Abstract
Background
The mechanism of sinoatrial node (SAN) automaticity is traditionally attributed to membrane ion currents. Recent evidence indicates spontaneous sarcoplasmic reticulum (SR) Ca2+ cycling also plays an important role.
Methods and Results
We performed computer simulation on SAN cell and 1D tissue model. In the SAN cells, SR Ca2+ cycling broadly modulated sinus rate from 1.74Hz to 3.87Hz. Shortening of the junctional SR refilling time and increase of SR Ca2+ release were responsible for sinus rate acceleration. However, under the fast SR Ca2+ cycling, decreased L-type Ca2+ current (ICaL) resulted in irregular firing. When Ca2+ cycling was suppressed, If and ICaT both acted to stabilize the pacemaker rhythm, but ICaT had less effect than If. At the 1D level, the electrical coupling between neighboring cells had little effect on the earliest pacemaker location. The leading pacemaking site always colocalized with the site with the highest SR Ca2+ cycling rate, but shifted to the site with less inhibited ICaL.
Conclusions
The rate of SR Ca2+ cycling can effectively and broadly modulate the sinus rate. If, ICaL and ICaT play integral roles to guarantee SAN cell rhythmic firing. The leading pacemaker site is determined by intracellular Ca2+ dynamics and membrane currents, indicating the synergistic dual automaticity not only exists in single SAN cells, but also at the tissue level.
Introduction
The sinoatrial node cells (SANC) are the primary pacemakers of the heart. They spontaneously exhibit slow diastolic depolarization (DD) to a threshold for firing, thereby periodically initiating action potentials to set the rhythm of the heart1, 2. Spontaneous DD has traditionally been attributed to a “membrane clock (M clock)” mechanism 3, 4, in which the membrane potential changes with the activation and inactivation of the membrane ion channel currents. Recently, the “Ca2+ clock” has been put forward as a complementary mechanism for SAN automaticity5, 6. Results from single SANC studies suggested that isolated sarcoplasmic reticulum (SR) could operate as a self-sustained Ca2+ oscillator and a small spontaneous Ca2+ release from the junctional SR to the intracellular subspace occurred first as the initiating event, which in turn activated Na+/Ca2+ exchanger current (INCX) and caused the accelerated DD. With these experimental results, a mathematical model of SANC was recently constructed to include the intracellular Ca2+ cycling together with the more traditional ionic channel currents 7. On the other hand, at the tissue level, the functional organization of SANC is still not very clear today. This makes evaluation of the interaction between the two clocks especially challenging.
The synergistic action of the M and Ca2+ clocks has been demonstrated by the Maltsev-Lakatta model7, but previous works did not extensively explore mechanisms of heart rate modulation. Furthermore, it is unclear whether the two clock synergy can be applied to multicelluar SAN structures to determine the dominant pacemaking site. In this paper, by introducing a variety of simulated interventions, such as β-adrenergic stimulation, ICaL and ICaT alteration, on a single cell and a constructed tissue model, we evaluated the synergistic effects of two clocks on the rhythmic firing and leading pacemaker site.
Method
The single cell model and its solution
We selected the Maltsev-Lakatta SAN rabbit model 7 for the current study because it is the latest and most advanced numerical formulations to address dynamic activities of the SR Ca2+ pump and ion buffering in all cell compartments, including Ca2+ buffering by calsequestrin in junctional SR (jSR). The model included twenty nine first-order differential equations with the following format to describe action potential of a single SANC:
| (1) |
where V was the transmembrane potential, Cm was the membrane capacitance, t was the time, Iion was the total ionic current.
In general Iion was a function of the voltage V and gating variables Y1,….Yi,….YM that satisfied the following ordinary differential equations:
| (2) |
where Yi_inf was the steady-state value of the gating variable Yi. τYi was its time constant. αi, βi were opening and closing rates of the channel gating.
Ca2+ release from the SR via ryanodine receptors (RyRs)8, its intracellular fluxes, buffering, and concentrations in cell compartments satisfied another set of ordinary differential equations.
| (3) |
where Zi, j, r were a variety of introduced variables, such as Ca2+ concentrations in different cell compartment or flux coefficients during dynamic processes.
The fourth-order Runge-Kutta method was applied to integrate the single cell model with a time step of 0.005ms. Decreasing the time step to 0.001ms was not found to change the simulation results.
One-dimensional SAN tissue and its numerical integration
Cardiac tissue could be treated as a continuous and excitable medium connected by gap junctions between adjacent cells. If only the intracellular space was taken into account, the tissue was considered as a monodomain system. Then Eq.4 could be used to describe the tissue, together with the no-flux boundary conditions imposed at both ends of the fiber as described by Eq.5.
| (4) |
| (5) |
where x was the spatial coordinates in the string of tissue, ρx was the bulk cytoplasmic resistivity, Sv was the surface-to-volume ratio, and l was the length of the whole fiber.
SAN central area is normally the dominant pacemaker location. To explore the role of SR Ca2+ dynamics in determining the leading pacemaker site, we performed simulation around the central SAN region. The fiber consisted of 30 cells with strand length of 0.3cm that was approximately half the length of SA node in the rabbit 9, and parallel to the direction of the superior to the inferior vena cava which was consistent with extension direction of the central SANC 10. The fiber was divided into three parts: cells from the first to the 10th were designated the inferior part, the 11th to 20th cells middle part, and the left 10 cells superior part.
