Abstract
Aims
Humanin (HN) is a 24-amino acid peptide that has been shown to have an anti-apoptotic function against neuronal cell death caused by Alzheimer's disease. Increased oxidative stress, one of the major factors contributing to this cell death, also plays an important role in the inflammatory process of atherosclerosis. The current study was designed to test the hypothesis that HN is expressed in the human vascular wall and may protect against oxidative stress.
Methods and results
HN expression in the vascular wall was detected by immunostaining in the endothelial cell layer of human internal mammary arteries (n = 5), atherosclerotic coronary arteries (n = 17), and sections of the greater saphenous vein (n = 3). HN mRNA was expressed in the human aortic endothelial cells (HAECs). Cytoprotective effects of HN against oxidative stress were tested in vitro in HAECs. Pre-treatment with 0.1 µM HN reduced oxidized LDL (Ox-LDL)-induced (i) formation of reactive oxygen species by 50%, (ii) apoptosis by ∼50% as determined by TUNEL staining, and (iii) formation of ceramide, a lipid second messenger involved in the apoptosis signalling cascade, by ∼20%.
Conclusion
The current study demonstrates for the first time the expression of HN in the endothelial cell layer of human blood vessels. Exogenous addition of HN to endothelial cell cultures was shown to be effective against Ox-LDL-induced apoptosis. These findings suggest that HN may play a role and may have a protective effect in early atherosclerosis in humans.
Keywords: Humanin, Atherosclerosis, Oxidized LDL, Oxidative stress
1. Introduction
Humanin (HN) is a recently discovered peptide isolated from brain tissue of patients with Alzheimer's disease (AD) where it was found to suppress neuronal cell death.1,2 The protective mechanism of HN is not yet known. However, in recent studies, the direct interaction of exogenously added HN with different receptors involved in survival pathways has been described.3,4 Other studies have shown that intracellular expression of HN interferes with the activation of pro-apoptotic proteins such as Bid and Bax,5 thereby suggesting that HN may act via different pathways in modulating programmed cell death.
Extensive literature links AD pathogenesis to oxidative stress damage.6 It has been shown that β-amyloid peptide (Abeta), which causes neuronal cell death in AD, results in an increase in radical mediated damage.7 Subsequent studies confirmed the cytoprotective effects of HN in various other cell types where oxidative stress-induced cell death was significantly reduced in the presence of HN.8
Similar to AD, oxidative stress in atherosclerosis has been shown to play an important role in mediating cell damage. During the early stages of atherosclerosis, the endothelial cell layer in arterial vessels is damaged resulting in penetration of LDL into the subendothelial space. Endothelial cells and smooth muscle cells are the initial source of reactive oxygen species (ROS), which oxidatively modifies LDL to form oxidized-LDL (Ox-LDL). Ox-LDL itself amplifies the generation of ROS, which finally results in enhanced oxidative stress, inflammation, and the formation of atherosclerotic plaques.9–11 Finally, high levels of ROS can result in the arrest of cell cycle and ultimately lead to apoptosis.12–14
Interestingly, Ox-LDL also increases the levels of cellular ceramide, a lipid second messenger that initiates a signalling cascade leading to apoptosis (for reviews, see refs15–17).
The role of HN as a survival factor in AD led us to the hypothesis that HN may also play a role in protecting endothelial cells under conditions of oxidative stress such as high ROS levels which related to atherogenesis. Thus, in order to address this hypothesis, we investigated the presence of HN in the human vascular wall and its potential to protect human aortic endothelial cells (HAECs) responding to oxidative stress.
2. Methods
2.1. Human vessel tissue
This study was approved by the Mayo Clinic Institutional Review Board and utilized coronary arteries obtained at autopsy at the Mayo Clinic from patients who died from fatal coronary events. Additional samples were obtained from the left internal mammary artery (LIMA) and the great saphenous vein (GSV) as non-diseased reference tissues. These were obtained from normal remnant vessels used for coronary bypass surgeries. The investigation conforms with the principles outlined in the Declaration of Helsinki.
