Accidental interruption of the hepatic artery or one of its principal branches is a feared surgical complication. Although vascular repair may be feasible, the injury is frequently so extensive that arterial reconstruction is not possible. In this situation, accurate knowledge of the consequences of ligation of the hepatic artery would be desirable.
In the dog and other experimental animals, hepatic arterial ligation has been well tolerated if antibiotics are given. Similar information is not available for the human. The last comprehensive survey of accidental hepatic artery ligation in the human was by Graham and Cannell [1] in 1933. Their review indicated that a mortality of approximately 60 per cent could be expected.
The present report documents five cases of injury to the common hepatic or right hepatic artery. Arterial reconstruction was impossible in four of the patients. In addition, the cases of ligation of the hepatic artery reported since 1933 have been compiled with particular attention to those patients treated during the antibiotic era. The results of this investigation suggest that ligation of the hepatic artery in the human is a less lethal complication than has been generally realized.
CASE REPORTS
CASE I
A thirty-five year old man was operated on for subacute cholecystitis in May 1962. Jaundice had not been present. Cholecystectomy was difficult. During dissection of the neck of the gallbladder, the right hepatic artery was lacerated. (Fig. IA.) Efforts at arterial reconstruction led to further loss of vessel substance and the proximal and distal stumps of the right hepatic artery were ligated. It was noted that the right lobe (Fig. 2) was deeply cyanotic compared to the remainder of the liver. Drains were placed in the subhepatic space and the incision closed. For the first three postoperative days 6 gm. of chloromycetin and 4 gm. of erythromycin were given intravenously every twenty-four hours. From the third to the eighth postoperative days the antibiotic dose was reduced and given orally.
FIG. I.
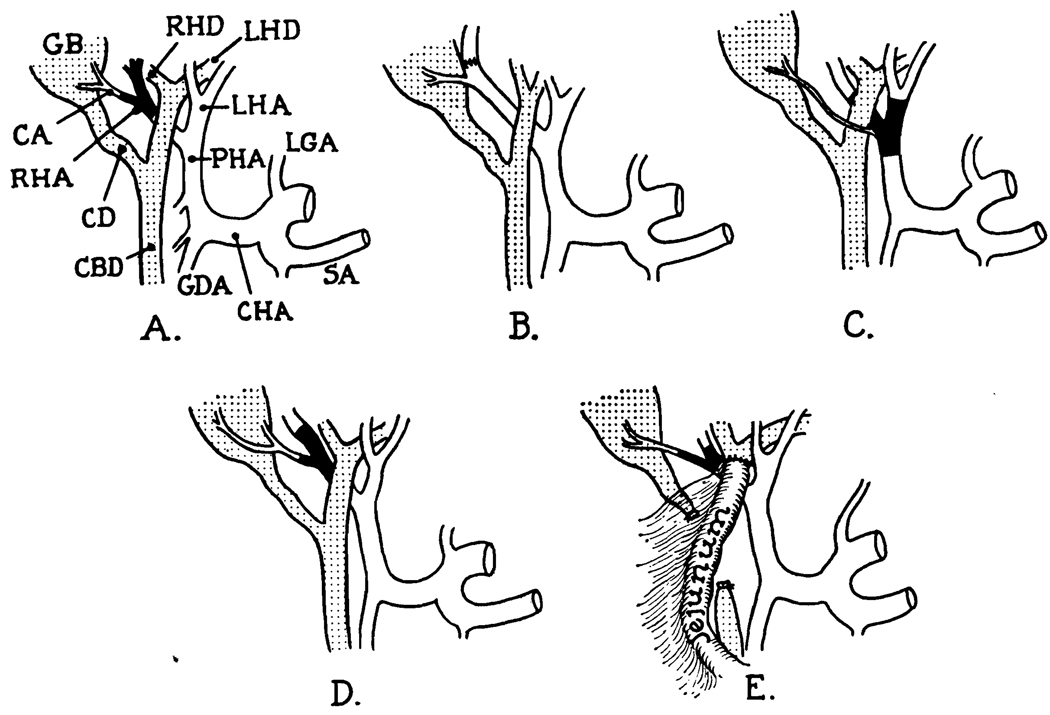
The nature of the arterial injury in the reported cases. The order of the diagrams is the same as the case reports. In B, reanastomosis of the right hepatic artery was done. In E, the common duct was also injured and repaired with a Roux-en-Y choledochojejunostomy. CA, cystic artery; CBD, common bile duct; CHA, common hepatic artery; GB, gallbladder; GDA, gastroduodenal artery; LGA, left gastric artery; LHA, left hepatic artery; LHD, left hepatic duct; RHA, right hepatic artery; RHD, right hepatic duct; SA, splenic artery.
FIG. 2.
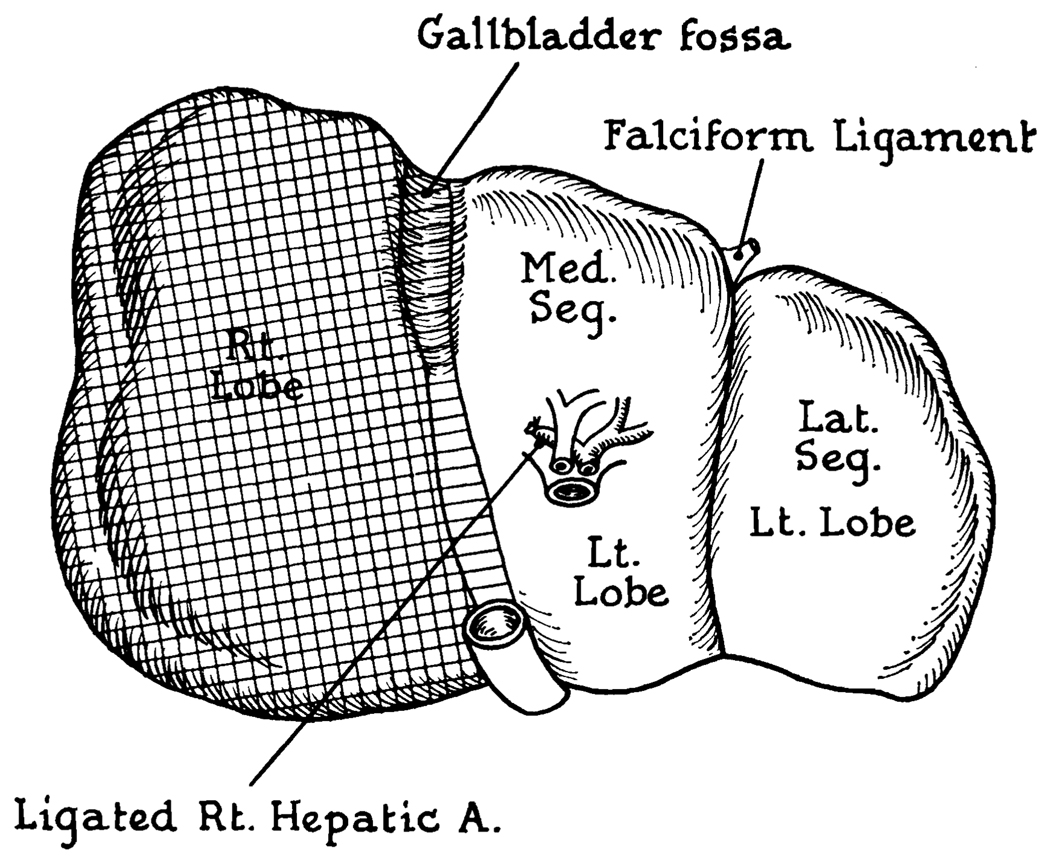
Area of cyanosis resulting from ligation of right hepatic artery in Cases I and IV. Note that the color change was essentially identical in both, involving only a few extra millimeters in Case I.