So far, two models (mosaic and gradient model) of the organization of SAN tissue have been put forward11. According to the mosaic model, all SANC had reasonably uniform properties, it was the peppered atrial myocytes that made them show the different morphology. Recently, a mix of atrial and SAN cells in the SAN tissue periphery was reported, but no atrial cells were found in the SAN central area 10, indicating that our concerned region could be considered homogeneous in the controlled condition. In the gradient model12, it was suggested that SANCs showed transition of electrophysiological properties from the center to the periphery. An empirical exponential formula was given to describe changes of parameters such as cellular size and current conductances. According to this formula, these parameters almost kept the same within half length of the fiber, and only started to show heterogeneity close to the defined periphery. This implied that the central region was also considered uniform in this organization. Therefore, based on these two models, our concerned region could be set homogeneous to the controlled condition.
It was reported that the leading pacemaker site changed dynamically within the central area because of interventions affecting different regions of the node differentially 13, 14, 15. For β-adrenergic stimulation, there has been little report about heterogeneous sensitivities among primary pacemakers. Our main purpose here is to investigate relationship between the leading pacemaker site and SR Ca2+ activity, therefore the exact form of its distribution has less significance on the results. In this case, we hypothesized three kinds of heterogeneity: one was a linear change of SERCA pump rate( Pup) from the superior to the inferior parts along the fiber. The second adopted an exponential change from the putative equation 80 12 shown in formula (6). The third was a discontinuous state: the middle cells had higher Pup than the superior and inferior cells. On the basis of such a discontinuity, Pup in different parts of the SAN received different percentage of increase to investigate whether the leading pacemaker site shifted with Pup.
| (6) |
where n stands for the numbered cell, cell_number is the total amount of cells in the tissue ( here cell_number = 30), Fcell is a scaling factor of Pup, which is directly based on the location of a cell relative to the firstSAN.
Assuming that all cells were cylindrical and adhered to the dimensions suggested in the Maltsev-Lakatta’s single cell model, a radius of 4.0 μm and length of 70μm were used for all cells in the fiber, resulting in a surface-to-volume ratio Sv = 5300cm−1. According to the experimental data obtained in pairs of rabbit SANC using the double whole-cell patch clamp technique 16, the mean conductivity value of the intercellular coupling was 7.5nS. Therefore, based on the Eq.86 of 12 in which at each grid point the conductivity tensor was determined from both the intercellular conductivity and the dimensions of the cell, we used ρx = 9.57 × 10−6 GΩ ··cm at each grid point in our tissue simulation. The model was considered a monodomain and continuum system, thus the geometry of the cells and gap junctions would not significantly affect the results of computation.
The three-point centered difference method with spatial step of Δ x = h = 0.01cm was used in the calculation. Source code was developed by us using C++ programming language and all simulations were performed on a personal computer.
Results
Intracellular SR Ca2+cycling
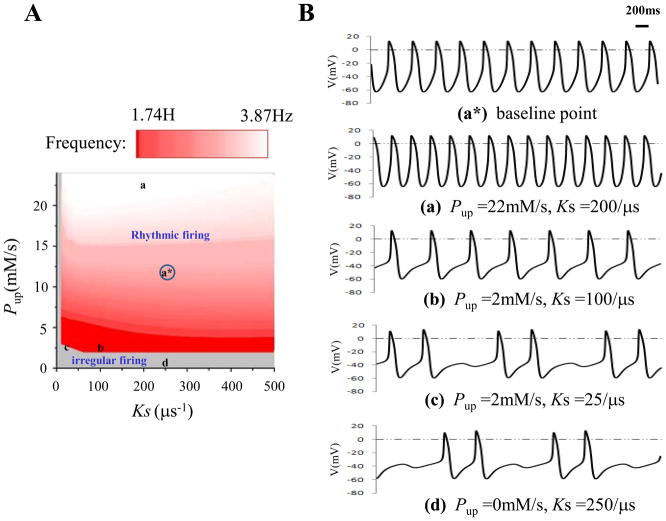
Two main parameters, a SERCA pump rate Pup and a Ca2+ release rate Ks, were included in the model to account for the operation of the cardiac SR. In Ref.7, by removing the membrane clock (all ion currents = 0), the investigators analyzed characteristics of the isolated SR Ca2+ in simultaneous Pup and Ks changes. We simulated their combined effects on sinus rate regulation with the intact two clocks (Fig. 1). Panel A showed that spontaneous action potential could be generated within a broad frequency ranging from 1.74Hz to 3.87Hz. Increasing Pup accelerated sinus rate whereas decreasing Ks had the opposite effect. Under slow sinus rate the diastolic depolarization was markedly prolonged with slight hump-like elevation during DD comparing (b) with (a)in Panel B. When either Pup or Ks was greatly reduced from their baseline values (Pup =12mM/s, Ks =250/μs), irregular firing and intermittent sinus pause (Fig. 1Bc and Fig. 1Bd) occurred in the grey zone.
Figure 1.
Effects of SR Ca2+ cycling on sinus rate. Panel A: Pup (Ca2+ pump rate) changed from 0.0mM/s to 24.0mM/s with the step size of 1.0mM/s. Ks (SR Ca2+ release rate) changed from 0.0/μs to 500/μs with the step size of 10/μs. Grey area represented the irregular firing while red area corresponded to the rhythmic firing. Panel B: some specific action potentials positioned in Panel A. a* was the baseline point (Pup =12mM/s, Ks =250/μs). a and b were two points which showed regular firings but with different sinus rates. c and d displayed two sites showing irregular firings.
