. Expression of SDF-1 by perivascular cells serves as a retention signal and is crucial for the maintenance of pro-angiogenic cells within the tissue, as demonstrated by the loss of cells when the SDF-1–CXCR4 axis is blocked and cell accumulation when SDF-1 is upregulated [20,21,30]. These data define another function for the SDF-1–CXCR4 pathway, in which expression of SDF-1 within the ischemic niche is essential for the maintenance of the pro-angiogenic cells stabilizing newly formed nascent neo-vessels.
Multiples roles of SDF-1 in governing neo-angiogenesis in vivo
In addition to its fundamental role in recruiting CXCR4+ cells at the site of neo-angiogenesis, SDF-1 has important functions in inducing, controlling and regulating vascularization of tumors and damaged tissues. SDF-1 directly participates in new blood vessel formation: SDF-1 has an angiogenic effect on endothelial cells by inducing cell proliferation, differentiation, sprouting and tube formation in vitro and by preventing the apoptosis of EPCs [28,30]. In vivo, SDF-1 placed in matrigel plugs induces angiogenesis [18]. However, SDF-1 exerts a more potent pro-angiogenic effect when delivered in combination with VEGF-A, entertaining the possibility that these two factors exert a synergistic effect [56,57]. The mechanism by which these pro-angiogenic factors collaborate to induce neo-angiogenesis is still unclear. VEGF-A and SDF-1 could augment angiogenesis by reciprocal mutually exclusive pathways (Box 1). Aghi et al. have shown that despite high levels of SDF-1 and VEGF-A secretion by vascular tumor cells, it is SDF-1 that is the main inducer of vasculogenesis [58]. In the ischemic limb model, VEGF-A induction of angiogenesis depends on the activation of the SDF-1–CXCR4 pathway [20]. Grunewald et al. also reported that VEGF-A-induced angiogenesis requires engagement of the CXCR4 signaling pathway [21]. Deciphering the relative contribution of the SDF-1–CXCR4 and VEGFA–VEGF-receptor signaling pathways is crucial to our understanding of the regulation of neo-angiogenesis during tumor development and tissue regeneration.
SDF-1 also modulates vascularization of ischemic tissues and tumors by influencing the expression of other angiogenic factors. Wang and colleagues have shown that pro-angiogenic factors such as VEGF-A, IL-6, IL-8 and tissue inhibitor of metalloproteinase-2 were secreted in prostate cancer cells in response to SDF-1 [59]. SDF-1 also decreases the production of the anti-angiogenic molecule angiostatin, whose effect is further amplified by the down-regulation of phosphoglycerate kinase-1, an enzyme that leads to angiostatin production in tumors [59,60]. In addition, SDF-1 induces the production of metalloproteinases, enzymes that are essential to deploying angiogenic factors, thereby accelerating tissue remodeling during vascularization [21,61,62].
In pre-clinical studies, the pro-angiogenic role of SDF-1 in tumor angiogenesis has been demonstrated by antagonizing SDF-1–CXCR4 signaling [58]. However, knocking down CXCR4 expression specifically on tumor cells in murine models has generated conflicting data regarding the role of SDF-1 signaling on tumor cells in regulating angiogenesis. SDF-1 has been shown to promote directly tumor vasculature development [59] or to support only the growth and metastasis of CXCR4+ tumors [63], underscoring distinct SDF-1 functions in different cancer types.
Finally, SDF-1 contributes to the stabilization of neo-vessel formation by recruiting CXCR4+PDGFR+ckit+ smooth muscle progenitor cells during recovery from vascular injury [64]. Although Aghi et al. suggested that SDF-1 did not recruit pericytes in glioma models [58], it is likely that specific tumor types could require recruitment of BM-derived CXCR4+ pericyte progenitors in a SDF-1-dependent manner [65].
By recruiting and entrapping diverse pro-angiogenic cells, including EPCs, CXCR4+VEGFR1+ hemangiocytes and CXCR4+PDGFR+cKit+ pericytes, and by promoting in situ neo-angiogenesis, SDF-1 clearly seems to be a master player, if not the principal conductor, in the angiogenic switch and de novo adult revascularization. Recently, a new SDF-1 receptor named CXCR7 has been discovered [66,67]. CXCR7 is also expressed on tumor cells and endothelium and regulates tumor growth [67,68]. As such, it is conceivable that CXCR4-independent effects of SDF-1 on angiogenesis could potentially be attributed to CXCR7 [69]. The role of SDF-1–CXCR7 signaling in mediating angiogenesis is not yet known and further investigation is required.