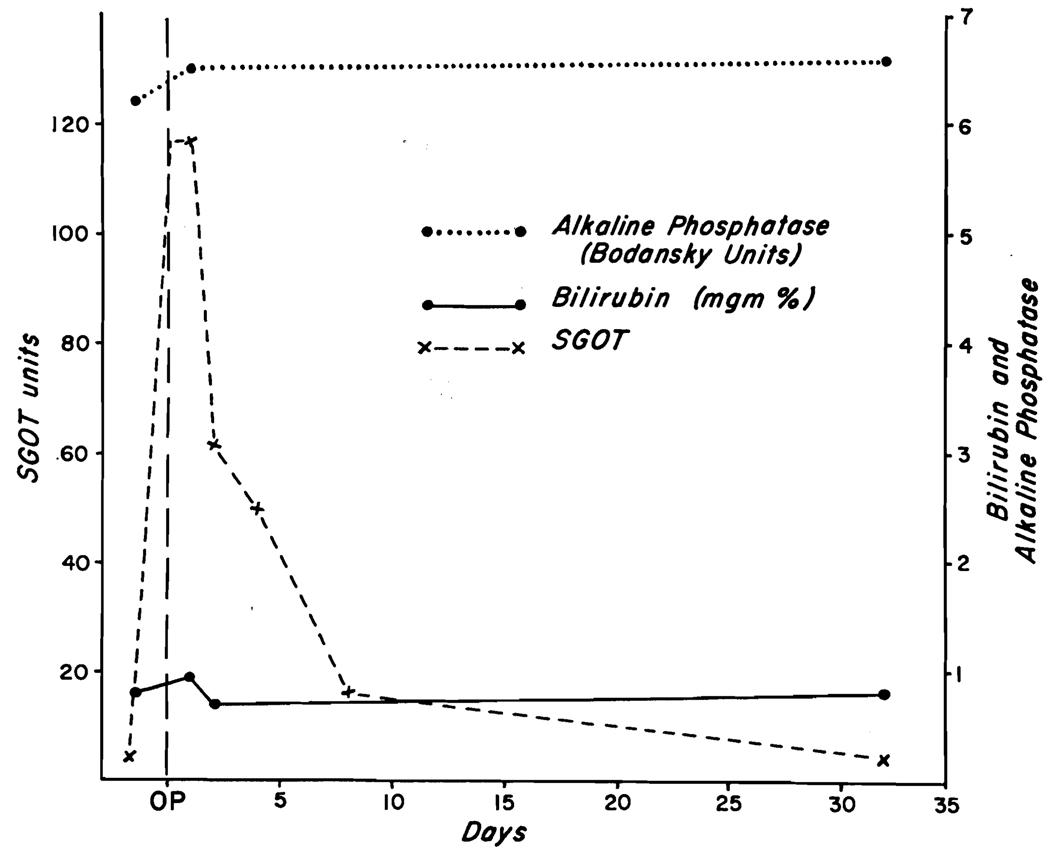
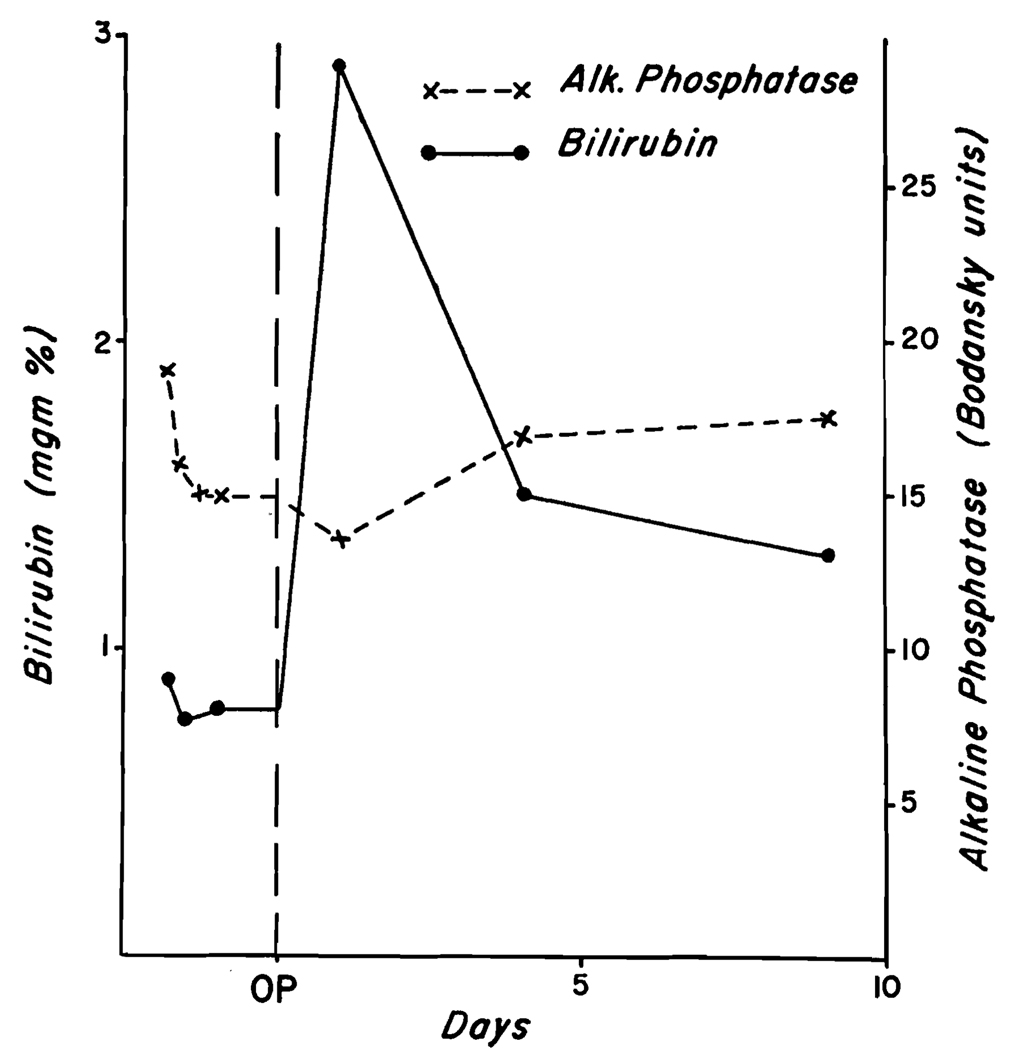
The serum glutamic-oxalacetic acid transaminase (SGOT) rose abruptly within twenty-four hours of surgery (Fig. 3) and declined thereafter. The prothrombin time on the first, second and third postoperative days was 39, 29 and 25 per cent and then returned to normal. The bilirubin and alkaline phosphatase did not rise. (Fig. 3.) On the eighth postoperative day, forty-five minute bromsulphalein retention was I per cent. The patient was discharged on the tenth postoperative day. Follow-up studies two and five weeks after surgery showed no abnormality of liver function.