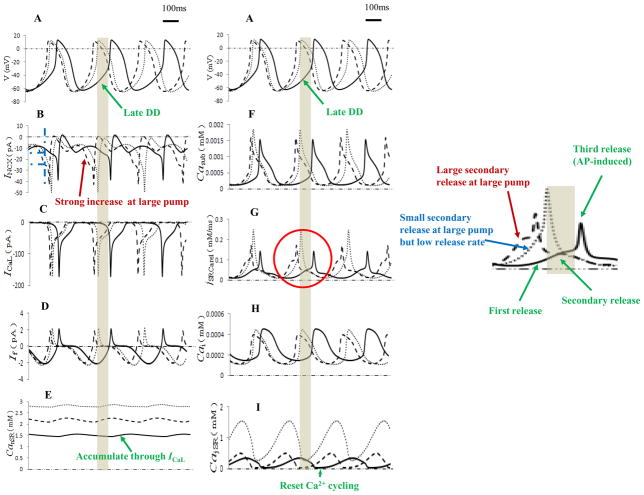
Fig. 2 displayed internal processes of sinus rate regulation by Pup and Ks. According to Ref.7, Ca2+ release from the jSR was divided into three stages (Fig. 2G and green arrows in the inset on the right): the first spontaneous primary release (during mid-DD), a secondary larger release (during late DD indicated by the grey bars) and the third AP-induced release (Ca2+-induced Ca2+ release or CICR17 due to activation of L-type Ca2+ current ICaL). Therefore, compared with baseline (all solid curves), with elevation of Pup to 20 mM/s (dashed curves), Ca2+ concentration in network SR (Fig. 2E) and junctional SR (Fig. 2I) both increased, leading to a faster rate of jSR refilling. Therefore, within a shorter time period that the higher jSR Ca2+ loads was attained, a secondary larger Ca2+ release during the DD period (red arrow in the inset of Fig. 2G) drove a strong and fast increase of INCX (red arrow in Fig. 2B) to bring the membrane potential to threshold and produced an action potential with a shorter cycle time than the baseline.
Figure 2.
Simulation traces of the primary dynamic interactions of the M clock and Ca2+ clock during spontaneous production of action potential. Action potentials, major ionic currents and Ca2+ behaviors in different compartments were shown. Solid curves denoted the baseline (Pup =12mM/s, Ks =250/μs). Dashed curves were under the high SERCA pump rate (Pup =20mM/s, Ks =250/μs) while dotted curves corresponded to the high SERCA pump but low Ca2+ release rate (Pup =20mM/s, Ks =12.5/μs). Grey bars indicated the period of late DD under the baseline conditions. The jSRCarel curves inside the red circle were enlarged at the right side.
When Ks was significantly reduced under the high Pup (all dotted curves), although accumulation of Ca2+ in jSR (Fig. 2I) and nSR (network SR, Fig. 2E) increased remarkably, Ca2+ release from the jSR (blue arrow in Fig. 2G) during the late DD decreased, resulting in smaller diastolic INCX than that during high Pup (about 35% decrease at the time indicated with the blue dashed lines in Fig. 2B), thereby increasing the time to bring the membrane potential to threshold and reducing the sinus rate. Of note, when ICaL was activated, because of the high Ca2+ concentration in the jSR during CICR process, the third release (AP-induced) was strong in this situation, resulting in a higher peak of subspace Ca2+ (dotted curve in Fig. 2F) and INCX (dotted curve in Fig. 2B) than with the normal Ks.
L-type Ca2+current
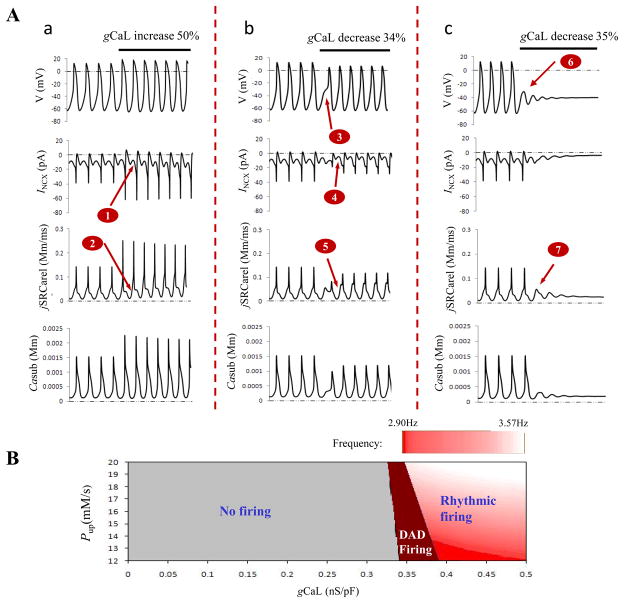
ICaL is one of the most important interactions between Ca2+ and M clocks because SR is reset and refueled by CICR process due to ICaL activation. Fig. 3A showed effects of ICaL on the electrical firing. To facilitate comparison, the baseline was plotted first with instant parameter changes (black bars) simulating various conditions explained with labels above the bars. Fig. 3Aa column corresponded to Pup 67% increase and gCaL 50% increase. As noted simultaneous increase Pup and gCaL resulted in fast sinus rate compared with baseline (15% rate increase). Large Pup led to the large INCX (arrow 1) and large Ca2+ release (arrow 2) during late DD. Increased gCaL further strengthened the CICR process, resulting in the large peak of Ca2+ release and INCX during fast firing of AP. However, if gCaL was reduced by 34% (Fig. 3Ab column), a notch (arrow 3) appeared in the phase 0 of the action potential with prolonged duration, implying delayed activation of ICaL. Although Ca2+ release and INCX during late DD was still larger than that of the baseline due to the larger Pup (arrows 4 and 5), reduced gCaL made their peak value become small because of the suppressed CICR process. Further reduction of gCaL by 35% (Fig. 3Ac column) resulted in membrane oscillation (arrow 6) and spontaneous Ca2+ release (arrow 7) with sinus rhythm halt characterized with delayed afterdepolarization (DAD).
