Abstract
OBJECTIVE:
To measure the impact of implementing an oral rehydration clinical pathway for children with mild to moderate dehydration from gastroenteritis in the paediatric emergency department (ED) on the indicators of health care utilization.
METHODS:
ED charts of children, six months to 17 years of age, meeting the criteria for the oral rehydration clinical pathway were reviewed. There were three 12-month periods of data collection: pre-implementation, transition and postimplementation. The clinical pathway consisted of a standard nursing assessment form and instructions on oral rehydration to be initiated and maintained by caregivers while waiting to see a physician. The primary outcome measure was ED length of visit (LOV) for children treated using the clinical pathway. This was compared with LOV for all other ED visits during the study periods to highlight the effect of the clinical pathway implementation. Secondary outcome measures included rate of intravenous rehydration, unscheduled return visits to the ED and hospital admission.
RESULTS:
During the three data collection periods, 11,816 children met the eligibility criteria. A decrease in the mean LOV of 24 min (95% CI 17 to 31) was observed, as well as a trivial decrease in the rate of intravenous rehydration therapy (14.6% to 12%) with implementation of the clinical pathway.
CONCLUSION:
The implementation of an oral rehydration clinical pathway in the ED led to a modest reduction in the ED LOV.
Keywords: Dehydration, Diarrhea, Gastroenteritis, Oral rehydration therapy, Paediatric emergency, Vomiting
Abstract
OBJECTIF :
Mesurer les répercussions de l’adoption d’une voie clinique de réhydratation orale au département d’urgence pédiatrique (DU) pour les enfants ayant une déshydratation légère à modérée causée par une gastroentérite sur les indicateurs d’utilisation des soins de santé.
MÉTHODOLOGIE :
Les chercheurs ont examiné les dossiers de DU d’enfants de six mois à 17 ans qui respectaient les critères de la voie clinique de réhydratation orale. Ils ont colligé des données pendant trois périodes de 12 mois : avant l’intervention, pendant la transition et après l’adoption. La voie clinique se composait d’un formulaire d’évaluation classique en soins infirmiers et de directives aux personnes qui s’occupent de l’enfant d’entreprendre et de maintenir la réhydratation orale en attendant de voir un médecin. La première mesure d’issue était la durée de la visite (DDV) au département d’urgence (DU) des enfants traités à l’aide de la voie clinique. Cette mesure se comparait à la DDV de toutes les autres visites au DU pendant les périodes d’étude, afin de faire ressortir l’effet de l’adoption de la voie clinique. Les mesures d’issue secondaires incluaient la réhydratation intraveineuse, de nouvelles visites non planifiées au DU et une hospitalisation.
RÉSULTATS :
Pendant les trois périodes de collecte de données, 11 816 enfants ont respecté les critères d’admissibilité. Les chercheurs ont observé une diminution de la DDV moyenne de 24 minutes (95 % IC 17 à 31), de même qu’une réduction non significative du taux de thérapie de réhydratation par voie intraveineuse (14,6 % à 12 %) après l’adoption de la voie clinique.
CONCLUSION :
L’adoption d’une voie clinique de réhydratation orale au DU a suscité une modeste réduction de la DDV au DU.
Acute gastroenteritis is one of the most common illnesses affecting infants and children. In developed countries, the average child younger than five years of age experiences 2.2 episodes of diarrhea per year, whereas children attending daycare centres may have even higher rates (1). These episodes result in a large number of visits to paediatric offices and emergency departments (EDs). In the United States (US), the treatment for dehydration as a result of acute gastroenteritis accounts for an estimated 200,000 hospitalizations and 300 deaths per year, with comparable rates occurring in Canada. Annually, costs of medical and nonmedical factors related to gastroenteritis in the US are US $600 million to US $1.0 billion (2).
The use of oral rehydration therapy (ORT) for dehydration resulting from acute gastroenteritis has been well established. ORT is an inexpensive, safe and highly effective means of rehydration, and has successfully helped reduce the number of deaths due to diarrheal diseases worldwide (3). Despite guideline recommendations from both the WHO and the American Academy of Pediatrics (4), ORT continues to be underused by paediatricians and paediatric emergency physicians (5,6). A 2002 survey (7) of ED physicians in the US revealed that although 70.5% used ORT for mild dehydration, only 15.3% used ORT for moderate dehydration.