FIG. 3.
Changes in laboratory values in Case I after ligation of the right hepatic artery. Note that the only change was a rise in SGOT.
Comment
The injury to the right hepatic artery occurred in a typical location where the artery passes close to the neck of the gallbladder. The striking color change in the right lobe was considered an ominous sign. However, the only demonstrable consequence was a short-lived rise in SGOT and a similar prolongation of the prothrombin time. The liver was extensively drained; but if evidence of necrosis had developed, immediate re-exploration would have been in order with a view to excision of the devitalized lobe.
CASE II
A seventy-four year old woman underwent cholecystectomy and common duct exploration in December 1961, for jaundice of several weeks’ duration. Preoperative liver function studies revealed SGOT of 150, bilirubin 7.2 mg. per cent, alkaline phosphatase of 123 King Armstrong (K-A) units, prothrombin time of 47 per cent and thymol turbidity of 6.0 units. During the course of cholecystectomy, the right hepatic artery was ligated and divided. (Fig. IB.) This was recognized almost immediately, and end to end anastomosis carried out with return of good pulsation throughout the course of the extrahepatic portion of the vessel. During the anastomosis, good back bleeding of what appeared to be arterial blood was noted from the distal cut end. Color changes in the liver itself were not specifically noted.
PostoperativeIy the patient did well clinically. There was a gradual return of the previously deranged liver chemistries to normal. Three days after surgery SGOT was 125, bilirubin 5.6 mg. per cent, alkaline phosphatase 86 K-A units, prothrombin time 58 per cent. Three weeks postoperatively the SGOT was 98, bilirubin 1.75 mg. per cent, alkaline phosphatase 37 K-A units, and prothrombin time 70 per cent. One week later the bilirubin was 1.3 mg. per cent and alkaline phosphatase 21 K-A units. Liver biopsy taken at the time of the operation revealed acute and chronic cholangitis of moderate degree. She has remained in good health during the subsequent year.
Comment
In this case injury occurred to the right hepatic artery as it crossed cephalad to the cystic duct, the operator mistaking it for the duct. This was promptly recognized and a satisfactory anastomosis apparently carried out. There was a period of ischemia, however, of approximately thirty minutes before blood flow was reinstituted. Of interest was the apparent arterial back bleeding. Postoperatively, liver function in the first week did not differ markedly from that noted prior to surgery. Gradual improvement over four weeks was noted, as would be expected with relief of jaundice.
CASE III
A seventy-nine year old jaundiced male was operated upon in June 1960, for chronic cholecystitis, cholelithiasis and choledocholithiasis. During removal of the gallbladder, the cystic artery was avulsed from its origin at the proper hepatic artery. Attempts at repair resulted in extensive tissue loss at the bifurcation necessitating proper ligation of the right and left hepatic artery. (Fig. IC.) The tissues of the gastrohepatic ligament were searched for collateral arteries passing to the liver. None were found. Choledochotomy was then performed and forty stones removed. A T-tube was placed in the common duct. Multiple drains were placed about the liver Intensive penicillin therapy was given for eight days. The T tube was removed on the fifteenth postoperative day, and he was discharged on the twenty-sixth day. He remains in good health thirty months after operation.
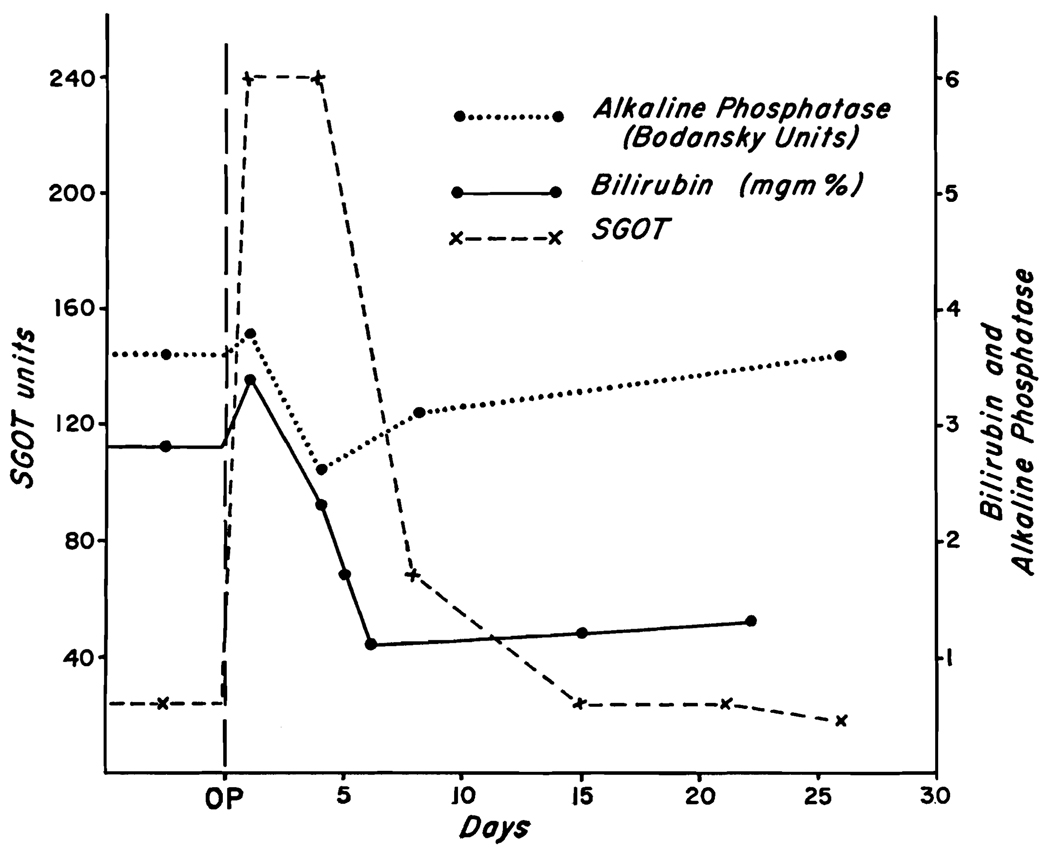
The most striking chemical alteration was a rise in SGOT to a maximum of 240 units. (Fig. 4.) This returned to normal in fifteen days. The pre-existing low grade jaundice was slightly increased at twenty-four hours, but was clearing by the fourth day. Alkaline phosphatase was unchanged (Fig. 4), as were the prothrombin time and serum proteins.
FIG. 4.
Biochemical changes in Case III after ligation of the proper hepatic artery. The rise in SGOT was prominent but had returned to normal in fifteen days.