Figure 3.
Effects of ICaL on rhythmic AP firing. Panel A: Action potentials, major ionic and Ca2+ changes during large Pup (67% higher than baseline) and large gCaL (50% increase) (a), or large Pup and small gCaL (34% decrease in (b) and 35% decrease in (c)). Panel B: exploration of AP firing with variations of Pup and gCaL at the same time. The steps for Pup and gCaL were 1.0mM/s and 0.001nS/pF, respectively.
Fig. 3B explored effects of decreased ICaL on AP firing under different values of Pup. If we define AP of Fig. 3Aa as rhythmic firing, Fig. 3Ab as firing with DAD, and Fig. 3Ac as no firing, Fig. 3B could be divided into three parts. Small gCaL led to failed firing even under the high Pup condition. Rhythmic firing existed only when the SR Ca2+ activity and ICaL dynamically and properly interacted with each other. With decreasing Pup, large ICaL was needed to sustain the rhythmic firing.
Hyperpolarization-activated “funny” current
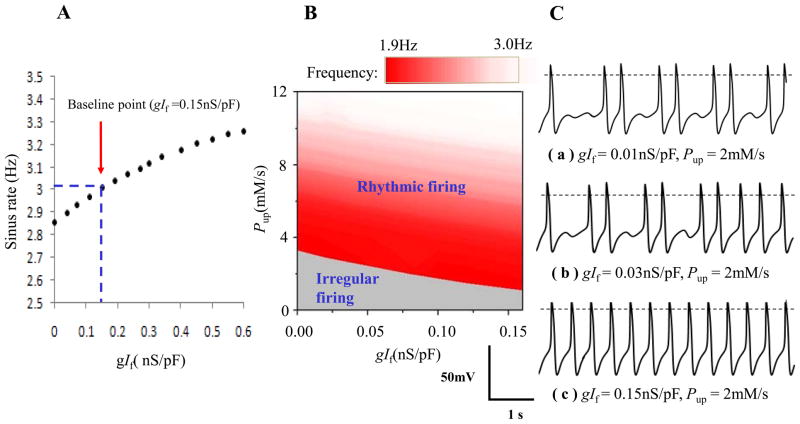
If has long been considered as the most important ion channel involved in the rate regulation of cardiac pacemaker cells 3. Fig. 4 showed change of sinus rate with gIf (panel A), and a parametric analysis of sinus rate versus gIf and Pup (panel B and C). Of note, complete blockade of gIf made sinus rate fall to 2.86Hz, but without appearance of pause or abnormal firing. The rate modulation range was less than 0.3Hz when gIf changed from 0.0nS/pF to two times of its baseline (0.30nS/pF). Even if gIf was elevated to 0.6nS/pF the change of rate was less than 0.5Hz. But in panel C, blocking If and down-regulating Pup caused intermittent sinus pause. At the same low Pup, the persistent irregular firing (a) changed to transient irregular firing (b) and finally to rhythmic firing (c) by increasing gIf. Also in B, increasing gIf reduced the area of irregular firing at low Pup.
Figure 4.
Role of If in the rhythmic firing of the whole system. A: sinus rate modulation by If. B: analysis of Pup and gIf. The step size was 0.01nS/pF for gIf and 0.2mM/s for Pup. Action potentials of the three specific points within the panel A were depicted in panel C.
T-type Ca2+current
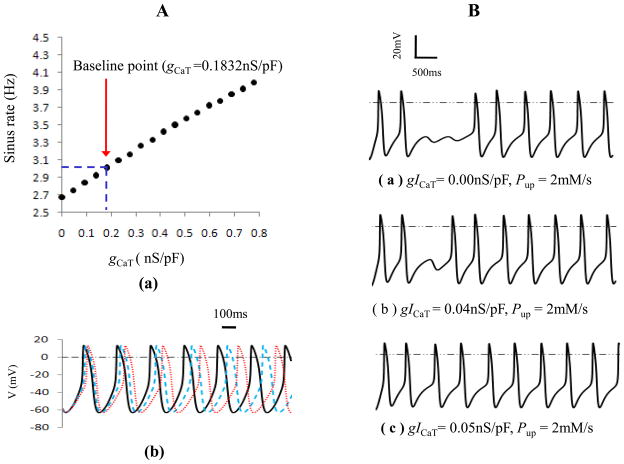
Since T-type Ca2+ current (ICaT ) has a low activation threshold (~-60 mV), its activation was suggested as an additional mechanism contributing to the mid-late DD. Fig. 5 showed the role of ICaT in sinus rate and rhythmic firing. As noted in the panel A, with decreasing of gCaT, sinus rate gradually reduced. When gCaT changed from 0.0nS/pF to two times of its baseline (0.3664nS/pF), the firing rate was smoothly regulated within the range of 2.67Hz to 3.33Hz. Four times of gCaT resulted in a rate of 3.98Hz which was larger than the effect of If on sinus rate. Additionally, if the SR Ca2+ uptake rate was greatly inhibited as in panel B, a transient firing pause occurred after the suppression of Pup. But with ICaT increased (from (a) to (c)), the time of sinus pause shortened and the firing recovered to the normal in the end.
