Abstract
We employed antibody pre-conditioning with alemtuzumab and posttransplant immunosuppression with low-dose tacrolimus monotherapy in 26 consecutive pediatric kidney transplant recipients between January 2004 and December 2005. Mean recipient age was 10.7 ± 5.8 years, 7.7% were undergoing retransplantation, and 3.8% were sensitized, with a PRA >20%. Mean donor age was 32.8 ± 9.2 years. Living donors were utilized in 65% of the transplants. Mean cold ischemia time was 27.6 ± 6.4 h. The mean number of HLA mismatches was 3.3 ± 1.3. Mean follow-up was 25 ± 8 months. One and 2 year patient survival was 100% and 96%. One and 2 year graft survival was 96% and 88%. Mean serum creatinine was 1.1 ± 0.6 mg/dL, and calculated creatinine clearance was 82.3 ± 29.4 mL/min/1.73 m2. The incidence of pre-weaning acute rejection was 11.5%; the incidence of delayed graft function was 7.7%. Eighteen (69%) of the children were tapered to spaced tacrolimus monotherapy, 10.5 ± 2.2 months after transplantation. The incidence of CMV, PTLD and BK virus was 0%; the incidence of posttransplant diabetes was 7.7%. Although more follow-up is clearly needed, antibody pre-conditioning with alemtuzumab and tacrolimus monotherapy may be a safe and effective regimen in pediatric renal transplantation.
Keywords: Immunosuppression, kidney transplantation, pediatric
In all transplant patients, but especially in pediatric recipients, minimizing the side affects of immunosuppression is perhaps as important as improving patient and graft survival. In our pediatric kidney recipients, we have utilized an immunosuppressive regimen based on a novel paradigm, emphasizing recipient pre-conditioning with minimal posttransplant immunosuppression (1–5). This regimen avoids posttransplant maintenance corticosteroids, which have had such deleterious effects on growth in children, and allows for low-dose tacrolimus monotherapy, to minimize nephrotoxicity. We recently reported on the early clinical experience with this regimen (4), and in this report describe a larger, more comprehensive experience with alemtuzumab pre-conditioning and tacrolimus monotherapy.
Patients and Methods
Recipient and donor demographics
Between January 2004 and December 2005, 26 consecutive pediatric patients underwent kidney transplantation alone at the Children's Hospital of Pittsburgh. The mean recipient age was 10.7 ± 5.8 years (range 1–19). Two 17.7%) patients were undergoing retransplantation, and one (3.8%) was sensitized, with a PRA >20%. The mean donor age was 32.8 ± 9.2 years (range 17–53), and 17 (65%) cases were with living donors. The mean cold ischemia time for the deceased donor cases was 27.6 ± 6.4 h and the mean number of HLA mismatches was 3.3 ± 1.3.
Immunosuppression
Immunosuppression was with alemtuzumab (6–10) 0.4–0.5 mg/kg. given as a single i.v. dose after induction of anesthesia over 2–3 h. Pre-medication was with i.v. methylprednisolone 10–20 mg/kg, famotidine, diphenhydramine and acetaminophen. A second dose of i.v. methylprednisolone was given during the arterial anastomosis. On postoperative day one, tacrolimus monotherapy was started at a dose of 1–3 mg twice daily, with a target 12 h trough of 10 ng/mL for the first 3.5–4 months after transplantation. In stable patients, twice daily tacrolimus was then consolidated to once daily tacrolimus, i.e. a patient on 2 mg twice daily would be converted to 3 or 4 mg once daily. Four to eight months later, stable patients on once daily immunosuppression would be taken to every other day immunosuppression, i.e. a patient on 4 mg once a day would be converted to 4 mg every other day. Further spaced weaning was generally not undertaken.
Rejections were generally biopsy proven, and were treated initially with steroids and an adjustment of the tacrolimus dosage.