Comment
This was the only injury to the proper hepatic artery in this series. After operation the characteristic transient rise in SGOT was observed. Despite this accident, the postoperative convalescence was quite uneventful.
CASE IV
A seventy year old man had a cholecystectomy in August 1961. There was no history of jaundice. At operation, cholelithiasis was found as well as a small carcinoma of the ampulla of the gallbladder. During cholecystectomy the right hepatic was irreparably injured. (Fig. ID.) The right lobe of the liver immediately became cyanotic (Fig. 2), with a sharp line of color demarcation in the gallbladder bed. The liver area was extensively drained. Three gm. per day of tetracycline were given intravenously for the first seventy-two hours, and 2 gm. per day orally for the ensuing seven days. Convalescence was uncomplicated except for atelectasis on the fourth postoperative day. He was discharged fourteen days after operation.
There was a transient rise in bilirubin (Fig. 5), chiefly in the unconjugated component. The alkaline phosphatase which was elevated to surgery did not change. (Fig. 5.) The SGOT, which had been 61 preoperatively, was 72 when first determined on the eighth postoperative day. Prothrombin time, cephalin flocculation, thymol turbidity and total proteins remained normal.
FIG. 5.
Response in Case IV to right hepatic artery ligation; transient bilirubinemia occurred.
Comment
The observations on this patient were similar to those of Case I. In both an alarming surgical accident was followed by prompt recovery with minimal biochemical evidence of hepatic parenchymal injury. In each case massive antibiotic therapy was given postoperatively.
CASE V
A jaundiced sixty-six year old man was operated upon in July 1961, with the diagnosis of cholecystitis and choledocholithiasis. Preoperative bilirubin was 2 to 7 mg. per cent, alkaline phosphatase 12 to 17 Bodansky units and SGOT 45. Cephalin flocculation, thymol turbidity, serum protein concentration and prothrombin time were all normal.
During operation a portion of the right hepatic artery was excised and the upper common duct was transected. (Fig. IE.) Repair of the artery was impossible. Biliary drainage was provided with a Roux-en-Y anastomosis to the jejunum. Postoperatively the patient became progressively more jaundiced, to a maximum bilirubin level of 14.4 mg. per cent. The first postoperative SGOT obtained four days after operation was 78 and frequent subsequent determinations were in the range of 70 to 140.
Despite large doses of tetracycline and penicillin, the patient had a febrile course, and death occurred on the nineteenth postoperative day. At autopsy the liver weighed 1,700 gm. Biliary cirrhosis was prominent. The right hepatic artery had been surgically excised. The right and left hepatic lobes were similar in appearance and necrosis had not occurred. The choledochojejunal anastomosis had disrupted with the formation of a subhepatic abscess. Bilateral lower lobe pneumonia was present.
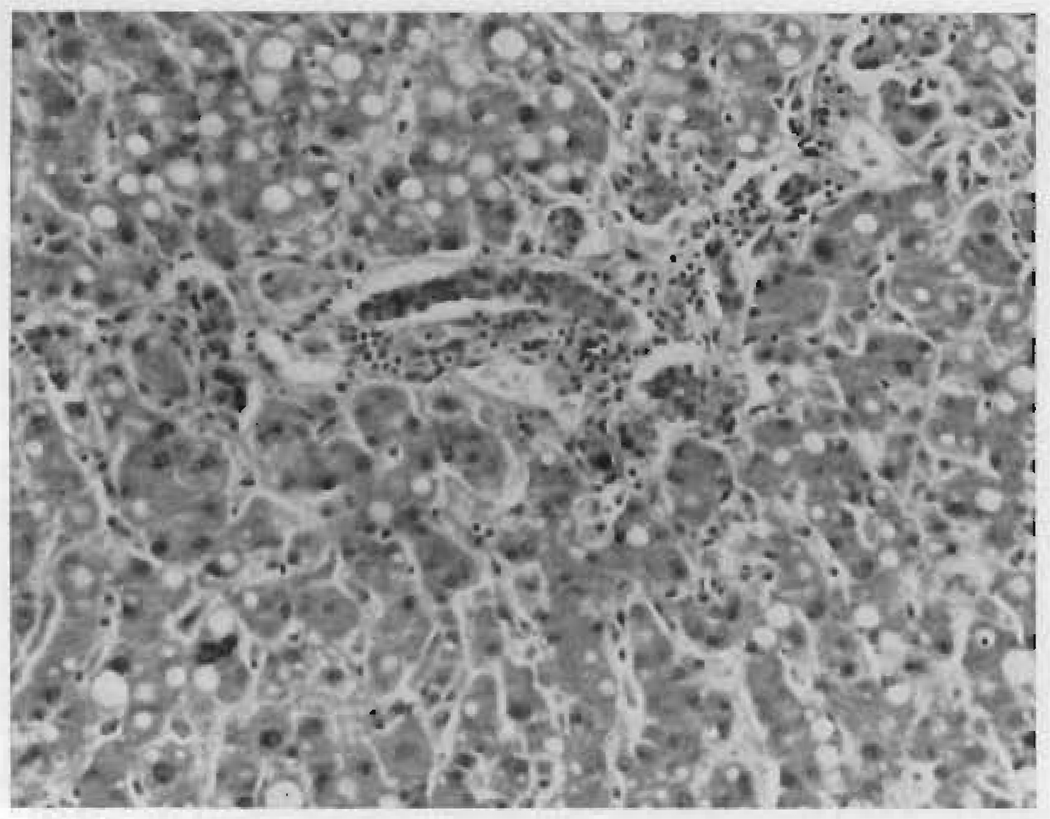
Histologic sections of the liver showed no difference between the two lobes. There was cholestasis, fatty metamorphosis and inflammatory reaction but no evidence of necrosis. (Fig. 6.)
FIG. 6.
Autopsy specimen of dearterialized right lobe of liver in Case v nineteen days after operation.
Comment
From an anatomic point of view, one would predict that damage to multiple structures in the portal triad would commonly involve the combination of right hepatic artery and common duct, as occurred in this patient. The indolent rise in SGOT suggested that hepatic necrosis had not occurred, and this was confirmed at autopsy. In similar reported cases ligation of the hepatic artery has often been indicted as the cause of death, especially in the older literature. The duct injury was undoubtedly responsible for the fatal outcome in this case, and the arterial ligation of secondary significance. In the past, consideration of similar cases as deaths due to ligation of the hepatic artery has created the impression that the arterial injury is a more grave complication than may be the case.
COMMENTS
The lethal consequences of accidental ligation of the hepatic artery or one of its principal branches has been emphasized to generations of surgeons. The exact incidence of this complication is unknown, although it is quite 1ikely that ligation of at least the right hepatic artery is not rare and at times may be unrecognized [2]. Much of the information in humans regarding the effect of ligation of the hepatic artery is drawn from experiences with patients with cirrhosis [3–5] or aneurysm of the hepatic artery [6,7]. The prior existence of vascular abnormalities may vitiate the value of these data when applied to patients with normal hepatic vasculature and function.