2.2. Plasma HN measurement
For human plasma HN level, samples were collected from 32 healthy volunteers. Plasma HN was measured using an in-house assay as reported previously.18
2.3. Immunohistochemistry
Immunohistochemistry (IHC) staining was performed as described.19 Briefly, following deparaffinization in xylene, the array sections were rehydrated in graded alcohols. The sections were placed for 20 min in a 95°C solution of citric acid, pH 6, for antigen retrieval. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol at room temperature for 30 min. Then, samples were incubated with ‘Protein Block Serum Free’ (DakoCytomation, Carpinteria, CA, USA) to block non-specific staining. The primary antibody (IgA column-purified polyclonal rabbit anti-human HN antibody4) was applied at a final concentration of 12.5 µg/mL and incubated at 4°C overnight.
Horseradish peroxidase-labelled polymer conjugated to a secondary anti-rabbit antibody was applied for 30 min followed by incubation with diaminobenzidine + substrate–chromogen (DakoCytomation) for 10 min. The sections were counterstained with haematoxylin followed by dehydration and mounting. The human testis was used as a positive staining control tissue.20 Rabbit serum (Sigma) was applied to negative controls instead of primary antibody.
2.4. Cell culture and reagents
HAECs were maintained in M200 medium containing low serum growth supplement and 1% penicillin–streptomycin (Invitrogen, Carlsbad, CA, USA). HAECs were used between Passages 4 and 7. Cells were seeded on gelatin-coated glass cover slips and incubated as required in media with or without HN.
2.5. Reverse transcription–polymerase chain reaction
Total RNA from HAECs was extracted with RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA) and reverse transcription (RT) was performed with Superscript® III First-Strand Synthesis System (Invitrogen). Polymerase chain reaction (PCR) was performed with forward primer: CCGCGGTACCCTAACCGTGC and reverse primer: ACGGGGGAAGGCGCTTTGTG. Primers were designed by using Primer-BLAST of NIH.
2.6. ROS production
Intracellular ROS production was monitored by following the conversion of the oxidant-sensitive dye dihydroethidine (DHE, Invitrogen) to fluorescent ethidium.21 HAECs were plated on glass cover slips and incubated overnight with HN (0.1 and 0.01 µM) or culture medium. For ROS measurement, cells were incubated with 10 µM DHE in Hanks' balanced salt solution buffer containing calcium, magnesium, and glucose for 30 min at 37°C. Then, cells were stimulated with Ox-LDL (100 µg/ml; Biomedical Technologies Inc., Stoughton, MA, USA) for 30 min at 37°C.21 Fluorescence images were taken with an inverted fluorescence microscope (IX70; Olympus) at ×15 magnification using appropriate filters for ethidium fluorescence. Ten to 12 non-overlapping images in each culture dish were acquired and quantified by image analysis using Metamorph software (Molecular Devices, Sunnyvale, CA, USA). Only cells whose fluorescence exceeded twice the basal level of fluorescence of non-treated cells were used for quantitation of DHE fluorescence. Values are expressed as average relative fluorescence units (RFU) for n ≥ 100 cells per experimental condition in two independent experiments.
2.7. Apoptosis
HAECs plated on glass cover slips were incubated overnight ±0.1 µM HN. Cells were then further incubated with 100 µg/mL Ox-LDL for 6 h at 37°C in the absence of HN. Apoptotic cells were quantified by TUNEL staining using the In Situ Cell Death Detection Kit (Roche Applied Science, Indianapolis, IN, USA) as described,22 mounted in SlowFade containing DAPI (Invitrogen), and observed under the fluorescence microscope. Apoptotic cells were defined based on the morphological changes of the nuclei and the fluorescence intensity after TUNEL staining. The apoptotic index = (number of apoptotic cells)/(total number of cells).
2.8. Lipid extraction and mass spectrometry of ceramide molecular species
HAECs were treated overnight with 0.1 µM HN or cell culture medium alone and subsequently exposed for 6 h to 100 µg/mL Ox-LDL as described above. Cells were then scraped in phosphate-buffered saline, pelleted, and frozen in liquid nitrogen. The samples were sent to MUSC-lipdomics core (Lipidomics Analytical Unit, Charleston, SC, USA) and processed as described previously in order to detect and quantify individual ceramide molecular species.23
2.9. Antibodies and peptides
Rabbit polyclonal anti-HN antibody was generated by Harlan Bioproducts of Science (Madison, WI, USA). The HN peptide (glycine variant1) and a scrambled HN peptide were synthesized by Peptide International (Louisville, KY, USA) or Genemed Synthesis Biotechnologies (South San Francisco, CA, USA).