There are many barriers to the use of ORT including unfamiliarity with ORT techniques, routine practice of intravenous (IV) rehydration and a belief that ORT is more time consuming than IV rehydration (8). Prospective, randomized controlled trials, however, showed shorter times spent in the ED for children receiving ORT compared with those receiving IV rehydration. In addition to reduced ED stay, children with moderate dehydration receiving ORT also required shorter nursing staff time compared with those receiving IV rehydration (9,10).
The objective of the present study was to determine the impact of implementing an ORT clinical pathway for children with mild to moderate dehydration on length of visit (LOV), admission rate and revisits to a tertiary care paediatric ED. We hypothesized that implementation of this clinical pathway would result in a reduction in the ED LOV of at least 30 min. Secondary objectives included rate of IV use, admission and ED revisits.
PATIENTS AND METHODS
Study design and setting
The present study was conducted in the ED at the BC Children’s Hospital (Vancouver, British Columbia) – a tertiary care paediatric referral centre that provides care for more than 40,000 children annually. The study protocol was approved by the Children’s & Women’s Health Centre (Vancouver, British Columbia) Research Review Committee as well as by the University of British Columbia (Vancouver, British Columbia) Clinical Research Ethics Board.
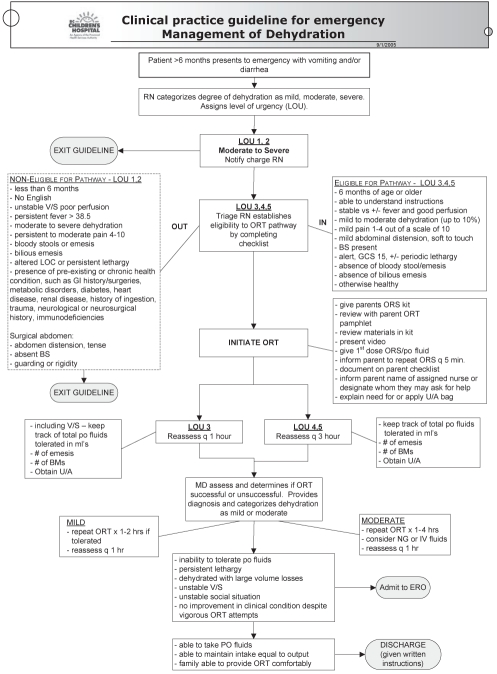
In December 2005, a clinical pathway for children with mild to moderate dehydration due to gastroenteritis symptoms was implemented in the ED of the BC Children’s Hospital. The clinical pathway was created and approved by the ED staff (nurse educator and physicians) following an extensive review of the literature. It promotes standardized assessment, emphasizes the early and routine use of ORT in the waiting room, and encourages parental education and participation in delivering ORT. Triage and bedside nurses were trained to assess children presenting to the ED with gastroenteritis-related dehydration, and were provided with a checklist of inclusion and exclusion criteria that determined a child’s eligibility to complete the clinical pathway (Figure 1). The main goals were to use time efficiently and to begin correcting dehydration within 1 h of presentation to the ED. Parents and/or caregivers of eligible children were then provided with teaching and guidelines for ORT administration. This included teaching with an ORT kit, which contained pamphlets, oral rehydration solution and required the parents to watch a video on ORT. The parents were also informed to repeat the administration of oral rehydration solution every 5 min.
Figure 1).
Clinical pathway for emergency department management of mild and moderate dehydration. BMs Bowel movements; BS Bowel sounds; ERO Emergency room observation; GCS Glasgow Coma Score; GI Gastrointestinal; IV Intravenous; LOC Level of consciousness; MD Physician; NG Nasogastric; ORS Oral rehydration solution; ORT Oral rehydration therapy; po Oral; q Every; RN Registered nurse; U/A Urine analyses; V/S Vital signs; vs Versus
A retrospective ED chart review was conducted to compare outcomes for patients presenting with gastroenteritis symptoms without severe dehydration over a four-year period surrounding the development and implementation of the ORT clinical pathway. This resulted in three annual data sets: one year before (January to December 2004), one year during (January to December 2006) and one year after stable implementation (January to December 2007) of the ORT clinical pathway. Outcomes of interest were LOV, rate of IV rehydration, rate of return visits to the ED for the same illness and hospital admission rates.
There were no other notable system changes in the ED during the three-year period (physical plant, information systems, registration or triage processes, staffing, point of care testing, etc) that would have impacted LOV.
