Abstract
We have modified the apparatus for isolated rat liver perfusion (IPRL) in order to be able to perform two perfusions simultaneously. In addition, we studied the quality and stability of livers by comparison of five different perfusates: Blood (Group A), Original Krebs Henseleit buffer (Group B), Krebs buffer with glucose (Group C) or bovine serum albumin (BSA) added, (Group D). In a last group (E) albumin, glucose, and taurocholic acid were added to Krebs. After 180 min of perfusion, livers perfused with solutions including 2% albumin (Group D, E) had a significantly higher release of hepatocellular and endothelial cell (purine nucleoside phosphorylase) enzymes and lower bile production as compared to Groups A, B, and C (P < 0.0001). Increasing levels of purine nucleoside phosphorylase (PNP), a reflection of damage to the microvascular endothelium preceded the increases in hepatocellular enzymes. Histologically, damages of sinusoidal endothelial cells and hepatocytes are appreciated moderate to severe in Groups D and E, slight to mild in Groups A and B, and not significant in Group C. These results suggest that BSA may have toxic effects to the perfused rat liver. These data also confirm that the IPRL modified for simultaneous perfusion of two livers is efficient, and that with this technique the rat liver can be optimally perfused for up to 3 hr with oxygenated Krebs Henseleit buffer without additives (Group B) and without blood. These two improvements should allow those performing studies with perfused rat livers to obtain data in a more efficient, accurate, and inexpensive fashion.
INTRODUCTION
Many modifications of the isolated perfused rat liver (IPRL) techniques have been described, but the majority of investigators have used variations of the original perfusion arrangement introduced by Brower and Miller [1–6]. At our laboratories this organ perfusion technique is performed to assess the viability of preserved livers prior to transplantation.
To enable us to perfuse livers more efficiently, we constructed an IPRL apparatus designed to perfuse two livers simultaneously. This perfusion arrangement allows more studies to be performed in less time and simultaneous comparisons of different treatment regimens. This manuscript concerns the practical aspects of this model as well as a study which compares stability of livers during perfusions with homologous blood, and various cell-free perfusates. The viability of hepatic endothelial cells during perfusions with various perfusates was studied by following the release of purine nucleoside phosphorylase (PNP) into the perfusate. This cytoplasmatic enzyme, most active in the endothelial and Kupffer cells, recently has been demonstrated to be a reliable index of injury to the endothelial cells after warm ischemia [7, 8]. Krebs–Henseleit buffer (KHB) with glucose, albumin, and taurocholic acid added has been the standard perfusate in our lab, as well as others [3, 5, 38]. In addition, some investigators add blood to KHB to improve the oxygen-carrying capacity of the perfusate [4, 5]. We have been interested in what the optimal perfusate truly is for the IPRL system, and whether these additives are necessary.
MATERIALS AND METHODS
The liver perfusion system is contained within a thermostatically controlled plexiglass cabinet (65 cm L × 32 cm W × 60 cm H), designed to perform two perfusions simultaneously. Mounted within the cabinet is a frame that fits all clamps and holders to stabilize the various parts of the system (Fisher Scientific, Pittsburgh, PA).
The perfusate is circulated by a Masterflex pump controller (No. 1, Fig. 1) (Cole Parmer Instrument Co., Chicago, IL, Model 7553-60), which acts on silicone tubing (Cole Parmer Instrument Co., Models 6411-16 and 6411-14). A separate pump is used in each of the perfusion circuits.
FIG. 1.
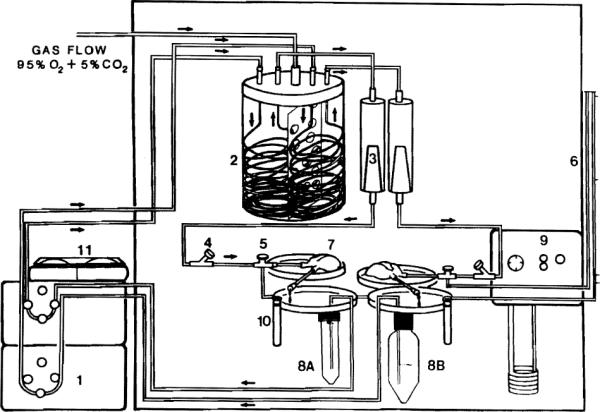
(1) Pulsatile pump. (2) Oxygenator (separated into two chambers). (3) Filter debubbler, upper perfusate reservoir. (4) Inflow perfusate sampling port, bubble trap. (5) Three-way stopcock. (6) Manometer. (7) Liver platform. (8A) Collection basin 50 cc. (8B) Collection basin, 250 cc. (9) Bile collector. (10) Constant temperature circulator. (11) Digital perfusate temperature control.