2.10. Statistical analysis
Data were expressed as mean ± SEM. A comparison of different groups was performed by one-way ANOVA. Two group comparisons were made by Student's t-test. A value of P < 0.05 was considered significant.
3. Results
3.1. HN is expressed in endothelial cells in human arteries and veins
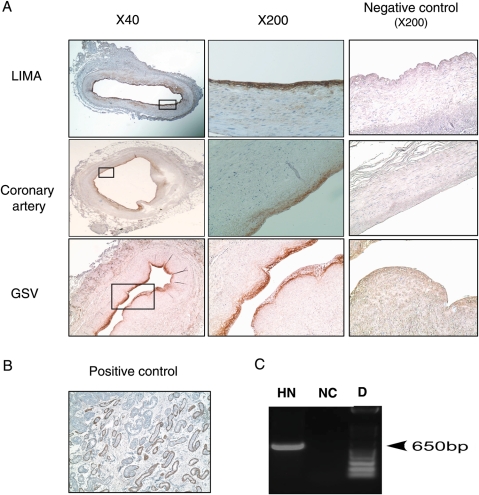
Because one of the hallmarks of atherosclerosis is the presence of enhanced oxidative stress, we were interested to determine whether HN is endogenously expressed in the atherosclerotic human vasculature. In IHC staining of sections of coronary arteries obtained from patients with fatal coronary events, we detected HN expression in 13 of 17 samples (Figure 1A). HN was also detected in all segments of the LIMAs (n = 5; Figure 1A), which served as non-diseased reference vessels, and in all of the segments of the saphenous vein (GSV) (n = 3; Figure 1A). Immunoreactivity was detected in the endothelial cell layer of both diseased and disease-free control samples, whereas no signal could be detected in the smooth muscle cell layer. As a positive control, we used young male testis. HN expression in Leydig cells of young male testis is shown in Figure 1B. LIMA, coronary artery, and GSV incubated with rabbit serum instead of HN antibody were used as negative controls (Figure 1A). To confirm HN expression in endothelial cells, we performed RT–PCR on cDNA from HAECs. Expression of HN mRNA was verified by RT–PCR in HAECs (Figure 1C). These results demonstrate that HN is present in the endothelial cell layer.
Figure 1.
HN expression in endothelial cells of human arteries and veins. (A) Immunostaining of HN (brown) at low and high magnification in normal LIMA, atherosclerotic coronary artery, and saphenous vein (GSV). Negative controls: LIMA, coronary artery, and GSV were incubated with rabbit serum instead of HN antibody. (B) HN is expressed in Leydig cells of young male testis (positive control). HN-positive cells are indicated by the brown staining at the site of the endothelium. (C) HN mRNA is expressed in HAECs. HN, RT–PCR performed on mRNA isolated from HAECs showed that HN mRNA is expressed in cultured endothelial cells. NC, negative control was performed whole reaction without DNA. D, DNA ladder.
3.2. Plasma HN level
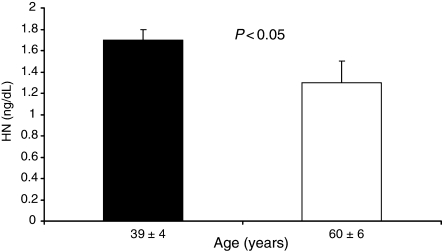
Healthy volunteers were divided into two groups according to age. The number of each group was 16. The average age of the younger group was 39 ± 4 and that of the older group was 60 ± 6. Plasma HN level was significantly lower in the older group (1.3 ± 0.2 ng/mL) than that of the younger group (1.7 ± 0.1 ng/mL) (Figure 2).
Figure 2.
Plasma HN level of healthy volunteers according to age. The older group showed significantly lower plasma HN level than the younger group.
