Abstract
Aims
Cardiac malformations are prevalent in trisomies of human chromosome 21 [Down's syndrome (DS)], affecting normal chamber separation in the developing heart. Efforts to understand the aetiology of these defects have been severely hampered by the absence of an accurate mouse model. Such models have proved challenging to establish because synteny with human chromosome Hsa21 is distributed across three mouse chromosomes. None of those engineered so far accurately models the full range of DS cardiac phenotypes, in particular the profound disruptions resulting from atrioventricular septal defects (AVSDs). Here, we present analysis of the cardiac malformations exhibited by embryos of the transchromosomic mouse line Tc(Hsa21)1TybEmcf (Tc1) which contains more than 90% of chromosome Hsa21 in addition to the normal diploid mouse genome.
Methods and results
Using high-resolution episcopic microscopy and three-dimensional (3D) modelling, we show that Tc1 embryos exhibit many of the cardiac defects found in DS, including balanced AVSD with single and separate valvar orifices, membranous and muscular ventricular septal defects along with outflow tract and valve leaflet abnormalities. Frequencies of cardiac malformations (ranging from 38 to 55%) are dependent on strain background. In contrast, no comparable cardiac defects were detected in embryos of the more limited mouse trisomy model, Dp(16Cbr1-ORF9)1Rhr (Ts1Rhr), indicating that trisomy of the region syntenic to the Down's syndrome critical region, including the candidate genes DSCAM and DYRK1A, is insufficient to yield DS cardiac abnormalities.
Conclusion
The Tc1 mouse line provides a suitable model for studying the underlying genetic causes of the DS AVSD cardiac phenotype.
Keywords: Down syndrome, Mouse model, 3D modelling, AVSD
1. Introduction
Down's syndrome (DS) results from trisomy of human chromosome 21 (Hsa21) and in the absence of first trimester screening and occurs in ∼1 in 800 live births.1 A complex disorder, DS, is characterized by many phenotypic features and 40–60% of individuals with DS have some congenital heart defect (CHD). These range from defects in chamber separation to more complex abnormalities affecting multiple aspects of cardiac anatomy (e.g. tetralogy of Fallot). The most common abnormalities affect the atrioventricular (AV) junction,2 indicating an effect of trisomy on early heart morphogenesis.
Understanding the aetiology of DS phenotypes has proved challenging not only because of the large number of trisomic genes, but also because the majority of DS phenotypes (including those affecting the heart) are partially penetrant, varying in severity between individuals. Rare cases of segmental trisomy 21 have been used in efforts to define a ‘critical region’ responsible for major DS phenotypes3,4 and to correlate trisomy of specific genes/regions with specific aspects of DS.5
Mouse models of DS offer an alternative approach, holding the promise of experimental intervention to test phenotype–genotype interactions. However, since the mouse orthologues to genes on Hsa21 are found on three different mouse chromosomes, Mus musculus (Mmu) 16, 17, and 10, generating mouse models of DS has proved complex.6 To overcome the difficulties inherent in this dispersal of synteny, a transchromosomic mouse containing Hsa21 has been generated.7 The Tc(Hsa21)1TybEmcf (Tc1) mouse contains a freely segregating, and near complete, copy of Hsa21 which is retained mosaically throughout the tissues of the mouse and exhibits a broad range of DS-like phenotypes, including craniofacial abnormalities, motor coordination deficits, and cognitive impairment.
A striking feature of the cardiac abnormalities associated with DS is the prevalence of malformations affecting the entire AV junction. In the DS AV septal defect (AVSD), the primary malformation is the retention of a common AV junction, balanced in its connection to the right and left ventricles. This disrupts the normal arrangement of leaflets that form the mitral and tricuspid valves and is generally associated with septal defects that result in shunting at the atrial or ventricular level.8 None of the mouse models based on complete or partial trisomies of Mmu16 described so far have been reported to recapitulate this most complex and common DS cardiac malformation.9,10
One difficulty in identifying and characterizing such defects is the complex and rapidly changing morphology of the mammalian heart during the period in which the looped heart tube is transformed into a chambered organ. Visualizing initial defects in AV cushion formation and fusion requires histological levels of resolution, but using a two-dimensional method to examine a complex, three-dimensional (3D) transformation has its own inherent limitations. Equally challenging, the variable penetrance of trisomy phenotypes requires analysis of significant embryo numbers. To overcome both problems, we have utilized high-resolution episcopic microscopy (HREM) as a rapid way to obtain highly detailed 3D models of mouse embryo hearts, while retaining the ability to examine individual tissue sections by histology.11 We have used this approach to analyse the frequency and type of cardiac malformations detected in transchromosomic Tc1 embryos.
2. Methods
2.1. Mouse lines
All animal work conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the NIMR Ethical Review Panel.
Transchromosomic Tc(Hsa21)1TybEmcf (Tc1) mouse embryos and their wild-type littermates were obtained by crossing 129S8:C57BL/6J (F1) Tc1 females with 129S8:C57BL/6J (F1) males or C57BL/6J males and genotyping by polymerase chain reaction (PCR).7
Ts1Rhr mice12 are trisomic only for the region of Mmu16 syntenic with the 33 gene-containing region of Hsa21 termed the Down's syndrome critical region (DSCR).3,4 Ts1Rhr embryos and their wild-type littermates were obtained for analysis from crosses of C57BL/6J females and 129S8:C57BL/6J (F1) Ts1Rhr males (genotypes determined by PCR; see Supplementary material online).