Immunologic monitoring
Beginning in October 2004, routine serial monitoring for donor specific antibodies (DSA) was undertaken. Patients on spaced weaning who developed evidence of DSA were returned to once daily tacrolimus. If necessary, mycophenolate mofetil was added several months later. In patients not yet on spaced weaning who developed donor specific antibody, immunosuppression was generally increased, from once daily to twice daily tacrolimus, with or without the addition of mycophenolate mofetil.
Institutional oversight (11)
The Pharmacy and Therapeutics Committee made the determination that the use of alemtuzumab was considered to be innovative clinical therapy, and requested that patients' families sign consent prior to the administration of alemtuzumab. Thus, all patients undergoing kidney transplantation during this time frame signed consent to receive alemtuzumab. Subsequently, in February 2006, the Pharmacy and Therapeutics Committee made the determination that the regimen seemed to be associated with adequate safety, and decided that parents no longer needed to sign consent for alemtuzumab. Data analysis was carried out under an IRB-approved protocol. Strong and enthusiastic institutional support remained in force throughout this time period.
Results
The mean follow-up was 25 ± 8 months.
One year actual patient survival was 100%, and 2 year actuarial patient survival was 96%. One patient died at home of a presumed mucus plug 18 months after transplantation, with a serum creatinine of 0.5 mg/dL. He had had a complicated course after transplantation and had required a tracheostomy. At the time of his death, he was at home recovering from elective bowel surgery. One year actual graft survival was 96% and 2 year actuarial graft survival was 88%. Two kidneys were lost, in addition to the one death, one 11 months after transplantation to recurrent focal segmental glomerulosclerosis, and one 20 months after transplantation to torsion of an intra-abdominal kidney.
Renal function
At 3 months, the serum creatinine was 1.1 ± 0.6 mg/dL, and the calculated creatinine clearance was 82.3 ± 27.9 mL/min/1.73 m2. At most recent follow-up, the serum creatinine was 1.1 ± 0.6 mg/dL, with a calculated creatinine clearance of 82.6 ± 29.4 mL/min/1.73 m2.
Rejection
Biopsy-proven acute rejection was demonstrated in four (11.5%) patients; in two of these patients, noncompliance (as admitted by the patients) was an important factor in the development of acute rejection. There were no cases of steroid resistant rejection.
Delayed graft function
Delayed graft function, defined as the need for dialysis in the first week after transplantation, was seen in two (7.7%) patients. Both patients ultimately recovered good kidney function.
Viral complications/posttransplant diabetes mellitus (PTDM)
There were no cases of cytomegalovirus disease, posttransplant lymphoproliferative disorder, or BK virus. Prophylactic valganciclovir was administered for 6–12 months. In the 15 (58%) CMV seropositive donor/seronegative recipient cases, CMV antigenemia (and beginning in 2006, PCR) was monitored weekly for the first 4 months, monthly for the next 4 months and then every 2–3 months, and was transiently positive at a low level (6 cells /200 000) in 1 (7%) patient. Seroconversion was documented in 3 of 13 (23%) cases, and was not assessed in 2 cases, both of whom remained antigenemia/PCR negative. In the 14 (54%) EBV seropositive donor/seronegative recipient cases (this group received 12 months of valganciclovir prophylaxis), EBV PCR's were monitored on monthly basis for the first year, and every 2–3 months thereafter, and were detectable in 2 (14%) cases, 1 transiently, and 1 at a low-grade level (peak 1500, current 285). Seroconversion was documented in 4/13 (31 %) cases, and was not assessed in 2 cases, both of whom remained PCR negative. There were two (7.7%) cases of PTDM, one occurring in a retransplant recipient who had developed posttransplant diabetes after his first transplant, and another in a teenager with spina bifida who became morbidly obese prior to becoming insulin dependent.
There was one (3.8%) case of autoimmune hemolytic anemia. This patient received corticosteroids and had a reduction of the tacrolimus dosage from once daily to every other day, with resolution of the anemia and eventual discontinuation of corticosteroids.