3.3. HN attenuates Ox-LDL-induced ROS production in HAECs
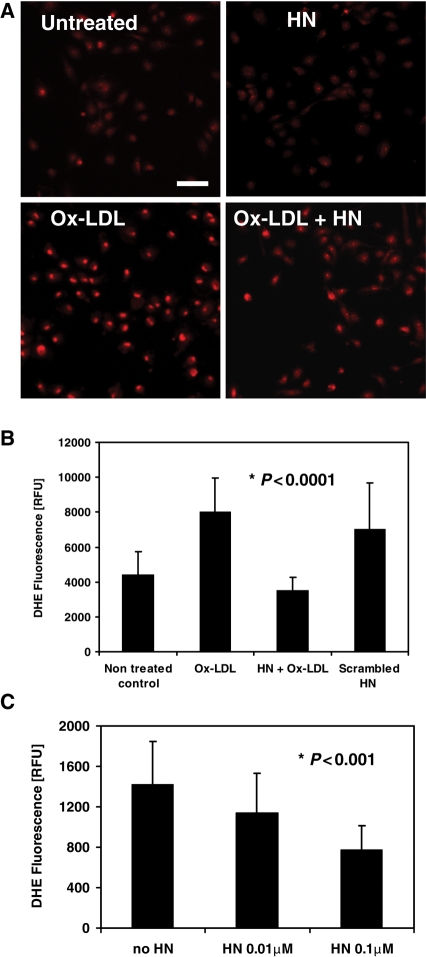
Previously, it was shown that treatment of vascular endothelial cells with Ox-LDL stimulates ROS formation.13 In order to test whether HN has any effect on Ox-LDL-induced ROS production in endothelial cells, we used the fluorescent probe DHE for detecting the presence of superoxide in living cells.24 DHE fluorescence intensity was measured in HAECs pre-incubated with HN or culture media overnight and were subsequently exposed to Ox-LDL to trigger ROS formation (Figure 3A). Pre-treatment with HN prior to Ox-LDL exposure caused a significant decrease in ROS production of ∼50%, whereas a scrambled HN peptide did not show a significant reduction of ROS formation (Figure 3B). In addition, we found that the decrease in Ox-LDL-induced ROS formation by HN occurred in a dose-dependent manner (Figure 3C).
Figure 3.
HN inhibits Ox-LDL-induced ROS formation in HAECs. HAECs were incubated overnight in the absence or presence of 0.1 µM HN, treated with DHE for 30 min, and exposed to 100 µg/mL Ox-LDL for another 30 min. Fluorescence images of 10–12 non-overlapping fields were taken at low magnification (×15). Bar, 100 µm. (B) Quantification of DHE fluorescence in individual HAECs photographed at high magnification (×90). Data are shown for non-treated control cells, or cells treated with 100 µg/mL Ox-LDL, 0.1 µM HN, or 0.1 µM scrambled HN peptide. (C) HAECs were pre-incubated overnight with 0.01 or 0.1 µM HN, treated with DHE and Ox-LDL as described in (B) and fluorescence was quantified. In (C), DHE fluorescence is expressed as average RFU (n ≥ 100 cells per experimental condition in two independent experiments).
3.4. HN attenuates Ox-LDL-induced apoptosis in HAECs
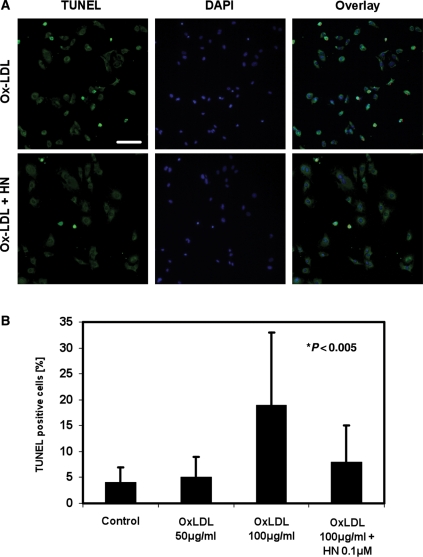
In the later stages of the atherosclerotic disease, the ongoing formation of ROS results in induction of apoptosis in the vascular wall. We next investigated whether HN could also prevent HAECs from undergoing apoptosis after Ox-LDL exposure. Using TUNEL staining, we detected a basal rate of apoptosis in untreated HAEC cultures of 1–5%. HAECs, which were incubated with Ox-LDL alone for 6 h, showed a concentration-dependent increase in apoptosis from 5 ± 4 to 19.4 ± 14% apoptotic cells after treatment with 50 or 100 µg/mL Ox-LDL, respectively. These findings are in agreement with previous reports.12 In contrast, HAECs that were pre-incubated overnight with 0.1 µM HN and subsequently exposed to 100 µg/mL Ox-LDL showed a decrease in apoptosis of more than 50% compared with cells without pre-treatment (Figure 4).