The oxygenator (No. 2, Fig. 1) is constructed from a single container which has been separated into two chambers by fenestrated plastic wall. The same oxygenator is used for both perfusion circuits. The perfusate is exposed to a gas mixture of 95% O2/5% CO2 while passing through a thin-walled silicone tubing (American Scientist, Warrendale, PA, T5715-6, I.D. 1.5 mm) which is 8 m long. This pressurized membrane oxygenator is developed from the “Hamilton Lung” and can generate a partial pressure of oxygen of 500–600 mm Hg in each perfusion circuit at a O2/CO2 flow of 5 liters/min [9, 10).
After the oxygenator, the perfusion medium passes through a disposable nylon filter (blood set 64, Abbott Lab) (No. 3, Fig. 1). The macrofilter is placed in-line within each inflow circuit as both a bubble trap and a perfusate filter.
The pressure within each circuit is continuously monitored through a three-way stop cock, placed immediately before the portal vein cannula (No. 5, Fig. 1). The manometer (Abbott Laboratories, North Chicago, IL, Model 4607) (No. 6, Fig. 1) is fixed to the side of the cabinet.
Male Lewis rats (250 to 350 g) are used as liver donors. Anesthesia is induced and maintained with inhalation methoxyflurane. Every rat receives 300 units of heparin via the penile vein prior to harvest of the liver. A transverse incision is made to expose the upper abdominal cavity, which allows optimal access to all the supporting ligaments of the liver and to the supra- and infrahepatic vena cava (IVC). For bile collection, the bile duct is intubated with polyethylene (Clay Adams, PE-10 tubing, I.D. 0.2 mm). Immediately after cannulation of the portal vein with a 16-gauge teflon angiocatheter, the liver is flushed gently with 50–60 cc of heparinized (10 U/cc) Ringer Lactate solution. After cannulation of the infrahepatic IVC with a 14 gauge catheter, the suprahepatic IVC is ligated. This larger angiocatheter in the IVC results in decreased perfusion pressures (10–15 cm H2O) at high flow rates (up to 3.5 cc/min/g/liver which improves oxygen delivery. Harvest of the second liver is staggered from 15 to 30 min for an adequate spacing of sample collection. Liver weights range from 9.37 to 12.10 g, and average 10.4 ± 1.3 g.
The liver is placed on a removable petri dish (No. 7, Fig. 1) (Fisher Scientific Corp., Pittsburgh, PA) after cannulation of the portal vein and IVC is completed. Grooves at the edge of the dishes stabilize the portal and IVC cannulae. The IVC effluent drains into a disposable, 50-cc centrifuge tube (No. 8A, Fig. 1) (Corning Laboratory Sciences Co., Corning, NY) which is attached to a second petri dish. If the perfusate will be sampled frequently, a larger reservoir (250-cc centrifuge tube) can be substituted (No. 8B, Fig. 1).
The temperature of the perfusate is maintained at 37°C [11] by a circulating water bath in the base of the cabinet (No. 9, Fig. 1). Each system is continuously monitored with a digital thermometer and a thermistor in the arterial inflow (Type T thermo couple thermometer, Cole Parmer Instrument Co.) (No. 11, Fig. 1).
Perfusates
The standard perfusate in our experiments used (Table 1) is based upon the original Krebs–Henseleit solution (Group B) (12). In Group A, we employed homologous whole rat blood diluted with KHB to a hematocrit value of 15–20% with a osmolarity ranging between 295–302 milliosmols (mosmol). In Group C, the livers were perfused with Krebs plus 5.5 mM glucose added as an energy source. Krebs buffer with 2% BSA added as an oncotic agent (Albumin, Fraction V, Sigma Chemical Co) was used in Group D. As anginous modified Krebs solution, which is commonly used in many laboratories, contains albumin (2%), glucose (5.5 mM), and taurocholic acid (0.073 g/liter). This perfusate was studied in Group E (3, 13, 14). The volume of medium used in each perfusion system was 180 ml. A separate group (F) was subjected to 90 min of warm ischemia via bypassing perfusate flow around the liver to show the effects of severe injury. This period of ischemia was followed by 120 min of reperfusion with KHB.
TABLE 1.
Perfusates Used in Experimental Groups A–E
| A | B | C | D | E | F | |
|---|---|---|---|---|---|---|
| Krebs | + | + | + | + | + | + |
| 5.5 mM Glucose | + | + | ||||
| 2% Albumin | + | + | ||||
| Taurocholic acid | + | |||||
| Blood | + |
Note. The Krebs medium consisted of NaCl 118.5 mM, KCl 4.7 mM, CaCL2 H2O 2.5 mM, KH2PO4 1.2 mM, MgSO4 1.2 mM, NaHCO3 24.9 mM. Livers were flushed with Ringer's solution and immediately placed on the perfusion apparatus. In Group F the livers were subjected to 90 min of warm ischemia (bypass ischemia).