Prior to the twentieth century, experimental studies on ligation of the hepatic artery had little meaning because of inadequately documented technical description and lack of aseptic technic. In 1905 Haberer reported, in a well controlled study, that ligation of the hepatic artery distal to the right gastric branch was usually fatal in dogs [8]. This early work was confirmed and extended to other animals by Behrend [9,10] and Cameron [11].
Considerable work has been done on the exact mechanism of death following interruption of the hepatic arterial supply. In 1936 Loeffler [12], on the basis of his work, believed it was ultimately due to thrombosis of the portal vein occurring as a result of interrupting the vasa vasorum to this structure. The following year Huggins and Post [13] found that ligation of the hepatic artery in stages was well tolerated. They postulated that gradual reduction of arterial blood supply allowed the liver to adjust to the lowered oxygen tension. In addition, they suggested that overgrowth of anaerobic bacteria which had been previously demonstrated to be normally present in many species [14,15] was the primary cause of death in dugs. The latter concept has been substantiated by Markowitz [16,17], Tanturi [18], and others [19–22] who showed that almost all dogs would tolerate ligation or extirpation of the hepatic artery providing antibiotics were given.
However, there is reason to doubt that the concept of a lethal bacterial factor can be freely extrapolated to higher species. Child [23], using monkeys, found that almost all animals tolerated complete extirpation of the hepatic artery whether or not antibiotics were given. In man there is good evidence that bacteria do not normally reside in the liver tissue [24–26]. It is thus possible that any harmful effects of dearterialization of the human liver are due to ischemia alone.
After ligation of the hepatic artery the portal venous flow is of obvious critical importance in maintaining tissue viability. The contribution of arterial collaterals is less certain. In early work on ligation of the hepatic artery in dogs, Haberer [8] alluded to a correlation between the size of the phrenic arteries and survival following radical extirpation of the hepatic artery and its branches. Popper and his associates [27] have more recently supported the view that some arterial collateral flow is necessary for survival after complete removal of the hepatic artery. They confirmed Markowitz’ experiments showing uniform survival after ligation of the hepatic artery when antibiotics were used. If the phrenic arteries were also ligated, however, most of the animals died. By means of injection technics, they were able to demonstrate filling of the entire intrahepatic arterial tree through the phrenic arteries, one month or more after operation [27].
Others have been in sharp disagreement about the role of collateral arterial flow. Loeffler [12], working with rats and rabbits, obtained evidence that portal blood alone was sufficient to prevent hepatic necrosis. Tanturi [18] was unable to demonstrate arterial collateral channels in dogs which survived hepatic arterial ligation. Similarly, Child [23] was unable to obtain any physiologic or morphologic evidence that collateral arterial flow played a significant role in survival after excision of the hepatic artery in monkeys.
Recent studies of segmental hepatic anatomy in humans contribute to an understanding of the regional ischemia which may occur following interruption of one of the major hepatic arterial branches. Healey and Schroy [28], and Goldsmith and Woodburne [29] have shown that the liver is divided into four major segments, two for each lobe. The true lobar division is near the gallbladder fossa and each lobe is supplied solely by its corresponding branch of the main hepatic artery. The sharp line of color demarcation noted in two patients in the present series following ligation of the right hepatic artery (Fig. 2) is in keeping with this concept. It is also strong evidence in favor of Michels’ contention that all hepatic arteries of significant size are end arteries [30]. In addition, the sharply defined lobar cyanosis also supports the belief that the principal factor in maintaining tissue viability is the portal venous flow. However, Segall [31], using injection technics in human cadavers, described collaterals from the diaphragm, the upper abdominal wall, intercostal and lumbar vessels, adrenal and renal arteries, as well as numerous other potential anstomotic channels. Michels has classified these collateral pathways and has listed twenty-six possible means by which collateral blood flow could reach the dearterialized liver [30].
Whether or not the existence of arterial collaterals is of importance in maintaining viability, there are several inherent features of the portal flow which would appear to be important in providing adequate hepatic perfusion. First, the portal flow accounts for approximately two-thirds of the total hepatic blood flow in humans [32,33]. In addition, the oxygen content of this blood has been reported to be slightly higher than found in systemic venous blood [34]. Restrepo and Warren [35] have reported that the volume of portal venous flow increases with hepatic arterial ligation in dogs. These data have not been confirmed in humans [32]. Finally, the concept of hepatic parenchymal structure as described by Elias [36] has shown the interrelated nature of tissue perfusion within the liver by blood from the two different sources. The arterioles terminate at various levels within the sinusoidal walls, thereby achieving the same ultimate distribution as the venous system.
Despite the vast amount of information from experimental animals, it is difficult to obtain a clear-cut picture of the effects of hepatic arterial ligation as an isolated event in man. The impression is gained from the excellent review in 1933 by Graham and Cannell [1], that ligation of an hepatic artery leads to death in more than 50 per cent of the cases. In their collected series the proper hepatic artery had been interrupted in nine, the right hepatic artery in eight, the left hepatic artery in eight, and the common hepatic artery in three patients. However, a somewhat different impression emerges with re-examination of the original descriptions of the twenty-eight cases upon which their paper was based. In the first place, the operations were all performed at a time when mortality was heavily influenced by lack of adequate anesthesia, transfusions or antibiotics. Another salient feature was the heterogeneity of the cases. In only six patients was there a definite relationship between ligation of the artery and death of the patient from necrosis of the liver [37–42]. Nine patients survived without known sequelae [38,43–48] although transient jaundice developed in one [47]. In another six [1,41,49–52] liver injury was not the cause of death, although areas of hepatic necrosis were noted in four cases at autopsy [1,50–52]. An additional patient who apparently died of hemorrhage eight hours after ligation of the common hepatic artery was included, although autopsy was not performed [53]. In another case in which autopsy was not performed jaundice preceded death [10]. A final group of five patients was included in whom a lobar artery was ligated in the course of partial hepatectomy [8,42,46,54,55]. Four patients survived.
Similarly, there is little basis for comparison within the fifteen cases reported since 1933 (Table I) in which the ligation was not performed for the treatment of cirrhosis or aneurysm of the hepatic artery. In five patients the ligation was performed deliberately in a desperate effort to control hemorrhage from the liver which was due to hemobilia in three [56–58] and traumatic laceration in two cases [59,60], conditions which of themselves cause varying degrees of hepatic parenchymal damage. In another, the proper hepatic artery was transected in order to reduce the vascularity of a hepatic hemangioma [56]. In four others the proper hepatic or a lobar artery was sacrificed incidentally during a radical operation upon the stomach for cancer [56,61], gallbladder [62], or esophagus [63]. Another patient had ligation of the proper hepatic artery during repair of a common duct stricture [56]. Four patients [64,65] had an isolated injury to the hepatic arterial supply during cholecystectomy. There were six deaths in the fifteen cases reported since 1933 (Table I), and in five of these [57,63,64] hepatic infarction appeared to be the cause of death. It is of interest that the over-all mortality is heavily weighted by the report of Bianchi [64], in which three deaths occurred within forty-eight hours after ligation of the right hepatic artery. In two of the three cases hypotension which occurred during operation may have contributed to the fatal outcome. In addition, none of the three patients received antibiotics.
TABLE I.