Figure 4.
HN inhibits Ox-LDL-induced apoptosis in HAECs. Cells were incubated overnight with 0.1 µM HN followed by Ox-LDL treatment for 6 h, fixed, and subjected to TUNEL staining. Nuclei were visualized by DAPI staining and cells were imaged by epifluorescence microscopy. TUNEL-positive cells are shown in green and nuclei in blue. (A) Upper panels: HAECs were treated with Ox-LDL without HN pre-incubation; (lower row) cells pre-treated overnight with HN followed by Ox-LDL incubation. Bar, 100 µm. (B) Quantification of TUNEL staining in cells that were left untreated (Control), or incubated with 50 or 100 µg/mL Ox-LDL, or with 100 µg/mL Ox-LDL after an overnight pre-incubation with HN. All incubations with Ox-LDL were for 6 h.
3.5. HN decreases cellular ceramide levels
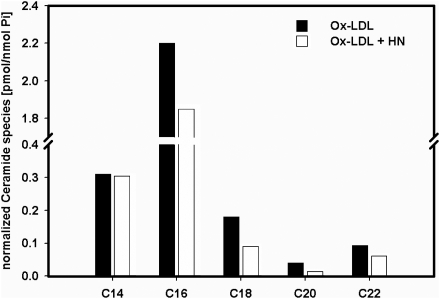
We also carried out experiments to determine whether HN pre-treatment would influence ceramide levels during Ox-LDL-triggered oxidative stress since Ox-LDL is known to increase the levels of cellular ceramide.15–17 HAECs were treated or untreated overnight with HN as above, followed by a subsequent incubation with Ox-LDL for 6 hrs. Cells were then harvested and the ceramide molecular species present in the lipid extracts of HAECs were determined by HPLC/MS/MS as described.23 Our results demonstrate a decrease in total cellular amount of ceramides in cells pre-treated overnight with HN (Figure 5). Furthermore, C16-ceramide which is known to be involved in apoptosis was decreased in cells pre-treated with HN and subsequently exposed to Ox-LDL.
Figure 5.
Mass spectrometry analysis of ceramide species in HAECs. HAECs were incubated overnight with or without with 0.1 µM HN followed by an incubation with 100 µg/mL Ox-LDL for 6 h. Cells were then harvested and ceramide species were analysed by mass spectrometry (HPLC/MS/MS) from lipid extracts of the cells (see Section 2).
4. Discussion
The current studies demonstrate for the first time that HN is expressed in the endothelial cell layer of the vascular wall of human arteries and veins. Moreover, in a cell culture model employing HAECs, pre-treatment with HN attenuated Ox-LDL-induced ROS formation and apoptosis by 50%. The current study may support a potential role for HN in the defence mechanism in cardiovascular disease.
HN was originally identified as an endogenous peptide that protects neuronal cells from apoptosis induced by various types of AD insults. Subsequently, a broad range of protective effects of HN against AD-related insults was discovered; yet, the detailed cytoprotective mechanism is still obscure. Although not aetiologically related to each other, AD and atherosclerosis have in common an enhanced oxidative stress that occurs during progression of the disease, leading to extensive cell damage, and finally resulting in cell death. Thus, the focus of our research was to investigate the protective effects of HN in human vasculature involved in atherosclerotic plaques and coronary events.
Here, we were able to demonstrate by IHC for the first time that HN is expressed in the endothelial cell layer of the vascular wall of human arteries and veins. HN is also expressed in cultured HAECs as shown by RT–PCR. These results confirm that HN is expressed in the endothelial cell layer. Moreover, HN attenuated the generation of Ox-LDL-induced ROS formation and apoptosis by 50% in HAECs treated with Ox-LDL. Taken together, our results on HN expression in human endothelial cells as well as the cytoprotective effect of exogenously added HN on HAECs suggest that HN may play a critical role in the defensive anti-oxidative mechanism of the vascular system.