Figure 5.
Role of ICaT in sinus rate and rhythmic firing. Panel A: (a) relationship between gCaT and sinus rate. (b) Action potentials at gCaT 0.1832nS/pF (solid lines), 0.09nS/pF (dashed lines) and 0.00 nS/pF (dotted lines), respectively. Panel B: Role of ICaT at the low Ca2+ pump rate.
Leading pacemaker site
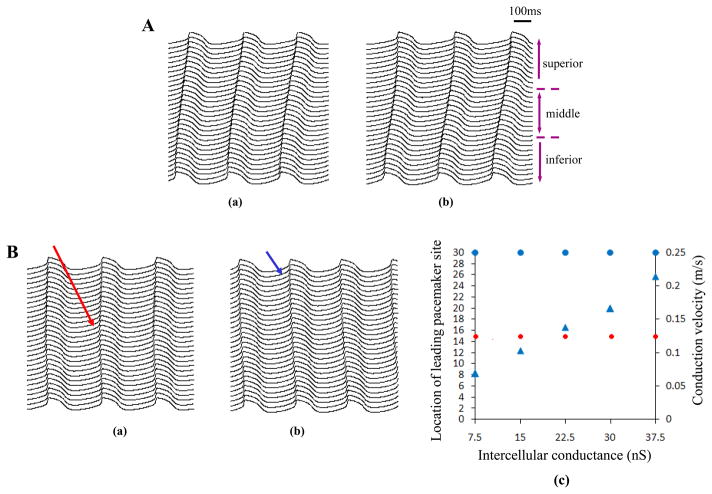
We performed simulation on the developed fiber. In the first situation, when Pup increased linearly from 10 mM/s (superior) to 15 mM/s (inferior) along the strand, the leading pacemaker site located at the inferior region (Fig. 6Aa). In the second situation in which Pup increased exponentially, the inferior part was also the leading pacemaker site (Fig. 6Ab). In the third discontinuous situation, when Pup of the middle cells was 13mM/s in contrast to 12mM/s in other parts, the cells in the middle region exhibited the earliest electrical excitation and propagated towards the superior and inferior regions (Fig. 6Ba). Therefore, the above three cases consistently showed colocolization of the leading pacemaker site and the highest SERCA pump activity. To further test this relationship, on the bases of the conditions in Fig. 6Ba, we increased Pup of the superior cells by 30% and 15% for the middle and inferior cells. The results demonstrated that the leading pacemaker site shifted from the 15th cell (red arrows) to the 30th cell in the superior side (blue arrows in Fig. 6Bb) where the SR Ca2+cycling was most active.
Figure 6.
Role of Ca2+clock in determining the leading pacemaker site in a sin us node fiber. Panel A: electrical propagation when Ca2+ uptake rate (Pup) was smoothly changed in a linear (a) or exponential (b) way along the strand from 10mM/s (superior) to 15mM/s (inferior). Along the strand the upper 10 cells was defined as the superior site, the lower 10 cells was defined as the inferior site as denoted. Panel B: leading pacemaker site shifted from the middle (red arrow in a,) to the superior (blue arrow in b,) when Pup of the superior cells increased 30% while other cells only had 15% increase. (c) showed relationship of the intercellular conductance with the location of leading pacemaker site and conduction velocity. Similar to the (a) and (b), the earliest excitation always shifted from the 15th cell in the middle (solid red circles) to the 30th cell in the superior site (solid blue circles) if Pup of the superior cells became the maximum no matter what value the coupling conductance was. But electrical conduction velocity from the superior to the inferior was greatly increased (solid blue triangles) if the coupling conductance elevated from its baseline (7.5nS) to five times (37.5nS).
Additionally, relationship between the pacemaker site location, conduction velocity and the intercellular coupling conductance was assessed and summarized in Fig. 6Bc, where location of the earliest excitation was designated with the cell number. Leading pacemaker site and velocity both were plotted as a function of the coupling conductance between two neighboring cells, but with regards to the different y-axis. As noted, similar to the Fig. 6Ba and Fig. 6Bb, the leading pacemaker site always shifted from the 15th cell in the middle (solid red circles) to the 30th cell in the superior site (solid blue circles) if Pup of the superior cells became the maximum no matter what value the coupling conductance was. However, electrical conduction velocity from the superior to the inferior was greatly increased if the coupling conductance elevated from its baseline (7.5nS) to five times (37.5nS) (solid blue triangles).