Modulating SDF-1 signals offers promising therapeutic strategies
Evidence for the central role of SDF-1 in organ repair and tumor growth has prompted investigators to examine the effect of manipulating SDF-1–CXCR4 signaling in a therapeutic setting. Indeed, favoring revascularization by increasing SDF-1 effects should be beneficial for organ repair, whereas pathological conditions, where blood vessel formation is detrimental, such as retinopathies, atherosclerosis or cancers, could benefit from an efficient SDF-1–CXCR4 blockade. Therefore, development of strategies aiming either to promote or inhibit SDF-1-driven signals should have tremendous therapeutic implications. Several CXCR4antagonists are currently being validated for clinical use [70,71]. Chronic treatment of AMD3100 efficiently blocks SDF-1-mediated vasculogenesis [20,21]. In tumor models, inhibition of the SDF-1–CXCR4 pathway with chronic administration of AMD3100 leads to efficient reduction of tumor growth and angiogenesis by targeting (i) proliferation of CXCR4-expressing tumor cells, (ii) diminishing recruitment of CXCR4+ angiogenic cells and (iii) promoting CXCR4-dependent metastasis [27,58,63]. In addition, CXCR4 antagonists might prevent vascular neointima formation and atherosclerosis [10].
Current clinical protocols employing anti-angiogenesis strategies have focused mainly on the inhibition of VEGF-A signaling. Modest benefits of such treatments in patients call for targeting other pro-angiogenic factors, such as the SDF-1–CXCR4 axis, that have a more prominent role in mediating postnatal and tumoral neo-angiogenesis. Indeed, the seminal role of SDF-1 in enhancing revascularization suggests that SDF-1 is an effective target of choice, which eventually might be used in therapeutic modalities involving VEGF-A to block tumor angiogenesis or promote revascularization [56]. Indeed, inhibition of both SDF-1 and VEGF-A signaling in murine ischemic models synergistically and efficiently impaired revascularization [20]. Combining CXCR4 blockade with conventional chemotherapy could also provide alternative therapeutic approaches for abrogating tumor neo-angiogenesis [72].
Delivery of SDF-1 for stem cell therapy and organ revascularization
Preliminary data from recent clinical trials with stem cell therapy using delivery of BM-derived cells for regenerating infarcted myocardium have revealed only marginal benefits [73]. These poor outcomes could have been owing to limited retention and survival of the transplanted cells within the ischemic myocardium. Therefore, deploying SDF-1 within the ischemic myocardium can support retention and proper positioning of the infused stem cells, thereby facilitating regenerative angiomyogenesis within the ischemic myocardium as suggested by studies in animal models [14,29, 57], although the efficacy of this approach has been questioned [74].
Widely used in clinics to induce HSC mobilization, G-CSF is an ideal candidate to promote BM cell recruitment. G-CSF is also efficient in inducing the mobilization of angiogenic BM cells, including CD34+CD133+VEGFR2+ EPCs, in addition to directly promoting angiogenesis, in part by upregulating SDF-1 [75–77]. The promising efficacy of G-CSF in supporting organ regeneration in animal studies is currently being tested in clinical trials [78]. Alternatively, promoting SDF-1 signaling could also be achieved by fucoidan-mediated SDF-1 elevation [79], or direct activation of the CXCR4 pathway with SDF-1 agonists [49], with FTY720, a sphingosine-1- phosphate analog that activates CXCR4 signaling [80] or soluble molecules priming CXCR4+ cells such as complement C3a [81].
Therefore, strategies aimed to amplify SDF-1–CXCR4 signaling could significantly promote angiogenic cell mobilization and vascularization in stem cell-based therapies. However, strategies using SDF-1 targeting agents should require careful pre-clinical and clinical design to avoid untoward side effects, such as excessive angiogenesis manifested by acceleration of atherosclerosis [82].
In conclusion, the SDF-1–CXCR4 pathway regulates several crucial aspects of stem cell recruitment, adult vasculogenesis and blood vessel development, and as such qualifies itself as one of the major molecular hubs regulating neo-vascularization. Future directions of research (see Outstanding questions) will be necessary to optimize novel strategies exploiting the potential of SDF-1–CXCR4 signaling in various therapeutic approaches.
Outstanding questions.