Monitoring of liver function
All perfusions in Groups A–E were done for 3 hr after an initial equilibration period of 15 min (t – 15). The samples were taken at t – 15, t0, and subsequently every 30 min during the 3-hr perfusion period. Six liver perfusions were performed in each group.
(A) The oxygen consumption of the liver was monitored by periodic determination (15 min) of the pO2 and pCO2 (mm Hg) of the portal vein inflow (PV) and lVC outflow using a pH-blood gas meter (ABL 2-Acid-Base Laboratory, Radiometer, Copenhagen, Denmark) (4). The oxygen consumption (O2/g wet liver/min) was calculated by
(B) All bile (microliter/g liver/min) secreted was collected into a graduate cylinder (No. 10, Fig. 1) and quantitated every 15 min.
(C) The release of hepatocellular enzymes (AST, ALT, and LDH) into the perfusate were determined using a Technicon RA-500 analyzer.
(D) Levels of (PNP) in the effluent were measured using the methods of Hoffee et al. (15). The increase in absorbance (293 nm) from uric acid produced by the breakdown of nucleoside was measured by a coupled spectrophotometric assay.
Histopathology
After a 3 hr perfusion, each liver specimen was fixed in 10% neutral buffered formalin and routinely stained with hematoxylin and eosin.
Histological evaluation was blindly and independently assessed for each specimen by one of the authors (K.N.). The histological features of the hepatic damage of the liver tissue examined were morphological changes of sinusoidal lining cells and hepatocytes as a whole, and graded from 0 to 4 with 0, indication nothing particular; 1, slight; 2, mild; 3, moderate; and 4, severe changes.
Statistical analysis
Data are presented as mean ± standard deviation (M ± SD). In order to investigate differences between the groups, a repeated measures analysis of variance was performed. The three hypotheses of interest were: (1) between group differences, (2) within time difference, and (3) the interaction between time and groups [16–18]. When indicated, the student's t test was performed.
RESULTS
Hemodynamic parameters
The rate of perfusate flow with cell-free perfusates and with the blood containing medium ranged from 2.3 to 3 mljg liver/min which resulted in a perfusion pressure between 8.4 ± 2.0 and 10.9 ± 1.0 mm H2O. The mean oxygen consumption (Table 2) in groups without blood was 1.5 ± 0.2 O2/g liver/min. Perfusion with homologous blood (Group A) resulted in a significantly greater oxygen consumption, 2.57 ± 0.5 mol O2/g liver/min (P < 0.0001) when compared to the blood free perfusions (Fig. 2). After 180 min, the oxygen uptake in this group declined from 2.87 ± 0.4 to 2.05 nmole/g liver/min (29%). In livers perfused without blood oxygen consumption averaged 1.51 ± 0.18 nmole/g liver/min and remained stable throughout the perfusions.
TABLE 2.
Comparison of Perfusions in IPRL Using Different Perfusates (A–F)
| A | B | C | D | E | |
|---|---|---|---|---|---|
| Flow rate (ml/g liver/min) | 2.2 | 3.0 | 3.0 | 3.0 | 2.3 |
| Portal vein pressure (cm H2O) | 10.1 ± 0.8 | 8.4 ± 2.0 | 9.9 ± 1.1 | 10.9 ± 1.0 | 8.73 ± 1.3 |
| Oxygen consumption (nmol/g liver/min) | |||||
| Time course | |||||
| 60 min | 2.87 ± 0.4 | 1.31 ± 0.1 | 1.41 ± 0.09 | 1.78 ± 0.04 | 1.42 ± 0.06 |
| 120 min | 2.25 ± 0.07 | 1.41 ± 0.0 | 1.63 ± 0.09 | 1.72 ± 0.04 | 1.48 ± 0.01 |
| 180 min | 2.05 ± 0.07 | 1.12 ± 0.01 | 1.67 ± 0.02 | 1.60 ± 0.14 | 1.55 ± 0.04 |
| Mean | 2.51 ± 0.51 | 1.28 ± 0.13 | 1.52 ± 0.1 | 1.70 ± 0.13 | 1.47 ± 0.07 |
| Osmolarity (mosmole) | 300 ± 4.4 | 287 ± 4.0 | 306 ± 7.6 | 296 ± 3.0 | 297 ± 9.0 |
Note. The experimental conditions were described under Materials and Methods. The values are means ± SD.
FIG. 2.

Effects of different perfusates on oxygen consumption using the IPRL. The experimental conditions and calculation of oxygen consumption were as described under Materials and Methods. In contrast to Group A, Groups B–E maintained stable consumption of oxygen and showed no differences throughout the perfusion period (P > 0.0001).