2.2. HREM imaging, 3D modelling, and histology
Embryonic hearts were dissected from surrounding tissue and fixed for 30 min in 4% paraformaldehyde followed by a 1 h wash in distilled water and secondary fixation overnight. Samples were dehydrated and embedded in modified JB4 methacrylate resin.11 E14.5 and E18.5 hearts were sectioned at 2 and 3 µm, respectively. Selected sections were stained with haematoxylin and eosin, as described by the manufacturer (Sigma-Aldrich). HREM imaging (isometric resolutions of 2 or 3 µm) used a Jenoptik C14 plus CCD camera (1360 × 1024 pixels). Data sets were normalized and subsampled prior to 3D volume rendering using OsiriX 3.13
2.3. Classification of VSDs
In humans, VSDs have historically been classified in a number of different ways based variously on the position of the septal lesion (as viewed from the right ventricle) or on the anatomic nature of the lesion borders. Classification of the VSD subtype using a combination of both approaches (led by the border classification) has been advocated to give a clear categorization that is particularly useful in a clinical setting when preparing for surgical repair.14,15 We have used this approach to analyse the septal defects in mouse embryos, dividing the right view of the ventricular septum into membranous (M), outlet (O), inlet (I), and trabeculated (T) regions and distinguishing three subtypes of VSD by the nature of their borders: (i) perimembranous VSD, in which part of the border of the hole is formed by fibrous continuity between leaflets of an arterial valve and an AV valve; (ii) muscular VSD in which the holes in the ventricular septum have exclusively muscular borders; and (iii) lesions in which part of the border is formed by fibrous continuity between the leaflets of the arterial valves.
2.4. Classification of AVDSs
In humans, AVSD covers a spectrum of disorders, all of which have the essential morphological hallmark of a common AV junction guarded by a five-leaflet valve, with two ‘bridging’ leaflets lying across the ventricular septum.8 The bridging leaflets can be anchored to the atrial septum, more commonly the ventricular septum (ostium primum defect) or neither. They may also be joined by a tongue of valvar tissue, resulting in separate left and right orifices that remain within a common AV junction. Finally, the common AV junction may be shared equally between left and right ventricles (or ‘balanced’), or the ventricular septum can be shifted to give a spectrum of left or right ventricular dominance (hence termed ‘unbalanced’).
3. Results
3.1. E14.5 Tc1 embryos exhibit a high incidence of heart defects
Establishing an accurate mouse model of the cardiac defects associated with DS would be an important step towards identifying their underlying molecular lesions. In the initial report describing the Tc1 mouse,7 abnormalities in the embryonic heart were observed but their character was largely unexplored. Although a single E14.5 embryo showed failure in fusion of superior and inferior AV cushions indicative of an AV junction defect, neither the structure of this abnormality nor its similarity to DS AVSD was assessed. By using high-resolution episcopic microscopy, we have now been able to undertake a comprehensive analysis of Tc1 heart morphology, using 3D volume rendering and digital resectioning to compare the full spectrum of defects with those found in DS.
Embryos generated with C57BL/6J males had a higher phenotype incidence (55%, n = 49) than those generated with 129S8:C57BL/6J (F1) males (38%, n = 29), suggesting a higher susceptibility to heart defects in mice with C57BL/6J rich backgrounds. Heart phenotypes affected ventricular septation, outflow tract position, and AV canal development (Table 1), either alone or in combination with no other differences (such as chamber size or wall thickness) distinguishing trisomic embryos from their control littermates.
Table 1.
Cardiovascular defects are observed in Tc1 but not Ts1Rhr embryos
| Stage | Paternal background | Heart defects |
Normal hearts | % heart defects | |||
|---|---|---|---|---|---|---|---|
| Genotype | VS | AVC | OFT | ||||
| E14.5 | 129S8:C57BL/6J (F1) | Wild-type (n = 30) | 3 | 0 | 0 | 27 | 10.0 |
| Tc1 (n = 29) | 11 | 1 | 5 | 18 | 37.9§ | ||
| E14.5 | C57BL/6J | Wild-type (n = 51) | 7 | 0 | 1 | 44 | 13.7 |
| Tc1 (n = 49) | 26 | 15 | 12 | 22 | 55.1* | ||
| E18.5 | C57BL/6J | Wild-type (n = 22) | 0 | 0 | 0 | 22 | 0.0 |
| Tc1 (n = 33) | 7 | 1 | 1 | 26 | 21.2# | ||
| E14.5 | Wild-type (n = 22) | 0 | 0 | 0 | 22 | 0.0 | |
| Ts1Rhr (n = 19) | 1 | 0 | 0 | 18 | 5.3 | ||
A spectrum of heart malformations is observed in Tc1 hearts at E14.5 and E18.5, affecting the ventricular septum (VS), atrioventricular canal (AVC), and outflow tract (OFT). Ts1Rhr hearts were no different to controls.
Significant difference to corresponding wild-type incidence (Fisher's exact test): §P < 0.05, *P < 0.0001, and #P = 0.034.
3.2. Septal defects and abnormal arterial trunk arrangements in Tc1 embryos
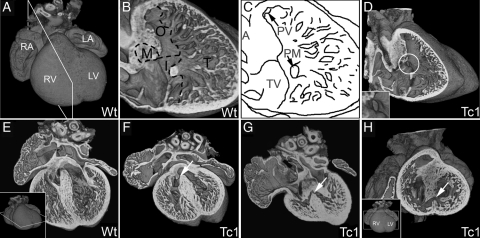
Forty-seven per cent of Tc1 samples (37 of 78 embryos) exhibited VSD (Table 1). Adopting the classification system15 advocated for clinical use (see Section 2), only two subtypes of VSD were observed in Tc1 embryonic hearts at E14.5: perimembranous VSD (Figure 1D and F; see Supplementary material online, Movie S1) positioned in the membranous region of the ventricular septum and muscular VSD (Figure 1G and I; see Supplementary material online, Movie S2) which always opened into the inlet region of the ventricular septum (Figure 1A–C). The two subtypes of VSD were observed alone, together, or in association with other heart malformations (see Supplementary material online, Table S1).
Figure 1.
E14.5 Tc1 embryos exhibit ventricular septal defects. Three-dimensional reconstructions of wild-type (A, B, and E) and Tc1 (D, F, G, and H) hearts, eroded in the planes indicated in insets. (B and C) Division of the ventricular septum (right view) into membranous (M), outlet (O), inlet (I), and trabeculated (T) regions. Many Tc1 samples have defects in the membranous region (white circle, D and inset). (E–G) Four-chambered view showing membranous VSD (white arrow, F) and muscular inlet VSD (white arrow, G; the defect has an exclusively muscular border and opens to the inlet region of the ventricular septum) in Tc1 hearts. (H) Short-axis view of a Tc1 muscular inlet VSD (white arrow, H). (LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; M, membranous region; O, outlet region; I, inlet region; T, trabeculated region; A, aorta; PV, pulmonary valve; PM, base of papillary muscle; TV, septal leaflet of tricuspid valve).