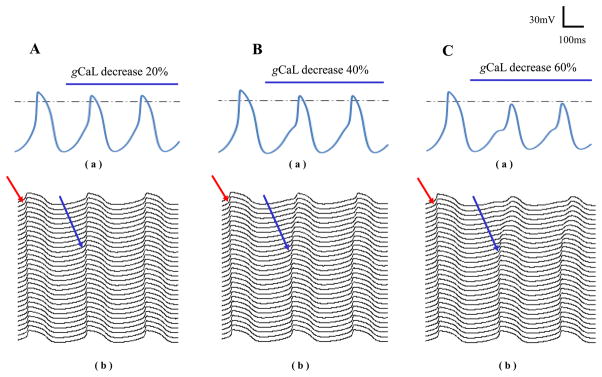
Besides SR Ca2+ cycling, we found that ICaL could also in some extent determine location of the earliest excitation. Based on the conditions in Fig. 6Bb, we suddenly decreased gCaL of the superior cells after the time notified with respective labels in Fig. 7. The action potential of the superior cell was shown in the upper panels while the lower panels displayed electrical propagations along the fiber. The leading pacemaker site was observed to shift from the superior (red arrows) towards the middle site (blue arrows) again. Comparing the gCaL effects, more suppressed gCaL caused more obvious shift of the leading pacemaker site.
Figure 7.
Effects of ICaL on the leading pacemaker site. gCaL of the superior cells was changed after the time indicated with respective labels in each panel. Their action potentials were shown in (a) while (b) displayed electrical propagations along the fiber. Before changing gCaL, action potential propagated from the superior to the inferior as indicated by the red arrows. After decreasing gCaL, leading pacemaker site started to shift towards the middle site indicated by the blue arrows.
Discussion
The present study focuses on the mechanistic investigation of recent experimental findings based on the latest mathematical model. Shortening of the jSR refilling time and increase of Ca2+ release from SR during DD were found to be responsible for the isoproterenol-induced sinus rate acceleration. In contrast with If and ICaT, SR Ca2+ cycling was a major contributor to the broad sinus rate modulation. However, appearance of DAD-like firing at down-regulated ICaL, and stabilizing effect of If and ICaT on sinus rhythm at suppressed SR Ca2+ cycling suggested the importance of membrane clock. In the simulated tissue, the leading pacemaker site was found to always colocalize with the site having the highest SR Ca2+ cycling, but ICaL down-regulation also showed an obvious effect on the location.
Mechanisms of rate modulation and irregular firing
When the membrane clock was removed (all ion currents = 0), SR was shown to be a release-pump-delay oscillator, but unable to generate high-amplitude firing at higher rates7. Within the full system of two clocks, it was demonstrated that the interaction between the secondary releases of Ca2+ and INCX was responsible for producing rhythmic firing. Based on these findings, we investigated the combined effects of Pup and Ks on firing rate and the mechanisms to explain experimental observations, in which heart rate increased with elevated concentration of isoproterenol and decreased with infusion of ryanodine18. Fig. 2 suggested that increased SERCA pump activity by isoproterenol could markedly accelerate the rate of jSR refilling and increase the amount of Ca2+ release from SR during DD, thus shortening the time of INCX to threshold and thereby the cycle length. On the contrary, Ca2+ release inhibition by ryanodine would attenuate effects of isoproterenol and delay activation of INCX, resulting in sinus rate reduction. When Pupor Ksdramatically decreased, Ca2+ release from jSR was too small to activate INCX. Then transient sinus pause occurred until accumulated Ca2+ was enough to trigger INCX and started a new action potential. In this case, the firings would most likely lose their intrinsic rhythm and became irregular as shown in Fig. 1.
We did the study under conditions of high Pup (higher than 12mM/s) and broad change of gCaL (from 0 to 0.5nS/pF) to investigate their combined effects on automaticity. Area of rhythmic firing was found to extend with Pup elevation. Such results are in line with the finding in Ref.7 and support the importance of Ca2+ clock. However, our results additionally suggested that even with higher Pup, lower contribution of ICaL could lead to DAD-like firing and persistent sinus pause, implying a key role of ICaL on rhythmic firing as well. Because ICaL is mainly responsible for refilling the SR through Ca2+ influx and triggering CICR process, our results illustrated that the Ca2+ cycling could not be sustained when ICaL was significantly down-regulated. Thus, after a spontaneous rhythmic Ca2+ release from the SR, depletion of Ca2+ in SR could cause sinus rhythm to completely stop after the DAD as observed in Fig. 3Ac. If down-regulation of ICaL could be compensated by elevation of SERCA pump activity, which meant Ca2+ refueled into SR was just enough to maintain their cycling, then firing with DAD occurred as shown in Fig. 3Ab. In this case, because of decreased Ca2+ influx due to reduced ICaL, increased SERCA pump would transport more Ca2+ into SR, thereby resulting in small Casub concentration in submembrane space as shown in the trace of Casub in Fig. 3Ab. This case was suggested to be a delicate balance between Ca2+ available for pumping and the speed of the SERCA pump, thus it only existed in a small area as shown in Fig. 3B. The above finding suggested importance of delicate corporation between SR Ca2+ cycling and ICaL in keeping the system robustness.
In addition, we observed different effects of SR Ca2+ cycling and ICaL on the action potential amplitude (APA). As observed in Fig. 1Ba and Fig. 1Bb, with increase of sinus rate, the maximum diastolic potential became more negative (from −57.5mV in Fig. 1Bb to −62.7mV in Fig. 1Ba), resulting in increase of APA. But 106.7% increase of the rate led to only 9.5% increase of APA. Therefore, the effect was only marginal. Nevertheless, as shown in Fig. 3, changes of ICaL had obviously greater effects on APA. It might due to their different phases of function: SR Ca2+ cycling was mainly responsible for the spontaneous diastolic depolarization after the previous action potential to bring the membrane potential to threshold and activated INCX, while ICaL was the primary current to generate the AP upstroke, thus should be the main factor to affect APA and overshoot.