Moreover, we demonstrated that HN is detected in human plasma and their levels were lower with age which is usually associated with an increase in oxidative stress and the decline in endothelial cell function.
The mechanism by which HN exerts its cytoprotective effect is unknown. Several studies describe the binding of exogenously supplied HN to cell surface receptors which are involved in survival pathways of the cells,3,4 but intracellular HN can also inhibit the pro-apoptotic effects of Bax by direct protein–protein interaction.25 This is of particular interest, since Bax is involved in the induction of Ox-LDL-mediated apoptosis in endothelial cells.26
Among other factors, Bax activation has been shown to be mediated by ceramide, a major sphingosine-based lipid second messenger.15–17 Ceramide is involved in several signalling pathways of cellular growth and differentiation and is a critical mediator involved in extracellular signalling cell apoptosis.15–17,27 Various exogenous oxidants or conditions known to promote elevated cellular levels of ROS also induce ceramide generation.28 Ceramide can be formed via the salvage pathway by sphingomyelinases, hydrolysing sphingomyelin to ceramide or the de novo synthetic pathway by ceramide synthases. Because ceramide has been suggested to play a role in Bax activation29,30 and HN was suggested to interfere with Bax, we examined the cross-talk between ceramide production and HN protection mechanism after a 6 h exposure to Ox-LDL.
We have shown that Ox-LDL causes the increase in several molecular species of ceramide, among them C16-ceramide, a molecular species that has been specifically associated with the signalling cascade leading to apoptosis.31,32 Pre-treatment of cells with HN before exposure to Ox-LDL decreased total cellular levels of ceramide, especially C16-ceramide. This result leads us to speculate that the anti-apoptotic mechanism for HN results from its inhibition of ceramide production. A possible mechanism could involve the inhibition of sphingomyelinase activation or alter its translocation to the plasma membrane, thereby inhibiting ceramide generation. However, we cannot exclude a direct inactivation of Bax by HN and more studies are needed to investigate the exact mechanism by which HN exerts its protective effect against Ox-LDL.
The observations in the current study that HN may exert cellular protective effect may have important clinical implications. It can be speculated that in vivo delivery of HN in situations associated with high oxidative stress may have a beneficial effect. Indeed, Xu et al.33 demonstrated that administration of HN in vivo protects against cerebral ischaemia/reperfusion injury in mice. Intracerebrovascular or intraperitoneal administration of HN was also shown to be potent in reversing learning and memory impairment in rats modelled for AD,34,35 whereas in the cardiovascular system, pre-treatment of mouse heart with HN protected against ischaemia/reperfusion injury.36
The current study suffers from several limitations. In this study we did not explore in details all the potential mechanisms by which HN may exerts its effect. Although we suggest that one of the mechanisms may be through a ceramide, other mechanisms of protective effect of HN in atherosclerosis may exist. The current study demonstrated the effect of HN in vitro and do not necessarily prove that acute or long-term administration of HN can reverse the disease process. Further studies are needed to clarify the mechanism of protective effect of HN and whether HN also has a protective effect against atherosclerosis in vivo.
In summary, our study is the first to demonstrate that HN is expressed at the vascular wall in humans and may have an important protective role in atherosclerosis by attenuating oxidative stress by reducing ROS production. HN may play an important role in the response of the vascular system to various insults and may open a window of opportunity to discover new therapeutic approaches to attenuate the process of atherosclerosis.
Conflict of interest: none declared.
Funding
The study was supported by the National Institutes of Health (R01 HL63911, R01 HL77131, RO1 DK73608, P01 HL85307, and GM-22942 to R.E.P.) and an American Heart Association post-doctoral fellowship to L.S.