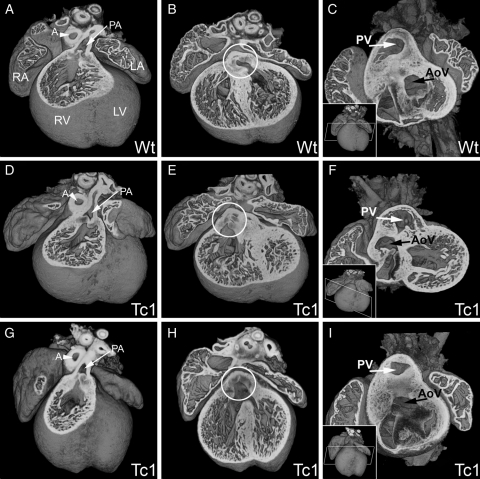
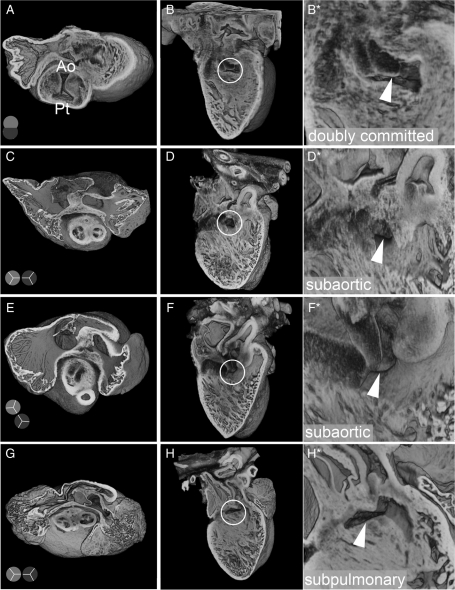
Defects in the outflow tract were also observed in 22% of Tc1 samples (17 of 78 embryos, Table 1). All of these defects were observed in association with VSD and the majority affected positioning of the aorta (Figure 2; see Supplementary material online, Movies S3 and S4). Several Tc1 samples showed an over-riding aorta, arising from a central position directly above a VSD (Figure 2E and F). Other Tc1 hearts exhibited double-outlet right ventricle (DORV), both pulmonary and aortic trunks arising from the right ventricle (Figure 2G–I). In all but one of the wild-type embryos from Tc1 litters, the septation and positioning of the outflow vessels was completed as expected by E14.5 (Figure 2A–C). Inspection of the relative positioning of arterial trunks, infundibular morphology, and relationship of interventricular communication to the subarterial outflow tracts revealed a variety of abnormal arrangements in samples exhibiting DORV (Figure 3). One Tc1 embryo showed failure of septation of the two outflow vessels (common arterial trunk), resulting in aortic-pulmonary continuity, with a doubly committed VSD (Figure 3A and B). Subaortic ventricular communication was associated with bilateral infundibulums and either side-by-side (Figure 3C and D) or normal arrangement of arterial trunks (Figure 3E and F). Subpulmonary communication was also found in association with side-by-side arterial trunks, equivalent to the Taussig–Bing anomaly in humans (Figure 3G and H).
Figure 2.
E14.5 Tc1 embryos exhibit outflow tract defects. Three-dimensional reconstructions of wild-type (A–C) and Tc1 (D–F, G–I) hearts. In all samples analysed, the pulmonary artery (PA, white arrow, A, D, and G) arose from the right ventricle (RV). In the wild-type, the aorta arises from the left ventricle (B and C), whereas in the Tc1 hearts, the position of the aortic valve (white circle, B, E, and H) is shifted rightwards. Tc1 embryos can exhibit DORV, both the pulmonary artery and the aorta arising from the right ventricle (E and F) and overriding aorta, the aortic valve (AV) sitting centrally above a VSD, resulting in subaortic interventricular communication (H and I). The models in C, F, and I have been eroded to the level of the white box indicated in the respective insets. (LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; PA, pulmonary artery; A, aorta; PV, pulmonary valve; AoV, aortic valve).
Figure 3.
Abnormal arterial trunk arrangements in E14.5 Tc1 embryos. Three-dimensional reconstructions of Tc1 hearts, showing abnormal arterial trunk arrangements associated with DORV. Models are eroded in the short axis through the atria at the level of the aortic valve (A, C, E, and G) and the relative position of the aortic and pulmonary trunks is shown in diagram (pale and dark grey, respectively). For each heart, a long-axis view of the interventricular septum from the right ventricle (B, D, F, and H) reveals the location of the septal defect (white circle), shown in detail in the adjacent panel (B*, D*, F*, and H*; white arrowhead). Abnormalities range from failure of outflow tract septation, resulting in aortic-pulmonary continuity, with a doubly committed VSD (A and B); subaortic ventricular communication with bilateral infundibulums and either side-by-side (C and D) or normal arrangement of arterial trunks (E and F); subpulmonary VSD with side-by-side arterial trunks, equivalent to the Taussig–Bing anomaly in humans (G and H). (Ao, aortic trunk; Pt, pulmonary trunk).
3.3. AV canal defects in Tc1 embryos
We also identified malformations of the AV junction (4%, 3 of 78 embryos) and valve leaflets of the AV canal (17%, 13 of 78 embryos) in E14.5 Tc1 embryos (21%, n = 78; Table 1). In mouse, septation of the AV junction separating the right and left sides of the AV canal should be complete by E14.5 (Table 1) with the outflow tract of the aorta occupying a ‘wedged’ position between the mitral valve and the AV septum (Figure 4A and D). Three of the Tc1 embryos examined showed disruption of these normal arrangements.
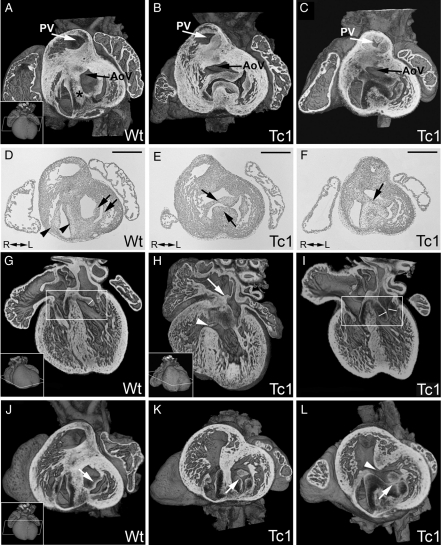
Figure 4.