What is the role of SDF-1 in establishing long-term stable blood vessels?
What is the role of the newly discovered SDF-1 receptor CXCR7 in SDF-1 angiogenic properties?
Will combined anti-VEGF-A and anti-SDF-1 therapies offer promising directions for cancer treatments?
References
- 1.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Aicher A, et al. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007;100:581–589. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]
- 3.De Palma M, Naldini L. Role of haematopoietic cells and endothelial progenitors in tumour angiogenesis. Biochim Biophys Acta. 2006;1766:159–166. doi: 10.1016/j.bbcan.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Bertolini F, et al. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 5.Lyden D, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 6.Rafii S, et al. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 7.Ratajczak MZ, et al. The pleiotropic effects of the SDF-1– CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 8.Ara T, et al. The role of CXCL12 in the organ-specific process of artery formation. Blood. 2005;105:3155–3161. doi: 10.1182/blood-2004-07-2563. [DOI] [PubMed] [Google Scholar]
- 9.Kollet O, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiba Y, et al. M-CSF accelerates neointimal formation in the early phase after vascular injury in mice: the critical role of the SDF-1– CXCR4 system. Arterioscler Thromb Vasc Biol. 2007;27:283–289. doi: 10.1161/01.ATV.0000250606.70669.14. [DOI] [PubMed] [Google Scholar]
- 11.Ponomaryov T, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceradini DJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 13.Lee SP, et al. Integrin-linked kinase, a hypoxia-responsive molecule, controls postnatal vasculogenesis by recruitment of endothelial progenitor cells to ischemic tissue. Circulation. 2006;114:150–159. doi: 10.1161/CIRCULATIONAHA.105.595918. [DOI] [PubMed] [Google Scholar]
- 14.Askari AT, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, et al. Evidence for ischemia induced host-derived bone marrow cell mobilization into cardiac allografts. J Mol Cell Cardiol. 2006;41:478–487. doi: 10.1016/j.yjmcc.2006.06.074. [DOI] [PubMed] [Google Scholar]
- 16.Pillarisetti K, Gupta SK. Cloning and relative expression analysis of rat stromal cell derived factor-1 (SDF-1)1: SDF-1 α mRNA is selectively induced in rat model of myocardial infarction. Inflammation. 2001;25:293–300. doi: 10.1023/a:1012808525370. [DOI] [PubMed] [Google Scholar]
- 17.Ma J, et al. Time course of myocardial stromal cell-derived factor 1 expression and beneficial effects of intravenously administered bone marrow stem cells in rats with experimental myocardial infarction. Basic Res Cardiol. 2005;100:217–223. doi: 10.1007/s00395-005-0521-z. [DOI] [PubMed] [Google Scholar]
- 18.De Falco E, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 19.Yang C, et al. Enhancement of neovascularization with cord blood CD133+ cell-derived endothelial progenitor cell transplantation. Thromb Haemost. 2004;91:1202–1212. doi: 10.1160/TH03-06-0378. [DOI] [PubMed] [Google Scholar]
- 20.Jin DK, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grunewald M, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 22.Schober A, et al. Crucial role of stromal cell-derived factor-1α in neointima formation after vascular injury in apolipoprotein E-deficient mice. Circulation. 2003;108:2491–2497. doi: 10.1161/01.CIR.0000099508.76665.9A. [DOI] [PubMed] [Google Scholar]
- 23.Urbich C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Kryczek I, et al. Stromal derived factor (SDF-1/CXCL12) and human tumor pathogenesis. Am J Physiol Cell Physiol. 2006;292:C987–C995. doi: 10.1152/ajpcell.00406.2006. [DOI] [PubMed] [Google Scholar]
- 25.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 26.Ohira S, et al. Possible regulation of migration of intrahepatic cholangiocarcinoma cells by interaction of CXCR4 expressed in carcinoma cells with tumor necrosis factor-α and stromal-derived factor-1 released in stroma. Am J Pathol. 2006;168:1155–1168. doi: 10.2353/ajpath.2006.050204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin JB, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A. 2003;100:13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salvucci O, et al. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99:2703–2711. doi: 10.1182/blood.v99.8.2703. [DOI] [PubMed] [Google Scholar]