Bile flow
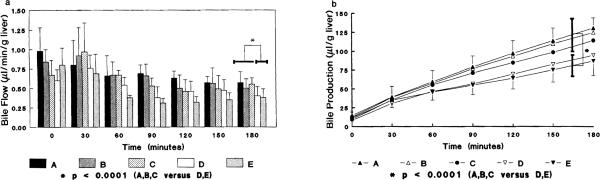
The volume of excreted bile was significantly lower in perfusions using albumin (P < 0.0001) (Fig. 3A). In Groups D and E, bile flow averaged 0.51 ± 0.13 and 0.46 ± 0.19 μl/g/min, respectively, which were significantly lower than the bile flow in all other groups (P < 0.0001). The livers perfused with Krebs solution either without glucose or with glucose only added (Group B, C) had bile flow comparable to that of livers perfused with blood. The cumulative bile production is also greater in those livers perfused without albumin (P < 0.0001) (Fig. 3B).
FIG. 3.
(a) Bile flow during a 180-min perfusion period. Each bar represent microliter bile per wet weight liver produced per min. A significantly greater bile flow (P < 0.0001) was seen in the groups without albumin (BSA) added to the perfusate. (b) The cumulative bile production during a 180-min perfusion. The excreted bile in Groups D and E was significantly less than that in Groups A–C (P < 0.0001). No statistical difference was seen between Groups D and E (P = 0.853).
pH
The pH of the perfusate fell slightly in all groups from 7.40 to 7.32 over the 3-hr period (data not shown).
Enzyme activity
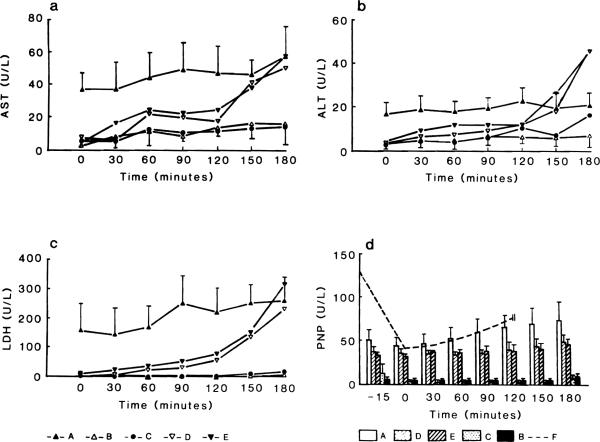
The release of hepatocellular enzymes (AST, ALT, LDH) was significantly elevated in perfusions containing albumin (Group D and E). The leakage of hepatocellular enzymes started between 90 and 120 min as compared to Groups Band C which showed no increase in AST, ALT, and LDH (Figs. 4A–4C). Throughout the perfusion with blood, enzyme levels were higher presumably due to hemolysis of red cells. The most sensitive indicator of a failing perfusion was an increase in LDH activity, as the perfusions with albumin, showed an exponential rise after 90 min.
FIG. 4.
(a–c) Enzyme release (AST, ALT, LDH) during the 3-hr perfusion period. In the perfusions with albumin (Groups D, E) the hepatocellular enzymes significantly (P < 0.0001) rose especially after the second hour of perfusion. (d) PNP levels in perfusions with albumin of blood were significantly higher (P < 0.0001). There was no significant difference between groups including albumen in the perfusate (P = 0.569). Also no significant P value could be seen in blood perfusates compared to the later groups (D, E) over the 180-min perfusion period. Higher hepatocellular enzymes and PNP levels using blood (Group A) coincide with progressive hemolysis. Dotted line (Group F) represents changes in PNP levels during reperfusions after 90 min of warm ischemia.
The release of PNP (Fig. 4D) was high in perfusates containing blood and cell-free perfusates with albumin. Only a mild activity was seen in the Groups Band C (without BSA) as PNP activity averaged 10.1 ± 3.5 and 7.1 ± 4.9 at the end of 180 min of perfusion. These were significantly lower than in Groups A (74 ± 21.3) and E (46 ± 7.08) (P < 0.0001).
In Group A, there was a gradual accumulation of PNP in the perfusate, while the livers perfused with albumin showed high levels of PNP early which were sustained throughout the perfusion. In livers subjected to 90 min warm ischemia, levels of PNP rose to 132 ± 22.5 mU/ml by the end of the ischemic period. After an initial decrease during the early reperfusion PNP levels steadily increased throughout reperfusion (P < 0.0001) (Fig.4D). In those livers showing gradual damage during a perfusion (D, E) the degree of PNP release correlated with reduced bile production (Fig. 5). In addition, the leakage of PNP preceded the increase of hepatocellular enzymes (Figs 4A–4D).
FIG. 5.