Stabilization of the membrane currents on rhythmic firing
Activation of If and ICaT were both suggested to be able to trigger a depolarization because of their low threshold 3. In Ref.7, the authors proved that inhibition of If resulted in rate reduction and the function of If in increasing the system robustness at low firing rates. On the other hand, our simulation in Fig. 4 demonstrated that compared with the rate modulation through SR Ca2+ cycling, If adjusted sinus rate within a narrow range (less than 0.3Hz) and without occurrence of firing pause at its blockade, suggesting a limited role of If in controlling pacemaker cell automaticity. Besides, as shown in Fig. 5 when gCaT changed from 0.0nS/pF to 0.3664nS/pF, sinus rate was modulated within a range of 0.66Hz which was larger than that of If, indicating the more important effect of ICaT than If on rate adjustment. As noted in Fig. 4 and Fig. 5, similar to If, increase of ICaT could stabilize the firing rhythm. But in contrast to If, complete elimination of ICaT could not result in persistent sinus pause. Therefore, as far as keeping the rhythm stabilization was concerned, If was suggested to be more important than ICaT. This might due to their different phases of activation, because If was mainly responsible for the earlier spontaneous DD while ICaT was the primary current during the mid-late DD.
Effects of SR Ca2+ cycling and ICaL on the leading pacemaker site
With the observation that the maximum LDCAE (late diastolic Cai elevation) slope always colocalized with the leading pacemaker site and that LDCAE could faithfully represent Ca2+ clock activity, the leading pacemaker site was proposed to be the site exhibiting the highest Ca2+ activity18. To test this proposal, we modified the distribution of Pup in various ways along the developed fiber. In either the linear, exponential or discontinuous situation, leading pacemaker site showed good consistency with the highest SR Ca2+cycling.
In an optical mapping experiment, after isoproterenol infusion, the leading pacemaker site was found to shift to the superior SAN18. The SERCA2a/phospholamban molar ratio was reported lower in the superior SAN than the inferior SAN and right atrial tissue. The key regulator of SR Ca2+ uptake was phospholamban that inhibited SERCA2a in its dephosphorylated state, thereby superior site was supposed to have more phospholamban molecules available to regulate SERCA2a molecules and more robust Ca2+ uptake. We simulated this response by increasing Pup more in the superior than in other regions of the 1D model based on the third situation. The results in Fig. 6B confirmed the shift of the leading pacemaker site with increase of Pup. The site with the highest SERCA pump activity was characterized with the fastest and earliest spontaneous firing, driving the surrounding cells to fire and resulting in shift of the dominant pacemaker. Additionally, the results showed that the coupling conductance between neighboring cells had little effect on the location of the earliest excitation but velocity of the electrical propagation increased with the conductance elevation.
Besides, we observed the role of ICaL in determining location of the earliest excitation. If ICaL was suppressed as we did in Fig. 7, even with the superior cells showed the highest Pup, the leading pacemaker site still shifted to the site with less inhibited ICaL. Note that in Fig. 7Aa and Fig. 7Ba, decreasing gCaL in the superior cells led to reduced amplitude of action potential, longer cycle length between the two neighboring action potentials and the appearance of DAD, thereby might suppress their dominant status in spontaneous activity. Consequently, in response to an intervention or diseased condition that suppresses the SAN spontaneous activity, the leading pacemaker is expected to shift to the site with the least suppressed intrinsic spontaneous activity, forming a hierarchical structure of pacemaker dominance.
Limitations
Because little is known about the heterogeneous distribution of the Ca2+ cycling in the SAN tissue, especially during β-adrenergic stimulation, we tested three cases and confirmed good consistency between the leading pacemaker site and the highest SR Ca2+ activity, and the site shift with change of Pup as well. This tissue model provided us with an opportunity to exclusively investigate role of Ca2+ and ICaL in determining the earliest pacemaker location. However, in reality, two-dimensional tissue would be more ideal and realistic in the study. Heterogeneities, such as cellular size, ionic currents and SR Ca2+cycling among cells are neededfor further consideration.
The interaction of intracellular calcium dynamics and membrane current is a complicated process. We provided theoretical analysis of the SR Ca2+ cycling and effects of several main currents ICaL, If and ICaT on automaticity. However, other ionic channels, such as non-selective sustained current (Ist), was also suggested as additional mechanisms contributing to DD 19. But due to its exhibition of many properties of ICaL and INCX, molecular origin and specific blockers of Ist have not been found yet, the identity of Istremains unclear.
Supplementary Material
Acknowledgments
This study was supported in part by National Institutes of Health grants P01 HL78931, R01 HL78932, 71140, Chinese National Natural Science Foundation 30870659, 30971221 and Health Foundation of Shaanxi Province 08D23 (HZ), Medtronic-Zipes Endowments (PSC) and American Heart Association Established Investigator Award (SFL).