- 29.Hiasa K, et al. Gene transfer of stromal cell-derived factor-1α enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–2461. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi J, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 31.Hattori K, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354–3360. doi: 10.1182/blood.v97.11.3354. [DOI] [PubMed] [Google Scholar]
- 32.Heissig B, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Togel F, et al. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 34.Massberg S, et al. Platelets secrete stromal cell-derived factor 1α and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med. 2006;203:1221–1233. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stellos K, Gawaz M. Platelets and stromal cell-derived factor-1 in progenitor cell recruitment. Semin Thromb Hemost. 2007;33:159–164. doi: 10.1055/s-2007-969029. [DOI] [PubMed] [Google Scholar]
- 36.Dar A, et al. Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat Immunol. 2005;6:1038–1046. doi: 10.1038/ni1251. [DOI] [PubMed] [Google Scholar]
- 37.Aicher A, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 38.Sbaa E, et al. Caveolin plays a central role in endothelial progenitor cell mobilization and homing in SDF-1-driven postischemic vasculogenesis. Circ Res. 2006;98:1219–1227. doi: 10.1161/01.RES.0000220648.80170.8b. [DOI] [PubMed] [Google Scholar]
- 39.Urbich C, et al. Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med. 2005;11:206–213. doi: 10.1038/nm1182. [DOI] [PubMed] [Google Scholar]
- 40.Kollet O, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 41.Reca R, et al. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003;101:3784–3793. doi: 10.1182/blood-2002-10-3233. [DOI] [PubMed] [Google Scholar]
- 42.Avigdor A, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 43.Wysoczynski M, et al. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105:40–48. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 44.Capoccia BJ, et al. G-CSF and AMD3100 mobilize monocytes into the blood that stimulate angiogenesis in vivo through a paracrine mechanism. Blood. 2006;108:2438–2445. doi: 10.1182/blood-2006-04-013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shepherd RM, et al. Angiogenic cells can be rapidly mobilized and efficiently harvested from the blood following treatment with AMD3100. Blood. 2006;108:3662–3667. doi: 10.1182/blood-2006-06-030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petit I, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 47.Levesque JP, et al. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Semerad CL, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106:3020–3027. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong R, et al. Small peptide analogs to stromal derived factor-1 enhance chemotactic migration of human and mouse hematopoietic cells. Exp Hematol. 2004;32:470–475. doi: 10.1016/j.exphem.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Vajkoczy P, et al. Multistep nature of microvascular recruitment of ex vivo-expanded embryonic endothelial progenitor cells during tumor angiogenesis. J Exp Med. 2003;197:1755–1765. doi: 10.1084/jem.20021659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao L, et al. Selective expression of stromal-derived factor-1 in the capillary vascular endothelium plays a role in Kaposi sarcoma pathogenesis. Blood. 2003;102:3900–3905. doi: 10.1182/blood-2003-02-0641. [DOI] [PubMed] [Google Scholar]
- 52.Butler JM, et al. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115:86–93. doi: 10.1172/JCI22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chavakis E, et al. Role of β2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Y, et al. Essential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ Res. 2006;99:315–322. doi: 10.1161/01.RES.0000235986.35957.a3. [DOI] [PubMed] [Google Scholar]
- 55.Duan H, et al. LFA-1 and VLA-4 involved in human high proliferative potential-endothelial progenitor cells homing to ischemic tissue. Thromb Haemost. 2006;96:807–815. [PubMed] [Google Scholar]
- 56.Kryczek I, et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005;65:465–472. [PubMed] [Google Scholar]
- 57.Carr AN, et al. Efficacy of systemic administration of SDF-1 in a model of vascular insufficiency: support for an endothelium-dependent mechanism. Cardiovasc Res. 2006;69:925–935. doi: 10.1016/j.cardiores.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 58.Aghi M, et al. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res. 2006;66:9054–9064. doi: 10.1158/0008-5472.CAN-05-3759. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, et al. Diverse signaling pathways through the SDF-1/CXCR4 chemokine axis in prostate cancer cell lines leads to altered patterns of cytokine secretion and angiogenesis. Cell Signal. 2005;17:1578–1592. doi: 10.1016/j.cellsig.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 60.Wang J, et al. A glycolytic mechanism regulating an angiogenic switch in prostate cancer. Cancer Res. 2007;67:149–159. doi: 10.1158/0008-5472.CAN-06-2971. [DOI] [PubMed] [Google Scholar]