Cases of Hepatic Artery Ligation Reported since 1833 in Patients without Cirrhosis or Hepatic Artery Aneurysm
| Name | Age (yr.) | Diagnosis | Operation | Vessel | Concomitant Injury |
Chemistries | Antibiotics | Outcome | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|
| Brunschwig, 1941 | 63 | Carcinoma, cystic duct | Cholecystectomy, excision common duct | PHA,* RHA,† LHA‡ | Common duct excised | Jaundice, depression of plasma proteins | None | Died, 22 days | Small infarcts of right lobe, intrahepatic cholestasis |
| Misnik, 1948 | 42 | Traumatic laceration, right lobe liver | Ligation proper hepatic artery | PHA | Liver laceration | Not presented | No mention | Lived | … |
| Edgemont, 1951 | 50 | Adenocarcinoma stomach | Radical subtotal gastrectomy | PHA | None | Transient elevation of bilirubin, alkaline phosphatase; prothrombin time, cephalin flocculation transiently deranged | Penicillin, chlortetracycline | Lived | … |
| Popper, 1953 | 43 | Cholecystitis | Cholecystectomy | CHA § and PHA | None | No jaundice | Penicillin and streptomycin | Lived | … |
| Bianchi, 1956 | 36 | Cholelithiasis | Cholecystectomy | RHA | None | Jaundice; prothrombin time, protein and cholesterol decreased | None | Died, 32 hr. | Necrosis, right lobe |
| Bianchi, 1956 | 41 | Cholelithiasis | Cholecystectomy | RHA | None | Jaundice; prothrombin time, protein and cholesterol decreased | None | Died, 41 hr. | Necrosis, right lobe |
| Bianchi, 1956 | 38 | Cholelithiasis | Cholecystectomy | RHA | None | Jaundice; prothrombin time, protein and cholesterol decreased | None | Died, 36 hr. | Necrosis, right lobe |
| Fireson, 1957 | 58 | Carcinoma middle esophagus | Palliative esophagectomy | Aberrant LHA | None | Not presented | Yes, type? | Died, 12 days | Infarction, left lobe |
| Spector, 1957 | 43 | Hemobilia | Ligation, right hepatic artery | RHA | None | Not presented | Penicillin, oxytetracycline | Lived | … |
| Breton, 1959 | 26 | Traumatic laceration right hepatic lobe | Ligation, right hepatic artery | RHA | Liver lacerations | Meaningless due to postoperative bile peritonitis | No mention | Lived | … |
| Guynn, 1961 | 63 | Hemobilia | Ligation, left hepatic artery | LHA | Ligation, left hepatic duct | Not presented | No mention | Died, 12 days | Septic necrosis, left lobe |
| Andreassen, 1962 | 64 | Hemobilia | Ligation porper hepatic artery | PHA | None | SGOT up for 12 days; proteins, bilirubin, alkaline phosphatase, thymol turbidity normal | Penicillin, streptomycin | Lived | … |
| Andreassen, 1962 | 8 (mo.) | Hemangioma of liver | Ligation, proper hepatic artery | PHA | None | SGOT up for 12 days; bilirubin up transiently | Penicillin | Lived | … |
| Andreassen, 1962 | 73 | Common duct stricture | Repair common duct stricture | PHA | None | Preexisting jaundice relieved | No mention | Lived | … |
| Andreassen, 1962 | 53 | Carcinoma stomach | Gastropancreatectomy | PHA, RHA, LHA | None | SGOT up 16 days; bilirubin and alkaline phosphatase up transiently | Penicillin | Lived | … |
| Authors, 1963 | 35 | Cholecystitis | Cholecystectomy | RHA | None | See text | Yes | Lived | … |
| Authors, 1963 | 74 | Cholecystitis and common duct stone | Cholecystectomy, choledochotomy | RHA | None | See text | Yes | Lived | … |
| Authors, 1963 | 79 | Cholecystitis and common duct stone | Cholecystectomy, choledochotomy | PHA | None | See text | Yes | Lived | … |
| Authors, 1963 | 70 | Cholecystitis, carcinoma gallbladder | Cholecystectomy | RHA | None | See text | Yes | Lived | … |
| Authors, 1963 | 66 | Cholecystitis | Cholecystectomy | RHA | Common duct | See text | Yes | Died | Subphrenic abscess; disrupted common duct repair |
PHA, distal to gastroduodenal artery.
RHA, right hepatic artery
LHA, left hepatic artery.
CHA, proximal to gastroduodenal artery.
All of the cases in the present study had accidental hepatic artery ligation during biliary tract surgery, and in four of the five patients the artery was the only structure injured. In these cases a consistent anatomic feature of the portal triad resulted in a highly characteristic arterial injury. The common duct divides at a more cephalic level than the artery (Fig. I), frequently within the hilum of the liver. The upper common duct is, therefore, constantly related to the right hepatic artery which originates more inferiorly. As a consequence, injuries to the right or left hepatic ducts are probably rare compared to common duct injury. Conversely, injury to the right hepatic artery would be expected to occur with much greater frequency than to the proper hepatic artery. Combined biliary and vascular injuries would be predicted to most frequently involve the right hepatic artery and the common duct, as in Case v.
The present study suggests that survival of dearterialized hepatic tissue usually occurs when either the proper hepatic or one of its lobar branches is interrupted, providing hypotension does not occur. The one patient who died in the present series after right hepatic artery ligation did not have parenchymal infarction. Death was caused by complications of the common bile duct reconstruction rather than by the vascular accident.
Considerable information on the acute physiologic reactions of hepatic artery occlusion in the human has recently been reported by Tygstrup and his associates [33]. These authors studied the splanchnic flow and metabolic alterations in eight patients who had temporary occlusion of the hepatic artery in the course of laparotomy for other purposes. They found that the arterial contribution to total hepatic flow was 35 per cent, and that 50 per cent of the oxygen consumption was derived from this source. With occlusion of the proper hepatic artery, extraction of oxygen from the portal venous blood increased, thereby compensating for the loss of the arterial supply. They also found that hepatic vein concentrations of SGOT, serum glutamicpyruvic acid transaminase (SGPT), and lactic acid dehydrogenase (LDH) were slightly increased. They concluded that, under ordinary circumstances, the hepatic artery could be ligated with impunity.
The foregoing data have made it clear that an effective compensatory mechanism of increased oxygen extraction from the portal venous blood is ordinarily sufficient to maintain hepatic viability. It suggested that many of the fatalities which have followed ligation of the hepatic artery are probably due to factors which either have further increased hepatic oxygen needs, or have reduced the volume of portal flow. Fever, atelectasis, cardiac failure or shock could all adversely effect the relationship between hepatic oxygen needs and an already reduced blood flow. Of all these possibilities, shock, which is known to reduce the volume of portal flow, would be the most likely to precipitate necrosis. It is significant that virtually all deaths from liver necrosis following hepatic artery ligation reported since 1933, have been in patients who have had extensive shocking procedures or who were critically ill before operation.