E14.5 Tc1 embryos exhibit AV canal defects. Three-dimensional reconstructions of wild-type (A, G, and J) and Tc1 (B, C, H, I, K, and L) hearts eroded in the short or long axis (as indicated). (D–F) The corresponding sections (haematoxylin and eosin stain) from (A–C). In wild-type hearts, the two AV junctions are separated by the AV septum (black asterix, A) and the left outflow tract (black arrow, A) sits in a ‘wedged’ position between the mitral valve and AV septum. The tricuspid and mitral valves (D: black arrowheads and arrows, respectively) are therefore separate. In Tc1 hearts with AVSD (B, C, E, and F), a common AV junction can be guarded by a single valve (B) containing bridging leaflets (black arrows, E), or the bridging leaflets can be joined along the crest of the ventricular septum (black arrow, F), giving two valvar orifices within the common AV junction (C). In both cases, the ‘unwedged’ position of the left outflow tract is evident (black arrows, B and C). The AV bridging leaflets in Tc1 samples with AVSD are anchored to the atrial septum (white arrow, H) but not the ventricular septum (white arrowhead, H). Other Tc1 samples show fat enlarged valves within the AV junctions (compare valve leaflets in white boxes, G and I; leaflet thickness marked) or a cleft (or failure of fusion) in the anterior leaflet of the mitral valve (white arrows, K and L) either with (white arrowhead, L) or without (K) a VSD. (PV, pulmonary valve; AoV, aortic valve; R, right; L, left. Scale bars in D, E, and F represent 500 µm.)
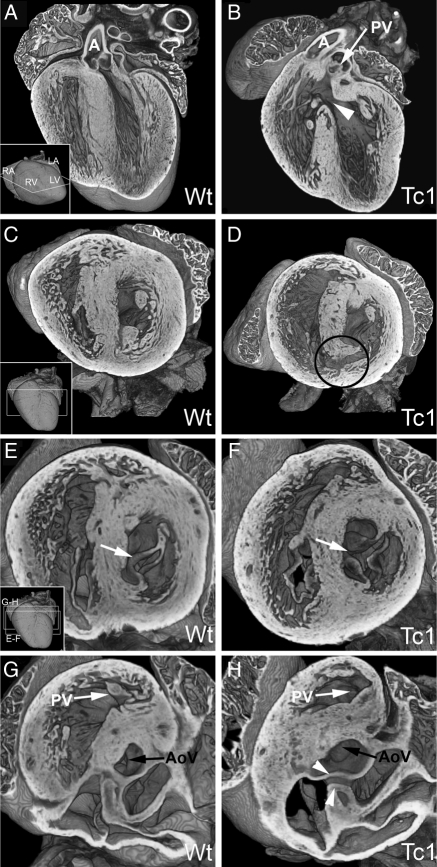
In Tc1 AVSD, we detected both balanced common (n = 1, Figure 4B and E; see Supplementary material online, Movie S5) and separate valvar orifices (n = 2; Figure 4C and F; see Supplementary material online, Movie S6). In all cases, the bridging leaflets were anchored to the atrial septum but not the ventricular septum, resulting in ventricular shunting (e.g. Figure 4H). Tc1 AVSD also showed the classic ‘unwedged’ morphology of the left outflow tract (n = 2; Figure 4B and C) compared with the ‘wedged’ position in a normal wild-type heart (Figure 4A and D). Other Tc1 embryos showed normal AV junction morphology but anomalies in valve leaflet morphology, showing either fat, enlarged leaflets (n = 7, Figure 4I), or a failure of fusion (cleft) in the aortic leaflet of the mitral valve (n = 6, Figure 4K and L). Together, these results demonstrate that at E14.5, Tc1 embryos show many of the AV canal defects associated with DS in humans.
3.4. DS-like heart defects in E18.5 Tc1 embryos
We also looked later in development at isolated hearts from E18.5 embryos, generated with C57BL/6J males. Again, a statistically significant number of Tc1 samples exhibited heart defects at E18.5 (21%, n = 33; Table 1) compared with wild-type littermates. All affected embryos had a VSD, either perimembranous (in the membranous region of the ventricular septum) or muscular (in the inlet region of the ventricular septum) (Figure 5B and D). One sample also showed a DORV with subpulmonary interventricular communication and an anterior aorta (Figure 5B; see Supplementary material online, Movie S7) and another had an AVSD comprising a balanced common AV junction, guarded by a common five-leaflet valve and an unwedged left outflow tract (Figure 5F and H; see Supplementary material online, Movies S8 and S9). As with AVSD at E14.5, bridging leaflets of the AV valve were anchored to the atrial but not the ventricular septum. The frequency of malformations detected at E18.5 was noticeably lower than that detected at E14.5 on the same genetic background (22 vs. 55%; Table 1). This difference is attributable at least in part to the reduced incidence of detectable VSD in the older hearts, suggesting that some of the septal defects detected at E14.5 are subsequently closed during later development. (In humans, small, asymptomatic VSDs have been detected by echocardiography in 5% of neonates, with the overwhelming majority of these closing spontaneously within 10 months.16) It may also be the result of reduced viability for embryos with more severely malformed hearts.
Figure 5.
E18.5 Tc1 embryos exhibit AVSD, ventricular septal, and outflow tract defects. Three-dimensional reconstructions of wild-type (A, C, E, and G) and Tc1 (B, D, F, and H) hearts eroded to the levels indicated in the insets. (B) A Tc1 membranous VSD (white arrowhead; four-chamber view) resulting in subpulmonary interventricular communication, both arterial roots arising side-by-side from the right ventricle (the Taussig–Bing malformation). (C–H) Short-axis view from apex. (D) A Tc1 muscular inlet VSD (black circle, D). The left AV valve in the Tc1 heart is trifoliate in comparison to the normal mitral valve (compare E and F, white arrows). This apparent cleft in the aortic leaflet can be traced in continuity with the two bridging leaflets (white arrowheads, H) guarding a common AV junction (see also Supplementary material online, Movie S9) (AoV, aortic valve; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; A, aorta; PV, pulmonary valve; R, right; L, left; VS, ventricular septum).