References
- 1.Chen PS, Joung B, Shinohara T, Das M, Chen ZH, Lin SF. The Initiation of the Heart Beat. Circ J. 2010;74(2):221–225. doi: 10.1253/circj.cj-09-0712. [DOI] [PubMed] [Google Scholar]
- 2.Imai S, Saito F, Takase H, Enomoto M, Aoyama H, Yamaji S, Yokoyama K, Yagi H, Kushiro T, Hirayama A. Use of bepridil in combination with Ic antiarrhythmic agent in converting persistent atrial fibrillation to sinus rhythm. Circ J. 2008;72(5):709–715. doi: 10.1253/circj.72.709. [DOI] [PubMed] [Google Scholar]
- 3.Baruscotti M, Bucchi A, DiFrancesco D. Physiology and pharmacology of the cardiac pacemaker (“funny”) current. Pharmacology & Therapeutics. 2005;107(1):59–79. doi: 10.1016/j.pharmthera.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Brown HF, DiFrancesco D, Noble SJ. How does adrenaline accelerate the heart? Nature. 1979;280:235–236. doi: 10.1038/280235a0. [DOI] [PubMed] [Google Scholar]
- 5.Maltsev VA, Vinogradova TM, Lakatta EG. The emergence of a general theory of the initiation and strength of the heartbeat. J Pharmacol Sci. 2006;100(5):338–369. doi: 10.1254/jphs.cr0060018. [DOI] [PubMed] [Google Scholar]
- 6.Vinogradova TM, Lyashkov AE, Zhu WZ, Ruknudin AM, Sirenko S, Yang DM, Deo S, Barlow M, Johnson S, Caffrey JL, Zhou YY, Xiao RP, Cheng HP, Stern MD, Maltsev VA, Lakatta EG. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circulation Research. 2006;98(4):505–514. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- 7.Maltsev VA, Lakatta EG. Synergism of coupled subsarcolemmal Ca2+ clocks and sarcolemmal voltage clocks confers robust and flexible pacemaker function in a novel pacemaker cell model. Am J Physiol Heart Circ Physiol. 2009;296(3):H594–615. doi: 10.1152/ajpheart.01118.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yano M. Ryanodine receptor as a new therapeutic target of heart failure and lethal arrhythmia. Circ J. 2008;72(4):509–514. doi: 10.1253/circj.72.509. [DOI] [PubMed] [Google Scholar]
- 9.Bleeker WK, Mackaay AJ, Masson-Pevet M, Bouman LN, Becker AE. Functional and morphological organization of the rabbit sinus node. Circ Res. 1980;46(1):11–22. doi: 10.1161/01.res.46.1.11. [DOI] [PubMed] [Google Scholar]
- 10.Dobrzynski H, Li J, Tellez J, Greener ID, Nikolski VP, Wright SE, Parson SH, Jones SA, Lancaster MK, Yamamoto M, Honjo H, Takagishi Y, Kodama I, Efimov IR, Billeter R, Boyett MR. Computer three-dimensional reconstruction of the sinoatrial node. Circulation. 2005;111(7):846–854. doi: 10.1161/01.CIR.0000152100.04087.DB. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Holden AV, Boyett MR. Gradient model versus mosaic model of the sinoatrial node. Circulation. 2001;103(4):584–588. doi: 10.1161/01.cir.103.4.584. [DOI] [PubMed] [Google Scholar]
- 12.Garny A, Kohl P, Hunter PJ, Boyett MR, Noble D. One-dimensional rabbit sinoatrial node models: benefits and limitations. J Cardiovasc Electrophysiol. 2003;14(10 Suppl):S121–132. doi: 10.1046/j.1540.8167.90301.x. [DOI] [PubMed] [Google Scholar]
- 13.Shibata N, Inada S, Mitsui K, Honjo H, Yamamoto M, Niwa R, Boyett MR, Kodama I. Pacemaker shift in the rabbit sinoatrial node in response to vagal nerve stimulation. Exp Physiol. 2001;86(2):177–184. doi: 10.1113/eph8602100. [DOI] [PubMed] [Google Scholar]
- 14.Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity - Promoting understanding of sick sinus syndrome. Circulation. 2007;115(14):1921–1932. doi: 10.1161/CIRCULATIONAHA.106.616011. [DOI] [PubMed] [Google Scholar]
- 15.Opthof T. Embryological development of pacemaker hierarchy and membrane currents related to the function of the adult sinus node: implications for autonomic modulation of biopacemakers. Medical & Biological Engineering & Computing. 2007;45(2):119–132. doi: 10.1007/s11517-006-0138-x. [DOI] [PubMed] [Google Scholar]
- 16.Verheule S, van Kempen MJ, Postma S, Rook MB, Jongsma HJ. Gap junctions in the rabbit sinoatrial node. Am J Physiol Heart Circ Physiol. 2001;280(5):H2103–2115. doi: 10.1152/ajpheart.2001.280.5.H2103. [DOI] [PubMed] [Google Scholar]
- 17.Eisner DA, Kashimura T, Venetucci LA, Trafford AW. From the ryanodine receptor to cardiac arrhythmias. Circ J. 2009;73(9):1561–1567. doi: 10.1253/circj.cj-09-0478. [DOI] [PubMed] [Google Scholar]
- 18.Joung B, Tang L, Maruyama M, Han S, Chen Z, Stucky M, Jones LR, Fishbein MC, Weiss JN, Chen PS, Lin SF. Intracellular calcium dynamics and acceleration of sinus rhythm by beta-adrenergic stimulation. Circulation. 2009;119(6):788–U751. doi: 10.1161/CIRCULATIONAHA.108.817379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maltsev VA, Lakatta EG. Dynamic interactions of an intracellular Ca2+ clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovasc Res. 2008;77(2):274–284. doi: 10.1093/cvr/cvm058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.