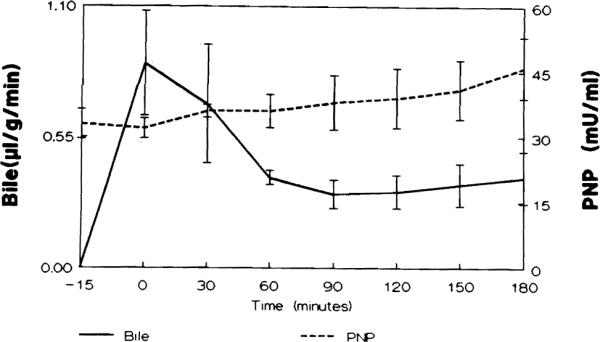
Comparison of bile flow (microliter/g liver/min) and PNP (U/liter) release in a perfusion using albumin (Group E). A significant correlation (Spearman rank, r = −0.738, P < 0.05) was observed between increase in the activity of PNP in the perfusate and decreasing bile flow.
Histopathology
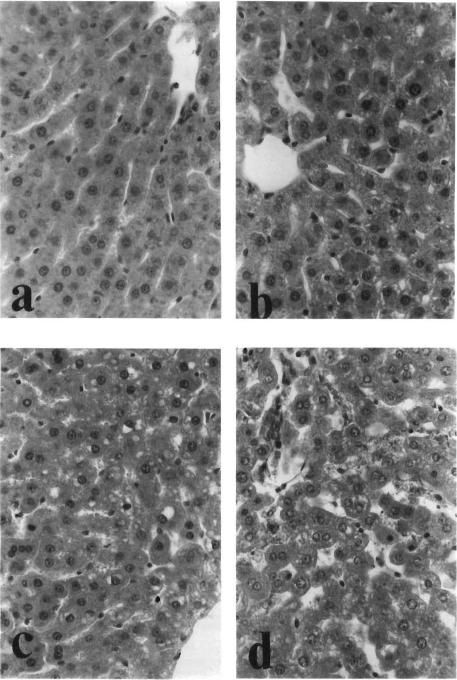
Sinusoidal lining cells are normally seen with slender and fusiform configuration, attached to the regularly arranged cords of hepatocytes (Fig. 6a). Slightly to mildly damaged (Fig. 6b), they appeared round and denudated in configuration, and detached from the cords. Moderately to severely damaged (Figs. 6c, 6d), sinusoidal lumen laced the lining endothelial cells but filled with sloughs of nuclear debris.
FIG. 6.
Histological grades of hepatic damage. (a) Not particular (Group A; H&E, ×500). (b) Slight damage (Group B; H&E, ×500). (c) Moderate damage (Group E; H&E, ×500). (d) Severe damage (Group E; H&E, ×500).
Damage of the hepatocytes was evaluated by focal and spotty necrosis, intracytoplasmic vacuolization, and irregular arrangement of cords. Severely damaged hepatic tissue often showed patchy areas of hepatocytic discoloration preferentially located in the centrilobular area accompanied by lender cords of hepatocytes with eosinophilic cytoplasm and pyknotic nuclei consistent with histological changes of ongoing necrosis.
As shown in Table 3, histological damages were most evident in Groups D and E, while Groups A and C retained near-normal architecture after 3 hr perfusion.
TABLE 3.
The Degree of Histological Damage of the Liver After a 3-hr Perfusion
| (N) | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|---|
| Group A | 5 | 3 | 2 | 0 | 0 | 0 |
| Group B | 7 | 1 | 3 | 1 | 1 | 1 |
| Group C | 4 | 4 | 0 | 0 | 0 | 0 |
| Group D | 6 | 0 | 3 | 2 | 1 | 0 |
| Group E | 10 | 0 | 0 | 3 | 5 | 2 |
Note. Grades from 0 to 4 were described under Material and Methods.
DISCUSSION
Various modifications of the IPRL technique have been reported, and a variety of perfusates are used (1–6). This makes comparison and reproduction of results from different laboratories difficult. Since the apparatus is so extensively used in biochemical and clinical studies, we set out to make IPRL more efficient and to standardize the technique. The first effort was to develop a system for simultaneous perfusion of two livers. The second goal was to systematically study various perfusates under identical conditions by comparing organ viability based on patterns of bile flow and production, leakage of hepatocellular enzymes and of PNP as a marker of endothelial integrity [7].
In the initial experiments, we compared KHB and a perfusate containing homologous blood. The addition of blood to perfusates is used by many investigators but is controversial because of the effect of inconstant levels of hematocrit on hepatic function. In addition, organ function is adversely affected by hemolysis and microemboli [10, 20, 21]. We found no difference as to the rate of bile flow or cumulative bile production between perfusions performed with (Group A) and without blood (Group B). The absence of blood allowed for more stable and lower enzyme levels in perfusate. The unpredictable effects of factors soluble in blood (hormones, immunoglobulins, etc.) are eliminated when blood-free perfusates are used. Finally, the sacrifice of animals for blood is obviated.