Study population
Patients were included in the study as ORT candidates if they were six months to 17 years of age, and presented to the ED with either vomiting and/or diarrhea for fewer than seven consecutive days during the three data collection periods. Patients younger than six months of age were excluded from the study because the nurse-initiated intervention, as directed by the clinical pathway, only applied to patients six months of age and older. This limitation was due to the concern that younger infants are at a higher risk of presenting with vomiting from causes other than gastroenteritis and, hence, are required to be seen by a physician before the start of any intervention. Patients were excluded if they presented with severe dehydration (unstable vital signs and poor perfusion), an altered level of consciousness (Glasgow Coma Score lower than 15 or persistent lethargy or acute head injury), possible surgical abdomen (bloody or bilious vomiting, bloody diarrhea, abdominal distension and tense, absent bowel sounds, guarding or rigidity, and right lower quadrant pain) or chronic health conditions (such as gastric or jejunal feeding tube dependence, known inflammatory bowel disease, known immunodeficiency syndrome, known metabolic disorders, insulin-dependent diabetes, heart or renal disorder, and neurosurgical history).
Data collection and processing
During each of the three study periods, a pair of chart abstractors was responsible for independently collecting data for ORT eligibility from emergency physician history and physical examination notes. In total, there were six different chart abstractors: two summer students per annual dataset. Each chart was reviewed in detail to confirm inclusion/exclusion based on the criteria mentioned. Patients’ age, date and time of registration at the ED, time of physician assessment, time of discharge from the ED and information regarding interventions during ED visits (ORT, IV rehydration and admission to ward) were collected. ED revisit data were gathered by searching the database for the patient name and unit number within the two weeks of initial presentation to the ED.
To improve accuracy and minimize inconsistencies in collecting data from ORT-eligible patients, all chart abstractors were trained by the primary investigator (QD). The research team for each data collection period communicated frequently during the study to resolve disputes and maintain consistency in ORT candidate selection and exclusion. All chart abstractor pairs performed a blinded review of random overlapping two-week periods of charts to determine interextractor agreement (regarding which subjects should be included as ORT candidates). This was assessed using Cohen’s Kappa statistic. The abstractors could not be blinded to the year of the database because the date of the ED visit was collected by the same abstractor.
Although pre- and postimplementation data would seem sufficient, it was postulated that a new clinical pathway in the ED would take six to 12 months to be fully and consistently established. Hence, by collecting data during the transition year (2006), it was possible to monitor chronological changes in outcome measures during the process of implementing the ORT guidelines. This would establish temporality and strengthen the association between the implementation of the ORT clinical pathway and the observed outcome.
Furthermore, to explore the potential for confounding by the period effect (variables that may differ in the pre- to postimplementation periods and affect the ED’s efficiency other than the implementation of the ORT clinical pathway), data were also collected for the LOV for all other patients treated in the ED (all patients not included in the ORT sample) during each of the study periods. Therefore, the primary outcome measure was ED LOV for children affected by the clinical pathway. This was contrasted with changes in the LOV for all other ED visits during the study periods to highlight the effect of the clinical pathway implementation. Secondary outcome measures included IV rehydration, unscheduled return visits to the ED and hospital admission.
Data analysis
Twelve-month study blocks were used to control for seasonal variations in viral infections within one year, which may be a confounder. This approach resulted in surpassing the sample size required to detect a 30 min reduction in the LOV between the pre- and the postimplementation periods with 90% power and significance level of 0.05, which was slightly less than 900 patients in each group.
Descriptive statistics were used to represent the progression in the outcome measures surrounding the implementation of the ORT clinical pathways for ORT candidates. The changes in the LOV of all other ED visits were assessed, excluding those included in the study sample, during those three years. The LOV was defined as time from registration to discharge from the ED. Mean LOVs were compared between the pre- and postimplementation periods using the Student’s t test because this comparison illustrates the impact of implementing the clinical pathway. An ANOVA was also conducted to test the differences in the LOVs among the three annual data sets (pre-implementation, transition and postimplementation).
Revisits were defined as a subsequent visit with a diagnosis of any gastrointestinal symptom within two weeks of one another.
RESULTS
A total of 11,816 children meeting the eligibility criteria were identified during the three study periods (preimplementation, 5057; transition, 3322 and postimplementation, 3437). The proportion of subjects with missing LOV data was 4%. Agreement on case inclusion between each data extractor pair was substantial, with Cohen’s Kappa coefficients of 0.8, 0.8 and 0.62 for the preimplementation, transition and postimplementation annual database, respectively. Baseline characteristics per study period are presented in Table 1. No significant differences were identified.
TABLE 1.