References
- 1.Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc Natl Acad Sci USA. 2001;98:6336–6341. doi: 10.1073/pnas.101133498. doi:10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niikura T, Hashimoto Y, Tajima H, Nishimoto I. Death and survival of neuronal cells exposed to Alzheimer's insults. J Neurosci Res. 2002;70:380–391. doi: 10.1002/jnr.10354. doi:10.1002/jnr.10354. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor α/WSX-1/gp130. Mol Biol Cell. 2009;20:2864–2873. doi: 10.1091/mbc.E09-02-0168. doi:10.1091/mbc.E09-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, et al. Interaction between the Alzheimer's survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci USA. 2003;100:13042–13047. doi: 10.1073/pnas.2135111100. doi:10.1073/pnas.2135111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi J, Zhai D, Zhou X, Satterthwait A, Reed JC, Marassi FM. Mapping the specific cytoprotective interaction of humanin with the pro-apoptotic protein bid. Chem Biol Drug Des. 2007;70:383–392. doi: 10.1111/j.1747-0285.2007.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beal MF. Oxidative damage as an early marker of Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2005;26:585–586. doi: 10.1016/j.neurobiolaging.2004.09.022. doi:10.1016/j.neurobiolaging.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Markesbery WR. The role of oxidative stress in Alzheimer disease. Arch Neurol. 1999;56:1449–1452. doi: 10.1001/archneur.56.12.1449. doi:10.1001/archneur.56.12.1449. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z, Zhang Q, Ge J, Tan Z. Protective effects of tetramethylpyrazine on rat retinal cell cultures. Neurochem Int. 2008;52:1176–1187. doi: 10.1016/j.neuint.2007.12.008. doi:10.1016/j.neuint.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. doi:10.1161/01.ATV.0000051384.43104.FC. [DOI] [PubMed] [Google Scholar]
- 10.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 11.Galle J, Hansen-Hagge T, Wanner C, Seibold S. Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis. 2006;185:219–226. doi: 10.1016/j.atherosclerosis.2005.10.005. doi:10.1016/j.atherosclerosis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Li D, Mehta JL. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells: evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler Thromb Vasc Biol. 2000;20:1116–1122. doi: 10.1161/01.atv.20.4.1116. [DOI] [PubMed] [Google Scholar]
- 13.Zmijewski JW, Moellering DR, Le Goffe C, Landar A, Ramachandran A, Darley-Usmar VM. Oxidized LDL induces mitochondrially associated reactive oxygen/nitrogen species formation in endothelial cells. Am J Physiol Heart Circ Physiol. 2005;289:852–861. doi: 10.1152/ajpheart.00015.2005. [DOI] [PubMed] [Google Scholar]
- 14.Yung LM, Leung FP, Yao X, Chen ZY, Huang Y. Reactive oxygen species in vascular wall. Cardiovasc Hematol Disord Drug Targets. 2006;6:1–19. doi: 10.2174/187152906776092659. [DOI] [PubMed] [Google Scholar]
- 15.Haimovitz-Friedman A, Kolesnick RN, Fuks Z. Ceramide signaling in apoptosis. Br Med Bull. 1997;53:539–553. doi: 10.1093/oxfordjournals.bmb.a011629. [DOI] [PubMed] [Google Scholar]
- 16.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. doi:10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 17.Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta. 2002;1585:114–125. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- 18.Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, et al. Humanin: a novel central regulator of peripheral insulin action. PLoS ONE. 2009;4:e6334. doi: 10.1371/journal.pone.0006334. doi:10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrmann J, Edwards WD, Holmes DR, Jr, Shogren KL, Lerman LO, Ciechanover A, et al. Increased ubiquitin immunoreactivity in unstable atherosclerotic plaques associated with acute coronary syndromes. J Am Coll Cardiol. 2002;40:1919–1927. doi: 10.1016/s0735-1097(02)02564-0. doi:10.1016/S0735-1097(02)02564-0. [DOI] [PubMed] [Google Scholar]