The flow rate was kept at 3.0 ml/g liver/min in blood-free perfusions, and between 2 to 2.3 ml/g liver in blood containing perfusions. A higher flow rate of 3 ml/g liver was the required oxygenation when perfusates without blood were used. We were able to accomplish this while keeping the portal pressure in the physiological range of 8.4 ± 2.0 to 10.9 ± 1.0 mM Hg [22, 23]. This perfusion pressure is necessary to deliver sufficient oxygen to the liver while avoiding barotrauma to the sinusoidal endothelial cells [3, 5, 24]. Oxygen consumption with homologous blood was nearly twice that of the cell-free perfusions, but gradually declined throughout the perfusion consistent with a decline in the viability of erythrocytes [25]. The stability of oxygen consumption in the perfusions without blood oxygen argues against the liver being compromised by the absence of erythrocytes. The higher flow rates compensated well for the lower oxygen-carrying capacity of cell-free perfusates.
The exclusion of albumin from the cell-free perfusates proved to be a critical improvement in the technique. Hepatic function was more stable as judged by constant enzyme levels and bile production. The leakage of hepatocellular enzymes in these perfusions were also considerably less than in other reports using cell-free or blood-containing perfusates [26, 27]. These findings may be related to a toxic effect of bovine albumin on the rat liver. In a model of kidney preservation, denaturation of BSA was shown to occur with the production of harmful substances which lead to severe organ damage [28, 29]. Hepatocellular enzymes can be released in toxic and metabolic injuries even in the absence of fulminant hepatic necrosis through shedding from viable hepatocytes of cytosol contained within cell-surface blebs [30]. The coincident decreasing bile production supports the theory that the livers are being damaged duringperfusions with albumin.
Histochemical studies have shown that PNP is an enzyme most active in the cytoplasm of endothelial and Kupffer cells (7). A correlation had been demonstrated between levels of PNP and the degree of microvascular endothelial cell damage after warm ischemia and reperfusion (8). This observation was also confirmed in this study in the perfusions following a 90-min period of warm ischemia. The degree of PNP release was comparable in the ischemic and blood containing perfusions (Groups F and A). Release of PNP was lower in the perfusions without albumin (P < 0.0001). The higher levels of transaminases and PNP in the perfusate using blood coincide with progressive hemolysis and blockage of the microvasculature. Leakage of enzymes from erythrocytes clouds the results of any perfusion, and the presence of erythrocytes did not yield higher or more constant bile productions (P = 0.404).
Our results show that an oncotic agent is not essential for successful isolated perfusion of the rat liver over a period of 3 hr. The rise in PNP routinely preceded the increase in AST, ALT, and LDH, which may reflect an initial endothelial cell injury with a secondary damage to the hepatocytes (Figs. 4A–4D). The relationship between endothelial injury and liver failure is confirmed by the correlation between decreasing bile production and increasing PNP release into the perfusate (Fig. 5).
Some reports have mentioned that bile production may be a less reliable parameter of liver function than the composition of secreted bile because of exposure to many factors, namely nervous, humoral, and ionic [31]. Sufficient bile production in our system was obtained without the continuous replacement of bile acids. This production was comparable to that reported by other authors, [3, 4, 14, 32, 33]. In standard IPRL systems, a gradual drop in bile production of up to 40% is expected after 2 hr of perfusion [34]. However, after an initial rise during the first 30 min of perfusions, the amount of bile produced during perfusions without albumin was stable and significantly higher than in the presence of albumin (P < 0.0001). In our system, the extent of hepatic injury can be assessed simply by monitoring the bile flow rate as a reflection of the cellular energy status [35–37].
In the past, isolated perfusion of organs has been a time-consuming procedure. However, we have further modified this technique so as to be able to efficiently perfuse two livers simultaneously. Simple KHB seemed to be the optimal perfusate and the addition of erythrocytes, glucose, albumin, and bile acids is unnecessary and potentially damaging. This model can be easily assembled from common laboratory supplies, and may allow less expensive, and more economical use of perfused organs in a variety of research fields [38].
ACKNOWLEDGMENTS
We express our appreciation to F. Sterz, M.D., R. E. Stauber, M.D., and Jo Smerdell for their assistance in processing the data and to Bernice Kula for typing the manuscript.
Dr. Hans J. Mischinger is supported by a Schroedinger Stipendium for the Fond zur Foerderung der Wissenschaftlichen Forschung, Austria (Jo-355-Med).