In human DS, phenotype apparently varies with the percentage of trisomic cells.17 In the Tc1 mouse, tissue retention of Hsa21 is also mosaic, with 20–50% of cells in the hearts of adult Tc1 mice retaining the Hsa21 transchromosome.7 Whether their distribution is clonal, dispersed, or localized preferentially to individual cardiac tissues is unknown since their identification by FISH has only proved possible with dispersed cells rather than tissue sections. Using expression of the Hsa21 gene SH3BGR18 as an alternative marker of Hsa21-retaining cells in the myocardium, we found no obvious association between distribution of Hsa21-expressing cells at either E14.5 or E18.5 and the presence of morphological abnormalities (see Supplementary material online, Figure S1).
3.5. Ts1Rhr mice do not exhibit cardiac malformations
Since Tc1 embryos show a range of cardiac malformations to those associated with DS, they provide a reference against which to compare the effect of more partial trisomies of Hsa21 genes or their murine counterparts. As a first step, we used HREM to assess the morphology of hearts isolated from Ts1Rhr embryos.12 Strikingly, in contrast to the Tc1 line, trisomy of only the 33 gene-containing region equivalent to the DSCR in the Ts1Rhr line results in no statistically significant increase in cardiac malformations compared with wild-type littermates (Table 1). This region is not therefore sufficient to cause the DS heart phenotype in mice.
4. Discussion
The nature of the cardiac abnormalities associated with DS suggests that trisomy affects cardiogenesis early, during chamber morphogenesis. Some of the existing mouse models for DS based on partial trisomies of Mmu16 do show cardiac abnormalities,9,10,19 but none show the hallmark DS malformation of AVSD. This is a critical test for a mouse model of DS, since the risk for this anomaly is increased 1000-fold among DS births and DS-associated AVSD accounts for 70% of all AVSD cases.20 AVSD abnormalities have been identified in embryos of Ts16,21 a complete Mmu16 trisomy, but careful analysis of AV junction morphology has revealed important differences with the malformations seen in DS. Most DS AVSD comprises both a common junction and common valve, which is balanced in its connection to both ventricular chambers.22 This arrangement has not been detected in any Ts16 embryos. Instead, most show connection of the common AV junction exclusively to the left ventricle, or with only minimal connection to the right ventricle. Additionally, although in DS, the aorta is generally connected exclusively to the left ventricle, none of the Ts16 hearts showed this arrangement.23,24 Together, these findings suggest that unlike DS, malalignment of the chambers plays an important role in the in Ts16 cardiac malformations.
Here, we show that in contrast to the various Mmu16 trisomy models, the Tc1 mouse replicates many of the specific features of AVSD found in DS. All the Tc1 AVSDs were balanced between the two ventricles, and in all but one case, the aorta was attached exclusively to the left ventricle. Half of the cases showed a single common AV orifice guarded by a five-leaflet valve typical of DS and several showed a cleft mitral valve.25 These results suggest that genetic interactions arising from genes outside of the region of synteny between Hsa21 and Mmu16 are decisive for DS-like AVSD phenotypes in mouse models.
4.1. Septal defects in Tc1 embryos
Similarities between the Tc1 model and DS extend to ventricular septal defects, which in both is the most common heart abnormality.2 Moreover, the location of the septal lesions (membranous or muscular inlet portions with none in the outlet or trabeculated regions) matches well those reported in DS.26 We have also detected several outflow tract defects in Tc1 mice, most commonly over-riding aorta, and DORV. DS infants have an elevated risk of both defects (risk ratios of 200 and 21, respectively),6,20 although their absolute frequency (3.46 and 1.7 per 1000, respectively) appears much lower than in the Tc1 mouse. One notable disparity between Tc1 malformations and those found in DS is the absence of defects affecting fusion of the atrial septum at the AV junction (the ‘ostium primum septal defect’). Although such atrial septal defects are of obvious clinical significance, from a developmental perspective, they remain secondary to the principal malformation, which is the failure to form separate left and right AV junctions.8 In DS, the resulting bridging leaflets of the AVSD can be attached either to the atrial septum, resulting in ventricular shunting (as we have found in Tc1 hearts); more commonly, they are attached to the crest of the muscular ventricular septum, shunting confined to the atrial level (the ostium defect). We have not yet encountered the latter type of AVSD in the Tc1 model. This may reflect important species differences in the relative contributions of the many tissues forming the AV junction formation. Other factors may be the incomplete nature of the Hsa21 transchromosome, mosaicism in transchromosome distribution, or the impact of mouse strain background.
The influence of genetic background on heart abnormalities in mutant mice is well established and our detection of strain-dependent variation in the prevalence of Tc1 cardiac malformations is reminiscent of the finding that the prevalence and subtypes of CHD associated with DS vary in different human populations.27 Furthermore, two genetic loci for familial AVSD have been identified outside of Hsa21,28–30 leading to the suggestion that a combination of polymorphisms/mutations on non-Hsa21 genes, environmental influences, and trisomy of Hsa21 are all involved in the susceptibility for DS individuals to develop cardiac malformations.31 One noteworthy observation from our study was the detection of cardiac defects (all but one, VSD) in ∼10% of the wild-type littermates of Tc1 embryos at E14.5. Overall heart size and the morphology of other organs indicate that this is unlikely to be due to inaccurate embryo staging, nor does it easily account for the detection of muscular VSDs in the trabecular portion of the interventricular septum in several wild-type hearts. Our finding may simply reflect the very large number of embryos examined in the study (71 wild-types at E14.5) and the sensitivity afforded by the HREM 3D modelling technique. In humans, small, asymptomatic VSDs have been detected by echocardiography in 5% of neonates, with the overwhelming majority of these closing spontaneously within 10 months.16 It will be of interest in future to establish the extent to which genetic background contributes to this basal level of cardiac malformations in the mouse and the proportion that is subsequently repaired during development. We cannot rule out the possibility that subtle developmental delay of the Tc1 embryos accounts for a proportion of the phenotypes we detect (e.g. some VSDs) but we have no evidence for such delay either in embryo size, heart size, or development of any other organ. Nor, importantly, is it possible for such a delay to provide a credible explanation for the persistence of a balanced, common AV junction in some Tc1 embryos.