Spaced weaning
Spaced weaning was attempted in 18 (69%) patients. At most recent follow-up, 12 (46%) patients were on spaced weaning. Weaning was started a mean of 10.5 ± 2.2 months after transplantation. A total of 5 (19%) patients resumed once daily tacrolimus, because of the development of DSA after spaced weaning. There were no patients who developed acute rejection on spaced weaning; there was one patient who had been taken back to once daily tacrolimus because of DSA, who developed an acute rejection episode related to noncompliance. He was treated with steroids and the addition of mycophenolate mofetil. The one other patient whose weaning was discontinued was the patient who lost her kidney to torsion.
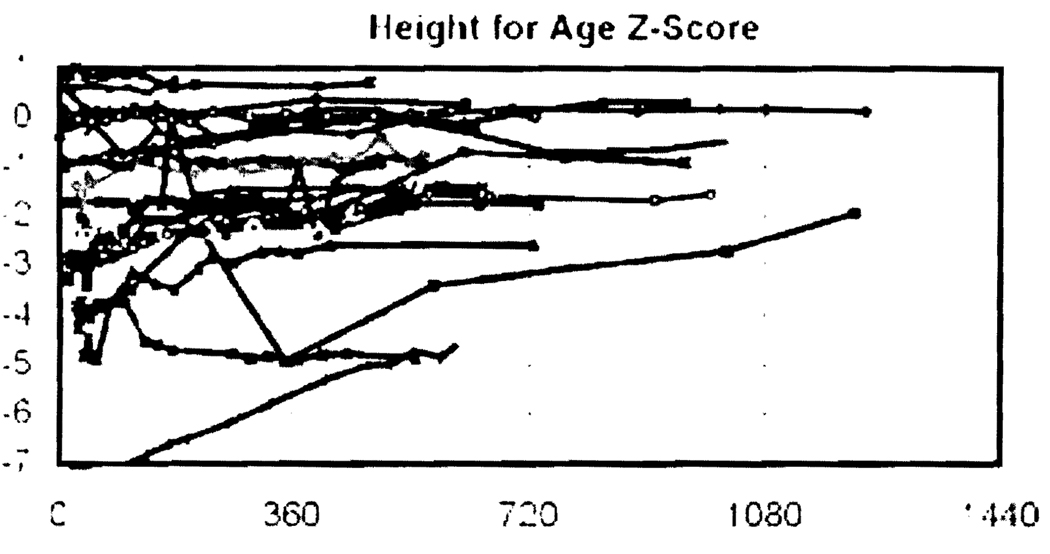
Growth (Figure 1)
Figure 1. Height for age and gender Z-scores after transplantation for the 26 recipients.
Reference population for Z-score calculations from the 2000 Center for Disease Control United States Growth Charts available at www.cdc.gov/growthcharts.
At the time of transplantation, the mean Z-score in height for age was −1.9 ± 1.9. After transplantation, catch up growth occurred as shown in Figure 1 and was most pronounced for those in the lower rankings of the Z-score scale. At the time of most recent follow up, the mean rise in the Z-scores for height was 0.6 ± 0.9. In the group of recipients aged 10 or less at the time of transplantation, the mean increase in the height Z-score was 1.3 ± 0.8.
Discussion
This report builds on the early experience with antibody pre-conditioning and tacrolimus (1–5) monotherapy, and continues to suggest that this regimen may be safe and effective in unselected pediatric kidney transplant recipients. Two year actuarial patient survival was 96%, and 2 year actuarial graft survival was 88%. The rate of early acute rejection was relatively low, and included two cases of recipient noncompliance. The quality of kidney function remained excellent, and there were no viral complications. A total of 46% of the patients were able to be tapered to and maintained on spaced weaning with tacrolimus monotherapy. Although the follow-up was short, reasonable catch-up growth was observed in this cohort of patients who were not receiving steroids after transplantation.