REFERENCES
- 1.Miller LL, Bly CG, Watson ML, Bale WF. The dominate role of the liver plasma protein synthesis. J. Exp. Med. 1951;94:431. doi: 10.1084/jem.94.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller LL. History of isolated liver perfusion and some still unsolved problems. In: Bartosek I, Guaitini A, Miller LL, editors. Isolated Liver Perfusion and Its Applications. Raven Press; New York: 1972. pp. 1–9. [Google Scholar]
- 3.Gores GJ, Kost LJ, Larusso NF. The isolated perfused rat liver: Conception and protocol considerations. Hepatology. 1986;6(3):511. doi: 10.1002/hep.1840060331. [DOI] [PubMed] [Google Scholar]
- 4.Ross B. Perfusion Techniques in Biochemistry. Claredon Press; Oxford: 1972. [Google Scholar]
- 5.Wolkoff AW, Johansen KL, Goeser T. The isolated perfused rat. liver: Preparation and application. Anal. Bio. Chem. 1987;167:1. doi: 10.1016/0003-2697(87)90127-8. [DOI] [PubMed] [Google Scholar]
- 6.Gayle WE. Isolated organ perfusion: Physiology and application. Med. Coll. Va. Q. 1969;5:1773. [Google Scholar]
- 7.Rubio R, Berne R. Localization of purine and pyrimidine nucleoside phosphorylases in heart, kidney, and liver. Am. J. Physiology. 1980;239:H721. doi: 10.1152/ajpheart.1980.239.6.H721. [DOI] [PubMed] [Google Scholar]
- 8.Rao PN, Walsh TR, Makowka L, Rubin R, Weber T, Snyder JT, Starzl TE. Purine nucleoside phosphorylase (PNP)—A new marker for free oxygen radical injury to endothelial cell. Hepatology. doi: 10.1002/hep.1840110206. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton RL, Berry MN, Williams ML, Severinghaus EM. A simple and inexpensive membrane “lung” for small organ perfusion. J. Lipid Res. 1974;15:182. [PubMed] [Google Scholar]
- 10.Melrose DG, Burns N, Singh MP, Elliot R, Read R, Williams FE, Becket J, Lamp MP, Adams JS. Oscillating silicone membrane tubes: A new principle of extracorporal respiration. Biomed. Eng. 1972;7:60. [PubMed] [Google Scholar]
- 11.Skibba JL, Collins FG. Effect of temperature on biochemical functions in the isolated perfused rat liver. J. Surg. Res. 1978;24:435. doi: 10.1016/0022-4804(78)90040-9. [DOI] [PubMed] [Google Scholar]
- 12.Krebs HA, Henseleit K. Untersuchungen uber die Harnstoffbildung im Tierkorper. Hoppe-Seyler's Z. Physiol. Chem. 1932;210:33. [Google Scholar]
- 13.Rosini S, Bennetti D, Kvetina J. The functional capacity of the isolated perfused rat liver in relation to the colloidal osmotic composition of the perfusion medium. Farmac. Prat. 1976;31:625. [PubMed] [Google Scholar]
- 14.Schmucker DL, Jones AL, Michielsen CE. An improved system for hemoglobin free perfusion of isolated rat livers. Lab. Invest. 1975;33:168. [PubMed] [Google Scholar]
- 15.Hoffee PA, May R, Robertson BC. Purine nucleoside phosphorylase from salmonella thyphimurium and rat liver. In: Hoffe P, Jones MA, editors. Methods in enzymology. Vol. 51. Academic Press; New York: 1978. pp. 517–524. [DOI] [PubMed] [Google Scholar]
- 16.Bock RD. Multivariated Statistical Methods in Behavioral Research. McGraw-Hill; New York: 1975. [Google Scholar]
- 17.Collier RO, Baker FB, Mandeville GK, Hayes TF. Estimates of test size for several test procedures based on conventional variance ratios in the repeated measures design. Psychometrika. 1967;32(3):339. doi: 10.1007/BF02289596. [DOI] [PubMed] [Google Scholar]
- 18.Davidson ML. Univariate versus multivariate tests in repeated-measures. Psyeholog. Bull. 1972;77(96):446. [Google Scholar]
- 19.Ashmore PG, Svitek V, Ambrose P. The incidence and effects of particulate aggregation and microembolism in pump oxygenator systems. J. Thorae. Cardiovase. Surg. 1968;55:691. [PubMed] [Google Scholar]
- 20.Felts JM. The metabolism of chylomicron triglyceride fatty acids by perfused rat livers and by intact rats. Ann. NY. Acad. Sci. 1965;131:24. doi: 10.1111/j.1749-6632.1965.tb34776.x. [DOI] [PubMed] [Google Scholar]
- 21.Riedel GL, Scholle JL, Shepherd AP, Ward WF. Effects of hematocrit on oxygenation of the isolated perfused rat liver. Am J. Physiol. 1983;245:769. doi: 10.1152/ajpgi.1983.245.6.G769. [DOI] [PubMed] [Google Scholar]