One potential difficulty with the Tc1 line as a model of DS is the mosaic and variable distribution of cells containing the Hsa21 transchromosome.7 In human mosaic DS cases, variation in phenotypes has been associated with the percentage of trisomic cells17 but appropriate tools for establishing whether a comparable link exists in the Tc1 mouse are not yet available. Species-specific in situ hybridization for SH3BGR expression indicates no obvious correlation between mosaicism and phenotype, but because of the tissue-specific pattern of its expression, using this marker can only reveal the extent of trisomy in the myocardium of the heart. Other tissues such as the endocardial cushions, the mesenchymal cap of primary atrial septum, and dorsal mesenchyme protrusion (DMP or vestibular spine) are important for septation and AV junction formation (see below) and methods to establish Hsa21 transchromosome distribution in these tissues will therefore be important in future studies.
4.2. Identification of causative genes
Since endocardial tissue is a major contributor to the AV junction, disruption of the normal genetic programme of the endocardial cushions has long been considered the most likely explanation for DS heart defects. One hypothesis is that trisomy of the two regulators of nuclear factor of activated T-cells (NFAT) signalling, Dyrk1a and Rcan1 (Dscr1), disrupts regulation of NFAT activity, leading to a number of DS phenotypes including those affecting the heart.32,33 However, RCAN1 is not present in the Hsa1 transchromosome of Tc1 mice, nor is it identified in the most recent phenotype mapping of DS using human partial Hsa21 trisomies,5 indicating that trisomy of this gene cannot be essential for the DS cardiac phenotype. The same mapping study also suggested that DYRK1A was unlikely to be necessary for DS cardiac phenotypes, a conclusion matched by our findings comparing the Tc1 and Ts1Rhr mouse models.
Another proposed candidate causative gene is DSCAM, which, unlike RCAN1 or DYRK1A, lies within the latest suggested critical region for DS–CHD5 and also within the DSCR syntenic region of Mmu16 in the Ts1Rhr mouse.12 Ts1Rhr has been used to identify a candidate gene for a DS phenotype,34 but the 33 gene-containing trisomic region does not yield any of the DS-like craniofacial defects12 or brain phenotypes exhibited by other partial trisomy 16 mouse models.35 Our study now demonstrates that this region is also insufficient for any of the DS-like cardiac phenotypes. Trisomy of DSCAM is therefore unlikely to be sufficient to yield DS cardiac abnormalities.
Recent studies suggest that rather than resulting from a defect in endocardial cushion tissue, AVSD results from abnormalities in the musculature. In particular, the dorsal mesenchymal protrusion (DMP or vestibular spine) has been identified as a critical component of the AV junction.36–40 Derived from ‘second heart field’ cells of the dorsal body wall rather than by epithelial to mesenchymal transition of cushion tissue, the DMP forms a wedge projecting into the atrial cavity and gives rise to the myocardial base of the primary atrial septum. Disruption of DMP development results in atrial and AV defects,41 as does the loss of Wnt2 signalling,20 which reduces proliferation of cardiac mesoderm progenitors of the cardiac inflow tract (including the DMP). These findings lend powerful support to the view that defects in the DMP underlie AVSD abnormalities such as those prevalent in DS.42,43
Whether the most fruitful way to identify genes responsible for DS-associated CHD is by identification of likely candidates or through the mapping of critical or susceptibility regions for particular DS phenotypes,5 it is evident that progress will depend on robust methods to correlate genotype and variably penetrant phenotypes in the mouse. Increasing numbers of partial trisomy models can now be combined with individual gene deletions to permit sophisticated manipulation of gene dosage, encompassing Hsa21-syntenic regions of Mmu10 and Mmu17, as well as Mmu16. Analysis of the embryos produced by such studies will be a formidable challenge. We have shown that systematic, high-resolution 3D modelling of embryo heart morphology using HREM provides an effective way to combine sensitive detection of DS-like cardiac abnormalities with the systematic screening that such studies will require.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the Wellcome Trust (grant number 080174/B/06/Z), the European Union (FP6 Integrated Project ‘AnEUploidy’), and the Medical Research Council (U117562103 and U117527252). Funding to pay the Open Access publication charges for this article was provided by the Medical Research Council (U117562103).
Supplementary Material
Acknowledgements
We thank Professor Robert Anderson for critical reading of the manuscript; staff of NIMR Biological Services Division for animal care; Lesley Vanes for help in maintaining the Tc1 mouse colony and Roger Reeves for providing the Ts1Rhr mice and PCR genotyping procedure.
Conflict of interest: none declared.