Patient demographics per study periods
| Preimplementation (2004) | Transition period (2006) | Postimplementation (2007) | |
|---|---|---|---|
| ORT candidates, n | 5057 | 3322 | 3437 |
| Sex, female to male ratio, n:n (female %) | 2308:2749 (46) | 1485:1837 (45) | 1937:1495 (56) |
| Mean age, years (range) | 3.97 (0.5–16) | 3.38 (0.5–16) | 3.65 (0.5–16) |
| All other emergency department visits, n | 37,132 | 34,704 | 35,389 |
ORT Oral rehydration therapy
A significant decrease was found in the average LOV for ORT clinical pathway candidates with initial implementation of the clinical pathway. No further changes were observed after full implementation was complete (the difference in the mean LOV preimplementation versus transition period was 24 min; 95% CI 17 min to 32 min). To address concerns regarding period effects and to control for such, the change in the mean LOV was also examined for all other patients treated in the ED during the study period. There were no significant differences between the ED LOV for all other ED visits during the three study years. The mean LOV through the study periods for the study sample and all other ED visits are presented and compared in Table 2.
TABLE 2.
Comparison of outcome measures before (2004), during (2006) and after (2007) implementing the oral rehydration therapy clinical pathway
| Preimplementation (95% CI) | Transition period (95% CI) | Postimplementation (95% CI) | Statistical three-group comparisons | Precomparison versus postcomparison | |
|---|---|---|---|---|---|
| Intravenous rate | 14.6% (13.6–15.6) | 13.3% (12.2–14.5) | 12% (10.9–13.1) | 0.0027* | 0.0006* |
| Mean LOV, min | 209 (204–214) | 185 (180–190) | 185 (180–190) | <0.0001† | <0.0001‡ |
| Admission rate | 0.95% | 0.54% | 0.76% | 0.081* | 0.35* |
| Revisit rate | 6.8% | 6.1% | 7.4% | 0.055* | 0.30* |
| All other ED visit mean | |||||
| LOV, min | 231 (201–261) | 278 (226–330) | 239 (208–269) | 0.193† | 0.71‡ |
ED Emergency department; LOV Length of visit.
χ2;
ANOVA;
Two-tailed t test
The rate of IV rehydration therapy changed from 14.6% (95% CI 13.6% to 15.6%) during the preimplementation period to 13.3% (95% CI 12.2% to 14.5%) during the transition period to 12% (95% CI 10.9% to 13.1%) after the clinical pathway was well established.
Hospital admission rates and unscheduled ED return visits for children with gastroenteritis without severe dehydration remained low throughout the study periods and did not vary significantly (Table 2).
DISCUSSION
ORT is a proven and effective means to safely and quickly rehydrate children with gastroenteritis. When compared with IV rehydration, ORT is painless, more physiological and less time consuming (11). Randomized controlled and efficacy trials (9,12) as well as a Cochrane meta-analysis (13) have found that the use of ORT resulted in comparable clinical outcomes to when IV rehydration was used, but significantly decreased the length of stay. Children who received ORT also had lower rates of hospital admissions compared with children who received IV therapy (10). Despite these benefits, ORT is underused in the ED (7,8).
We evaluated the effectiveness of implementing an ORT clinical pathway in the ED, and found a moderate decrease in the mean LOV and a clinically negligible reduction in IV use following the implementation of an ORT clinical pathway.
We believe that the reduction in the LOV, despite not affecting the rate of IV use, is due to using ORT early in the ED visit. The implementation of this clinical pathway allows nurses and parents to administer ORT while in the waiting room, before physician assessment. This not only addresses delays in what would be the treatment ordered by the physician after a 2 h to 4 h wait period, but also prevents the progression of dehydration.
It is possible that a moderate proportion of our subjects were only believed to be dehydrated by their caregivers, but not by the physicians’ clinical assessment. The clinical pathway was designed to be very inclusive (children with just one episode of vomiting or diarrhea were included) because the intervention is not invasive and is initiated by nurses and caregivers. Furthermore, creators of this clinical pathway believed that the ORT education and experience would be valuable to the families, considering that the care-givers were compelled to visit the ED regardless of the child’s actual severity of illness. If the proportion of non-dehydrated subjects were substantial, it would introduce a conservative bias because the LOV for children without dehydration would not be reduced by the implementation of the ORT pathway. We have no indication whether this proportion would be systematically different among the three study periods. It is, therefore, possible that the true effect size is larger than what we observed.