These results in our pediatric patients mirror the results that we have obtained in the adult renal transplant recipients, and confirm that this seems to be a reasonable regimen for recipients of all ages (1–3,5). There is a paucity of clinical experience elsewhere with alemtuzumab in pediatric renal transplant recipients, and most of the published experience is from the University of Wisconsin (12,13). Their published case series of four patients (12) has recently been augmented by a report on 10 living donor kidney recipients given two doses of alemtuzumab, tacrolimus, and mycophenolate mofetil, with no steroids administered after transplantation (13). A 100% 1-year patient and graft survival was obtained, with no evidence of clinical acute rejection and two cases of sub-clinical acute rejection. These data confirm the fundamental safety and efficacy of alemtuzumab given at the time of transplantation.
The important limitations of this report include both a relatively short follow-up and the fact that it is a nonrandomized trial. Thus, while the data are encouraging, additional follow-up will be required, and further accrual of patients will be needed to confirm these relatively favorable short-term outcomes. In the meantime, this regimen appears to be a reasonably effective and attractive one, with good patient acceptance, excellent patient and graft survival rates and good catch-up growth.
Acknowledgment
The authors thank Melissa Connell for manuscript preparation.
References
- 1.Starzl TE, Murase N, Abu-Elmagd K, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003;361:1502–1510. doi: 10.1016/s0140-6736(03)13175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro R, Jordan ML, Basu A, et al. Kidney transplantation under a tolerogenic regimen of recipient pretreatment and low-dose post- operative immunosuppression with subsequent weaning. Ann Surg. 2003;238:520–525. doi: 10.1097/01.sla.0000089853.11184.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro R, Basu A, Tan H, et al. Kidney transplantation under minimal immunosuppression after pretransplant lymphoid depletion with Thymoglobulin or Campath. J Am Coli Surg. 2005;200:505–515. doi: 10.1016/j.jamcollsurg.2004.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro R, Ellis D, Tan HP, et al. Antilymphoid antibody preconditioning and tacrolimus monotherapy for pediatric kidney transplantation. J Pediatr. 2006;148:813–818. doi: 10.1016/j.jpeds.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan HP, Kaczorowski DJ, Basu A, et al. Living donor renal transplantation using alemtuzumab induction and tacrolimus monotherapy. Am J Transplant. 2006;6:2409–2417. doi: 10.1111/j.1600-6143.2006.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waldmann H. A personal history of the CAMPATH-1H antibody. Med Oncol. 2002;19 suppl:3–9. doi: 10.1385/mo:19:2s:s03. [DOI] [PubMed] [Google Scholar]
- 7.Calne R, Friend P, Moffatt S, et al. Prope tolerance, perioperative campath 1H, and low-dose cyclosporine Monotherapy in renal allograft recipients. Lancet. 1998;351:1701–1702. doi: 10.1016/S0140-6736(05)77739-4. [DOI] [PubMed] [Google Scholar]
- 8.Stuart FP, Leventhal JR, Kaufman DB. Alemtuzumab facilitates prednisone free immunosuppression in kidney transplant recipients with no early rejection. Am J Transplant. 2002;2 suppl:397–399. [Google Scholar]
- 9.Knechtle SJ, Pirsch JD, H Fechner J, Jr, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am J Transplant. 2003;3:722–730. doi: 10.1034/j.1600-6143.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 10.Kirk AD, Hale DA, Mannon RB, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52- specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76:120–129. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 11.University of Pittsburgh Institutional Review Board (homepage on the internet) Jurisdiction, Structure, and Responsibilities of the Institutional Review Board. Reference Manual for the Use of Human Subjects in Research. Pittsburgh: University of Pittsburgh; 2006. [Accessed 2006]. (updated 2006 January 17; cited 2006 February 10). Available online at http://www.irb.pitt.edu/manual/preface.pdf. [Google Scholar]
- 12.Bartosh SM, Knechtle SJ, Sollinger HW. Campath-1H use in pediatric renal transplantation. Am J Transplant. 2005;5:1569–1573. doi: 10.1111/j.1600-6143.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- 13.Bartosh SM, Sanchez CP, Sollinger HW, Knechtle SJ. Pilot trial of steroid free immunosuppression with Campath-1H in low risk pediatric kidney transplants. Presented at the 4th International Congress, IPTA; March 2007; Cancun, Mexico. [Google Scholar]