- 22.Bartosek I, Guaitani A, Miller LL. Isolated Liver Perfusion and Its Applications. Raven Press; New York: 1973. [Google Scholar]
- 23.Fraser R, Bowler LM, Day WA, et al. High perfusion pressure damages the sieving ability of sinusoidal endothelium in rat livers. Br. J. Exp. Pathol. 1980;61:222. [PMC free article] [PubMed] [Google Scholar]
- 24.Schmucker DL, Curtis JC. A correlated study of fine structure and physiology of the perfused rat liver. Lab. Invest. 1974;30:201. [PubMed] [Google Scholar]
- 25.Schimassek H. Perfusion of isolated rat liver with semisynthetic medium and control of liver function. Life Sci. 1962;11:629. doi: 10.1016/0024-3205(62)90096-6. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt E, Schmidt FW, Mohr J, Otto P, Vido I, Wrogemann K, Herfarth CM. Liver morphology and enzyme release: Further studies in the isolated perfused rat liver. In: Keppler D, editor. Pathogenesis and Mechanisms of Liver Cell Necrosis. University Park Press; Baltimore: 1975. pp. 147–162. [Google Scholar]
- 27.Erikson H. A compact perfusion system for studies on the control of metabolic processes in isolated organs from small animals. Acta. Physiol. Scand. 1975;93:206. doi: 10.1111/j.1748-1716.1975.tb05811.x. [DOI] [PubMed] [Google Scholar]
- 28.Southard JH, Senzig KA, Hoffman R, Belzer FO. Denaturation of albumin: A critical factor in long term kidney preservation. J. Surg. Res. 1981;30:80. doi: 10.1016/0022-4804(81)90073-1. [DOI] [PubMed] [Google Scholar]
- 29.Cohen R, Iles R, Lloyd M. Perfusion of the isolated rat liver. In: Ritchie H, Hardcastle J, editors. Isolated Organ Perfusion. University Park Press; Baltimore, London, Tokyo: 1973. p. 120. [Google Scholar]
- 30.Lemasters JJ, Stemkowski CJ, Ji S, Thurman RG. Cell surface changes and enzyme release during hypoxia and reoxygenation in the isolated, perfused rat liver. J. Cell Biol. 1983;97:778. doi: 10.1083/jcb.97.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyer JL, Scheig RL, Klatskin G. The effect of sodium taurocholate on the hepatic metabolism of sulfobromophathalein sodium (BSP). The role of the bile flow. J. Clin. Invest. 1970;49:206. doi: 10.1172/JCI106229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavolini N, Reed S, Boyer JL. Hemodynamic effects on determinants of bile determinants in isolated rat liver. Am. J. Physiol. 1978;234:584. doi: 10.1152/ajpendo.1978.234.6.E584. [DOI] [PubMed] [Google Scholar]
- 33.Sugano T, Suda K, Shimada M, Oshimo N. Biochemical and ultrastructural evaluation of isolated rat liver systems perfused with hemoglobin free medium. J. Biochem. 1978;83:995. doi: 10.1093/oxfordjournals.jbchem.a132028. [DOI] [PubMed] [Google Scholar]
- 34.Meijer DK, Keulemans K, Mulder GJ. Isolated perfused rat liver technique. In: Jakoby WB, editor. Methods in Enzymology. Vol. 77. Academic Press; San Diego: 1981. p. 81. [DOI] [PubMed] [Google Scholar]
- 35.Nakazawa T, Nunokawa T. Energy transduction and adenine nucleotides in mitochondria from rat liver after hypoxic perfusion. J. Biochem. 82:1575–1977. doi: 10.1093/oxfordjournals.jbchem.a131852. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe F, Kamiikie W, Nishimura T, Hashimoto T, Tagawa K. Decrease in mitochondrial levels of adenine nucleotides and concomitant mitochondrial dysfunction in ischemic rat liver. J. Biochem. 1983;94:483. doi: 10.1093/oxfordjournals.jbchem.a134380. [DOI] [PubMed] [Google Scholar]
- 37.Kamiikie W, Nakahara M, Nakao K, Koseki M, Nishida T, Kawashima T, Watanabe F, Tagawa K. Correlation between cellular ATP level and bile excretion in the rat liver. Transplantation. 1985;39:50. doi: 10.1097/00007890-198501000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Ontell SJ, Collella MS, Horowitz J, Makowka L, Trager J, Starzl TE. Applications of the isolated perfused rat liver in transplant research. J. Invest. Surg. 1988;1:25. doi: 10.3109/08941938809141072. [DOI] [PubMed] [Google Scholar]