We found a statistically significant, but clinically trivial, reduction in the rate of IV use over the three study periods, which is a result of our very large sample size (Table 2). Although the reduction was not clinically meaningful, the trend is encouraging.
A pilot study by Boyd (14) found a substantially larger reduction in the ED LOV (5 h and 37 min; P=0.017) and hospital admission rates (22.5% to 5.1% [P=0.048]. The ORT clinical pathway was similar to ours, with ORT initiated by ED nurses before physician assessment. The main differences between our study and the 2005 pilot was that we evaluated the effectiveness of an ORT clinical pathway over a period of three years, and we only provided education to the ED staff about the pathway followed by its institution without the presence of a research nurse monitoring its uptake. Boyd’s pilot study was conducted over a period of two months following education and implementation of an ORT protocol. Evaluation of the ED staff regarding the effect of a recent educational intervention for a relatively short period may be susceptible to Hawthorne effects (15).
Our study’s strengths lie in our large study population (n=11,816) accumulated over the three-year study period, allowing for adequate implementation and stabilization of the ORT clinical pathway. To our knowledge, the present study is the only one with a large sample size that evaluates the effect of implementing an ORT clinical pathway in a paediatric ED. We also addressed period effects inherent to the pre-and poststudy designs spanning over such a long study period. If any departmental or population change (above and beyond the implementation of the ORT clinical pathway) occurred during the time of the study, potentially affecting our primary objective, these would be reflected in a change in the LOV for all ED patients during that period. Comparing the pre- to postmean LOV for all other ED visits, there was an increase in the LOV, which was not statistically significant. Over this same period, the mean difference in LOV for children with gastroenteritis without severe dehydration from pre- to postimplementation was 24 min (95% CI 17 to 31). In light of a decreased LOV for children affected by the ORT clinical pathway, while LOV for all other ED visits increased during the same period, it is likely that the change in our gastroenteritis treatment was responsible for this reduction in LOV.
The main limitations of the present study were due to its retrospective nature. We were dependent on treating physicians completing the patient charts; however, there were instances in which some data were not retrievable from the charts. These concerns – mainly the documentation of the time of physician assessment – primarily affected the wait time, but rarely the overall LOV because the time of discharge was often charted by the nurses and not by the physicians. Having gathered such a large sample size, we believe that the missing data were unlikely to alter the findings.
In addition, information regarding therapy (rehydration attempts) before the ED visit, which could affect success of ORT in the ED, was not captured. It is, however, difficult to predict the validity of parental reports of fluid (quantities) ingested at home. Furthermore, the effect of previous attempts on ORT trial in the ED has not been established.
Another limitation we encountered was the fact that data abstractors were not blind to the research question. When we conducted an interobserver agreement assessment with regard to subject selection on a random sample, abstractor reliability for all outcome measures was found to correlate perfectly. Although the Kappa score was imperfect, the discordance was not based on outcome measure entry but rather related to inclusions of subjects into the database.
Because this was an effectiveness study and not a controlled trial, the nursing staff was trained to implement the ORT clinical pathway at triage; however, we could not ensure that the clinical pathway was strictly adhered to. Sporadic monitoring of ORT initiated by the nursing staff before ED physician assessment was performed in the third year of our study to examine uptake one year after the pathway was introduced. We found that 60% to 80% of children who were candidates for ORT as per our pathway had chart documentation of having received ORT. Incomplete implementation of the ORT clinical pathway may have resulted in an underestimation of its potential impact.
In addition to the limitations associated with the study design, we did not fully address the use of ondansetron in our study. Use of antiemetic agents was not part of the ORT clinical pathway due to its nature: nursing-initiated intervention before physician assessment. It is possible that the recent availability of ondansetron will interact with an ORT clinical pathway in the future, but is unlikely to have played a major role in our study because the use of ondansetron was very rare. Review of the pharmacy supply records for ondansetron for our ED showed that ondansetron was only stocked in our ED since 2006, and its use in the study population was scarce. In 2006, only 1% of our subjects received ondansetron, and in 2007, only 3.5% received ondansetron.
Similarly, the impact of rotavirus vaccination on ED visit rate is likely trivial because the vaccine did not become available in our province until late in 2006, and is not universally covered (cost to patients is $200 to $250). Although not published, the consensus from discussions with infectious diseases specialists at the BC Children’s Hospital and at the Vaccine Evaluation Center in Vancouver is that there has been almost no uptake for this new vaccine in British Columbia. Data regading rotavirus vaccination in our study population were not available.