- 61.Heissig B, et al. Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Curr Opin Hematol. 2003;10:136–141. doi: 10.1097/00062752-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Petit I, et al. Atypical PKC-ζ regulates SDF-1-mediated migration and development of human CD34+ progenitor cells. J Clin Invest. 2005;115:168–176. doi: 10.1172/JCI21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith MC, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 64.Zernecke A, et al. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–791. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 65.Lamagna C, Bergers G. The bone marrow constitutes a reservoir of pericyte progenitors. J Leukoc Biol. 2006;80:677–681. doi: 10.1189/jlb.0506309. [DOI] [PubMed] [Google Scholar]
- 66.Balabanian K, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 67.Burns JM, et al. A novel chemokine receptor for SDF-1 and ITAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Madden SL, et al. Vascular gene expression in nonneoplastic and malignant brain. Am J Pathol. 2004;165:601–608. doi: 10.1016/s0002-9440(10)63324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hatse S, et al. Stromal cell-derived factor 1 (CXCL12) binds to endothelial cells and signals through a receptor different from CXCR4. Biochem Biophys Res Commun. 2006;348:192–199. doi: 10.1016/j.bbrc.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 70.De Clercq E. Potential clinical applications of the CXCR4 antagonist bicyclam AMD3100. Mini Rev Med Chem. 2005;5:805–824. doi: 10.2174/1389557054867075. [DOI] [PubMed] [Google Scholar]
- 71.Tsutsumi H, et al. The therapeutic potential of the chemokine receptor CXCR4 antagonists as multi-functional agents. Biopolymers. 2006;88:279–289. doi: 10.1002/bip.20653. [DOI] [PubMed] [Google Scholar]
- 72.Redjal N, et al. CXCR4 inhibition synergizes with cytotoxic chemotherapy in gliomas. Clin Cancer Res. 2006;12:6765–6771. doi: 10.1158/1078-0432.CCR-06-1372. [DOI] [PubMed] [Google Scholar]
- 73.Stamm C, et al. Stem cell therapy for ischemic heart disease: beginning or end of the road? Cell Transplant. 2006;15(Suppl. 1):S47–S56. doi: 10.3727/000000006783982313. [DOI] [PubMed] [Google Scholar]
- 74.Koch KC, et al. Effect of catheter-based transendocardial delivery of stromal cell-derived factor 1α on left ventricular function and perfusion in a porcine model of myocardial infarction. Basic Res Cardiol. 2006;101:69–77. doi: 10.1007/s00395-005-0570-3. [DOI] [PubMed] [Google Scholar]
- 75.Ohki Y, et al. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J. 2005;19:2005–2007. doi: 10.1096/fj.04-3496fje. [DOI] [PubMed] [Google Scholar]
- 76.Korbling M, et al. Recombinant human granulocyte-colony-stimulating factor-mobilized and apheresis-collected endothelial progenitor cells: a novel blood cell component for therapeutic vasculogenesis. Transfusion. 2006;46:1795–1802. doi: 10.1111/j.1537-2995.2006.00985.x. [DOI] [PubMed] [Google Scholar]
- 77.Misao Y, et al. Importance of recruitment of bone marrow-derived CXCR4+ cells in post-infarct cardiac repair mediated by G-CSF. Cardiovasc Res. 2006;71:455–465. doi: 10.1016/j.cardiores.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Kovacic JC, et al. Actions and therapeutic potential of G-CSF and GM-CSF in cardiovascular disease. J Mol Cell Cardiol. 2007;42:19–33. doi: 10.1016/j.yjmcc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 79.Luyt CE, et al. Low-molecular-weight fucoidan promotes therapeutic revascularization in a rat model of critical hindlimb ischemia. J Pharmacol Exp Ther. 2003;305:24–30. doi: 10.1124/jpet.102.046144. [DOI] [PubMed] [Google Scholar]
- 80.Walter DH, et al. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptor. Arterioscler Thromb Vasc Biol. 2007;27:275–282. doi: 10.1161/01.ATV.0000254669.12675.70. [DOI] [PubMed] [Google Scholar]
- 81.Ratajczak MZ, et al. Modulation of the SDF-1–CXCR4 axis by the third complement component (C3) – implications for trafficking of CXCR4+ stem cells. Exp Hematol. 2006;34:986–995. doi: 10.1016/j.exphem.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 82.Schober A, et al. SDF-1α-mediated tissue repair by stem cells: a promising tool in cardiovascular medicine? Trends Cardiovasc Med. 2006;16:103–108. doi: 10.1016/j.tcm.2006.01.006. [DOI] [PubMed] [Google Scholar]