When accidental ligation of the hepatic artery is discovered at operation, the appropriate therapy is immediate reconstruction of the injured vessel when feasible. If repair is impossible, the liver should be adequately drained. Further treatment is essentially supportive. In view of the known physiologic events which follow hepatic artery occlusion, the critical features of therapy would appear to be strict avoidance of hypotension and the maintenance of adequate oxygenation. To achieve the latter, the administration of oxygen, or confinement to a high pressure oxygen chamber might be of value. Prophylactic measures to prevent atelectasis are indicated.
In the cases under study in this report antibiotics were given. The rationale for this therapy is not firmly established in man, inasmuch as the human liver is usually sterile, and the central problem is not bacteriologic, as is the case with some carnivores. Nevertheless, until proof is forthcoming concerning the inefficacy of antibiotics under these circumstances, this form of therapy should not be withheld.
SUMMARY
Despite the vast amount of information from experimental animals, it has been difficult to obtain a clear-cut picture of the effects of ligation of the hepatic artery in humans with relatively normal livers. The last complete review of this subject in 1933 indicated that a mortality in excess of 50 per cent could be expected in non-cirrhotic patients with injury of the hepatic artery or its principal branches.
Five cases of dearterialization of the normal human liver have been observed. These were due to accidental interruption of the right hepatic artery in four and the proper hepatic artery in one. The injured vessel was repaired in one case and ligated in the others. In four of the five patients the vascular disruption was the sole injury. In the other the common bile duct was also lacerated. There was no evidence of hepatic necrosis in any case although one patient died from complications of common duct repair. Transient changes in SGOT and temporary low grade bilirubinemia were commonly noted.
In addition, all cases of ligation of the hepatic artery reported since 1933 have been compiled. On the basis of reviewed, as well as the presently reported cases, it is concluded that ligation of the hepatic artery or one of its branches in the patient with relatively normal hepatic function is not ordinarily fatal in the otherwise uncomplicated case. Adeauate perfusion of the liver can usually be provided by the remaining portal venous flow and whatever arterial collaterals are present, unless additional factors further reduce the portal venous flow or increase hepatic oxygen need. These factors include fever, shock and anoxia. The key to therapy in unreconstructed injuries to the hepatic artery is avoidance of these secondary influences.
Acknowledgments
Aided by Grants A-6344, A-6283, HE-07735 and AM-07772 from the United States Public Health Service.
REFERENCES
- 1.Graham RR, Cannell D. Accidental ligation of the hepatic artery. Brit. J. Surg. 1933;20:566. [Google Scholar]
- 2.Woolling KR, Baggenstoss AH, Weir JF. Infarcts of the liver. Gastroenterology. 1951;17:479. [PubMed] [Google Scholar]
- 3.Berman JK, Fields DC. Advanced atrophic cirrhosis; present status of hepatic, splenic and left gastric arterial occlusion as aid in control of its complications. Arcb. Surg. 1954;68:432. [PubMed] [Google Scholar]
- 4.Mcfadzean AJS, Cook J. Ligation of the splenic and hepatic arteries in portal hypertension. Lancet. 1953;1:615. doi: 10.1016/s0140-6736(53)91748-6. [DOI] [PubMed] [Google Scholar]
- 5.Rienhoff WF, Jr, Woods AC. Ligation of hepatic and splenic arteries in treatment of cirrhosis with ascites. J. A. M. A. 1953;152:687. doi: 10.1001/jama.1953.03690080031009. [DOI] [PubMed] [Google Scholar]
- 6.Browning LD, Clauss RH, Macfee WF. Aneurysm of the hepatic artery: report of two cases. Ann. Surg. 1959;150:320. doi: 10.1097/00000658-195908000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansbrough ET, Lipin RJ. Hepatic artery aneurysm with excision of celiac axis. Ann. Surg. 1959;149:273. doi: 10.1097/00000658-195902000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Von Haberer H. Experimental ligation of the hepatic artery. Arcb. klin. Cbir. 1905;78:557. [Google Scholar]
- 9.Behrend M. Experimental ligation of the hepatic artery. Surg. Gynec. & Obst. 1920;31:182. [Google Scholar]
- 10.Behrend M, Radasch HE, Kershner AG. Comparative results of the ligation of the hepatic artery in animals; its application to man. Arcb. Surg. 1922;4:661. [Google Scholar]
- 11.Cameron GR, Mayes BT. Ligation of the hepatic artery. J. Patb. & Bact. 1930;33:799. [Google Scholar]
- 12.Loeffler L. Factors determining necrosis or survival of liver tissue after ligation of hepatic artery. Arcb. Patb. 1936;21:496. [Google Scholar]
- 13.Huggins C, Post J. Experimental subtotal ligation of the arteries supplying the liver. Arcb. Surg. 1937;35:878. [Google Scholar]
- 14.Ford WW. Bacteriology of healthy organs. Tr. A. Am. Pbysicians. 1900;15:389. [Google Scholar]
- 15.Wolbach SB, Saiki T. A new anaerobic spore bearing bacterium commonly present in livers of healthy dogs and believed to be responsible for many changes attributed to aseptic autolysis of liver tissue. J. M. Researcb. 1909;21:267. [PMC free article] [PubMed] [Google Scholar]
- 16.Markowitz J, Rappaport AM. The hepatic artery. Physiol. Rev. 1951;31:188. doi: 10.1152/physrev.1951.31.2.188. [DOI] [PubMed] [Google Scholar]
- 17.Markowitz J, Rappaport AM, Scott AC. Prevention of liver necrosis following ligation of hepatic artery. Proc. Soc. Exper. Biol. & Med. 1949;70:305. doi: 10.3181/00379727-70-16907. [DOI] [PubMed] [Google Scholar]
- 18.Tanturi C, Swigart LL, Canepa JF. Prevention of death from experimental ligation of the liver (hepatic proper) branches of the hepatic artery. Surg. Gynec. & Obst. 1950;91:680. [PubMed] [Google Scholar]
- 19.Chaw AYS, Goldbloom VO, Gurd FN. Clostridial infection as cause of death after ligation of hepatic artery. Arcb. Surg. 1951;63:390. doi: 10.1001/archsurg.1951.01250040396014. [DOI] [PubMed] [Google Scholar]
- 20.Fahmy AR, Talaat M, Zachary MA. Serum glutamic oxaloacetic transaminase after hepatic artery ligature. Experientia. 1960;16:375. doi: 10.1007/BF02157915. [DOI] [PubMed] [Google Scholar]
- 21.Horvath SM, Farrand EA, Larsen R. Effect of hepatic arterial ligation on hepatic blood flow and related metabolic function. Arcb. Surg. 1957;74:565. doi: 10.1001/archsurg.1957.01280100083014. [DOI] [PubMed] [Google Scholar]