- 20.Colon E, Strand ML, Carlsson-Skwirut C, Wahlgren A, Svechnikov KV, Cohen P, et al. Anti-apoptotic factor humanin is expressed in the testis and prevents cell-death in leydig cells during the first wave of spermatogenesis. J Cell Physiol. 2006;208:373–385. doi: 10.1002/jcp.20672. doi:10.1002/jcp.20672. [DOI] [PubMed] [Google Scholar]
- 21.Deng T, Xu K, Zhang L, Zheng X. Dynamic determination of Ox-LDL-induced oxidative/nitrosative stress in single macrophage by using fluorescent probes. Cell Biol Int. 2008;32:1425–1432. doi: 10.1016/j.cellbi.2008.08.013. doi:10.1016/j.cellbi.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Hasdai D, Sangiorgi G, Spagnoli LG, Simari RD, Holmes DR, Jr, Kwon HM, et al. Coronary artery apoptosis in experimental hypercholesterolemia. Atherosclerosis. 1999;142:317–325. doi: 10.1016/s0021-9150(98)00249-4. doi:10.1016/S0021-9150(98)00249-4. [DOI] [PubMed] [Google Scholar]
- 23.Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. doi:10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Ermak N, Lacour B, Drüeke TB, Vicca S. Role of reactive oxygen species and Bax in oxidized low density lipoprotein-induced apoptosis of human monocytes. Atherosclerosis. 2007;200:247–256. doi: 10.1016/j.atherosclerosis.2007.12.052. doi:10.1016/j.atherosclerosis.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 25.Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, et al. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. doi:10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Mehta JL, Haider N, Zhang X, Narula J, Li D. Role of caspases in Ox-LDL-induced apoptotic cascade in human coronary artery endothelial cells. Circ Res. 2004;94:370–376. doi: 10.1161/01.RES.0000113782.07824.BE. doi:10.1161/01.RES.0000113782.07824.BE. [DOI] [PubMed] [Google Scholar]
- 27.Ruvolo PP. Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol Res. 2003;47:383–392. doi: 10.1016/s1043-6618(03)00050-1. doi:10.1016/S1043-6618(03)00050-1. [DOI] [PubMed] [Google Scholar]
- 28.Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic Biol Med. 2001;31:717–728. doi: 10.1016/s0891-5849(01)00655-4. doi:10.1016/S0891-5849(01)00655-4. [DOI] [PubMed] [Google Scholar]
- 29.Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem J. 2005;386:445–451. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh HL, Seok JY, Kwon CH, Kang SK, Kim YK. Role of MAPK in ceramide-induced cell death in primary cultured astrocytes from mouse embryonic brain. Neurotoxicology. 2006;27:31–38. doi: 10.1016/j.neuro.2005.05.008. doi:10.1016/j.neuro.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Osawa Y, Uchinami H, Bielawski J, Schwabe RF, Hannun YA, Brenner DA. Roles for C16-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-alpha. J Biol Chem. 2005;280:27879–27887. doi: 10.1074/jbc.M503002200. doi:10.1074/jbc.M503002200. [DOI] [PubMed] [Google Scholar]
- 32.Seumois G, Fillet M, Gillet L, Faccinetto C, Desmet C, Francois C, et al. De novo C16- and C24-ceramide generation contributes to spontaneous neutrophil apoptosis. J Leukoc Biol. 2007;81:1477–1486. doi: 10.1189/jlb.0806529. doi:10.1189/jlb.0806529. [DOI] [PubMed] [Google Scholar]
- 33.Xu X, Chua CC, Gao J, Hamdy RC, Chua BH. Humanin is a novel neuroprotective agent against stroke. Stroke. 2006;37:2613–2619. doi: 10.1161/01.STR.0000242772.94277.1f. doi:10.1161/01.STR.0000242772.94277.1f. [DOI] [PubMed] [Google Scholar]
- 34.Kunesova G, Hlavacek J, Patocka J, Evangelou A, Zikos C, Benaki D, et al. The multiple T-maze in vivo testing of the neuroprotective effect of humanin analogues. Peptides. 2008;29:1982–1987. doi: 10.1016/j.peptides.2008.06.019. doi:10.1016/j.peptides.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Mamiya T, Ukai M. [Gly14]-Humanin improved the learning and memory impairment induced by scopolamine in vivo. Br J Pharmacol. 2001;134:1597–1599. doi: 10.1038/sj.bjp.0704429. doi:10.1038/sj.bjp.0704429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao J, Chua CC, Wang H, Hamdy RC, Chua BH. Abstract 1612: humanin is a novel protector against myocardial infarction. (Abstract) Circulation. 2006;114:II131. [Google Scholar]