CONCLUSION
The implementation of an ORT clinical pathway in the ED led to a modest reduction in the ED LOV for children with gastroenteritis symptoms without severe dehydration. Implementing protocols for safe interventions before physician assessment allows for standardized treatments for common childhood problems to begin earlier, thus potentially decreasing the LOVs and health care resources required. Adoption of an ORT clinical pathway can be a time-saving strategy and should be strongly considered with the caveat that proper implementation is ensured and monitored. Since the conclusion of the present study, the ORT clinical pathway has been well integrated into our ED system, and we will soon engage in further evaluation of these efforts.
Acknowledgments
The authors thank the Provincial Health Services Authority decision services office for their contribution, and the BC Children’s Hospital pharmacy department in providing necessary data for secondary analyses.
REFERENCES
- 1.Canadian Paediatric Society, Nutrition Committee Oral rehydration therapy and early refeeding in the management of childhood gastroenteritis. Can J Paediat. 1994;1:160–4. [Google Scholar]
- 2.Avendano P, Matson DO, Long J, Whitney S, Matson CC, Pickering LK. Costs associated with office visits for diarrhea in infants and toddlers. Pediatr Infect Dis J. 1993;12:897–902. doi: 10.1097/00006454-199311000-00001. [DOI] [PubMed] [Google Scholar]
- 3.The United Nations Children’s Fund/World Health Organization WHO/UNICEF joint statement: Clinical management of acute diarrhea. < www.who.int/child_adolescent_health/documents/who_fch_cah_04_7/en/index.html> (Accessed on April 2008).
- 4.Practice parameter: The management of acute gastroenteritis in young children American Academy of Pediatrics, provisional committee on quality improvement, subcommittee on acute gastroenteritis. Pediatrics. 1996;97:424–35. [PubMed] [Google Scholar]
- 5.Reis EC, Goepp JG, Katz C, Santosham M. Barriers to use of oral rehydration therapy. Pediatrics. 1994;93:708–11. [PubMed] [Google Scholar]
- 6.Snyder JD. Use and misuse of oral therapy for diarrhea: Comparison of US practices with American Academy of Pediatrics recommendations. Pediatrics. 1991;87:28–33. [PubMed] [Google Scholar]
- 7.Ozuah PO, Avner JR, Stein RE. Oral rehydration, emergency physicians, and practice parameters: A national survey. Pediatrics. 2002;109:259–61. doi: 10.1542/peds.109.2.259. [DOI] [PubMed] [Google Scholar]
- 8.Conners GP, Barker WH, Mushlin AI, Goepp JG. Oral versus intravenous: Rehydration preferences of pediatric emergency medicine fellowship directors. Pediatr Emerg Care. 2000;16:335–8. doi: 10.1097/00006565-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Atherly-John YC, Cunningham SJ, Crain EF. A randomized trial of oral vs intravenous rehydration in a pediatric emergency department. Arch Pediatr Adolesc Med. 2002;156:1240–3. doi: 10.1001/archpedi.156.12.1240. [DOI] [PubMed] [Google Scholar]
- 10.Spandorfer PR, Alessandrini EA, Joffe MD, Localio R, Shaw KN. Oral versus intravenous rehydration of moderately dehydrated children: A randomized, controlled trial. Pediatrics. 2005;115:295–301. doi: 10.1542/peds.2004-0245. [DOI] [PubMed] [Google Scholar]
- 11.Canadian Paediatric Society, Nutrition and Gastroenterology Committee Oral rehydration therapy and early refeeding in the management of childhood gastroenteritis. Paediatr Child Health. 2006;11:527. [Google Scholar]
- 12.Listernick R, Zieserl E, Davis AT. Outpatient oral rehydration in the United States. Am J Dis Child. 1986;140:211–5. doi: 10.1001/archpedi.1986.02140170037024. [DOI] [PubMed] [Google Scholar]
- 13.Hartling L, Bellemare S, Wiebe N, Russell K, Klassen TP, Craig W. Oral versus intravenous rehydration for treating dehydration due to gastroenteritis in children. Cochrane Database Syst Rev. 2006;3:004390. doi: 10.1002/14651858.CD004390.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyd R, Busuttil M, Stuart P. Pilot study of a paediatric emergency department oral rehydration protocol. Emerg Med J. 2005;22:116–7. doi: 10.1136/emj.2003.010801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holden JD. Hawthorne effects and research into professional practice. J Eval Clin Pract. 2001;7:65–70. doi: 10.1046/j.1365-2753.2001.00280.x. [DOI] [PubMed] [Google Scholar]