- 22.Popper HL, Jefferson NC, Necheles H. Liver necrosis following complete interruption of hepatic artery and partial ligation of the portal vein. Am. J. Surg. 1953;86:309. doi: 10.1016/0002-9610(53)90408-5. [DOI] [PubMed] [Google Scholar]
- 23.Child CG, III, Hayes DM, Mcclure RD., Jr Studies of the hepatic circulation in the Macaco mulatta monkey and in man. S. Forum. 1952;2:140. [PubMed] [Google Scholar]
- 24.From P, Alli JH. Bacteriologic study of human liver in l00 cases. Gastroenterology. 1956;31:33. [PubMed] [Google Scholar]
- 25.Lewis FJ, Wangensteen OH. Penicillin in treatment of peritonitis due to liver autolysis in dogs. Proc. Soc. Exper. Biol. & Med. 1950;73:533. doi: 10.3181/00379727-73-17734. [DOI] [PubMed] [Google Scholar]
- 26.Shann H, Fradkin WZ. Liver sequestration after cholecystectomy, with review of experimental and clinical observations. J. A. M. A. 1933;101:829. [Google Scholar]
- 27.Jefferson NC, Hassan MI, Popper HL, Necheles H. Formation of effective collateral circulation following excision of hepatic artery. Am. J. Physiol. 1956;184:589. doi: 10.1152/ajplegacy.1956.184.3.589. [DOI] [PubMed] [Google Scholar]
- 28.Healey JE, Schroy PC. Anatomy of biliary ducts within human liver; analysis of prevailing pattern of branchings and major variations of biliary ducts. Arcb. Surg. 1953;66 doi: 10.1001/archsurg.1953.01260030616008. [DOI] [PubMed] [Google Scholar]
- 29.Goldsmith NA, Woodburne RT. The surgical anatomy pertaining to liver resection. Surg. Gynec. & Obst. 1957;105:310. [PubMed] [Google Scholar]
- 30.Michels NA. Newer anatomy of liver-variant blood supply and collateral circulation. J. A. M. A. 1960;172:125. doi: 10.1016/0002-9610(66)90201-7. [DOI] [PubMed] [Google Scholar]
- 31.Segall HN. An experimental anatomical investigation of the blood and bile channels of the liver. Surg. Gynec. & Obst. 1923;37:152. [Google Scholar]
- 32.Schenk WG, Jr, Mcdonald JC, McDonald K, Drapanes T. Direct measurement of hepatic blood flow in surgical patients with related observations on hepatic flow dynamics in experimental animals. Ann. Surg. 1962;156:463. doi: 10.1097/00000658-196209000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tygstrup N, Winkler K, Mellemgaard K, Andreassen M. Determination of the hepatic arterial blood flow and oxygen supply in man by clamping the hepatic artery during surgery. J. Clin. Invest. 1962;41:447. doi: 10.1172/JCI104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradley SE, Smythe CM, Fitzpatrick HF, Blakemore AH. The effect of a portacaval shunt on estimated hepatic blood flow and oxygen uptake in cirrhosis. J. Clin. Invest. 1953;32:526. doi: 10.1172/JCI102768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Restrepo JE, Warren WD. Total liver blood flow after portacaval shunts, hepatic artery ligation and 70 per cent hepatectomy. Ann. Surg. 1962;156:719. doi: 10.1097/00000658-196211000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elias H. A re-examination of the structure of the mammalian liver; parenchymal architecture. Am. J. Anat. 1949;84:311. doi: 10.1002/aja.1000840206. [DOI] [PubMed] [Google Scholar]
- 37.Grube, Herrenschmidt Quoted by Ritter [38] [Google Scholar]
- 38.Ritter A. Ligation of hepatic artery. Mitt. Grenz. d. Med. u. Cbir. 1922;35:76. [Google Scholar]
- 39.Sprengel Quoted by Ritter [38] [Google Scholar]
- 40.Tichau Quoted by Ritter [38] [Google Scholar]
- 41.Tuffier T. Aneurysms of the hepatic artery. Presse méd. 1909;18:153. [Google Scholar]
- 42.Wendel W. Resection of the liver. Arcb. klin. Cbir. 1920;114:982. [Google Scholar]
- 43.Alessandri R. Aneurysm of the hepatic artery; consideration of surgery. Bull. Accad. Med di Roma. 1906;76:63. [Google Scholar]
- 44.Bakes FL. Discussion and remarks. Verbandl. deutscb. Gesellscb. Cbir. 1904;33:82. [Google Scholar]
- 45.Bertrand Quoted by Ritter [38] [Google Scholar]
- 46.Hofmeister F. Ligation of the hepatic artery without injury to the liver. Zentralb. Cbir. 1922;49:154. [Google Scholar]
- 47.Kehr H. Ligation of the hepatic artery proper for aneurysms. Miincben. med. Wcbnscbr. 1903;43:1861. [Google Scholar]
- 48.Smith RE. Ligature of the hepatic artery. Brit. J. Surg. 1921;8:532. [Google Scholar]
- 49.Eiselberg AF. Gastrectomy and gastroenterostomy. Arcb. klin. Cbir. 1885;39:785. [Google Scholar]
- 50.Holst SF. Ligation of the hepatic artery. Norsk mag. laegevidensk. 1920;81:1182. [Google Scholar]
- 51.Kausch Quoted by Ritter [38] [Google Scholar]
- 52.Wilms Quoted by Ritter [38] [Google Scholar]
- 53.Socin Quoted by Ritter [38] [Google Scholar]
- 54.Klose Quoted by Ritter [38] [Google Scholar]
- 55.Palacia-Ranam Quoted by Ritter [38] [Google Scholar]
- 56.Andreassen M, Lindenberg J, Winkler K. Peripheral ligation of the hepatic artery during surgery in noncirrhotic patients. Gut. 1962;3:167. doi: 10.1136/gut.3.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guynn VL, Reynolds JT. Surgical management of hemobilia. Arcb. Surg. 1961;83:73. doi: 10.1001/archsurg.1961.01300130077009. [DOI] [PubMed] [Google Scholar]
- 58.Spector N. Ligation of the right hepatic artery in hemobilia—report of a case with recovery. Ann. Surg. 1957;145:244. doi: 10.1097/00000658-195702000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Breton GJ, Gordon DF, Cookston TS, Nicholson DP. Liver injury involving right hepatic artery ligation, complicated by survival. Canad. M. A. J. 1959;81:836. [PMC free article] [PubMed] [Google Scholar]
- 60.Misnik IL. Case of emergency ligature of the right hepatic artery in the rupture of the liver. Kbirurgüja. 1948;9:171. [PubMed] [Google Scholar]
- 61.Edgecomb PW, Gardner C. A case of accidental ligation of the hepatic artery. Canad. M. A. J. 1951;64:518. [PMC free article] [PubMed] [Google Scholar]
- 62.Brunschwig A, Clark DE. Carcinoma of cystic duct, report of case and comments on ligation of hepatic artery in man. Arcb. Surg. 1941;42:1094. [Google Scholar]
- 63.Frieson SR. The significance of the anomalous origin of the left hepatic artery from the left gastric artery in operations upon the stomach and esophagus. Am. Surgeon. 1957;23:1103. [PubMed] [Google Scholar]
- 64.Bianchi RG. Hepatic necrosis caused by accidental ligation of the right branch of the hepatic artery. Prensa méd. argent. 1956;43:3202. [PubMed] [Google Scholar]
- 65.Popper HL. Ligation of accidentally torn hepatic artery. Am. J. Surg. 1953;85:113. doi: 10.1016/0002-9610(53)90408-5. [DOI] [PubMed] [Google Scholar]