References
- 1.Sherman SL, Allen EG, Bean LH, Freeman SB. Epidemiology of Down syndrome. Ment Retard Dev Disabil Res Rev. 2007;13:221–227. doi: 10.1002/mrdd.20157. doi:10.1002/mrdd.20157. [DOI] [PubMed] [Google Scholar]
- 2.Freeman SB, Taft LF, Dooley KJ, Allran K, Sherman SL, Hassold TJ, et al. Population-based study of congenital heart defects in Down syndrome. Am J Med Genet. 1998;80:213–217. doi:10.1002/(SICI)1096-8628(19981116)80:3<213::AID-AJMG6>3.0.CO;2-8. [PubMed] [Google Scholar]
- 3.Korenberg JR, Chen XN, Schipper R, Sun Z, Gonsky R, Gerwehr S, et al. Down syndrome phenotypes: the consequences of chromosomal imbalance. Proc Natl Acad Sci USA. 1994;91:4997–5001. doi: 10.1073/pnas.91.11.4997. doi:10.1073/pnas.91.11.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delabar JM, Theophile D, Rahmani Z, Chettouh Z, Blouin JL, Prieur M, et al. Molecular mapping of twenty-four features of Down syndrome on chromosome 21. Eur J Hum Genet. 1993;1:114–124. doi: 10.1159/000472398. [DOI] [PubMed] [Google Scholar]
- 5.Korbel JO, Tirosh-Wagner T, Urban AE, Chen XN, Kasowski M, Dai L, et al. The genetic architecture of Down syndrome phenotypes revealed by high-resolution analysis of human segmental trisomies. Proc Natl Acad Sci USA. 2009;106:12031–12036. doi: 10.1073/pnas.0813248106. doi:10.1073/pnas.0813248106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delabar JM, Aflalo-Rattenbac R, Creau N. Developmental defects in trisomy 21 and mouse models. Sci World J. 2006;6:1945–1964. doi: 10.1100/tsw.2006.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Doherty A, Ruf S, Mulligan C, Hildreth V, Errington ML, Cooke S, et al. An aneuploid mouse strain carrying human chromosome 21 with Down syndrome phenotypes. Science. 2005;309:2033–2037. doi: 10.1126/science.1114535. doi:10.1126/science.1114535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahle WT, Shirali GS, Anderson RH. Echo-morphological correlates in patients with atrioventricular septal defect and common atrioventricular junction. Cardiol Young. 2006;16(Suppl. 3):43–51. doi: 10.1017/s1047951106000758. [DOI] [PubMed] [Google Scholar]
- 9.Williams AD, Mjaatvedt CH, Moore CS. Characterization of the cardiac phenotype in neonatal Ts65Dn mice. Dev Dyn. 2008;237:426–435. doi: 10.1002/dvdy.21416. doi:10.1002/dvdy.21416. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Yu T, Morishima M, Pao A, LaDuca J, Conroy J, et al. Duplication of the entire 22.9 Mb human chromosome 21 syntenic region on mouse chromosome 16 causes cardiovascular and gastrointestinal abnormalities. Hum Mol Genet. 2007;16:1359–1366. doi: 10.1093/hmg/ddm086. doi:10.1093/hmg/ddm086. [DOI] [PubMed] [Google Scholar]
- 11.Weninger WJ, Geyer SH, Mohun TJ, Rasskin-Gutman D, Matsui T, Ribeiro I, et al. High-resolution episcopic microscopy: a rapid technique for high detailed 3D analysis of gene activity in the context of tissue architecture and morphology. Anat Embryol (Berl) 2006;211:213–221. doi: 10.1007/s00429-005-0073-x. doi:10.1007/s00429-005-0073-x. [DOI] [PubMed] [Google Scholar]
- 12.Olson LE, Richtsmeier JT, Leszl J, Reeves RH. A chromosome 21 critical region does not cause specific Down syndrome phenotypes. Science. 2004;306:687–690. doi: 10.1126/science.1098992. doi:10.1126/science.1098992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205–216. doi: 10.1007/s10278-004-1014-6. doi:10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soto B, Becker AE, Moulaert AJ, Lie JT, Anderson RH. Classification of ventricular septal defects. Br Heart J. 1980;43:332–343. doi: 10.1136/hrt.43.3.332. doi:10.1136/hrt.43.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson RH, Spicer DE, Yoo SJ, Jacobs JP, Aiello VD. Innovation and the role of the cardiac morphologist. Cardiol Young. 2009;19(Suppl. 2):11–25. doi: 10.1017/S1047951109991570. [DOI] [PubMed] [Google Scholar]
- 16.Roguin N, Du ZD, Barak M, Nasser N, Hershkowitz S, Milgram E. High prevalence of muscular ventricular septal defect in neonates. J Am Coll Cardiol. 1995;26:1545–1548. doi: 10.1016/0735-1097(95)00358-4. doi:10.1016/0735-1097(95)00358-4. [DOI] [PubMed] [Google Scholar]
- 17.Papavassiliou P, York TP, Gursoy N, Hill G, Nicely LV, Sundaram U, et al. The phenotype of persons having mosaicism for trisomy 21/Down syndrome reflects the percentage of trisomic cells present in different tissues. Am J Med Genet A. 2009;149A:573–583. doi: 10.1002/ajmg.a.32729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egeo A, Di Lisi R, Sandri C, Mazzocco M, Lapide M, Schiaffino S, et al. Developmental expression of the SH3BGR gene, mapping to the Down syndrome heart critical region. Mech Dev. 2000;90:313–316. doi: 10.1016/s0925-4773(99)00253-1. doi:10.1016/S0925-4773(99)00253-1. [DOI] [PubMed] [Google Scholar]
- 19.Moore CS. Postnatal lethality and cardiac anomalies in the Ts65Dn Down syndrome mouse model. Mamm Genome. 2006;17:1005–1012. doi: 10.1007/s00335-006-0032-8. doi:10.1007/s00335-006-0032-8. [DOI] [PubMed] [Google Scholar]
- 20.Torfs CP, Christianson RE. Anomalies in Down syndrome individuals in a large population-based registry. Am J Med Genet. 1998;77:431–438. doi:10.1002/(SICI)1096-8628(19980605)77:5<431::AID-AJMG15>3.0.CO;2-J. [PubMed] [Google Scholar]
- 21.Miyabara S, Gropp A, Winking H. Trisomy 16 in the mouse fetus associated with generalized edema and cardiovascular and urinary tract anomalies. Teratology. 1982;25:369–380. doi: 10.1002/tera.1420250314. doi:10.1002/tera.1420250314. [DOI] [PubMed] [Google Scholar]
- 22.Al-Hay AA, MacNeill SJ, Yacoub M, Shore DF, Shinebourne EA. Complete atrioventricular septal defect, Down syndrome, and surgical outcome: risk factors. Ann Thorac Surg. 2003;75:412–421. doi: 10.1016/s0003-4975(02)04026-2. [DOI] [PubMed] [Google Scholar]
- 23.Webb S, Brown NA, Anderson RH. Cardiac morphology at late fetal stages in the mouse with trisomy 16: consequences for different formation of the atrioventricular junction when compared to humans with trisomy 21. Cardiovasc Res. 1997;34:515–524. doi: 10.1016/s0008-6363(97)00064-3. doi:10.1016/S0008-6363(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 24.Anderson RH, Webb S, Brown NA. The mouse with trisomy 16 as a model of human hearts with common atrioventricular junction. Cardiovasc Res. 1998;39:155–164. doi: 10.1016/s0008-6363(98)00037-6. doi:10.1016/S0008-6363(98)00037-6. [DOI] [PubMed] [Google Scholar]
- 25.Fraisse A, Massih TA, Bonnet D, Sidi D, Kachaner J. Cleft of the mitral valve in patients with Down's syndrome. Cardiol Young. 2002;12:27–31. doi: 10.1017/s1047951102000057. doi:10.1017/S1047951102000057. [DOI] [PubMed] [Google Scholar]
- 26.Marino B, Papa M, Guccione P, Corno A, Marasini M, Calabro R. Ventricular septal defect in Down syndrome. Anatomic types and associated malformations. Am J Dis Child. 1990;144:544–545. doi: 10.1001/archpedi.1990.02150290038021. [DOI] [PubMed] [Google Scholar]
- 27.Vida VL, Barnoya J, Larrazabal LA, Gaitan G, de Maria Garcia F, Castaneda AR. Congenital cardiac disease in children with Down's syndrome in Guatemala. Cardiol Young. 2005;15:286–290. doi: 10.1017/S1047951105000582. doi:10.1017/S1047951105000582. [DOI] [PubMed] [Google Scholar]
- 28.Phipps ME, Latif F, Prowse A, Payne SJ, Dietz-Band J, Leversha M, et al. Molecular genetic analysis of the 3p- syndrome. Hum Mol Genet. 1994;3:903–908. doi: 10.1093/hmg/3.6.903. doi:10.1093/hmg/3.6.903. [DOI] [PubMed] [Google Scholar]
- 29.Sheffield VC, Pierpont ME, Nishimura D, Beck JS, Burns TL, Berg MA, et al. Identification of a complex congenital heart defect susceptibility locus by using DNA pooling and shared segment analysis. Hum Mol Genet. 1997;6:117–121. doi: 10.1093/hmg/6.1.117. doi:10.1093/hmg/6.1.117. [DOI] [PubMed] [Google Scholar]
- 30.Green EK, Priestley MD, Waters J, Maliszewska C, Latif F, Maher ER. Detailed mapping of a congenital heart disease gene in chromosome 3p25. J Med Genet. 2000;37:581–587. doi: 10.1136/jmg.37.8.581. doi:10.1136/jmg.37.8.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maslen CL, Babcock D, Robinson SW, Bean LJ, Dooley KJ, Willour VL, et al. CRELD1 mutations contribute to the occurrence of cardiac atrioventricular septal defects in Down syndrome. Am J Med Genet A. 2006;140:2501–2505. doi: 10.1002/ajmg.a.31494. [DOI] [PubMed] [Google Scholar]
- 32.Arron JR, Winslow MM, Polleri A, Chang CP, Wu H, Gao X, et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature. 2006;441:595–600. doi: 10.1038/nature04678. doi:10.1038/nature04678. [DOI] [PubMed] [Google Scholar]
- 33.de la Luna S, Estivill X. Cooperation to amplify gene-dosage-imbalance effects. Trends Mol Med. 2006;12:451–454. doi: 10.1016/j.molmed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Sussan TE, Yang A, Li F, Ostrowski MC, Reeves RH. Trisomy represses Apc(Min)-mediated tumours in mouse models of Down's syndrome. Nature. 2008;451:73–75. doi: 10.1038/nature06446. doi:10.1038/nature06446. [DOI] [PubMed] [Google Scholar]
- 35.Olson LE, Roper RJ, Sengstaken CL, Peterson EA, Aquino V, Galdzicki Z, et al. Trisomy for the Down syndrome ‘critical region’ is necessary but not sufficient for brain phenotypes of trisomic mice. Hum Mol Genet. 2007;16:774–782. doi: 10.1093/hmg/ddm022. doi:10.1093/hmg/ddm022. [DOI] [PubMed] [Google Scholar]
- 36.Webb S, Anderson RH, Lamers WH, Brown NA. Mechanisms of deficient cardiac septation in the mouse with trisomy 16. Circ Res. 1999;84:897–905. doi: 10.1161/01.res.84.8.897. [DOI] [PubMed] [Google Scholar]
- 37.Mommersteeg MT, Soufan AT, de Lange FJ, van den Hoff MJ, Anderson RH, Christoffels VM, et al. Two distinct pools of mesenchyme contribute to the development of the atrial septum. Circ Res. 2006;99:351–353. doi: 10.1161/01.RES.0000238360.33284.a0. doi:10.1161/01.RES.0000238360.33284.a0. [DOI] [PubMed] [Google Scholar]
- 38.Snarr BS, O'Neal JL, Chintalapudi MR, Wirrig EE, Phelps AL, Kubalak SW, et al. Isl1 expression at the venous pole identifies a novel role for the second heart field in cardiac development. Circ Res. 2007;101:971–974. doi: 10.1161/CIRCRESAHA.107.162206. doi:10.1161/CIRCRESAHA.107.162206. [DOI] [PubMed] [Google Scholar]
- 39.Snarr BS, Wirrig EE, Phelps AL, Trusk TC, Wessels A. A spatiotemporal evaluation of the contribution of the dorsal mesenchymal protrusion to cardiac development. Dev Dyn. 2007;236:1287–1294. doi: 10.1002/dvdy.21074. doi:10.1002/dvdy.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goddeeris MM, Rho S, Petiet A, Davenport CL, Johnson GA, Meyers EN, et al. Intracardiac septation requires hedgehog-dependent cellular contributions from outside the heart. Development. 2008;135:1887–1895. doi: 10.1242/dev.016147. doi:10.1242/dev.016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann AD, Peterson MA, Friedland-Little JM, Anderson SA, Moskowitz IP. Sonic hedgehog is required in pulmonary endoderm for atrial septation. Development. 2009;136:1761–1770. doi: 10.1242/dev.034157. doi:10.1242/dev.034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blom NA, Ottenkamp J, Wenink AG, Gittenberger-de Groot AC. Deficiency of the vestibular spine in atrioventricular septal defects in human fetuses with Down syndrome. Am J Cardiol. 2003;91:180–184. doi: 10.1016/s0002-9149(02)03106-5. doi:10.1016/S0002-9149(02)03106-5. [DOI] [PubMed] [Google Scholar]
- 43.Snarr BS, Kern CB, Wessels A. Origin and fate of cardiac mesenchyme. Dev Dyn. 2008;237:2804–2819. doi: 10.1002/dvdy.21725. doi:10.1002/dvdy.21725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.